Abstract
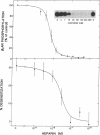
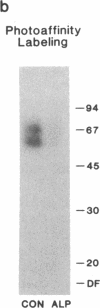
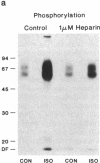
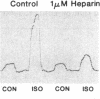
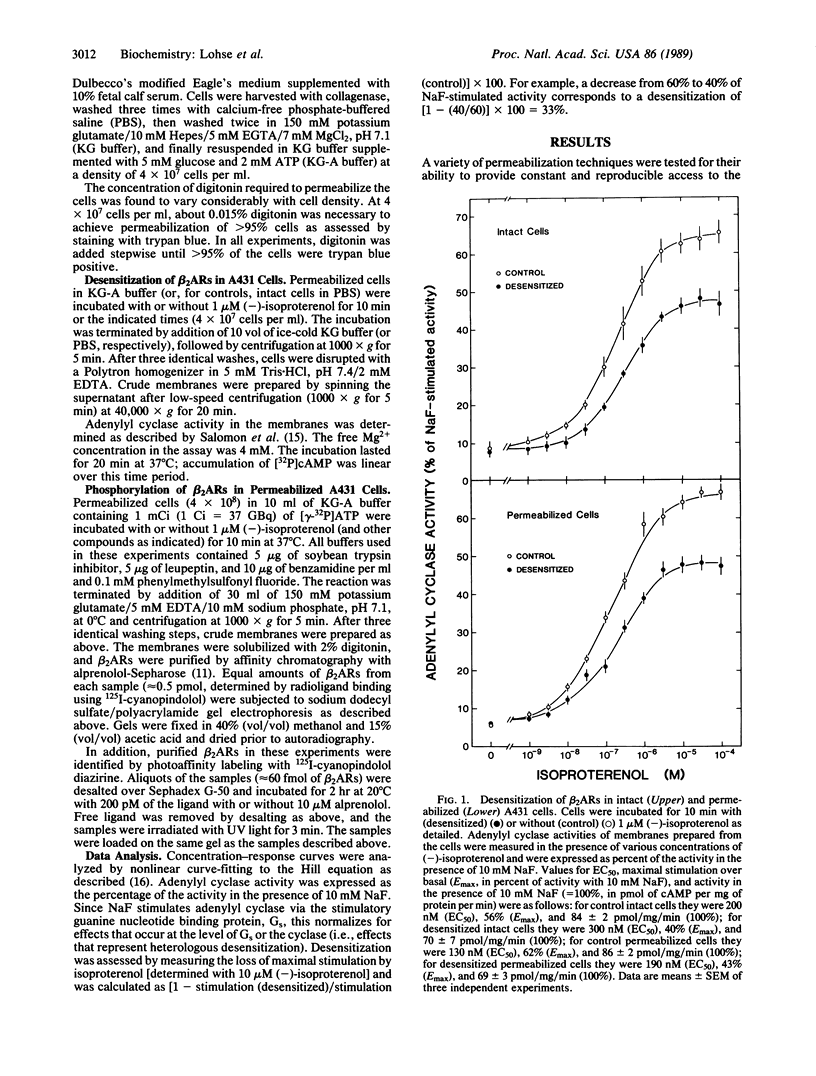
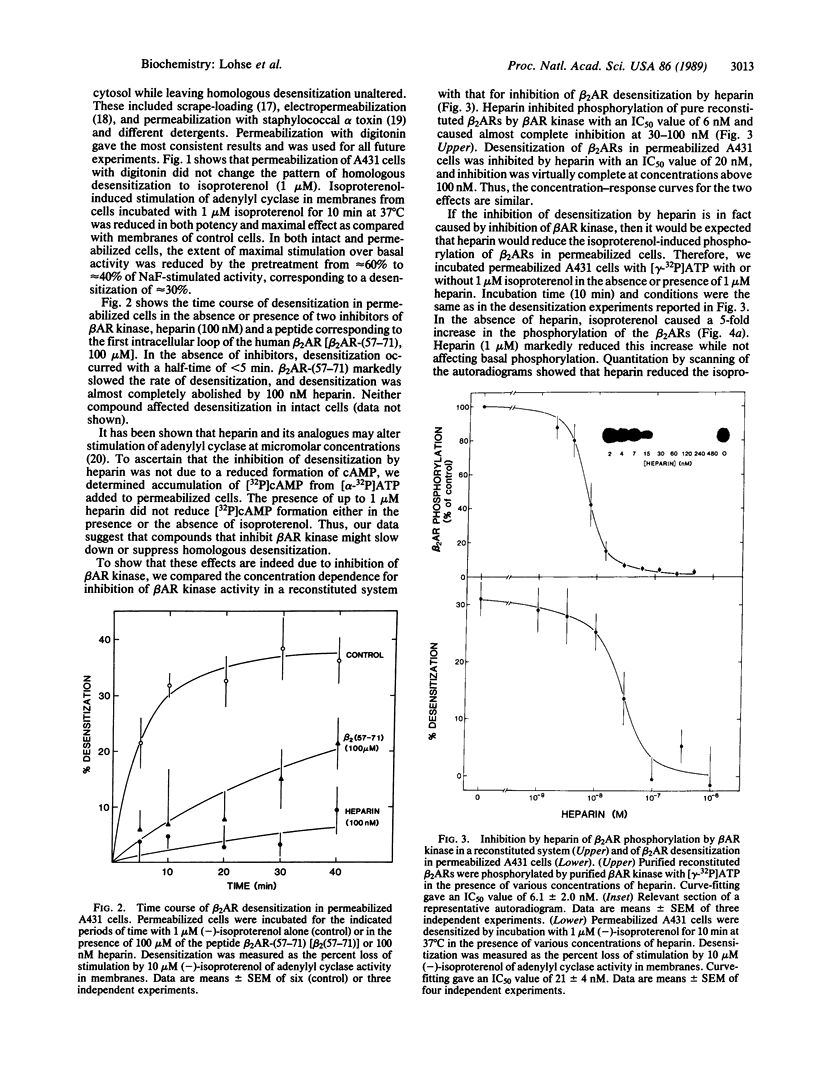
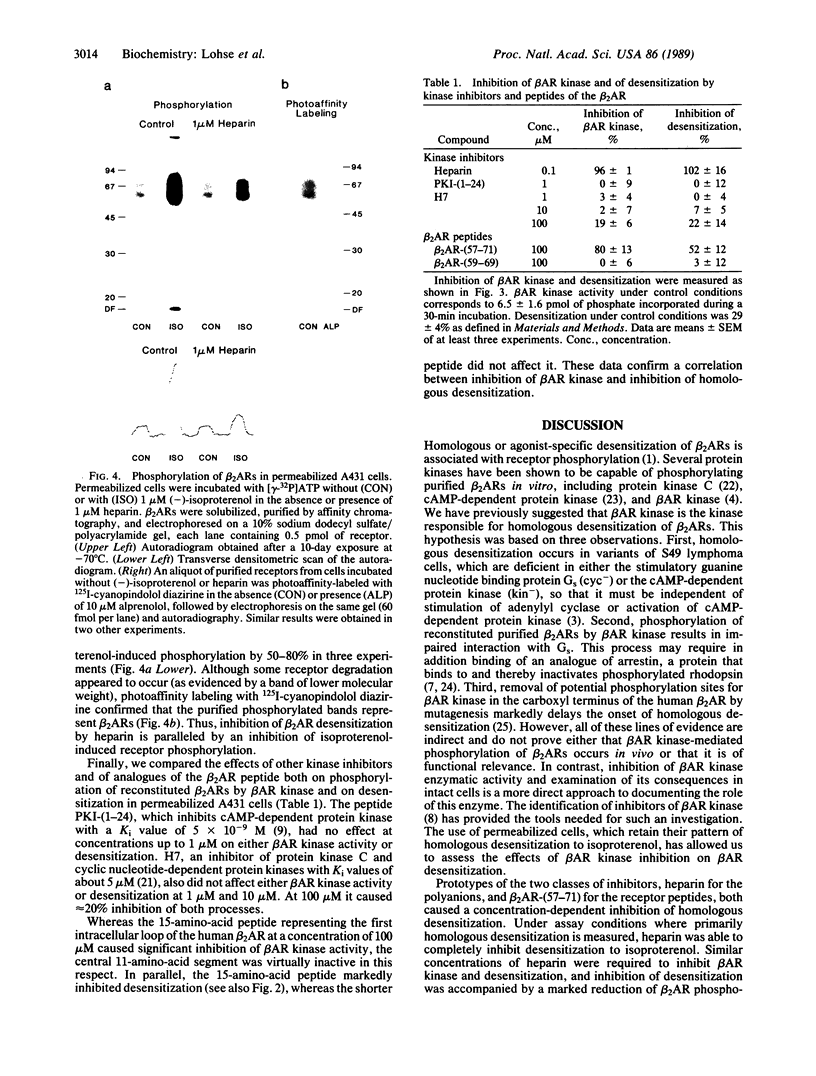
Homologous (agonist-specific) desensitization of beta-adrenergic receptors (beta ARs) is accompanied by and appears to require phosphorylation of the receptors. We have recently described a novel protein kinase, beta AR kinase, which phosphorylates beta ARs in vitro in an agonist-dependent manner. This kinase is inhibited by two classes of compounds, polyanions and synthetic peptides derived from the beta 2-adrenergic receptor (beta 2AR). In this report we describe the effects of these inhibitors on the process of homologous desensitization induced by the beta-adrenergic agonist isoproterenol. Permeabilization of human epidermoid carcinoma A431 cells with digitonin was used to permit access of the charged inhibitors to the cytosol; this procedure did not interfere with the pattern of isoproterenol-induced homologous desensitization of beta 2AR-stimulated adenylyl cyclase. Inhibitors of beta AR kinase markedly inhibited homologous desensitization of beta 2ARs in the permeabilized cells. Inhibition of desensitization by heparin, the most potent of the polyanion inhibitors of beta AR kinase, occurred over the same concentration range (5-50 nM) as inhibition of purified beta AR kinase assessed in a reconstituted system. Inhibition of desensitization by heparin was accompanied by a marked reduction of receptor phosphorylation in the permeabilized cells. Whereas inhibitors of beta AR kinase inhibited homologous desensitization, inhibitors of protein kinase C and of cyclic-nucleotide-dependent protein kinases were ineffective. These data establish that phosphorylation of beta ARs by beta AR kinase is an essential step in homologous desensitization of the receptors. They further suggest a potential therapeutic value of inhibitors of beta AR kinase in inhibiting agonist-induced desensitization.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amsterdam A., Reches A., Amir Y., Mintz Y., Salomon Y. Modulation of adenylate cyclase activity by sulfated glycosaminoglycans. II. Effects of mucopolysaccharides and dextran sulfate on the activity of adenylate cyclase derived from various tissues. Biochim Biophys Acta. 1978 Dec 1;544(2):273–283. doi: 10.1016/0304-4165(78)90096-x. [DOI] [PubMed] [Google Scholar]
- Benovic J. L., Bouvier M., Caron M. G., Lefkowitz R. J. Regulation of adenylyl cyclase-coupled beta-adrenergic receptors. Annu Rev Cell Biol. 1988;4:405–428. doi: 10.1146/annurev.cb.04.110188.002201. [DOI] [PubMed] [Google Scholar]
- Benovic J. L., Kühn H., Weyand I., Codina J., Caron M. G., Lefkowitz R. J. Functional desensitization of the isolated beta-adrenergic receptor by the beta-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein). Proc Natl Acad Sci U S A. 1987 Dec;84(24):8879–8882. doi: 10.1073/pnas.84.24.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benovic J. L., Mayor F., Jr, Staniszewski C., Lefkowitz R. J., Caron M. G. Purification and characterization of the beta-adrenergic receptor kinase. J Biol Chem. 1987 Jul 5;262(19):9026–9032. [PubMed] [Google Scholar]
- Benovic J. L., Pike L. J., Cerione R. A., Staniszewski C., Yoshimasa T., Codina J., Caron M. G., Lefkowitz R. J. Phosphorylation of the mammalian beta-adrenergic receptor by cyclic AMP-dependent protein kinase. Regulation of the rate of receptor phosphorylation and dephosphorylation by agonist occupancy and effects on coupling of the receptor to the stimulatory guanine nucleotide regulatory protein. J Biol Chem. 1985 Jun 10;260(11):7094–7101. [PubMed] [Google Scholar]
- Benovic J. L., Regan J. W., Matsui H., Mayor F., Jr, Cotecchia S., Leeb-Lundberg L. M., Caron M. G., Lefkowitz R. J. Agonist-dependent phosphorylation of the alpha 2-adrenergic receptor by the beta-adrenergic receptor kinase. J Biol Chem. 1987 Dec 25;262(36):17251–17253. [PubMed] [Google Scholar]
- Benovic J. L., Shorr R. G., Caron M. G., Lefkowitz R. J. The mammalian beta 2-adrenergic receptor: purification and characterization. Biochemistry. 1984 Sep 25;23(20):4510–4518. doi: 10.1021/bi00315a002. [DOI] [PubMed] [Google Scholar]
- Benovic J. L., Strasser R. H., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci U S A. 1986 May;83(9):2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier M., Hausdorff W. P., De Blasi A., O'Dowd B. F., Kobilka B. K., Caron M. G., Lefkowitz R. J. Removal of phosphorylation sites from the beta 2-adrenergic receptor delays onset of agonist-promoted desensitization. Nature. 1988 May 26;333(6171):370–373. doi: 10.1038/333370a0. [DOI] [PubMed] [Google Scholar]
- Bouvier M., Leeb-Lundberg L. M., Benovic J. L., Caron M. G., Lefkowitz R. J. Regulation of adrenergic receptor function by phosphorylation. II. Effects of agonist occupancy on phosphorylation of alpha 1- and beta 2-adrenergic receptors by protein kinase C and the cyclic AMP-dependent protein kinase. J Biol Chem. 1987 Mar 5;262(7):3106–3113. [PubMed] [Google Scholar]
- Cerione R. A., Codina J., Benovic J. L., Lefkowitz R. J., Birnbaumer L., Caron M. G. The mammalian beta 2-adrenergic receptor: reconstitution of functional interactions between pure receptor and pure stimulatory nucleotide binding protein of the adenylate cyclase system. Biochemistry. 1984 Sep 25;23(20):4519–4525. doi: 10.1021/bi00315a003. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Kunkel M. W., Friedman J., Goka T. J., Johnson J. A. Activation of cAMP-dependent protein kinase is required for heterologous desensitization of adenylyl cyclase in S49 wild-type lymphoma cells. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1442–1446. doi: 10.1073/pnas.85.5.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Brown M. S., Slaughter C. A., Goldstein J. L. Phosphorylation of serine 833 in cytoplasmic domain of low density lipoprotein receptor by a high molecular weight enzyme resembling casein kinase II. J Biol Chem. 1987 Jan 25;262(3):1344–1351. [PubMed] [Google Scholar]
- Knight D. E., Scrutton M. C. Gaining access to the cytosol: the technique and some applications of electropermeabilization. Biochem J. 1986 Mar 15;234(3):497–506. doi: 10.1042/bj2340497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilka B. K., Dixon R. A., Frielle T., Dohlman H. G., Bolanowski M. A., Sigal I. S., Yang-Feng T. L., Francke U., Caron M. G., Lefkowitz R. J. cDNA for the human beta 2-adrenergic receptor: a protein with multiple membrane-spanning domains and encoded by a gene whose chromosomal location is shared with that of the receptor for platelet-derived growth factor. Proc Natl Acad Sci U S A. 1987 Jan;84(1):46–50. doi: 10.1073/pnas.84.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Klotz K. N., Schwabe U. Agonist photoaffinity labeling of A1 adenosine receptors: persistent activation reveals spare receptors. Mol Pharmacol. 1986 Oct;30(4):403–409. [PubMed] [Google Scholar]
- McNeil P. L., Murphy R. F., Lanni F., Taylor D. L. A method for incorporating macromolecules into adherent cells. J Cell Biol. 1984 Apr;98(4):1556–1564. doi: 10.1083/jcb.98.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäenpä P. H. Effects of polyamines and polyanions on a cyclic nucleotide-independent and a cyclic AMP-dependent protein kinase. Biochim Biophys Acta. 1977 Jul 21;498(1):294–305. doi: 10.1016/0304-4165(77)90267-7. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Scott J. D., Glaccum M. B., Fischer E. H., Krebs E. G. Primary-structure requirements for inhibition by the heat-stable inhibitor of the cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1613–1616. doi: 10.1073/pnas.83.6.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley D. R., Strasser R. H., Caron M. G., Lefkowitz R. J. Homologous desensitization of adenylate cyclase is associated with phosphorylation of the beta-adrenergic receptor. J Biol Chem. 1985 Apr 10;260(7):3883–3886. [PubMed] [Google Scholar]
- Strasser R. H., Sibley D. R., Lefkowitz R. J. A novel catecholamine-activated adenosine cyclic 3',5'-phosphate independent pathway for beta-adrenergic receptor phosphorylation in wild-type and mutant S49 lymphoma cells: mechanism of homologous desensitization of adenylate cyclase. Biochemistry. 1986 Mar 25;25(6):1371–1377. doi: 10.1021/bi00354a027. [DOI] [PubMed] [Google Scholar]
- Toews M. L., Liang M., Perkins J. P. Agonists and phorbol esters desensitize beta-adrenergic receptors by different mechanisms. Mol Pharmacol. 1987 Dec;32(6):737–742. [PubMed] [Google Scholar]
- Wilden U., Hall S. W., Kühn H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]