Abstract
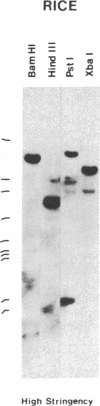
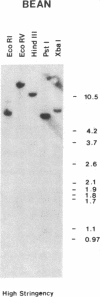
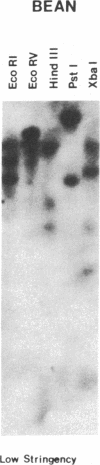
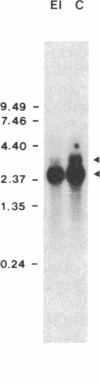
Oligonucleotides, corresponding to conserved regions of animal protein-serine/threonine kinases, were used to isolate cDNAs encoding plant homologs in the dicot bean (Phaseolus vulgaris L.) and the monocot rice (Oryzae sativa L.). The C-terminal regions of the deduced polypeptides encoded by the bean (PVPK-1) and rice (G11A) cDNAs, prepared from mRNAs of suspension cultures and leaves, respectively, contain features characteristic of the catalytic domains of eukaryotic protein-serine/threonine kinases, indicating that these cDNAs encode plant protein kinases. The putative catalytic domains are most closely related to cyclic nucleotide-dependent protein kinases and the protein kinase C family, suggesting the plant homologs may likewise transduce extracellular signals. However, outside these domains, PVPK-1 and G11A exhibit no homology either to each other or to regulatory domains of other protein kinases, indicating the plant homologs are modulated by other signals. PVPK-1 corresponds to a 2.4-kb transcript in suspension cultured bean cells. Southern blots of genomic DNA indicate that PVPK-1 and G11A correspond to single copy genes that form part of a family of related plant sequences.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Corbin D. R., Sauer N., Lamb C. J. Differential regulation of a hydroxyproline-rich glycoprotein gene family in wounded and infected plants. Mol Cell Biol. 1987 Dec;7(12):4337–4344. doi: 10.1128/mcb.7.12.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L., Parker P. J., Rhee L., Yang-Feng T. L., Chen E., Waterfield M. D., Francke U., Ullrich A. Multiple, distinct forms of bovine and human protein kinase C suggest diversity in cellular signaling pathways. Science. 1986 Aug 22;233(4766):859–866. doi: 10.1126/science.3755548. [DOI] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hanks S. K. Homology probing: identification of cDNA clones encoding members of the protein-serine kinase family. Proc Natl Acad Sci U S A. 1987 Jan;84(2):388–392. doi: 10.1073/pnas.84.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Hunter T. A thousand and one protein kinases. Cell. 1987 Sep 11;50(6):823–829. doi: 10.1016/0092-8674(87)90509-5. [DOI] [PubMed] [Google Scholar]
- Joshi C. P. Putative polyadenylation signals in nuclear genes of higher plants: a compilation and analysis. Nucleic Acids Res. 1987 Dec 10;15(23):9627–9640. doi: 10.1093/nar/15.23.9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopf J. L., Lee M. H., Sultzman L. A., Kriz R. W., Loomis C. R., Hewick R. M., Bell R. M. Cloning and expression of multiple protein kinase C cDNAs. Cell. 1986 Aug 15;46(4):491–502. doi: 10.1016/0092-8674(86)90874-3. [DOI] [PubMed] [Google Scholar]
- Krebs E. G. The phosphorylation of proteins: a major mechanism for biological regulation. Fourteenth Sir Frederick Gowland Hopkins memorial lecture. Biochem Soc Trans. 1985 Oct;13(5):813–820. doi: 10.1042/bst0130813. [DOI] [PubMed] [Google Scholar]
- Levin D. E., Hammond C. I., Ralston R. O., Bishop J. M. Two yeast genes that encode unusual protein kinases. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6035–6039. doi: 10.1073/pnas.84.17.6035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin L. R., Kuret J., Johnson K. E., Powers S., Cameron S., Michaeli T., Wigler M., Zoller M. J. A mutation in the catalytic subunit of cAMP-dependent protein kinase that disrupts regulation. Science. 1988 Apr 1;240(4848):68–70. doi: 10.1126/science.2832943. [DOI] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M., Sclafani R. A., Fangman W. L., Rosamond J. Molecular characterization of cell cycle gene CDC7 from Saccharomyces cerevisiae. Mol Cell Biol. 1986 May;6(5):1590–1598. doi: 10.1128/mcb.6.5.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Gierl A., Klösgen R. B., Wienand U., Peterson P. A., Saedler H. The Spm (En) transposable element controls the excision of a 2-kb DNA insert at the wx allele of Zea mays. EMBO J. 1984 May;3(5):1021–1028. doi: 10.1002/j.1460-2075.1984.tb01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. D., Glaccum M. B., Zoller M. J., Uhler M. D., Helfman D. M., McKnight G. S., Krebs E. G. The molecular cloning of a type II regulatory subunit of the cAMP-dependent protein kinase from rat skeletal muscle and mouse brain. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5192–5196. doi: 10.1073/pnas.84.15.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji S., Ericsson L. H., Walsh K. A., Fischer E. H., Titani K. Amino acid sequence of the catalytic subunit of bovine type II adenosine cyclic 3',5'-phosphate dependent protein kinase. Biochemistry. 1983 Jul 19;22(15):3702–3709. doi: 10.1021/bi00284a025. [DOI] [PubMed] [Google Scholar]
- Takio K., Blumenthal D. K., Edelman A. M., Walsh K. A., Krebs E. G., Titani K. Amino acid sequence of an active fragment of rabbit skeletal muscle myosin light chain kinase. Biochemistry. 1985 Oct 22;24(22):6028–6037. doi: 10.1021/bi00343a002. [DOI] [PubMed] [Google Scholar]
- Takio K., Wade R. D., Smith S. B., Krebs E. G., Walsh K. A., Titani K. Guanosine cyclic 3',5'-phosphate dependent protein kinase, a chimeric protein homologous with two separate protein families. Biochemistry. 1984 Aug 28;23(18):4207–4218. doi: 10.1021/bi00313a030. [DOI] [PubMed] [Google Scholar]
- Taylor S. S. Protein kinases: a diverse family of related proteins. Bioessays. 1987 Jul;7(1):24–29. doi: 10.1002/bies.950070106. [DOI] [PubMed] [Google Scholar]
- Uhler M. D., Chrivia J. C., McKnight G. S. Evidence for a second isoform of the catalytic subunit of cAMP-dependent protein kinase. J Biol Chem. 1986 Nov 25;261(33):15360–15363. [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]