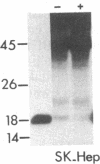
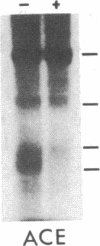
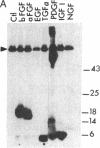
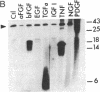
Abstract
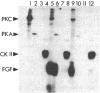
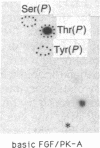
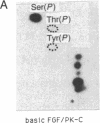
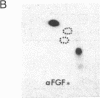
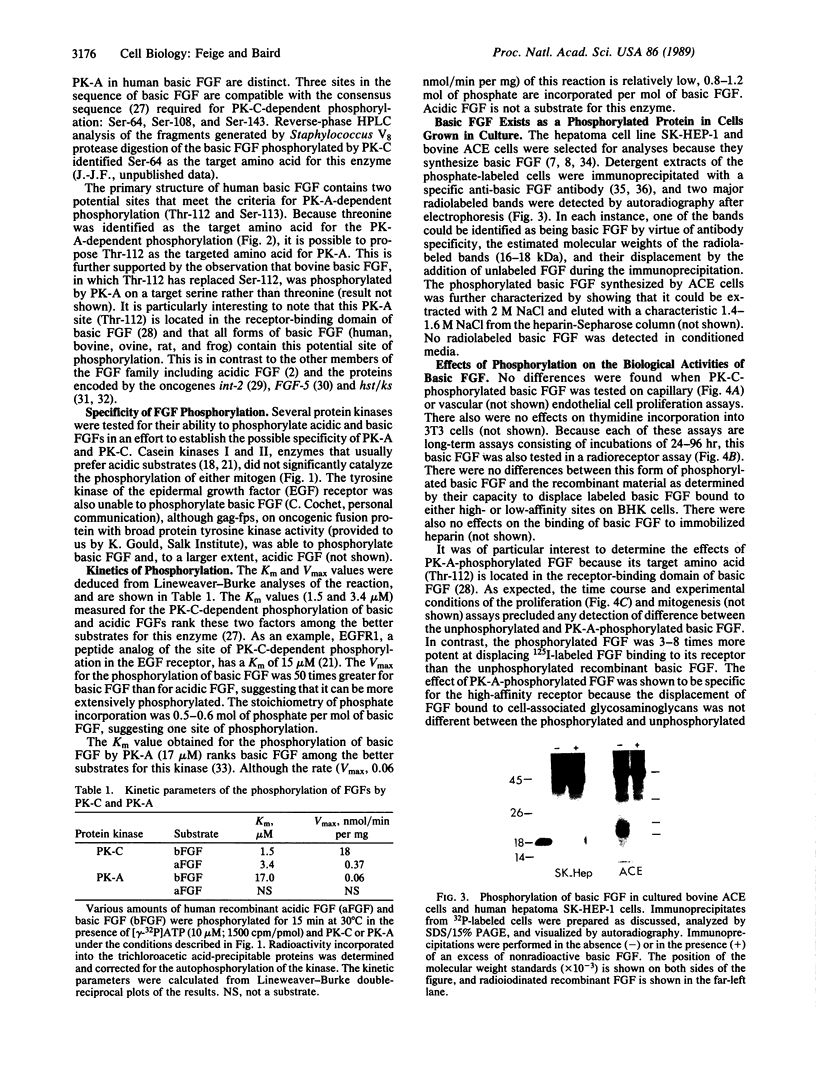
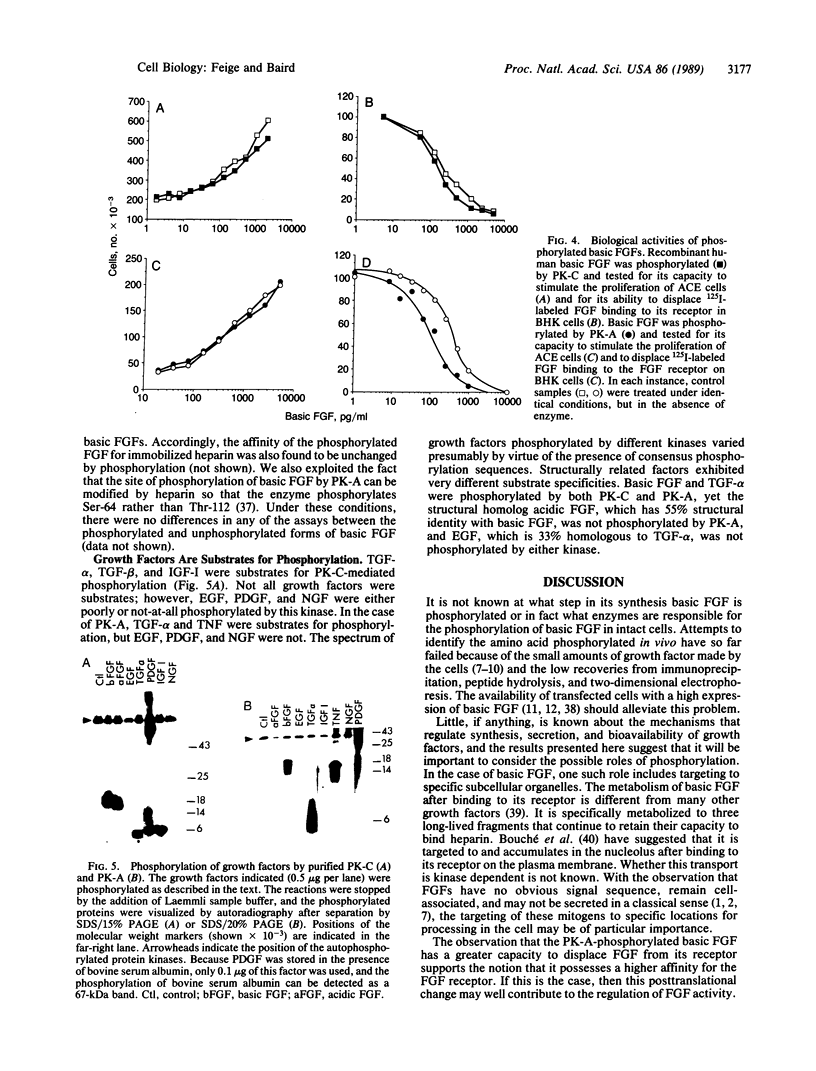
A phosphorylated basic fibroblast growth factor (FGF) can be detected in extracts of bovine capillary endothelial cells and human hepatoma cells. Accordingly, human basic FGF contains consensus sequences that account for its phosphorylation on Thr-112 by the catalytic subunit of the cAMP-dependent protein kinase A (PK-A) and on Ser-64 by the calcium- and phospholipid-dependent protein kinase C (PK-C). A kinetic analysis of both of these reactions revealed that basic FGF is among the better substrates for these enzymes. Although the kinase responsible for the phosphorylation in vivo has not yet been identified, we examined the effects of phosphorylation on the biological activity, heparin-binding capacity, and receptor-binding capacity of phosphorylated basic FGF. No effects of phosphorylation were observed when the mitogen was phosphorylated by PK-C. In contrast, when basic FGF was phosphorylated in the receptor-binding domain with PK-A, the growth factor was 3-8 times better at displacing radiolabeled basic FGF in the radioreceptor assay. No effects were seen on the binding of this FGF to immobilized heparin or cell-associated glycosaminoglycans, suggesting that this phosphorylation modifies the affinity of basic FGF for its receptor. Biological assays for basic FGF failed to identify differences between the phosphorylated and unphosphorylated forms of recombinant basic FGFs presumably because of the presence of ectophosphatases and the experimental conditions of proliferation and mitogenic assays (37 degrees C, 24-96 hr). Because the relative affinity of basic FGF for its receptor and cell-associated glycosaminoglycans may regulate its activity, the identification of a modified form of basic FGF may be of particular importance in understanding the mechanisms that regulate its biological activity, bioavailability, and processing to and from the extracellular matrix.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Baird A., Ling N. Fibroblast growth factors are present in the extracellular matrix produced by endothelial cells in vitro: implications for a role of heparinase-like enzymes in the neovascular response. Biochem Biophys Res Commun. 1987 Jan 30;142(2):428–435. doi: 10.1016/0006-291x(87)90292-0. [DOI] [PubMed] [Google Scholar]
- Baird A., Schubert D., Ling N., Guillemin R. Receptor- and heparin-binding domains of basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2324–2328. doi: 10.1073/pnas.85.7.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr P. J., Cousens L. S., Lee-Ng C. T., Medina-Selby A., Masiarz F. R., Hallewell R. A., Chamberlain S. H., Bradley J. D., Lee D., Steimer K. S. Expression and processing of biologically active fibroblast growth factors in the yeast Saccharomyces cerevisiae. J Biol Chem. 1988 Nov 5;263(31):16471–16478. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bouche G., Gas N., Prats H., Baldin V., Tauber J. P., Teissié J., Amalric F. Basic fibroblast growth factor enters the nucleolus and stimulates the transcription of ribosomal genes in ABAE cells undergoing G0----G1 transition. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6770–6774. doi: 10.1073/pnas.84.19.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson G. M., Bechtel P. J., Graves D. J. Chemical and regulatory properties of phosphorylase kinase and cyclic AMP-dependent protein kinase. Adv Enzymol Relat Areas Mol Biol. 1979;50:41–115. doi: 10.1002/9780470122952.ch2. [DOI] [PubMed] [Google Scholar]
- Cochet C., Feige J. J., Chambaz E. M. Catalytic and molecular properties of a highly purified G type casein kinase from bovine lung tissue. Biochim Biophys Acta. 1983 Feb 28;743(1):1–12. doi: 10.1016/0167-4838(83)90411-9. [DOI] [PubMed] [Google Scholar]
- Cochet C., Job D., Pirollet F., Chambaz E. M. Adenosine 3',5'-monophosphate-independent protein kinase activities in the bovine adrenal cortex cytosol. Endocrinology. 1980 Mar;106(3):750–757. doi: 10.1210/endo-106-3-750. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Delli Bovi P., Curatola A. M., Kern F. G., Greco A., Ittmann M., Basilico C. An oncogene isolated by transfection of Kaposi's sarcoma DNA encodes a growth factor that is a member of the FGF family. Cell. 1987 Aug 28;50(5):729–737. doi: 10.1016/0092-8674(87)90331-x. [DOI] [PubMed] [Google Scholar]
- Delli-Bovi P., Curatola A. M., Newman K. M., Sato Y., Moscatelli D., Hewick R. M., Rifkin D. B., Basilico C. Processing, secretion, and biological properties of a novel growth factor of the fibroblast growth factor family with oncogenic potential. Mol Cell Biol. 1988 Jul;8(7):2933–2941. doi: 10.1128/mcb.8.7.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Peters G. Potential oncogene product related to growth factors. 1987 Apr 30-May 6Nature. 326(6116):833–833. doi: 10.1038/326833a0. [DOI] [PubMed] [Google Scholar]
- Esch F., Baird A., Ling N., Ueno N., Hill F., Denoroy L., Klepper R., Gospodarowicz D., Böhlen P., Guillemin R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6507–6511. doi: 10.1073/pnas.82.19.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige J. J., Chambaz E. M. Membrane receptors with protein-tyrosine kinase activity. Biochimie. 1987 Apr;69(4):379–385. doi: 10.1016/0300-9084(87)90029-0. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M., Sasse J., Wadzinski M., Ingber D., Vlodavsky I. A heparin-binding angiogenic protein--basic fibroblast growth factor--is stored within basement membrane. Am J Pathol. 1988 Feb;130(2):393–400. [PMC free article] [PubMed] [Google Scholar]
- Friesel R., Burgess W. H., Mehlman T., Maciag T. The characterization of the receptor for endothelial cell growth factor by covalent ligand attachment. J Biol Chem. 1986 Jun 15;261(17):7581–7584. [PubMed] [Google Scholar]
- Gimenez-Gallego G., Rodkey J., Bennett C., Rios-Candelore M., DiSalvo J., Thomas K. Brain-derived acidic fibroblast growth factor: complete amino acid sequence and homologies. Science. 1985 Dec 20;230(4732):1385–1388. doi: 10.1126/science.4071057. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Ferrara N., Schweigerer L., Neufeld G. Structural characterization and biological functions of fibroblast growth factor. Endocr Rev. 1987 May;8(2):95–114. doi: 10.1210/edrv-8-2-95. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Massoglia S., Cheng J., Fujii D. K. Effect of fibroblast growth factor and lipoproteins on the proliferation of endothelial cells derived from bovine adrenal cortex, brain cortex, and corpus luteum capillaries. J Cell Physiol. 1986 Apr;127(1):121–136. doi: 10.1002/jcp.1041270116. [DOI] [PubMed] [Google Scholar]
- Halaban R., Ghosh S., Baird A. bFGF is the putative natural growth factor for human melanocytes. In Vitro Cell Dev Biol. 1987 Jan;23(1):47–52. doi: 10.1007/BF02623492. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Jaye M., Howk R., Burgess W., Ricca G. A., Chiu I. M., Ravera M. W., O'Brien S. J., Modi W. S., Maciag T., Drohan W. N. Human endothelial cell growth factor: cloning, nucleotide sequence, and chromosome localization. Science. 1986 Aug 1;233(4763):541–545. doi: 10.1126/science.3523756. [DOI] [PubMed] [Google Scholar]
- Jaye M., Lyall R. M., Mudd R., Schlessinger J., Sarver N. Expression of acidic fibroblast growth factor cDNA confers growth advantage and tumorigenesis to Swiss 3T3 cells. EMBO J. 1988 Apr;7(4):963–969. doi: 10.1002/j.1460-2075.1988.tb02902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moscatelli D. High and low affinity binding sites for basic fibroblast growth factor on cultured cells: absence of a role for low affinity binding in the stimulation of plasminogen activator production by bovine capillary endothelial cells. J Cell Physiol. 1987 Apr;131(1):123–130. doi: 10.1002/jcp.1041310118. [DOI] [PubMed] [Google Scholar]
- Neufeld G., Gospodarowicz D. The identification and partial characterization of the fibroblast growth factor receptor of baby hamster kidney cells. J Biol Chem. 1985 Nov 5;260(25):13860–13868. [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Plouët J., Mascarelli F., Loret M. D., Faure J. P., Courtois Y. Regulation of eye derived growth factor binding to membranes by light, ATP or GTP in photoreceptor outer segments. EMBO J. 1988 Feb;7(2):373–376. doi: 10.1002/j.1460-2075.1988.tb02823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogelj S., Weinberg R. A., Fanning P., Klagsbrun M. Basic fibroblast growth factor fused to a signal peptide transforms cells. Nature. 1988 Jan 14;331(6152):173–175. doi: 10.1038/331173a0. [DOI] [PubMed] [Google Scholar]
- Schubert D., Ling N., Baird A. Multiple influences of a heparin-binding growth factor on neuronal development. J Cell Biol. 1987 Mar;104(3):635–643. doi: 10.1083/jcb.104.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweigerer L., Neufeld G., Friedman J., Abraham J. A., Fiddes J. C., Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987 Jan 15;325(6101):257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Beemon K., Hunter T. Comparison of the expression of the src gene of Rous sarcoma virus in vitro and in vivo. J Virol. 1978 Dec;28(3):957–971. doi: 10.1128/jvi.28.3.957-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. A. Transforming potential of fibroblast growth factor genes. Trends Biochem Sci. 1988 Sep;13(9):327–328. doi: 10.1016/0968-0004(88)90098-9. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Fridman R., Sullivan R., Sasse J., Klagsbrun M. Aortic endothelial cells synthesize basic fibroblast growth factor which remains cell associated and platelet-derived growth factor-like protein which is secreted. J Cell Physiol. 1987 Jun;131(3):402–408. doi: 10.1002/jcp.1041310312. [DOI] [PubMed] [Google Scholar]
- Walton G. M., Bertics P. J., Hudson L. G., Vedvick T. S., Gill G. N. A three-step purification procedure for protein kinase C: characterization of the purified enzyme. Anal Biochem. 1987 Mar;161(2):425–437. doi: 10.1016/0003-2697(87)90471-4. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R., Gould K. L., Hunter T. Substrate specificity of protein kinase C. Use of synthetic peptides corresponding to physiological sites as probes for substrate recognition requirements. Eur J Biochem. 1986 Nov 17;161(1):177–184. doi: 10.1111/j.1432-1033.1986.tb10139.x. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Miyagawa K., Odagiri H., Sakamoto H., Little P. F., Terada M., Sugimura T. Genomic sequence of hst, a transforming gene encoding a protein homologous to fibroblast growth factors and the int-2-encoded protein. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7305–7309. doi: 10.1073/pnas.84.20.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Bates B., Hu X. G., Goldfarb M. The human FGF-5 oncogene encodes a novel protein related to fibroblast growth factors. Mol Cell Biol. 1988 Aug;8(8):3487–3495. doi: 10.1128/mcb.8.8.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]