Abstract
Olfaction and some forms of taste (including bitter) are mediated by G protein-coupled signal transduction pathways. Olfactory and gustatory ligands bind to chemosensory G protein-coupled receptors (GPCRs) in specialized sensory cells to activate intracellular signal transduction cascades. G protein-coupled receptor kinases (GRKs) are negative regulators of signaling that specifically phosphorylate activated GPCRs to terminate signaling. Although loss of GRK function usually results in enhanced cellular signaling, Caenorhabditis elegans lacking GRK-2 function are not hypersensitive to chemosensory stimuli. Instead, grk-2 mutant animals do not chemotax toward attractive olfactory stimuli or avoid aversive tastes and smells. We show here that loss-of-function mutations in the transient receptor potential vanilloid (TRPV) channels OSM-9 and OCR-2 selectively restore grk-2 behavioral avoidance of bitter tastants, revealing modality-specific mechanisms for TRPV channel function in the regulation of C. elegans chemosensation. Additionally, a single amino acid point mutation in OCR-2 that disrupts TRPV channel-mediated gene expression, but does not decrease channel function in chemosensory primary signal transduction, also restores grk-2 bitter taste avoidance. Thus, loss of GRK-2 function may lead to changes in gene expression, via OSM-9/OCR-2, to selectively alter the levels of signaling components that transduce or regulate bitter taste responses. Our results suggest a novel mechanism and multiple modality-specific pathways that sensory cells employ in response to aberrant signal transduction.
TO survive, organisms must be able to recognize and respond appropriately to chemical cues in their environment that indicate the presence or absence of food, reproductive partners, or predators. Chemosensation is the fundamental process by which chemical signals, in the form of gustatory (taste) and olfactory (smell) stimuli, are detected. The sense of taste is particularly vital to ensure survival as it confers the ability to distinguish favorable food sources from hazardous compounds before they are ingested (Herness and Gilbertson 1999; Perez et al. 2003). Bitter or sour tastes usually indicate the presence of toxic compounds that would be rejected, whereas salty, sweet, and umami (amino acid) reflect the presence of valuable nutrients (Herness and Gilbertson 1999).
Olfaction and gustatory responses to bitter, sweet, and umami stimuli are generally mediated by G protein-coupled signal transduction pathways that are conserved across species (Dryer and Berghard 1999; Chandrashekar et al. 2006; Palmer 2007). Signaling is initiated when a ligand (odorant or tastant) binds to a seven-transmembrane G protein-coupled receptor (GPCR), inducing a conformational change in the receptor that activates the associated heterotrimeric G proteins. The Gα subunit exchanges GDP for GTP and, now activated, dissociates from the Gβ and Gγ (Gβγ) subunits. Both the free Gα-GTP and Gβγ subunits can stimulate intracellular signaling cascades by interacting with downstream effectors such as adenylate cyclases, phospholipases, and ion channels (McCudden et al. 2005).
Following the activation of G protein-coupled signaling, a negative feedback mechanism known as desensitization is initiated (Hausdorff et al. 1990; Metaye et al. 2005). G protein-coupled receptor kinases (GRKs) recognize and phosphorylate activated GPCRs (Freedman and Lefkowitz 1996; Pitcher et al. 1998; Penn et al. 2000; Premont and Gainetdinov 2007). The phosphorylated GPCRs can then be bound by cytosolic arrestin proteins (Freedman and Lefkowitz 1996; Metaye et al. 2005; Premont and Gainetdinov 2007). GRK phosphorylation and arrestin binding result in the cessation of G protein signaling, even in the continued presence of agonist (Freedman and Lefkowitz 1996; Penn et al. 2000; Premont and Gainetdinov 2007). This desensitization process is necessary to avoid the potentially harmful effects that can result from excessive stimulation through activated GPCRs (Metaye et al. 2005). For example, loss-of-function mutations in human GRK1 (rhodopsin kinase) lead to Oguchi disease (Cideciyan et al. 1998; Yamada et al. 1999). GRK1 is required for rod recovery after photoactivation, and in patients with this disease, prolonged rod photoreceptor responses and slow recovery following light exposure result in night blindness. In a mouse model for Oguchi disease, loss of GRK1 function leads to retinal degeneration (Chen et al. 1999).
In most instances, the absence of GRK-mediated desensitization causes prolonged, exaggerated responses to GPCR agonists (Jaber et al. 1996; Rockman et al. 1998; Gainetdinov et al. 1999, 2003; Premont and Gainetdinov 2007). However, there are unique situations in which loss of a particular GRK can lead to decreased signaling and responsiveness in a cell-specific manner. For example, while GRK6−/− mice are hypersensitive to psychostimulants such as cocaine (Gainetdinov et al. 2003), T cells from GRK6-deficient mice are significantly impaired in their chemotactic response to CXCL12, a stimulatory chemokine that wild-type T cells migrate toward (Fong et al. 2002). Additionally, loss of GRK3, which is highly expressed in mouse olfactory epithelium (Schleicher et al. 1993), significantly reduces odorant-induced generation of the second messenger cAMP in cilia preparations (Peppel et al. 1997), in addition to the expected lack of agonist-induced desensitization following odorant exposure (Schleicher et al. 1993; Boekhoff et al. 1994; Peppel et al. 1997).
As soil dwelling nematodes, Caenorhabditis elegans depend heavily upon their ability to detect volatile (olfactory) and soluble (gustatory) chemicals to find food, avoid noxious environments, develop appropriately, and mate (Bargmann 2006a). Despite their small nervous system, consisting of just 302 neurons, C. elegans have a remarkable chemosensory repertoire. Using a limited number of head and tail sensory neurons, C. elegans are able to detect hundreds of chemicals as well as discriminate among multiple chemosensory stimuli when they are presented simultaneously (Bargmann and Horvitz 1991; Bargmann et al. 1993; Troemel 1999; Bargmann 2006a). The 11 pairs of chemosensory neurons located in the head each respond to a defined subset of stimuli (Bargmann 2006a). The AWA and AWC olfactory neurons detect chemicals that C. elegans are attracted to and chemotax toward, while the ASH, ADL, AWB, and ASK sensory neurons detect aversive odorants and tastants that C. elegans avoid by initiating backward locomotion upon stimulus detection (Bargmann 2006a). Furthermore, the ASH nociceptive neurons are polymodal and detect a range of aversive stimulants, including the odorant octanol, the bitter tastant quinine, SDS, high osmolarity, heavy metals such as copper, and light touch to the nose (Bargmann et al. 1990; Kaplan and Horvitz 1993; Hart et al. 1999; Sambongi et al. 1999; Troemel 1999; Hilliard et al. 2002, 2004, 2005).
The C. elegans genome encodes >500 predicted chemosensory GPCRs (Bargmann 2006a) and 21 Gα, 2 Gβ, and 2 Gγ subunits (Jansen et al. 1999; Cuppen et al. 2003). The Gα proteins ODR-3 and GPA-3 have a stimulatory role in chemical detection in AWA, AWC, and ASH (Roayaie et al. 1998; Troemel 1999; Hilliard et al. 2004; Lans et al. 2004; Bargmann 2006a). Downstream of G proteins, two distinct channels appear to be involved in chemosensory signal transduction. A cyclic nucleotide-gated channel encoded by the tax-2 and tax-4 genes is a sensory transduction channel in the AWC neurons (Coburn and Bargmann 1996; Komatsu et al. 1996). In contrast, the OSM-9 and OCR-2 transient receptor potential vanilloid (TRPV) channel subunits participate in primary signal transduction in the AWA and ASH neurons (Colbert et al. 1997; Hilliard et al. 2002, 2005; Tobin et al. 2002). Accordingly, loss of OSM-9 or OCR-2 results in mild to severe defects in AWA-mediated olfactory responses and ASH-mediated avoidance behaviors (Colbert et al. 1997; Tobin et al. 2002; Hilliard et al. 2004, 2005). In addition, FRET-based imaging revealed that Ca2+ transients, which are likely downstream of GPCR signaling in all sensory neurons, are reduced or eliminated in ASH in response to a variety of stimuli (including quinine) in osm-9 mutants (Hilliard et al. 2005). However, osm-9 and ocr-2 mutants retain a substantial behavioral response to the bitter tastant quinine (Hilliard et al. 2004; this work), which is detected primarily by ASH. This indicates that while OSM-9/OCR-2 contribute to bitter taste transduction, other channels are also likely involved.
Although GRKs have classically been described as negative regulators of GPCR signal transduction, C. elegans lacking GRK-2 function are not hypersensitive to chemical stimuli due to increased sensory signaling (Fukuto et al. 2004). Instead, grk-2 mutant animals neither chemotax toward attractive odorants detected by AWA and AWC nor avoid aversive odorants and tastants detected primarily by ASH (Fukuto et al. 2004). Consistent with defective chemosensory behavioral responses, loss of GRK-2 function leads to a decrease in stimulus-evoked Ca2+ signaling in the ASH sensory neurons (Fukuto et al. 2004). Taken together, loss of GRK-2 function leads to decreased signaling in C. elegans sensory neurons, similar to loss of mammalian GRK3 in olfactory epithelia (Peppel et al. 1997; Fukuto et al. 2004). However, the mechanism by which loss of mammalian GRK3 leads to decreased stimulus-induced cAMP levels is not known.
It was proposed that loss of C. elegans GRK-2 may initially result in excessive chemosensory signaling, but that this activates compensatory mechanisms to downregulate G protein-coupled signal transduction and terminate signaling (Fukuto et al. 2004) (supporting information, Figure S1). In this model, the inhibitory compensatory mechanism, which serves to protect against the potentially harmful effects of excessive neuronal stimulation, renders grk-2 mutants unable to respond to a range of chemosensory stimuli. Consistent with this model, loss of the regulator of G protein signaling (RGS) GTPase-activating protein EAT-16, a negative regulator of Gα activity, restores grk-2 chemotaxis to diacetyl (AWA) (Fukuto et al. 2004). However, loss of EAT-16 has no effect on ASH-mediated behaviors. grk-2;eat-16 double mutants remain defective for octanol (odorant) and quinine (tastant) avoidance. Furthermore, we found that loss of the other neuronally expressed RGS proteins, EGL-10, RGS-1, RGS-2, RGS-3, RGS-6, or RGS-10/11 (Koelle and Horvitz 1996; Dong et al. 2000; Kunitomo et al. 2005; Ferkey et al. 2007; M. Koelle, personal communication), does not restore ASH-mediated behaviors (data not shown). These results indicate that there are diverse, cell-specific responses to aberrant G protein-coupled signaling.
We sought to identify mechanisms responsible for regulating chemosensory GPCR signaling in the absence of GRK function in the ASH sensory neurons. Using C. elegans grk-2 mutant animals, we performed a forward genetic screen to isolate animals with the restored ability to respond to quinine, an aversive bitter tastant detected by ASH (Hilliard et al. 2004). We isolated eight mutants in which the quinine response of grk-2 animals was restored; three of the mutations were found to be in the TRPV-related channels OSM-9 and OCR-2. Surprisingly, we find that complete loss of OSM-9/OCR-2 channel function restores response of grk-2 mutants in both a cell-specific and modality-specific manner, as grk-2;TRPV double mutants have a wild-type response to bitter tastants, but remain defective for other chemosensory stimuli detected by ASH and AWA.
Downstream of their roles in primary signal transduction, OSM-9 and OCR-2 affect activity-dependent gene expression pathways to regulate the long-term transcriptional levels of sensory genes. Loss of these TRPV channels reduces expression of ODR-10, a GPCR expressed in the AWA olfactory neurons that detects diacetyl (Sengupta et al. 1996; Tobin et al. 2002) and selectively decreases expression of the serotonin biosynthetic enzyme TPH-1 in the ADF sensory neurons (Zhang et al. 2004). Furthermore, OCR-2 appears to use distinct structural motifs for primary chemosensory signal transduction and modulation of pathways that control transcriptional activity (Sokolchik et al. 2005). Specifically, the OCR-2(G36E) N-terminal point mutation, encoded by ocr-2(yz5), results in decreased TPH-1 expression in ADF, but does not diminish AWA olfaction (Sokolchik et al. 2005). Unexpectedly, we also find that the OCR-2(G36E) point mutation is sufficient to restore the response of grk-2 mutants to bitter tastants. This suggests that a unique output of TRPV function, transmitted via the OCR-2(G36) N-terminal structural motif, leads to the bitter taste defects of grk-2 animals.
MATERIALS AND METHODS
Strains:
Strains were maintained under standard conditions on NGM agar plates seeded with OP50 Escherichia coli bacteria (Brenner 1974). Strains used in this study include: N2 Bristol wild type, CB4856 Hawaiian, FG7 grk-2(gk268), FG78 grk-2(gk268);ocr-2(ud21), FG87 grk-2(gk268);osm-9(ud23), FG91 grk-2(gk268);osm-9(ud19), CX10 osm-9(ky10), FG60 grk-2(gk268);osm-9(ky10), CX4544 ocr-2(ak47), FG99 grk-2(gk268);ocr-2(ak47), LX748 osm-9(ky10)ocr-2(ak47), FG118 grk-2(gk268);osm-9(ky10)ocr-2(ak47), JY243 ocr-2(yz5), FG140 grk-2(gk268);ocr-2(yz5), CX7265 osm-9(ky10);yzEx53[osm-10∷osm-9,elt-2∷gfp], FG130 grk-2(gk268);osm-9(ky10);yzEx53[osm-10∷osm-9,elt-2∷gfp], FG166 udEx15[osm-10∷ocr-2,elt-2∷gfp], FG167 udEx16[osm-10∷ocr-2,elt-2∷gfp], FG168 udEx17[osm-10∷ocr-2,elt-2∷gfp], FG169 grk-2(gk268);ocr-2(yz5);udEx18[osm-10∷ocr-2,elt-2∷gfp], FG170 grk-2(gk268);ocr-2(yz5);udEx19[osm-10∷ocr-2,elt-2∷gfp], FG171 grk-2(gk268);ocr-2(yz5);udEx20[osm-10∷ocr-2,elt-2∷gfp], FG173 udEx22[srb-6∷ocr-2,elt-2∷gfp], FG174 udEx23[srb-6∷ocr-2,elt-2∷gfp], FG175 udEx24[srb-6∷ocr-2,elt-2∷gfp], FG176 grk-2(gk268);ocr-2(yz5);udEx25[srb-6∷ocr-2,elt-2∷gfp], FG177 grk-2(gk268);ocr-2(yz5);udEx26[srb-6∷ocr-2,elt-2∷gfp], FG179 grk-2(gk268);ocr-2(yz5);udEx28[srb-6∷ocr-2,elt-2∷gfp], FG180 grk-2(gk268);ocr-2(ak47);udEx29[osm-10∷ocr-2,elt-2∷gfp], FG181 grk-2(gk268);ocr-2(ak47);udEx30[osm-10∷ocr-2,elt-2∷gfp], FG182 grk-2(gk268);ocr-2(ak47);udEx31[osm-10∷ocr-2,elt-2∷gfp], FG184 grk-2(gk268);ocr-2(ak47);udEx33[srb-6∷ocr-2,elt-2∷gfp], FG185 grk-2(gk268);ocr-2(ak47);udEx34[srb-6∷ocr-2,elt-2∷gfp], FG186 grk-2(gk268);ocr-2(ak47);udEx35[srb-6∷ocr-2,elt-2∷gfp], FG187 udEx36[sra-6∷ocr-2,elt-2∷gfp], FG188 udEx37[sra-6∷ocr-2,elt-2∷gfp], FG189 udEx38[sra-6∷ocr-2,elt-2∷gfp], FG190 grk-2(gk268);ocr-2(ak47);udEx39[sra-6∷ocr-2,elt-2∷gfp], and FG191 grk-2(gk268);ocr-2(ak47);udEx40[sra-6∷ocr-2,elt-2∷gfp].
Plasmid construction:
osm-10∷ocr-2 (pFG14):
The ocr-2 promoter was removed from pAJ35 (gift of Cori Bargmann) using SphI and XmaI, leaving the ocr-2 cDNA in the vector backbone. The ∼900-bp upstream promoter region of osm-10 was removed from CR142 (Rongo et al. 1998) using SphI and XmaI and was placed into these sites upstream of the ocr-2 cDNA in the remaining fragment of pAJ35.
srb-6∷ocr-2 (pFG15):
The ∼1.3 kb srb-6 promoter was first isolated from pHA#355 (Fukuto et al. 2004) using PstI and BamHI and inserted into the same sites of Fire vector pPD49.26 to create pFG10. The srb-6 promoter was then removed from pFG10 using SphI and XmaI and was placed into these sites upstream of the ocr-2 cDNA (remaining fragment of pAJ35, as described above).
hsp∷ocr-2 (pFG16):
The hsp16-2 promoter was removed from Fire vector pPD49.78 using SphI and XmaI and was placed into these sites upstream of the ocr-2 cDNA (remaining fragment of pAJ35, as described above).
sra-6∷ocr-2:
This construct was the kind gift of Cori Bargmann and was described previously (de Bono et al. 2002).
Genetic analysis:
grk-2(gk268) animals were mutagenized with EMS (ethyl methanesulfonate) as previously described (Brenner 1974). Using this deletion allele decreased the likelihood of isolating intragenic suppressors or revertants, as might have been more likely if the grk-2(rt97) animals, which contain a single point mutation, were used. F2 animals were assayed for avoidance of 10 mm quinine using the drop assay with the quinine drop placed in front of the animal (Hilliard et al. 2002; Fukuto et al. 2004). Animals that responded by initiating backward locomotion within 4 sec of encountering the drop were selected. A total of 25,000 F2 animals were screened. Eight mutant strains were isolated.
To generate the mapping strain, the grk-2(gk268) allele (which was in the N2 wild-type background) was crossed into the Hawaiian single nucleotide polymorphism (SNP) strain CB4856. Animals were then extensively recrossed (10 times) to CB4856 and PCR and restriction enzyme digests were used to verify that all of the chromosomes had been replaced by CB4856 DNA, except for the left arm of chromosome III, where grk-2(gk268) now resided (map position −26.34). In short, grk-2(gk268) was crossed into the CB4856 Hawaiian background. Since the grk-2(gk268) allele was in both the animals that were used in the EMS screen and in the mapping strain, it remained homozygous during all mapping crosses. This allowed the use of the CB4856 restriction fragment length polymorphisms (RFLPs) to map the grk-2(gk268) suppressor mutations. In genetic mapping experiments, ud19 and ud23 were linked to LG IV near SNP C09G12 and ud21 was linked to LG IV between the two SNPs C06A6 and D2096. Following genetic linkage analysis, complementation assays using the previously defined alleles of osm-9(ky10) and ocr-2(ak47) confirmed that ud19 and ud23 were alleles of osm-9, while ud21 represented an allele of ocr-2. All subsequent behavioral experiments were performed with previously characterized alleles of osm-9 and ocr-2, and the molecular lesions in ud19, ud23, and ud21 have not been determined.
Behavioral assays:
Well-fed young adult animals were used for analysis, and all behavioral assays were performed on at least 2 separate days, along with controls. Behavioral assays were performed as previously described. Response to octanol was scored as the amount of time it took an animal to initiate backward locomotion when presented with a hair dipped in octanol (Troemel et al. 1995; Hart et al. 1999). (Assays were stopped at 20 sec.) Response to soluble tastants was scored as the percentage of animals that initiated backward locomotion within 4 sec of encountering a drop of tastant placed on the agar plate (Hilliard et al. 2002, 2004; Fukuto et al. 2004). Tastants were dissolved in M13, pH 7.4 (Wood 1988). The drop was placed in front of a forward moving animal. For octanol and taste avoidance assays, animals were tested 10–20 min after transfer to NGM plates lacking bacteria (“off food”). Chemotaxis assays were performed as previously described (Bargmann et al. 1993). After 1 hr, the chemotaxis index (C.I.) was calculated as the number of animals that had accumulated at the attractant, minus the number of animals at the control, divided by the total number of animals (Bargmann et al. 1993). For heat-shock experiments, animals were raised to young adulthood and then shifted to 33° for 2 hr. They were allowed to recover for 4 hr at 25° prior to testing. All data are presented as ± standard error of the mean (SEM). Student's t-test was used for statistical analysis.
RESULTS
Loss of OSM-9 or OCR-2 TRPV channel function restores quinine response to grk-2 mutant animals:
To identify the mechanisms that regulate sensory signaling in the absence of GRK-2 function, we performed a forward genetic screen to identify second-site suppressor mutations that restored the response of grk-2 animals to quinine. We used grk-2(gk268), a deletion allele that removes 608 nucleotides of the 5′ untranslated region and the first three exons of grk-2 coding sequence (930 additional nucleotides); it is a predicted grk-2 null and animals are phenotypically identical to the previously characterized grk-2(rt97) severe loss-of-function animals (Fukuto et al. 2004).
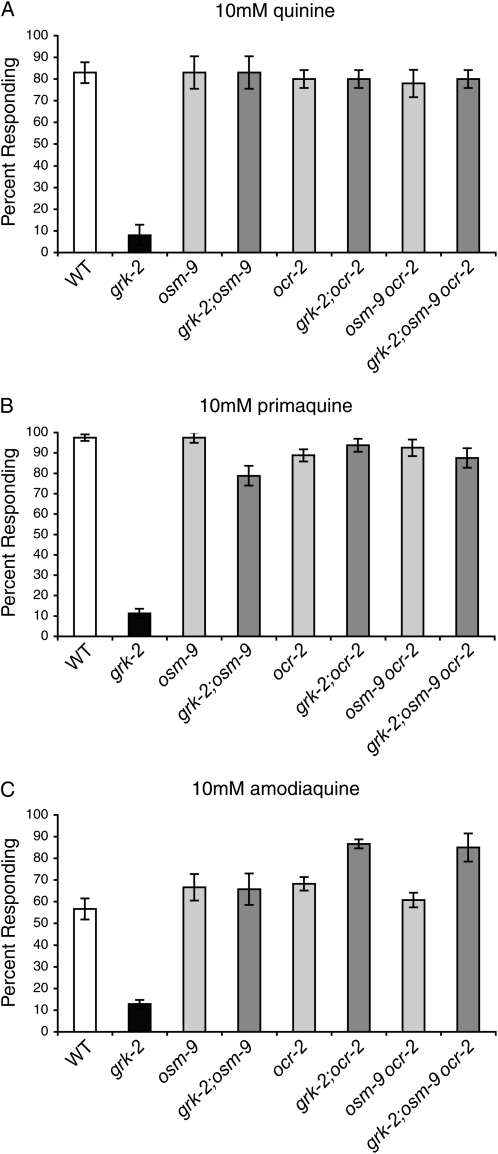
grk-2(gk268) mutant animals were EMS mutagenized by standard protocol (Brenner 1974) and second generation (F2) progeny of mutagenized animals were tested for restoration of normal quinine response. Single nucleotide polymorphism (SNP) mapping (Wicks et al. 2001) and genetic complementation analysis identified ud19 and ud23 as alleles of osm-9 and ud21 as an allele of ocr-2. The previously defined null alleles of these genes, osm-9(ky10) (Colbert et al. 1997) and ocr-2(ak47) (Tobin et al. 2002), were used in subsequent experiments. Loss of either OSM-9 or OCR-2 TRPV channel function restored the response of grk-2 mutants to 10 mm quinine to wild-type levels (Figure 1A). In addition, because OSM-9 and OCR-2 require each other for their mutual subcellular localization and function, simultaneous loss of OSM-9 and OCR-2 should affect behavioral responses similarly to the individual loss of these channels (Tobin et al. 2002). Consistent with this prediction, grk-2;osm-9ocr-2 triple mutants responded to 10 mm quinine similarly to grk-2;osm-9 and grk-2;ocr-2 double mutants (Figure 1A).
Figure 1.—
Loss of TRPV channel function restores grk-2 bitter taste response. While grk-2 mutant animals do not respond to bitter taste stimuli, loss of OSM-9 and OCR-2 TRPV channel function, alone or in combination, restored response to (A) 10 mm quinine, (B) 10 mm primaquine, and (C) 10 mm amodiaquine (P ≤ 0.001 when compared to grk-2 for each). Loss of OSM-9 and OCR-2 also partially or completely restored response to 10 mm quinacrine, 10 mm chloroquine, and 10 mm shikimic acid (not shown). Alleles used: grk-2(gk268), osm-9(ky10), and ocr-2(ak47). WT, the N2 wild-type strain. All tastants were dissolved in M13 buffer, pH 7.4. The percentage of animals responding is shown. N ≥ 40. Error bars represent the standard error of the mean (SEM).
Loss of TRPV channel function restores bitter taste response generally:
C. elegans show an avoidance response to several soluble, bitter chemicals in addition to quinine (Hilliard et al. 2004). We therefore tested whether loss of the TRPV channels OSM-9 and OCR-2 selectively restored quinine avoidance (Figure 1A) or whether decreased TRPV channel function could restore grk-2 bitter taste response more generally. We assayed the response of grk-2;osm-9, grk-2;ocr-2, and grk-2;osm-9ocr-2 animals to five additional bitter tastants. Similar to quinine, loss of OSM-9 and OCR-2, alone or in combination, completely or partially restored the response of grk-2 animals to primaquine (Figure 1B), amodiaquine (Figure 1C), quinacrine, chloroquine, and shikimic acid (data not shown). Taken together, decreased TRPV channel function broadly restored bitter taste response to grk-2 mutant animals.
Loss of TRPV channel function does not restore grk-2 response to other ASH-detected stimuli:
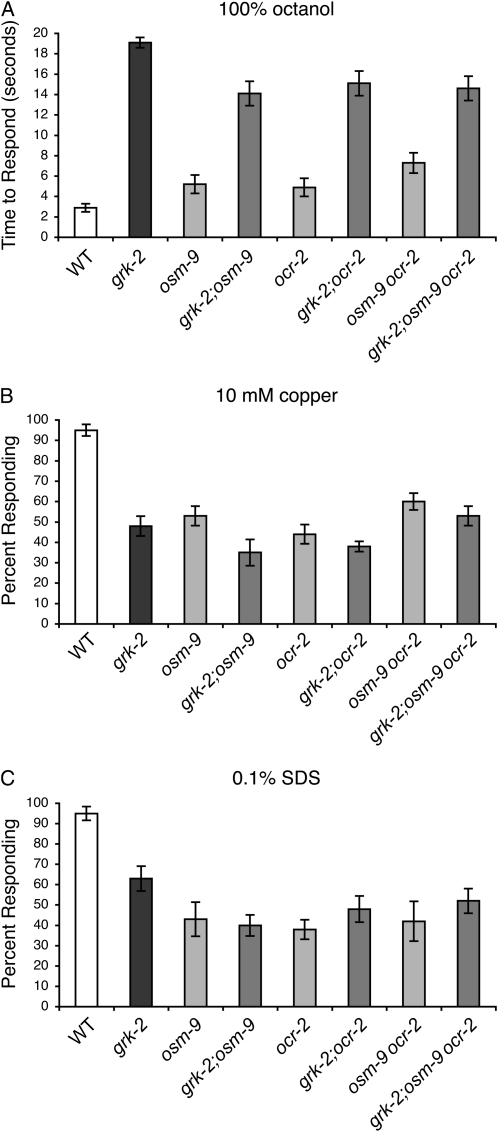
Loss of OSM-9 or OCR-2 TRPV function in grk-2 mutant animals restored response to soluble bitter compounds that likely act through G protein-coupled receptors (Chandrashekar et al. 2006; Palmer 2007) expressed in the ASH sensory neurons. Olfactory receptors are also G protein-coupled receptors (Dryer and Berghard 1999) and grk-2 animals are severely defective in their response to the aversive odorant octanol (Fukuto et al. 2004). Octanol is detected primarily by ASH, with contributions from the ADL and AWB neurons (Troemel et al. 1995; Chao et al. 2004). However, unlike bitter taste response, loss of the OSM-9/OCR-2 TRPV channels did not restore the octanol response of grk-2 mutants back to wild-type levels (Figure 2A).
Figure 2.—
Loss of TRPV channel function does not restore grk-2 response to other ASH-detected stimuli. The ASH sensory neurons also detect the volatile odorant octanol, the heavy metal copper, and the detergent SDS. grk-2 mutant animals are defective in their avoidance response to each of these stimuli. (A) Loss of OSM-9 and OCR-2 TRPV channel function had only a minimal, although statistically significant (P ≤ 0.01 when compared to grk-2) effect on 100% octanol response. However, grk-2;osm-9, grk-2;ocr-2, and grk-2;osm-9ocr-2 responses were not restored to the level of wild-type animals or to that of the TRPV channel mutants (P ≤ 0.00001). Time to respond is shown. N ≥ 40. (B) Loss of the OSM-9 or OCR-2 TRPV channels, alone or in combination, did not restore grk-2 response to 10 mm copper (P ≥ 0.1 when compared to grk-2). The percentage of animals responding is shown. N ≥ 40. (C) Loss of the OSM-9 or OCR-2 TRPV channels, alone or in combination, did not restore grk-2 response to 0.1% SDS. (P ≥ 0.01 for grk-2;osm-9 double mutants when compared to grk-2, with the double mutant response being somewhat worse than grk-2. P ≥ 0.1 for grk-2;ocr-2 and grk-2;osm-9ocr-2 when compared to grk-2). The percentage of animals responding is shown. N ≥ 60. Alleles used: grk-2(gk268), osm-9(ky10), and ocr-2(ak47). WT, the N2 wild-type strain. Error bars represent the standard error of the mean (SEM).
grk-2 animals are also partially defective for avoidance of several soluble, ASH-detected stimuli that are not thought to be detected by G protein-coupled receptors (Fukuto et al. 2004), including copper and SDS (Sambongi et al. 1999; Hilliard et al. 2002, 2005). As bitter tastants are soluble stimuli, we asked whether the TRPV suppression of grk-2 defects extended to include these additional soluble compounds detected by ASH. Loss of neither OSM-9 nor OCR-2 restored the response of grk-2 mutants to copper or SDS (Figure 2, B and C). We conclude that loss of TRPV channel function selectively restores the response of grk-2 animals to soluble, bitter tastants, and does not restore ASH signaling in general.
Loss of TRPV channel function does not restore chemotaxis toward attractive odorants:
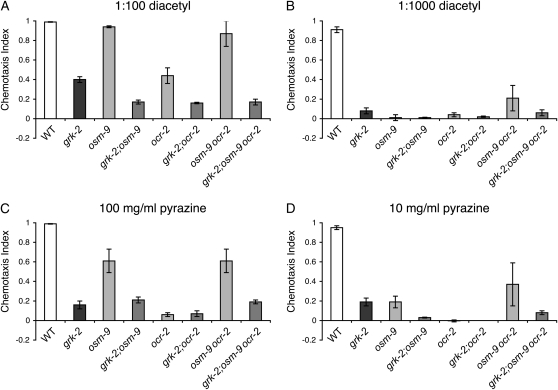
The ASH and AWA sensory neurons share a common signal transduction pathway, with OSM-9 and OCR-2 being part of the primary signaling cascade in both neurons (Colbert et al. 1997; Tobin et al. 2002; Hilliard et al. 2004). Having established that loss of either OSM-9 or OCR-2 function restored ASH-mediated avoidance of bitter tastants to grk-2 mutant animals, we wondered whether the loss of either TRPV channel would restore AWA-mediated attractive chemosensory behavior. grk-2;osm-9 and grk-2;ocr-2 double mutant animals and grk-2;osm-9ocr-2 triple mutants were assayed for their ability to chemotax toward the AWA-detected attractive odorants diacetyl and pyrazine (Bargmann et al. 1993). A range of concentrations for each odorant was tested (diacetyl, 10°–10−4; pyrazine, 100 mg/ml–1 mg/ml). Loss of neither TRPV channel, alone or in combination, restored AWA-mediated chemotaxis to grk-2 mutant animals at any concentration (Figure 3 and data not shown). We conclude that although the ASH and AWA sensory neurons utilize many of the same primary signal transduction components, including OSM-9 and OCR-2, the mechanism by which decreased TRPV channel function restores chemosensory behavior to grk-2 mutants is unique to the ASH-mediated avoidance of bitter tastants.
Figure 3.—
Loss of TRPV channel function does not restore AWA-mediated chemotaxis. The ASH and AWA sensory neurons share common signaling molecules to transduce chemosensory signals. The OSM-9 and OCR-2 TRPV channels are part of the primary signal transduction cascade in both neurons. (A and B) Loss of the OSM-9 or OCR-2 TRPV channels, alone or in combination, did not restore grk-2 chemotaxis toward diacetyl over a range of concentrations tested. Chemotaxis to 1 μl of (A) 1:100 and (B) 1:1000 diacetyl is shown; 100%, 1:10 and 1:10,000 not shown. (C and D) Loss of the OSM-9 or OCR-2 TRPV channels, alone or in combination, did not restore grk-2 chemotaxis toward any concentration of pyrazine tested. Chemotaxis to 1 μl of (C) 100 mg/ml and (D) 10 mg/ml pyrazine is shown; 1 mg/ml not shown. Chemotaxis index = (number of animals at odorant − number of animals at control) ÷ total number of animals on the assay plate. Each bar represents the average of four or more assays with 50–150 animals per trial. Alleles used: grk-2(gk268), osm-9(ky10), and ocr-2(ak47). WT, the N2 wild-type strain. Error bars represent the standard error of the mean (SEM).
The TRPV channels function in the ASH sensory neurons:
The OSM-9 and OCR-2 TRPV channels have very restricted expression patterns (Colbert et al. 1997; Tobin et al. 2002). OSM-9 is expressed in 10 pairs of head neurons, with OCR-2 being coexpressed in only 4 pairs of head sensory neurons: ASH, ADL, ADF, and AWA (Tobin et al. 2002). Although GRK-2 is expressed throughout the C. elegans nervous system, it appears to function in the sensory neurons to regulate chemosensory signaling (Fukuto et al. 2004). Importantly, loss of GRK-2 severely diminishes or eliminates stimulus-evoked Ca2+ fluxes in the ASH sensory neurons (Fukuto et al. 2004). In addition, laser ablation studies revealed that ASH is the main sensory neuron responsible for quinine detection, although the ASK sensory neurons also contribute (Hilliard et al. 2004).
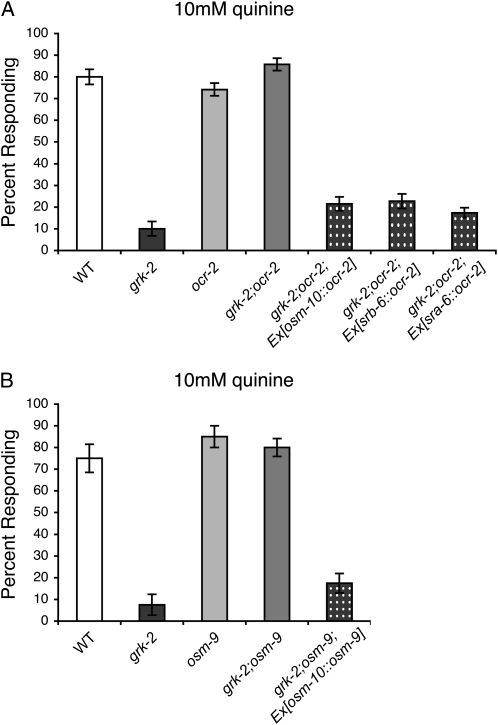
As GRK-2 and the TRPV channels function in the sensory neurons (Colbert et al. 1997; Tobin et al. 2002; Fukuto et al. 2004; Hilliard et al. 2005), it suggests that loss of TRPV channel function in the ASH neurons themselves may restore quinine avoidance to grk-2;osm-9 and grk-2;ocr-2 double mutant animals. To determine where TRPV function contributes to the defective quinine avoidance response of grk-2 mutant animals, the osm-10 promoter (Hart et al. 1999), srb-6 promoter (Troemel et al. 1995), and sra-6 promoter (Troemel et al. 1995; de Bono et al. 2002) were used to express wild-type ocr-2 in grk-2;ocr-2 null mutant animals. ASH is the only sensory neuron in which all three promoters are expressed. While grk-2;ocr-2 double mutant animals respond robustly to quinine (Figure 4A), double mutant animals expressing the osm-10∷ocr-2, srb-6∷ocr-2, or sra-6∷ocr-2 transgene were returned to the grk-2 quinine response defective phenotype (Figure 4A). Transgene expression had no effect in wild-type animals, indicating that transgene expression did not disrupt ASH function (data not shown). In addition, the osm-10 promoter (Hart et al. 1999) was used to express osm-9 cDNA in the ASH sensory neurons of grk-2;osm-9 animals. While grk-2;osm-9 double mutants also respond robustly to quinine (Figure 4B), double mutant animals expressing the osm-10∷osm-9 transgene yzEx53 (Zhang et al. 2004; Chang et al. 2006) were defective in their response to quinine (Figure 4B). We conclude that TRPV channel function in the ASH sensory neurons is sufficient for the defective quinine response of grk-2 mutant animals, and that grk-2;TRPV animals may have restored bitter tastant chemosensory responses due to changes in ASH sensory neuron signaling in the absence of OSM-9/OCR-2 TRPV channel function.
Figure 4.—
The OSM-9/OCR-2 TRPV channels function in ASH. The ASH sensory neurons are the primary neurons used to detect quinine. OSM-9/OCR-2 TRPV channel function is required in ASH for the defective quinine avoidance response of grk-2 animals. (A) The osm-10 (Hart et al. 1999), srb-6 (Troemel et al. 1995), and sra-6 (Troemel et al. 1995; de Bono et al. 2002) promoters were used to drive expression of wild-type ocr-2 in the quinine-detecting ASH neurons of grk-2;ocr-2 double mutant animals. The osm-10 promoter expresses in ASH, ASI, PHA, and PHB, while the srb-6 promoter drives expression in ASH, ADL, ADF, PHA, and PHB and the sra-6 promoter expresses in ASH, ASI, and PVQ. ASH is the only sensory neuron common to all three promoters. While grk-2;ocr-2 animals avoid 10 mm quinine, restoring OCR-2 function in ASH returned grk-2;ocr-2 animals to the quinine response defective phenotype (P ≤ 0.0001 for each transgene when compared to grk-2;ocr-2). (B) The osm-10 promoter (Hart et al. 1999) was used to drive expression of osm-9 cDNA in the quinine-detecting ASH neurons of grk-2;osm-9 double mutant animals. While grk-2;osm-9 animals avoid 10 mm quinine, restoring OSM-9 function in ASH returned grk-2;osm-9 animals to the grk-2 quinine response defective phenotype (P = 0.2 when compared to grk-2). Ex[osm-10∷osm-9] = the extrachromosomal transgenic array yzEx53 (Zhang et al. 2004; Chang et al. 2006). The percentage of animals responding is shown. N ≥ 80 transgenic animals. Alleles used: grk-2(gk268), ocr-2(ak47), and osm-9(ky10). WT, the N2 wild-type strain. Error bars represent the standard error of the mean (SEM).
The OCR-2(G36E) point mutation restores grk-2 bitter taste response:
The ocr-2(yz5) mutation encodes a single nucleotide change that creates a glycine-to-glutamate (G36E) substitution in the N-terminal cytoplasmic tail of OCR-2 (Zhang et al. 2004; Sokolchik et al. 2005). While the product of the ocr-2(ak47) deletion allele cannot form a functional channel, leading to severe disruption of AWA-mediated chemosensory transduction (Tobin et al. 2002), the G36E substitution produces a protein with correct subcellular localization and ocr-2(yz5) mutants have wild-type AWA-mediated chemotaxis (Sokolchik et al. 2005). Interestingly, the ocr-2(yz5) point mutation decreases expression of tph-1, which encodes a serotonin biosynthetic enzyme, in the ADF neurons as strongly as the ocr-2(ak47) predicted null (Zhang et al. 2004). Thus, the N-terminal G36E mutation appears to selectively disrupt the ability of the OCR-2 TRPV channel to direct changes in gene expression, while leaving channel function in primary chemosensory signal transduction intact (Zhang et al. 2004; Sokolchik et al. 2005). In addition, the OCR-2 N terminus is sufficient to increase TPH-1 expression when it is part of a chimeric channel (Sokolchik et al. 2005).
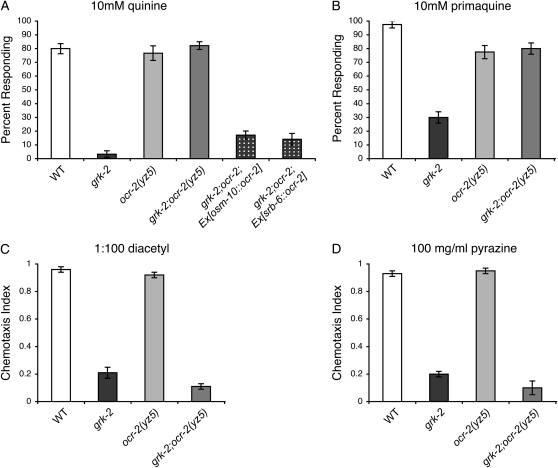
To determine whether the ocr-2(yz5) mutation was also able to suppress the bitter tastant avoidance defects of grk-2 animals, grk-2(gk268);ocr-2(yz5) double mutants were assayed for their response to quinine and primaquine. The ocr-2(yz5) single mutants have a wild-type response to these bitter tastants, and in the grk-2 mutants, the ocr-2(yz5) point mutation completely restored bitter tastant avoidance (Figure 5, A and B). Importantly, the mechanism by which the OCR-2 N-terminal G36E mutation restores quinine response appears to operate within the ASH sensory neurons. Similar to the ASH rescue of ocr-2 in grk-2;ocr-2 null animals (Figure 4A), expression of wild-type ocr-2 in the ASH neurons of grk-2(gk268);ocr-2(yz5) animals resulted in defective quinine responses (Figure 5A). Furthermore, the OCR-2 N-terminal point mutation appears to selectively restore the response to ASH-detected bitter tastants, as grk-2(gk268);ocr-2(yz5) double mutants remained defective for chemotaxis toward diacetyl and pyrazine (Figure 5, C and D), mediated by the AWA neurons, although ocr-2(yz5) animals displayed wild-type chemotaxis toward both odorants (Sokolchik et al. 2005).
Figure 5.—
The OCR-2(G36E) point mutation functions cell autonomously to restore grk-2 bitter taste responses mediated by ASH. (A and B) The OCR-2(G36E) point mutation, encoded by the ocr-2(yz5) allele, restored grk-2 mutant animals' response to (A) 10 mm quinine and (B) 10 mm primaquine (P ≤ 0.001 when compared to grk-2 for each). The osm-10 (Hart et al. 1999) and srb-6 (Troemel et al. 1995) promoters were used to drive wild-type ocr-2 expression in the ASH sensory neurons of grk-2(gk268);ocr-2(yz5) double mutant animals. Although grk-2;ocr-2 animals avoid 10 mm quinine, restoring OCR-2 function in ASH returned grk-2;ocr-2 animals to the grk-2 quinine response defective phenotype (P ≤ 0.0001 for each transgene when compared to grk-2;ocr-2). N ≥ 130 transgenic animals. Tastants were dissolved in M13 buffer, pH 7.4. The percentage of animals responding is shown. N ≥ 40. (C and D) grk-2;ocr-2(yz5) mutant animals remained defective for AWA-mediated chemotaxis toward (C) 1:100 diacetyl and (D) 100 (mg/ml) pyrazine (P ≥ 0.08 when compared to grk-2). Chemotaxis index = (number of animals at odorant − number of animals at control) ÷ total number of animals on the assay plate. Each bar represents the average of four or more assays with 50–150 animals per trial. Alleles used: grk-2(gk268) and ocr-2(yz5). WT, the N2 wild-type strain. Error bars represent the standard error of the mean (SEM).
By decreasing expression of TPH-1, mutations in the TRPV channels would also cause a reduction in serotonin levels. To ensure that disruption of TRPV channel function did not restore grk-2 bitter tastant avoidance by decreasing serotonin levels in a non-cell-autonomous manner, we assayed tph-1(mg280);grk-2(gk268) double mutants for their response to quinine. Loss of serotonin synthesis, via the tph-1 mutation, did not restore the response of grk-2(gk268) mutants to quinine (percentage responding to 10 mm quinine: N2 = 85 ± 2.9%, grk-2 = 5 ± 5%, tph-1 = 75 ± 2.9%, and tph-1;grk-2 = 7.5 ± 4.9%).
Taken together our results suggest that alterations in downstream regulatory pathways that couple to the N terminus of OCR-2 in the ASH sensory neurons may account for the restored response to bitter stimuli in grk-2;TRPV double mutant animals.
DISCUSSION
As C. elegans lack the ability to see and hear, they have evolved to rely heavily on their ability to detect chemical cues to successfully navigate their environment. This is reflected in the fact that >5% of their genome is dedicated to recognizing environmental chemicals (Bargmann 2006a). C. elegans must properly respond to gustatory and olfactory cues to initiate chemotaxis toward favorable conditions or rapid avoidance to evade harmful environments. Therefore, signals through chemosensory GPCRs must be precisely transduced and regulated to ensure continued survival.
Loss of the GPCR negative regulator GRK-2 results in an intriguing phenotype; grk-2 mutant animals are defective in chemosensory signaling and behavioral responses both to attractive and aversive chemosensory stimuli (Fukuto et al. 2004). To better understand how loss of receptor regulation can lead to decreased signaling in different cell types, we sought to identify mechanisms responsible for dampening ASH signaling in the absence of GRK-2 function. Three of the mutations found in our grk-2 suppressor screen were identified as alleles of the TRPV channels encoded by osm-9 and ocr-2. Using the previously characterized null alleles osm-9(ky10) and ocr-2(ak47), we found that complete loss of OSM-9/OCR-2 channel function fully restored the response of grk-2 mutant animals to quinine and other bitter tastants. This suggests that these channels contribute to multiple bitter taste responses, in addition to quinine (Hilliard et al. 2004, 2005). Surprisingly, though, grk-2;TRPV mutants remain defective in their avoidance of octanol, an odorant detected by ASH, the same neuron primarily responsible for quinine detection. In addition, loss of these TRPV channels failed to restore grk-2 responses to attractive chemosensory stimuli detected by the AWA sensory neurons. Together, these results reveal that C. elegans TRPV channels can regulate chemosensory signaling in both a cell-specific and modality-specific manner.
Furthermore, the ability of the ocr-2(yz5) N-terminal point mutation to restore bitter taste response in grk-2 mutants suggests that it may not be loss of TRPV channels as primary signal transduction components that restores grk-2 bitter taste avoidance. Rather, loss of a downstream function or pathway coupled to the N-terminal structural motif of OCR-2 may restore bitter responses in the absence of GRK-2 function. For example, the G36E change may disrupt interactions with adaptor proteins or signaling components required for TRPV-modulated changes in gene expression (Sokolchik et al. 2005); to date, no other function has yet been ascribed to this region of OCR-2. Therefore, one possibility is that loss of TRPV channels may decrease the expression of components used in primary signal transduction, thereby reducing the strength of signals being transduced (Figure S1). Reducing neuronal activity in this manner could circumvent the activation of inhibitory pathways that act to dampen signaling in the absence of GRK-2, thus restoring behavioral response. The Gα proteins are one possible target for transcriptional regulation. ODR-3 Gα and GPA-3 Gα contribute to a broad range of chemosensory responses, including bitter taste avoidance. However, ODR-3 protein levels are not altered in grk-2 mutants and loss of neither ODR-3 nor GPA-3 restores grk-2 response to bitter tastants (Fukuto et al. 2004; Ferkey et al. 2007). In addition, TRPV channel-mediated regulation of transcription in the ADF neurons was shown to occur independently of Gα activity (Zhang et al. 2004).
As loss of TRPV channel function selectively restored bitter taste response, and not chemosensory responses in general, it suggests that signaling components specific to bitter taste response may be regulated by the OSM-9/OCR-2 channels in the ASH sensory neurons. For example, the expression levels of the receptors for bitter tastants could be regulated by TRPV channels. If loss of TRPV channel function decreases expression of bitter GPCRs, then perhaps a signal being transduced through a reduced number of GPCRs would no longer be perceived as aberrant. This could also avert activation of compensatory inhibition in the absence of GRK-2 function, thereby restoring behavioral response in grk-2;TRPV double mutants.
Modulation of GPCR expression by sensory activity is a well-documented phenomenon in C. elegans (Lanjuin and Sengupta 2002; Nolan et al. 2002; van der Linden et al. 2008). Unlike vertebrates, C. elegans express multiple receptors in each chemosensory neuron (Bargmann 2006b). To selectively modify behavioral response to a single chemical, C. elegans may rely on changing the expression of a particular chemoreceptor gene, rather than altering signaling efficacy of the entire neuron, which would inadvertently affect the response to many chemicals (Peckol et al. 2001; Nolan et al. 2002). Selectively modulating distinct populations of receptors in this manner may allow C. elegans to fine tune their chemosensory GPCR repertoire and respond appropriately to their environment. This may be particularly important in a polymodal sensory neuron like ASH, which detects diverse stimuli including odorants, tastants, high osmolarity, and mechanical touch. Although the C. elegans genome encodes ∼500 chemosensory GPCRs (Bargmann 2006a), no receptors have yet been identified as bitter responsive. Thus, we were not able to directly examine the expression levels of this receptor class in animals lacking OSM-9 or OCR-2 function. However, we note that although levels of the diacetyl receptor ODR-10 are regulated by OSM-9/OCR-2 (Tobin et al. 2002), loss of neither TRPV channel restored grk-2 chemotaxis toward diacetyl (Figure 3).
Another possible target of TRPV-mediated transcriptional regulation is a compensatory pathway. Loss of GRK-2 may initially result in increased, aberrant signaling that is translated, via the TRPV channels, into changes in gene expression of inhibitory molecules (Figure S1). TRPV-mediated transcriptional upregulation of a compensatory pathway, which specifically dampens signals being transduced through bitter GPCRs, would result in the loss of behavioral response to bitter tastants. Loss of TRPV channel-regulated gene transcription could prevent the increased expression of an inhibitory molecule(s), thereby restoring bitter GPCR signal transduction and behavioral response in the absence of GRK-2 function. Interestingly, prior studies have not identified a role for the OSM-9 and OCR-2 TRPV channels in regulating expression of sensory components in ASH (Sokolchik et al. 2005). Perhaps TRPV channels do not affect expression of primary signal transduction molecules, but rather only regulatory components necessary to maintain normal signaling. Thus, it may be only in a sensitized background, like grk-2 where ordinary signaling has been compromised, that a role for the TRPV channels in modulating expression of ASH sensory components can be revealed.
Although regulated expression of sensory signaling components has not yet been described in mammals, exposure to chemosensory stimuli has been shown to activate the transcription factor CREB in both rat olfactory receptor neurons and taste receptor cells (Moon et al. 1999; Cao et al. 2002). CREB is a key mediator in translating transient neuronal activity into long-term changes in gene expression (Ooi and Wood 2008). Therefore, it has been proposed that in addition to initiating the immediate response of membrane depolarization, gustatory and olfactory stimuli also generate a delayed response that may modulate gene transcription in their respective sensory neurons (Moon et al. 1999; Cao et al. 2002). Thus, in addition to sharing components of chemosensory signal transduction, the long-lasting cellular consequences of odorant and tastant detection may also be conserved among invertebrates and vertebrates.
While it is possible that the N terminus of OCR-2 may have additional roles in cellular events downstream of the TRPV channels that have not yet been identified, our results suggest that misregulated signaling, such as in the absence of C. elegans GRK-2 function, may also lead to long-term transcriptional changes that alter signaling levels and behavioral responses. Importantly, temporal rescue of GRK-2 function in adult grk-2 mutant animals is sufficient to restore chemosensory response to octanol and quinine (Fukuto et al. 2004 and data not shown), indicating that the mechanisms that dampen signaling in the absence of GRK-2 function are under ongoing regulatory control. Similarly, adult rescue of OCR-2 function in grk-2;ocr-2 double mutants is sufficient to return animals to the grk-2 quinine response defective phenotype within hours (Figure S2). It will be interesting to determine whether similar TRPV-mediated mechanisms are at work in mammalian cells that show decreased signaling and responsiveness in the absence of GRK function (Peppel et al. 1997; Fong et al. 2002). Future studies to identify OCR-2 interacting proteins and the downstream targets regulated by activity through the TRPV channels will also further our understanding of the modality-specific mechanisms used by cells to modulate intracellular signaling.
Acknowledgments
We thank Richard Gronostajski, Douglas Portman, Sean M. Rumschik, and Jordan Wood for valuable feedback on this manuscript. We thank Cori Bargmann, Michael Chao, Anne Hart, Andrew Fire, Michael Koelle, Noelle L'Etoile, and the Caenorhabditis Genetics Center for reagents, and we are grateful to Marina Ezcurra and William Schafer for help with experiments not included in this manuscript. This work was supported by the National Science Foundation (MCB-0917896, to D.M.F.).
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.110.115188/DC1.
References
- Bargmann, C. I., 2006. a Chemosensation in C. elegans. WormBook, 1–29. [DOI] [PMC free article] [PubMed]
- Bargmann, C. I., 2006. b Comparative chemosensation from receptors to ecology. Nature 444 295–301. [DOI] [PubMed] [Google Scholar]
- Bargmann, C. I., and H. R. Horvitz, 1991. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7 729–742. [DOI] [PubMed] [Google Scholar]
- Bargmann, C. I., J. H. Thomas and H. R. Horvitz, 1990. Chemosensory cell function in the behavior and development of Caenorhabditis elegans. Cold Spring Harb. Symp. Quant. Biol. 55 529–538. [DOI] [PubMed] [Google Scholar]
- Bargmann, C. I., E. Hartwieg and H. R. Horvitz, 1993. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74 515–527. [DOI] [PubMed] [Google Scholar]
- Boekhoff, I., J. Inglese, S. Schleicher, W. J. Koch, R. J. Lefkowitz et al., 1994. Olfactory desensitization requires membrane targeting of receptor kinase mediated by βγ-subunits of heterotrimeric G proteins. J. Biol. Chem. 269 37–40. [PubMed] [Google Scholar]
- Brenner, S., 1974. The genetics of Caenorhabditis elegans. Genetics 77 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, Y., C. Shreffler and S. Herness, 2002. Localization and functional investigation of the transcription factor CREB in taste receptor cells. Neuroreport 13 1321–1325. [DOI] [PubMed] [Google Scholar]
- Chandrashekar, J., M. A. Hoon, N. J. Ryba and C. S. Zuker, 2006. The receptors and cells for mammalian taste. Nature 444 288–294. [DOI] [PubMed] [Google Scholar]
- Chang, A. J., N. Chronis, D. S. Karow, M. A. Marletta and C. I. Bargmann, 2006. A distributed chemosensory circuit for oxygen preference in C. elegans. PLoS Biol. 4 e274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao, M. Y., H. Komatsu, H. S. Fukuto, H. M. Dionne and A. C. Hart, 2004. Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc. Natl. Acad. Sci. USA 101 15512–15517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C. K., M. E. Burns, M. Spencer, G. A. Niemi, J. Chen et al., 1999. Abnormal photoresponses and light-induced apoptosis in rods lacking rhodopsin kinase. Proc. Natl. Acad. Sci. USA 96 3718–3722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan, A. V., D. C. Hood, Y. Huang, E. Banin, Z. Y. Li et al., 1998. Disease sequence from mutant rhodopsin allele to rod and cone photoreceptor degeneration in man. Proc. Natl. Acad. Sci. USA 95 7103–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn, C. M., and C. I. Bargmann, 1996. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron 17 695–706. [DOI] [PubMed] [Google Scholar]
- Colbert, H. A., T. L. Smith and C. I. Bargmann, 1997. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J. Neurosci. 17 8259–8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuppen, E., A. M. van der Linden, G. Jansen and R. H. Plasterk, 2003. Proteins interacting with Caenorhabditis elegans Gα subunits. Comp. Funct. Genomics 4 479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bono, M., D. M. Tobin, M. W. Davis, L. Avery and C. I. Bargmann, 2002. Social feeding in Caenorhabditis elegans is induced by neurons that detect aversive stimuli. Nature 419 899–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, M. Q., D. Chase, G. A. Patikoglou and M. R. Koelle, 2000. Multiple RGS proteins alter neural G protein signaling to allow C. elegans to rapidly change behavior when fed. Genes Dev. 14 2003–2014. [PMC free article] [PubMed] [Google Scholar]
- Dryer, L., and A. Berghard, 1999. Odorant receptors: a plethora of G-protein-coupled receptors. Trends Pharmacol. Sci. 20 413–417. [DOI] [PubMed] [Google Scholar]
- Ferkey, D. M., R. Hyde, G. Haspel, H. M. Dionne, H. A. Hess et al., 2007. C. elegans G protein regulator RGS-3 controls sensitivity to sensory stimuli. Neuron 53 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong, A. M., R. T. Premont, R. M. Richardson, Y. R. Yu, R. J. Lefkowitz et al., 2002. Defective lymphocyte chemotaxis in β-arrestin2- and GRK6-deficient mice. Proc. Natl. Acad. Sci. USA 99 7478–7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman, N. J., and R. J. Lefkowitz, 1996. Desensitization of G protein-coupled receptors. Recent Prog. Horm. Res. 51 319–351; discussion 352–313. [PubMed] [Google Scholar]
- Fukuto, H. S., D. M. Ferkey, A. J. Apicella, H. Lans, T. Sharmeen et al., 2004. G protein-coupled receptor kinase function is essential for chemosensation in C. elegans. Neuron 42 581–593. [DOI] [PubMed] [Google Scholar]
- Gainetdinov, R. R., L. M. Bohn, J. K. Walker, S. A. Laporte, A. D. Macrae et al., 1999. Muscarinic supersensitivity and impaired receptor desensitization in G protein-coupled receptor kinase 5-deficient mice. Neuron 24 1029–1036. [DOI] [PubMed] [Google Scholar]
- Gainetdinov, R. R., L. M. Bohn, T. D. Sotnikova, M. Cyr, A. Laakso et al., 2003. Dopaminergic supersensitivity in G protein-coupled receptor kinase 6-deficient mice. Neuron 38 291–303. [DOI] [PubMed] [Google Scholar]
- Hart, A. C., J. Kass, J. E. Shapiro and J. M. Kaplan, 1999. Distinct signaling pathways mediate touch and osmosensory responses in a polymodal sensory neuron. J. Neurosci. 19 1952–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff, W. P., M. G. Caron and R. J. Lefkowitz, 1990. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 4 2881–2889. [PubMed] [Google Scholar]
- Herness, M. S., and T. A. Gilbertson, 1999. Cellular mechanisms of taste transduction. Annu. Rev. Physiol. 61 873–900. [DOI] [PubMed] [Google Scholar]
- Hilliard, M. A., C. I. Bargmann and P. Bazzicalupo, 2002. C. elegans responds to chemical repellents by integrating sensory inputs from the head and the tail. Curr. Biol. 12 730–734. [DOI] [PubMed] [Google Scholar]
- Hilliard, M. A., C. Bergamasco, S. Arbucci, R. H. Plasterk and P. Bazzicalupo, 2004. Worms taste bitter: ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. EMBO J. 23 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard, M. A., A. J. Apicella, R. Kerr, H. Suzuki, P. Bazzicalupo et al., 2005. In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO J. 24 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber, M., W. J. Koch, H. Rockman, B. Smith, R. A. Bond et al., 1996. Essential role of β-adrenergic receptor kinase 1 in cardiac development and function. Proc. Natl. Acad. Sci. USA 93 12974–12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen, G., K. L. Thijssen, P. Werner, M. van der Horst, E. Hazendonk et al., 1999. The complete family of genes encoding G proteins of Caenorhabditis elegans. Nat. Genet. 21 414–419. [DOI] [PubMed] [Google Scholar]
- Kaplan, J. M., and H. R. Horvitz, 1993. A dual mechanosensory and chemosensory neuron in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 90 2227–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelle, M. R., and H. R. Horvitz, 1996. EGL-10 regulates G protein signaling in the C. elegans nervous system and shares a conserved domain with many mammalian proteins. Cell 84 115–125. [DOI] [PubMed] [Google Scholar]
- Komatsu, H., I. Mori, J. S. Rhee, N. Akaike and Y. Ohshima, 1996. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron 17 707–718. [DOI] [PubMed] [Google Scholar]
- Kunitomo, H., H. Uesugi, Y. Kohara and Y. Iino, 2005. Identification of ciliated sensory neuron-expressed genes in Caenorhabditis elegans using targeted pull-down of poly(A) tails. Genome Biol. 6 R17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanjuin, A., and P. Sengupta, 2002. Regulation of chemosensory receptor expression and sensory signaling by the KIN-29 Ser/Thr kinase. Neuron 33 369–381. [DOI] [PubMed] [Google Scholar]
- Lans, H., S. Rademakers and G. Jansen, 2004. A network of stimulatory and inhibitory Gα-subunits regulates olfaction in Caenorhabditis elegans. Genetics 167 1677–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCudden, C. R., M. D. Hains, R. J. Kimple, D. P. Siderovski and F. S. Willard, 2005. G-protein signaling: back to the future. Cell. Mol. Life Sci. 62 551–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metaye, T., H. Gibelin, R. Perdrisot and J. L. Kraimps, 2005. Pathophysiological roles of G-protein-coupled receptor kinases. Cell Signal 17 917–928. [DOI] [PubMed] [Google Scholar]
- Moon, C., Y. K. Sung, R. Reddy and G. V. Ronnett, 1999. Odorants induce the phosphorylation of the cAMP response element binding protein in olfactory receptor neurons. Proc. Natl. Acad. Sci. USA 96 14605–14610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan, K. M., T. R. Sarafi-Reinach, J. G. Horne, A. M. Saffer and P. Sengupta, 2002. The DAF-7 TGF-beta signaling pathway regulates chemosensory receptor gene expression in C. elegans. Genes Dev. 16 3061–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi, L., and I. C. Wood, 2008. Regulation of gene expression in the nervous system. Biochem. J. 414 327–341. [DOI] [PubMed] [Google Scholar]
- Palmer, R. K., 2007. The pharmacology and signaling of bitter, sweet, and umami taste sensing. Mol. Interv. 7 87–98. [DOI] [PubMed] [Google Scholar]
- Peckol, E. L., E. R. Troemel and C. I. Bargmann, 2001. Sensory experience and sensory activity regulate chemosensory receptor gene expression in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 98 11032–11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn, R. B., A. N. Pronin and J. L. Benovic, 2000. Regulation of G protein-coupled receptor kinases. Trends Cardiovasc. Med. 10 81–89. [DOI] [PubMed] [Google Scholar]
- Peppel, K., I. Boekhoff, P. McDonald, H. Breer, M. G. Caron et al., 1997. G protein-coupled receptor kinase 3 (GRK3) gene disruption leads to loss of odorant receptor desensitization. J. Biol. Chem. 272 25425–25428. [DOI] [PubMed] [Google Scholar]
- Perez, C. A., R. F. Margolskee, S. C. Kinnamon and T. Ogura, 2003. Making sense with TRP channels: store-operated calcium entry and the ion channel Trpm5 in taste receptor cells. Cell Calcium 33 541–549. [DOI] [PubMed] [Google Scholar]
- Pitcher, J. A., N. J. Freedman and R. J. Lefkowitz, 1998. G protein-coupled receptor kinases. Annu. Rev. Biochem. 67 653–692. [DOI] [PubMed] [Google Scholar]
- Premont, R. T., and R. R. Gainetdinov, 2007. Physiological roles of G protein-coupled receptor kinases and arrestins. Annu. Rev. Physiol. 69 511–534. [DOI] [PubMed] [Google Scholar]
- Roayaie, K., J. G. Crump, A. Sagasti and C. I. Bargmann, 1998. The Gα protein ODR-3 mediates olfactory and nociceptive function and controls cilium morphogenesis in C. elegans olfactory neurons. Neuron 20 55–67. [DOI] [PubMed] [Google Scholar]
- Rockman, H. A., D. J. Choi, S. A. Akhter, M. Jaber, B. Giros et al., 1998. Control of myocardial contractile function by the level of β-adrenergic receptor kinase 1 in gene-targeted mice. J. Biol. Chem. 273 18180–18184. [DOI] [PubMed] [Google Scholar]
- Rongo, C., C. W. Whitfield, A. Rodal, S. K. Kim and J. M. Kaplan, 1998. LIN-10 is a shared component of the polarized protein localization pathways in neurons and epithelia. Cell 94 751–759. [DOI] [PubMed] [Google Scholar]
- Sambongi, Y., T. Nagae, Y. Liu, T. Yoshimizu, K. Takeda et al., 1999. Sensing of cadmium and copper ions by externally exposed ADL, ASE, and ASH neurons elicits avoidance response in Caenorhabditis elegans. Neuroreport 10 753–757. [DOI] [PubMed] [Google Scholar]
- Schleicher, S., I. Boekhoff, J. Arriza, R. J. Lefkowitz and H. Breer, 1993. A β-adrenergic receptor kinase-like enzyme is involved in olfactory signal termination. Proc. Natl. Acad. Sci. USA 90 1420–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta, P., J. H. Chou and C. I. Bargmann, 1996. odr-10 encodes a seven transmembrane domain olfactory receptor required for responses to the odorant diacetyl. Cell 84 899–909. [DOI] [PubMed] [Google Scholar]
- Sokolchik, I., T. Tanabe, P. F. Baldi and J. Y. Sze, 2005. Polymodal sensory function of the Caenorhabditis elegans OCR-2 channel arises from distinct intrinsic determinants within the protein and is selectively conserved in mammalian TRPV proteins. J. Neurosci. 25 1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin, D., D. Madsen, A. Kahn-Kirby, E. Peckol, G. Moulder et al., 2002. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron 35 307–318. [DOI] [PubMed] [Google Scholar]
- Troemel, E. R., 1999. Chemosensory signaling in C. elegans. Bioessays 21 1011–1020. [DOI] [PubMed] [Google Scholar]
- Troemel, E. R., J. H. Chou, N. D. Dwyer, H. A. Colbert and C. I. Bargmann, 1995. Divergent seven transmembrane receptors are candidate chemosensory receptors in C. elegans. Cell 83 207–218. [DOI] [PubMed] [Google Scholar]
- van der Linden, A. M., S. Wiener, Y. J. You, K. Kim, L. Avery et al., 2008. The EGL-4 PKG acts with KIN-29 salt-inducible kinase and protein kinase A to regulate chemoreceptor gene expression and sensory behaviors in Caenorhabditis elegans. Genetics 180 1475–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks, S. R., R. T. Yeh, W. R. Gish, R. H. Waterston and R. H. Plasterk, 2001. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat. Genet. 28 160–164. [DOI] [PubMed] [Google Scholar]
- Wood, W. B., 1988. The Nematode Caenorhabditis elegans. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Yamada, T., M. Matsumoto, C. Kadoi, Y. Nagaki, Y. Hayasaka et al., 1999. 1147 del A mutation in the arrestin gene in Japanese patients with Oguchi disease. Ophthalmic Genet. 20 117–120. [DOI] [PubMed] [Google Scholar]
- Zhang, S., I. Sokolchik, G. Blanco and J. Y. Sze, 2004. Caenorhabditis elegans TRPV ion channel regulates 5HT biosynthesis in chemosensory neurons. Development 131 1629–1638. [DOI] [PubMed] [Google Scholar]