Abstract
The long-term objective of this study is to develop neural prostheses for people with spinal cord injuries who are unable to voluntarily control their bladder. This feasibility study was performed in 22 adult cats. We implanted an array of microelectrodes into locations in the sacral spinal cord that are involved in the control of micturition reflexes. The effect of microelectrode stimulation was studied under light Propofol anesthesia at monthly intervals for up to 14 months. We found that electrical stimulation in the sacral parasympathetic nucleus at S2 level or in adjacent ventrolateral white matter produced bladder contractions insufficient for inducing voiding, while stimulation at or immediately dorsal to the dorsal gray commissure at S1 level produced strong (at least 20 mmHg) bladder contractions as well as strong (at least 40 mm Hg) external urethral sphincter relaxation, resulting in bladder voiding in 14 animals. In a subset of three animals, spinal cord transection was performed. For several months after the transection, intraspinal stimulation continued to be similarly or even more effective in inducing the bladder voiding as before the transection. We speculate that in the absence of the supraspinal connections, the plasticity in the local spinal circuitry played a role in the improved responsiveness to intraspinal stimulation.
Introduction
The objective of this project is to develop intraspinal prosthesis for inducing micturition in people with spinal cord injuries. In recent years, electrical stimulation of the ventral sacral spinal roots and pudendal nerve has been applied with the goal of restoring micturition in people with spinal cord injury. Ventral sacral roots contain mixed efferent projections to the bladder, the external urethral sphincter (EUS) and other somatic and visceral muscles, and therefore their continuous stimulation produces little voiding due to a coincident contraction of the bladder and constriction of the EUS (Schmidt 1986). The intermittent stimulation employed in the Brindley–Finetech system (Brindley 1995) circumvents this problem by utilizing the difference in the relaxation time of the bladder detrusor muscle and the EUS and thereby producing a transient post-stimulus voiding. However, the voiding is of short duration (voiding in spurts) and may elevate bladder pressure to supra-normal levels (Jonas and Tanagho 1975, Egon et al 1998). In addition, coincident activation of the leg musculature is a common complaint from patients (Egon et al 1998). An alternative approach of intermittent stimulation of the deep perineal afferents in the pudendal nerve is potentially more efficient in bladder emptying than ventral sacral root stimulation, yet it still produces inappropriate intermittent activation of the EUS (Boggs et al 2006). Stimulation protocols aimed at reducing urinary incontinence, another major problem of spinal cord injured patients, are also available but are not discussed here.
Our approach is to selectively stimulate the spinal cord neuronal populations specifically involved in micturition reflexes, thereby avoiding the drawbacks associated with stimulation of spinal roots. This is not a novel approach and was first attempted clinically in the 1970s (Nashold et al 1971, 1972) but was abandoned due to the inability of the large penetrating electrodes (0.4 mm in diameter with 0.5 mm of exposed tip) to selectively stimulate small volumes of spinal cord tissue, resulting in undesired activation of the EUS motoneurons in some patients. In our approach, small penetrating microelectrode arrays are designed to target the regions of the sacral spinal cord containing bladder-activating neurons, such as the sacral parasympathetic nucleus (SPN) (de Groat 1993, 2006), and EUS-inhibiting neuronal circuitry, such as the dorsal gray commissure (DGC) (Blok et al 1998, Sie et al 2001, Buss and Shefchyk 2003). In the acute cat studies, contraction of the bladder can be induced by microstimulating in or near the SPN (Carter et al 1995, Grill et al 1999, Tai et al 2004) and at least a partial relaxation of the EUS can be induced by microstimulating in the DGC (Grill et al 1999, Blok et al 1998). Recently (McCreery et al 2004), we demonstrated that minimal tissue damage is associated with long-term implantation of the intraspinal microelectrodes and identified stimulus parameters that are effective and do not induce tissue injury. The intraspinal electrodes in this study were placed dorsal of the central canal. This minimized possible adverse effects, such as damage to the ependymal cell lining of the central canal and stimulation in the ventral horn, containing the EUS-projecting and other somatic motoneurons, activation of which is counterproductive to achieving voiding. The implantation technique has been standardized and is applicable to eventual translation to clinical practice. Using this approach, we examined the effect of stimulation in the intermediate zone of the S1–S2 sacral spinal cord on the bladder and EUS tone before and after spinal cord transection.
Methods
Microelectrode array fabrication
The shafts of the discrete iridium microelectrodes were fabricated from iridium wire, 75 µm in diameter. One end of each shaft was etched electrolytically to a cone terminating in a blunt tip with a radius of curvature of 4 to 5 µm. A wire lead was micro-welded near the upper end of the shaft. The shafts and leads were insulated with 3–3.5 µm of Parylene-C, and the insulation was ablated from the tips of the shafts by an excimer laser operating at 248 nm. The surface area of the exposed tips ranged from 1600 to 2400 µm2.
The individual microelectrodes were assembled into arrays of 9, 12 or 15, extending from an ovoid superstructure cast from epoxy (EpoTek 301), which had a width of 3 mm and a length of 3–6 mm, depending on the number of microelectrodes (McCreery et al 2004). The microelectrodes were spaced 0.5 mm apart in the rostral–caudal dimension, and 0.5–0.8 mm in the mediolateral direction. The electrodes comprising the two lateral (outboard) rows extended 1.4 to 2.4 mm beneath the epoxy superstructure, and the electrodes in the medial row extended 1.3–1.8 mm beneath the superstructure. The lateral electrodes were intended to target the SPN or the SPN axonal bundle, while the medial electrodes were intended to target the DGC.
A second type of array was based on multi-site silicon substrate probes, designed to our specifications by Jamille Hetke at the University of Michigan. Each silicon probe had cross-sectional dimensions of 50 µm × 15 µm (width × thickness) and contained three electrode sites sputter-coated with iridium. These sites had a geometric surface area of approximately 2000 µm2 each and were located 1.2, 1.5 and 1.8 mm below an epoxy (EpoTek 301) superstructure. This array contained four probes (a total of 12 electrode sites), positioned in pairs laterally at a distance 1.4 mm, which is somewhat less than the distance between the SPNs. These probes have been previously tested for long-term stability after chronic implantation in the feline spinal cord (McCreery et al 2004).
After assembly, the microelectrodes of both array types were ‘activated’ (a layer of high-valence iridium oxide formed by anodic conversion) by potentiodynamic cycling between −0.8 and +0.7 volts with respect to a saturated calomel electrode in saturated sodium phosphate solution until a total charge capacity reached approximately 200 nC. The arrays were then cleaned using the modified Clemson protocol (Rowland et al 1995) and sterilized in ethylene oxide before implantation into the spinal cord.
Experimental procedure
Twenty-one male cats and one female cat, 1–2 years in age, were used. The animal studies were conducted according to NIH guidelines and were approved by the HMRI Animal Care and Use Committee. The arrays were implanted using an aseptic technique with the animals anesthetized with Isoflurane and nitrous oxide. The sacral cord was exposed using a dorsal laminectomy and the dura over the sacral cord was cut longitudinally. To locate the junction of the S1 and S2 segments, the perigenital skin was stimulated using a pair of needle electrodes inserted approximately 20 mm apart, while the evoked responses were recorded at several rostral to caudal locations along the dorsal surface of the sacral cord. The arrays were implanted near the maximum of the second component of the evoked response (the dorsal cord potential) (McCreery et al 2004). At autopsy, the position of the array relative to the spinal cord level was validated by a complete dissection of the sacral spinal roots.
The electrode cable and attached microelectrode array was routed subcutaneously from a percutaneous connector mounted on the skull. The array was inserted into the cord using a custom-made inserter tool, at a velocity of approximately 1 m s−1 (McCreery et al 2004). After the array was implanted, a stabilizing pad of polyester mesh, attached to the cable 10 mm from the array superstructure, was taped onto the dura in order to prevent the transmission of torque and longitudinal forces from the cable to the array. The dura was then closed over the array. For the first two weeks after surgery, the animals were housed in a room without perches to reduce spinal mobility and allow the array superstructure and cable to become stabilized by connective tissue.
At approximately one-month intervals, assessment of urodynamic responses to the intraspinal microstimulation was conducted. At the end of the study, the animals were deeply anesthetized and sacrificed by transcardial perfusion. The spinal cord was removed, and transverse sections were cut through the sacral cord and processed for immunohistochemistry to identify the location of the microelectrode tips relative to the neuronal populations of interest.
Two to four months after the array implantation, the spinal cord was transected at the T12–T13 junction in three cats that had good urodynamic responses to the microstimulation. A dorsal laminectomy was performed at the caudal end of T12 and rostral part of T13 vertebral processes, and the dura was exposed for a length of about 10 mm. A strip of Teflon was passed under the cord inside the dura. The spinal cord was cut with microscissors, and the strip was lifted through the cut to ensure the completeness of the transection. The dura was sutured and the incision was closed.
Urodynamic testing during intraspinal microstimulation
The cats were anesthetized with Propofol and a transurethral bladder catheter was inserted via the urethral orifice (McCreery et al 2004). The low level of Propofol anesthesia (5–7 mg kg−1 h−1, i.v.) was maintained with the animal breathing on its own and responding to strong sensory stimulation. This allowed monitoring of possible undesired effects of stimulation, such as hindlimb flexion or movement of the tail, or possible painful sensations as indicated by generalized body movement and vocalization. The bladder was filled with sterile saline through the distal port of the 2-lumen catheter (OD = 1.33 mm) until equilibrium was reached with the hydrostatic pressure in the open-top reservoir elevated approximately 20 cm above the bladder. When the bladder contracted and the EUS relaxed, urine was able to flow out around the catheter. During bladder pressure measurements, lasting up to 30 min, the connection to the reservoir was closed. Bladder pressure changes were measured via a pressure transducer (Model 041500503A, Maxxim Medical, Athens, TX). The inner lumen diameter of the catheter is large enough (1 mm) and short enough (10 cm) to convey dynamic pressure changes up to 15 Hz without phase or amplitude distortions (Li et al 1976). This is acceptable for monitoring bladder and EUS changes, which occur at frequencies below 5 Hz. All animals were tested with resting bladder pressures of 5–10 mmHg, which is below the threshold for reflex contractions (20–25 mmHg), and reflect the storage phase of the micturition cycle. In addition, two animals were also tested with high bladder pressure (above 25 mmHg), which induced spontaneous reflex bladder contractions, as occur in the voiding phase of the micturition cycle. The constrictive force, or tone, within the EUS was measured with a transducer (Maxxim Medical) as an ‘infusion pressure’, the resistance to the infusion of saline through a side port of the second lumen of the catheter positioned within the EUS as saline was infused continuously at a rate of 100 ml h−1 (Brown and Wickham 1969). The EUS was localized by slowly moving the catheter along the urethra until the region of maximum infusion pressure was found. During EUS tone measurements, the connection from the bladder lumen to open-top reservoir, set at 5 mmHg, was opened to allow infused saline to escape from the bladder. EUS tone also can be measured directly by a microtransducer, with both methods providing a similarly high degree of reliability (Wang and Chen 2002). Infusion and microtransducer measurements were compared in the condition of resting EUS pressure and infusion pressure was consistently higher than microtransducer-measured pressure by ∼22 mmHg. This should be taken into account when comparing the hydrostatic IVP data and infusion-measured EUS tone data in this study.
The data from the pressure transducers were amplified using a physiological monitor (Model 78534A, Hewlett-Packard, Palo Alto, CA), digitized at a rate of ten samples per second using a 12-bit data acquisition system (Model PCI-6070E, National Instruments, Austin, TX), and displayed and stored on a computer using custom software, written in Visual Basic (Microsoft Corporation, Redmont, WA) and the Measurement Studio ActiveX components (National Instruments, Austin, TX). After the pressure data were collected, the transurethral catheter was removed and amounts of voided and residual urine were measured following stimulation with effective electrodes. Voiding efficiency was calculated as a ratio of the voided urine to the total bladder volume (voided plus residual volumes).
The train of stimuli was delivered for 30 s either to one microelectrode or to multiple electrodes in sequence, followed by an interval of 60 s before the next stimulus train was delivered. We used cathodic-first biphasic current-controlled pulses with a duration of 0.4 ms, amplitude of 0.1 mA (unless noted otherwise in the text), at a frequency of 20 Hz. This frequency was chosen as optimal for the stimulation of bladder and EUS-controlling neurons based on our earlier acute study (Carter et al 1995). When multiple electrodes were stimulated at the same time, the interleaved mode of pulsing was used to avoid the excessive spread of effective stimulation due to current summation. Changes in bladder pressure and EUS tone were calculated as a difference of average pressures measured during 30 s of stimulation and during 30 s immediately before the stimulation (the baseline period).
Histologic procedures
The cats were deeply anesthetized by i.v. injection of pentobarbital (50 mg kg−1), then i.v. heparinized (5000 units) and perfused through the aorta for 30 s with a pre-wash solution consisting of phosphate-buffered saline, and 0.05% procaine HCl, followed by 4% paraformaldehyde. Tissue blocks containing both the microelectrodes and areas rostral and caudal to the electrodes were post-fixed overnight in 4% paraformaldehyde, dehydrated and embedded in paraffin. The paraffin-embedded tissue blocks were cut at a thickness of 6–7 µm. The sections were stained with Cresyl Violet (Nissl stain), and/or for the immunohistochemical marker NeuN. Tissue sections were photographed using a digital microscope camera (Spot RT, Diagnostic Instruments Inc, Sterling Heights, MI).
NeuN immunohistochemistry
Immunohistochemistry for the neuron-specific protein NeuN was done using the monoclonal antibody (MAB377, 1:40, Chemicon, Temecula, CA), which selectively binds to proteins within the nucleus (primarily) and cytosol of neurons throughout the nervous system. We employed an antigen retrieval technique in which the sections on glass slides were microwaved for 12 min at 560 W in acidic citrate buffer. The immunoreactivity was visualized using biotinylated secondary antibody and peroxidase chromogen (DAB). At the end, the tissue sections were lightly counterstained with Cresyl Violet.
Statistics
Values of data sets are expressed as mean ± standard deviation. In evaluation of sub-acute and chronic effects of the spinal transection on voiding, we used one-way repeated-measures ANOVA followed by Bonferroni’s multiple comparison post-hoc test on predefined comparisons (Prism 4.02, GraphPad Software, San Diego, CA). Significance was set at p < 0.05 after Bonferroni’s correction.
Results
Twenty-two cats were used in the present study. We implanted the arrays of discrete iridium microelectrodes into the sacral cords of 20 cats, and arrays of multi-site silicon-substrate probes into two additional cats. All of the cats displayed normal behavior and no neurological disorders after the implantation until their sacrifice at 2–14 months after the procedure.
After the microelectrode arrays were implanted in the sacral spinal cord, the impedance of individual electrodes was measured at monthly intervals. The impedances remained relatively unchanged (less than a 20% difference from the initial value) for the duration of implantation, indicating the long-term stability of the electrode-tissue interface and allowing sufficient charge capacity for stimulating at up to 40 nC per phase.
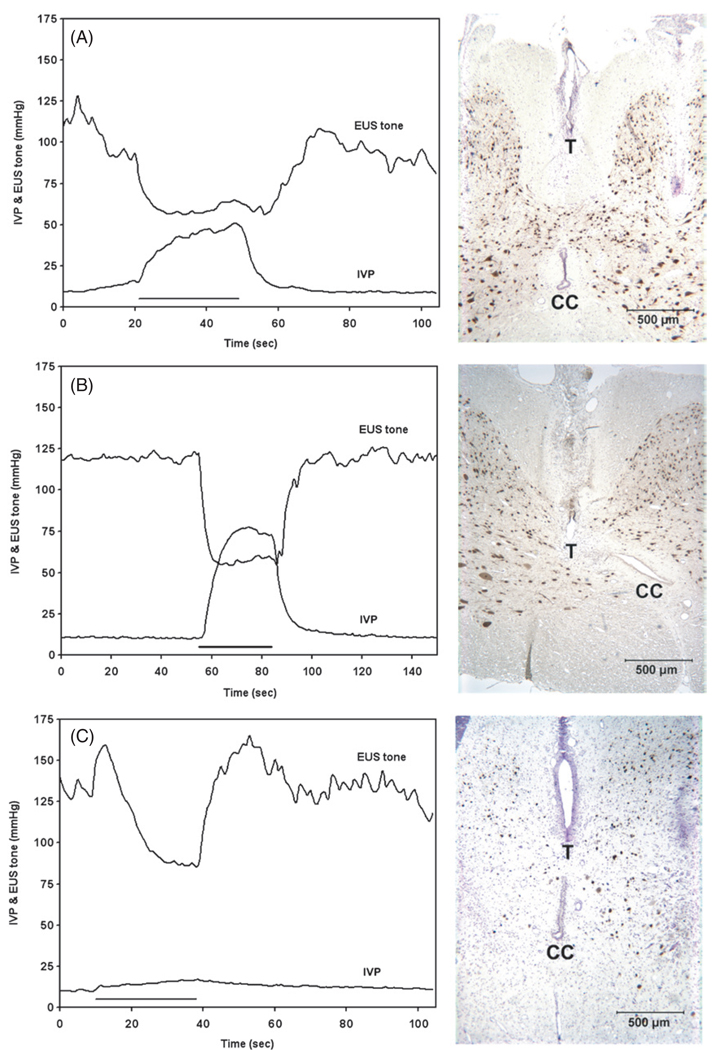
Figure 1 illustrates some typical responses to microstimulation. The most desirable effect of the stimulation was a coincident sustained elevation of bladder pressure and reduction of EUS tone (figures 1(A) and (B)), with no significant fatigue of the bladder or EUS responses during three cycles of 30 s of stimulation and 60 s without stimulation. This coordinated response of the bladder and EUS, often seen during stimulation with individual electrodes, was the most effective for the induction of voiding; and in the examples shown in figures 1(A) and (B), after the catheter was removed from the urethra, stimulation with the microelectrodes produced a continuous stream of urine leading to emptying of 70% of the bladder volume. Histological analysis showed that the microelectrode tip was located in the medial part of the dorsal white matter (the fasciculus gracilis) at the middle of the S1 level in figure 1(A) and in the DGC at the caudal S1 level in figure 1(B).
Figure 1.
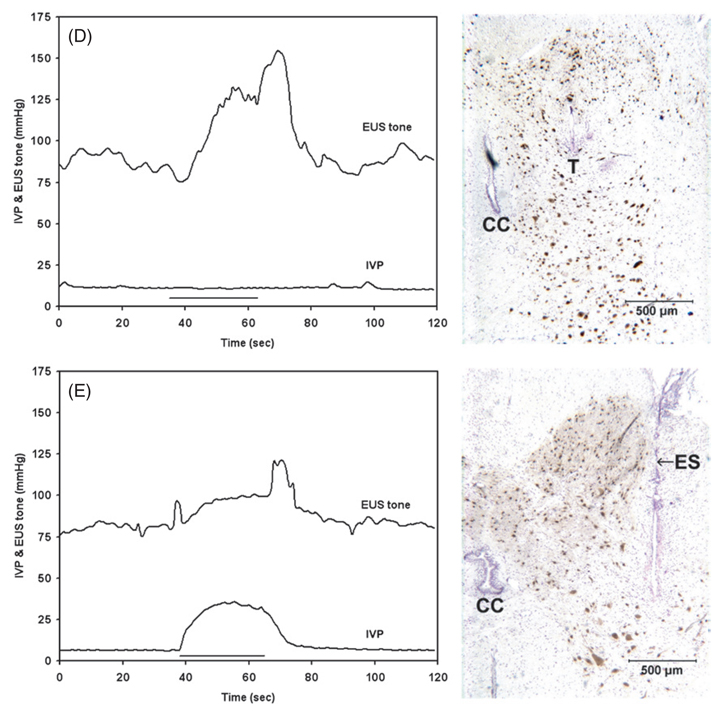
Changes in intravesical pressure (IVP) and EUS tone during stimulation with individual intraspinal microelectrodes. The left panels show IVP and EUS tone as a function of time, where the duration of the applied stimulation train is indicated by a solid horizontal line. The right panels are the photomicrographs of the sites of the microelectrodes in the sacral spinal cord. The tips of discrete electrodes (T) or electrode sites of the silicon probes (ES), as well as the central canal (CC), are indicated. The spinal cord tissue was immunostained with NeuN and counterstained with Cresyl Violet. Panels (A)–(E) represent five examples of evoked bladder and EUS activities. The stimulus current amplitude in all cases was 100 µA. IVP and EUS tone responses were examined simultaneously in (A) and (C)–(E), and separately in (B). The microelectrode sites in the right panels were located in the middle S1 (A), (C), caudal S1 (B), (E) and rostral S2 levels (D). See results for further details.
Some microelectrodes produced a significant sustained inhibition of EUS tone, but no effect on IVP (figure 1(C)). In the example shown, the microelectrode tip was located close to the DGC immediately above the central canal. During three cycles of stimulation, there was no significant fatigue in the EUS response. When the catheter was removed from the urethra, stimulating with this microelectrode produced drop-by-drop voiding, which could be augmented by applying gentle manual pressure to the bladder.
Stimulation with just two microelectrodes located in the intermediate zone at the S1 level produced an unusual marked increase in the tone of the EUS and no significant effect on the bladder (one of these electrodes is shown in figure 1(D)). After the withdrawal of the catheter from the urethra, stimulation with these microelectrodes produced no voiding.
Stimulating with microelectrodes located in the dorsal horn sometimes produced bladder and EUS co-contraction (figure 1(E)). This sometimes was accompanied by bilateral hindlimb flexion, indicating a potentially painful stimulus. In the example shown in figure 1(E), the active electrode site on the silicon probe was located in the lateral edge of the middle of the dorsal horn. Since the electrodes were targeted to the intermediate zone of gray matter, only a few electrodes were located in the dorsal part of the dorsal horn which contains the neuronal circuitry that mediates nociception, and such responses to stimulation were not seen frequently. We have never observed non-specific responses stronger than slight body movement or bilateral hindlimb contraction. Several electrode sites were located either in or lateral to the ventral horns of the sacral spinal cord and produced what appeared to be direct activation of the motoneurons innervating the hind limb muscles (data not shown). This probably was due to direct excitation of the axons of the motoneurons from the lumbar segments that pass close to the ventrolateral surface of the sacral cord. The stimulation in all of these 23 cases (11% of all electrodes) was immediately terminated.
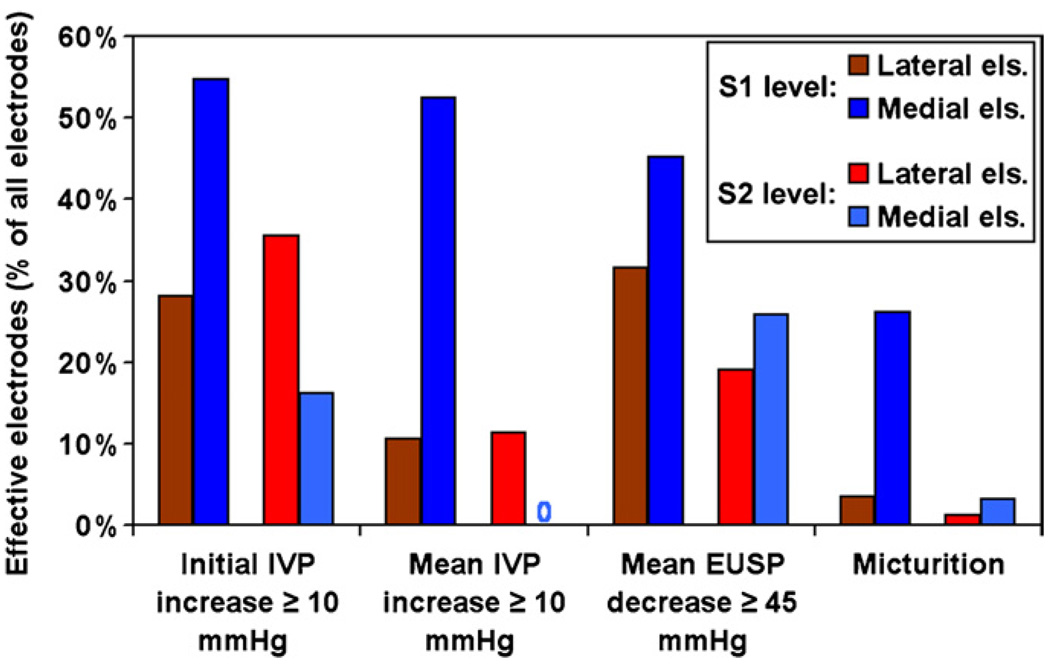
Stimulating with individual microelectrodes produced different effects on the bladder and EUS activity depending on their location in the sacral spinal cord. These effects were reproducible from session to session, indicating the stability and lack of movement of chronic implants in the tissue. As presented in our earlier study, microelectrodes separated by as little as 300 µm produced markedly different effects on the bladder and EUS (McCreery et al 2004). In the present study, the responses to intraspinal stimulation at the middle and caudal S1 and rostral, middle and caudal S2 spinal levels were also quite different and, therefore, have been analyzed separately. In addition, at each level, the efficiency of stimulation was analyzed separately for laterally and medially located electrodes. Numbers and percentages of the effective individually pulsed microelectrodes at various rostral–caudal and medial–lateral levels in the sacral cord are summarized in table 1 and figure 2 and figure 3. In our analysis of the IVP responses, we evaluated both transient and sustained IVP increases. About 30% of the lateral electrodes induced initial IVP increases and only 10% (or one third of electrodes that induced an initial IVP increase) induced sustained IVP increases. Medial electrodes were most effective at the S1 spinal level, where more than 50% of them induced an increase in IVP, which was almost always sustained for the duration of the stimulus train. At the S2 spinal level, none of the medial electrodes induced a sustained IVP increase.
Table 1.
Counts of effective microelectrodes implanted into the S1–S2 sacral cord of 22 cats and stimulated individually. Electrode counts are shown as the total count (lateral count + medial count).
| Spinal cord level |
||||||
|---|---|---|---|---|---|---|
| mS1 | cS1 | rS2 | mS2 | cS2 | Total | |
| # of animals | 5 | 6 | 5 | 2 | 4 | 22 |
| # of electrodes | 36 (21+15) | 63 (36+27) | 45 (36+9) | 20 (12+8) | 45 (31+14) | 209 (136+73) |
| # of electrodes ind. initial IVP increase ≥10 mmHg | 17 (9+8) | 22 (7+15) | 13 (10+3) | 2 (2+0) | 18 (16+2) | 72 (44+28) |
| # of electrodes ind. mean IVP increase ≥10 mmHg | 7 (0+7) | 21 (6+15) | 2(2+0) | 2(2+0) | 5 (5+0) | 37 (15+22) |
| # of electrodes ind. mean EUSP decrease ≥45 mmHg | 21 (11+10) | 16 (7+9) | 13 (10+3) | 3 (2+1) | 7 (3+4) | 60 (33+27) |
| # of electrodes ind. micturition | 3 (0+3) | 10 (2+8) | 0 | 0 | 2 (1+1) | 15 (3+12) |
Abbreviations: #—count, ind.—inducing, ≥—not less than, IVP—intravesical pressure, EUSP—external urethral sphincter pressure, m—middle, c—caudal, r—rostral.
Figure 2.
Counts of effective lateral/medial microelectrodes at S1 and S2 spinal levels from 22 cats, presented as a percentage of all lateral/medial microelectrodes at that level. An ‘effective’ electrode was defined as being able to induce an initial and mean intravesical pressure (IVP) increase ≥10 mmHg, or a mean EUS pressure (EUSP) decrease ≥45 mmHg, or micturition after the withdrawal of the transurethral catheter.
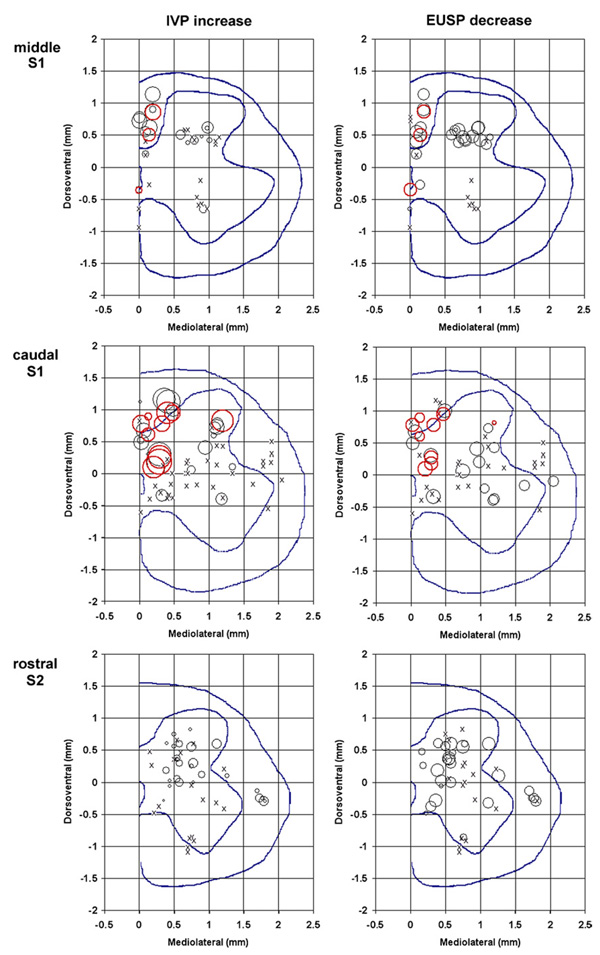
Figure 3.
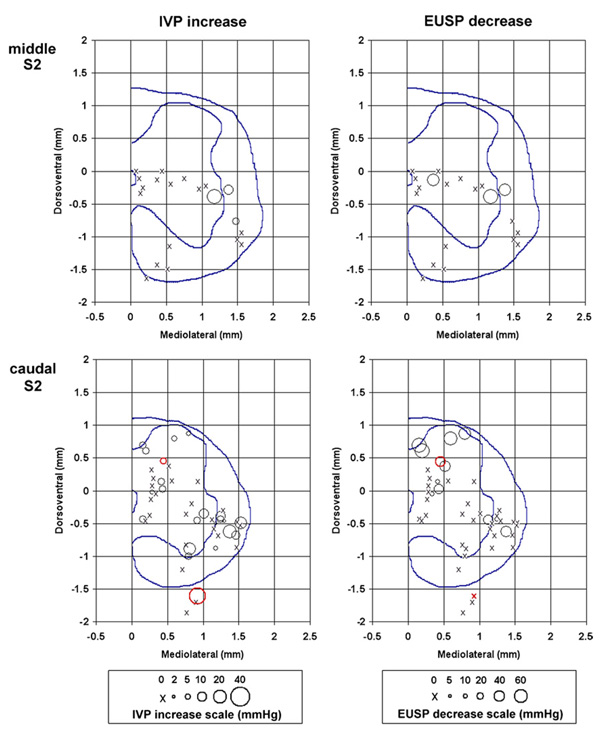
Tip/electrode site locations in the sacral spinal cord of the microelectrodes that produced micturition-related effects. The left panels show the increases in the intravesical pressure (IVP) and the right panels show decreases in the EUS pressure (EUSP) for different microelectrode locations. Red circles (online) indicate electrodes that were effective in producing at least drop-by-drop voiding. The circle size indicates the amount of pressure change from the baseline level. The scales relating the circle sizes and pressure changes are presented below the panels. The mediolateral and dorsomedial coordinates are given in reference to the dorsal edge of the central canal. The dotted lines in each panel represent the boundary of the gray matter and the location of the central canal.
The decreases in EUS tone were almost always sustained throughout the duration of the stimulus pulse. Large (≥45 mmHg) decreases in EUS tone were more easily obtained at the S1 spinal level for both lateral and medial electrodes (32% and 45% of electrodes were effective, respectively) than at the S2 level (19% and 26% of electrodes effective, respectively). More electrodes induced large decreases in EUS tone than induced moderate increases (≥10 mmHg) in IVP.
Only a small percentage (1–4%, depending upon the level) of the lateral electrodes induced micturition. Micturition was induced by 26% of the medial electrodes at the S1 level, and by 3% of the medial electrodes at the S2 level. At all levels, micturition was induced less frequently than either an IVP increase or EUS tone decrease alone, emphasizing the importance of coordinated bladder contraction and EUS inhibition in order for successful micturition to occur. The better performance of electrodes in the middle and caudal S1 levels as compared to other levels correlated with the results of the acute dorsal cord potential mapping during implantation, where the same levels were noted to receive maximal sensory input from the perigenital skin area (data not shown).
After the animals were sacrificed, the intraspinal locations of the stimulation sites were determined and entered into a series of profiles for each spinal cord level. Figure 3 shows the micturition-related responses at each spinal cord level. The profiles of the spinal cord for each spinal level were created by computerized averaging of the tracings of the spinal cord outlines and the boundaries between the gray and white matter from several animals. The locations of the stimulation sites are marked as circles of different diameters, indicating the amplitudes of intravesical pressure (IVP) increases and of EUS pressure (EUSP) decreases, or as an ‘X’ (ineffective sites). At the S1 spinal segment, 26% of the microelectrodes immediately dorsal to the DGC produced a strong coincident contraction of the bladder and inhibition of the EUS tone, and produced voiding upon withdrawal of the transurethral catheter (figure 1(A) and figure 2). At the S2 segment, some microelectrodes located in the white matter just lateral to the SPN also produced a moderate increase in the bladder pressure and suppression of the EUS tone. Fifteen electrodes were effective in producing at least drop-by-drop voiding (shown in red (online) in figure 3). Of those, nine were located in the intermediate zone of the spinal cord, spanning from the DGC medially to the SPNs laterally. Five electrodes were located in the ventral portion of the dorsal column, adjacent to the intermediate zone. The 15th electrode was located in the ventral root.
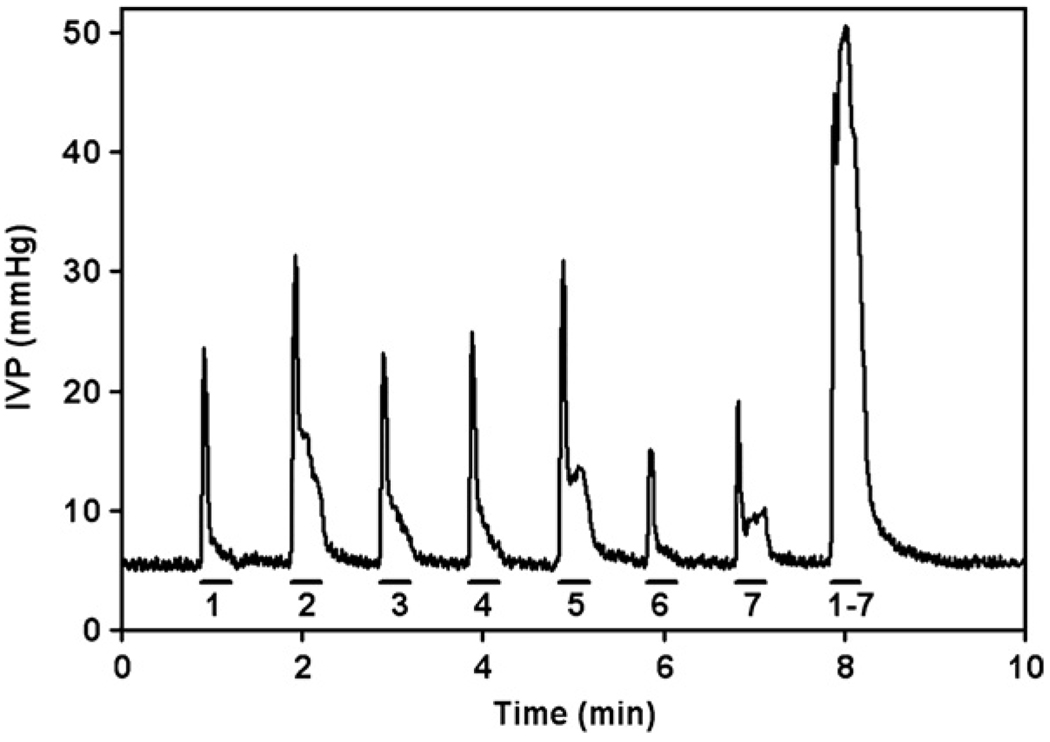
In most cats, interleaved pulsing with two or more of the lateral microelectrodes produced a larger (≥20 mmHg) and more sustained increase in bladder pressure than could be elicited with any one microelectrode. For example, in one cat (figure 4), the maximal sustained increase in bladder pressure that could be elicited by any one electrode was no more than 10 mmHg, but when seven electrodes were pulsed in the interleaved mode, bladder pressure increased by more than 40 mmHg and continuous micturition was induced with a residual volume of about 15 ml. This type of synergy was never observed for the relaxation of the EUS by stimulation with the electrodes near the midline of the spinal cord, and in most instances, pulsing with more than one of the effective medial electrodes produced less relaxation of the EUS than the most effective of these microelectrodes when it was pulsed alone.
Figure 4.
Changes in intravesical pressure (IVP) during stimulation with microelectrodes individually or with multiple electrodes, the latter in the interleaved mode. The solid horizontal lines indicate durations of the applied stimulation and the numbers below them indicate the pulsed electrode(s).
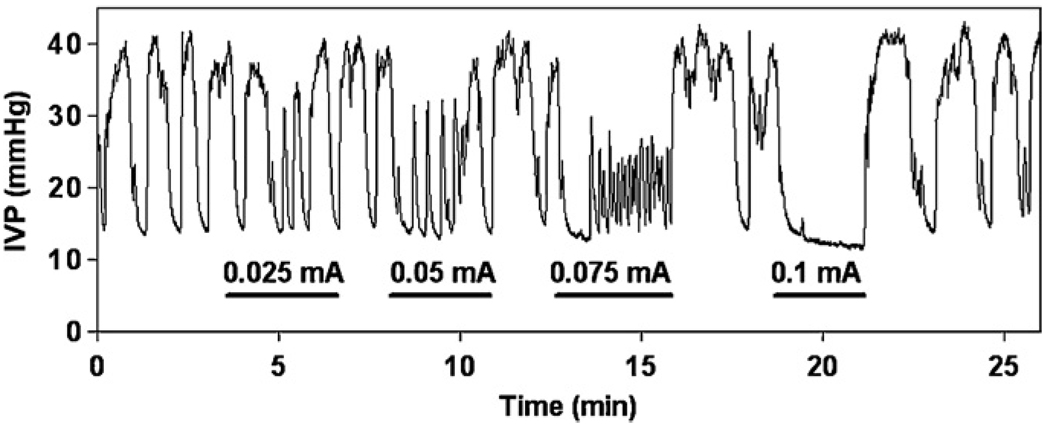
In two animals, we have also examined the effect of the intraspinal stimulation on the spontaneous bladder contractions, which began to occur when the baseline bladder pressure exceeded 25 mmHg. In the example shown (figure 5), stimulation with the microelectrode located in the middle of the dorsal horn produced a marked inhibition of spontaneous bladder contractions. When the bladder was filled to a pressure below 25 mmHg and in the absence of spontaneous contractions, the same microelectrode produced a small increase (7 mmHg) of bladder pressure. Similar suppression of the spontaneous bladder activity was seen during stimulation with almost all microelectrodes that produced contraction of the bladder at a low baseline pressure.
Figure 5.
Inhibition of spontaneous bladder activity by intraspinal stimulation in animals with intact spinal cords as measured by intravesical pressure (IVP). The duration of the applied stimulation is indicated by a solid line. Current amplitudes are indicated above the lines.
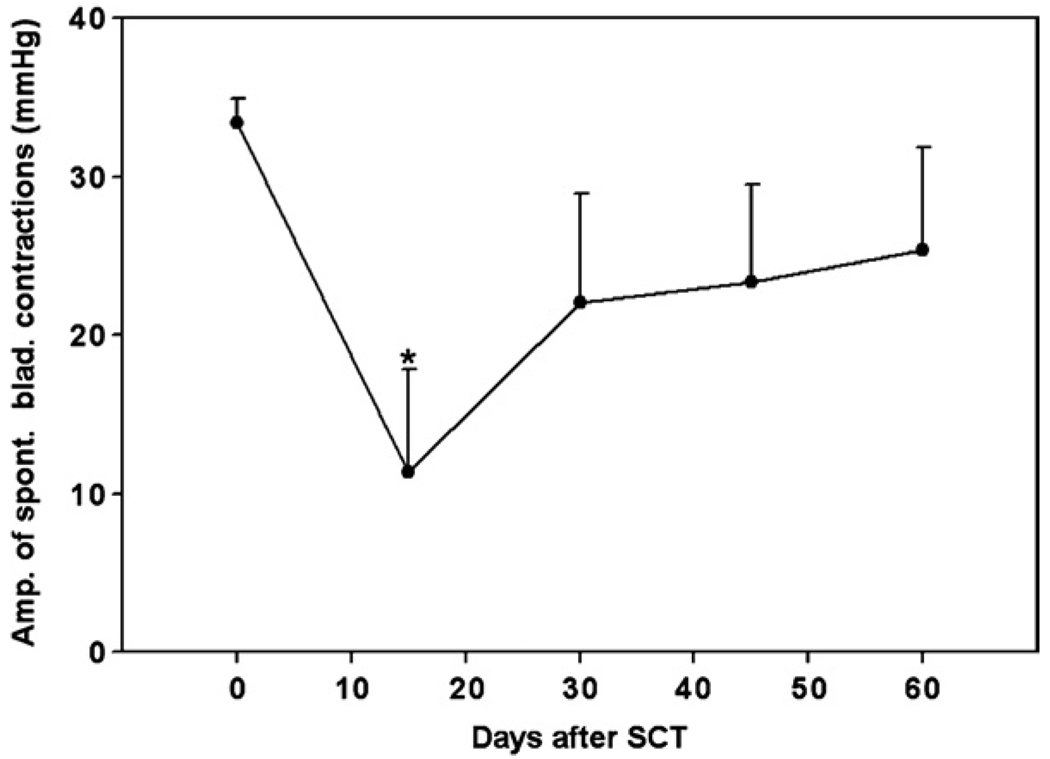
In three cats, we performed complete spinal transection (SCT) at the low thoracic level (T12–T13 junction) and maintained the animals for up to 352 days. Following SCT, all three cats were initially unable to empty their bladder, and bladder evacuation was performed twice a day by intermittent catheterization. During the first week after SCT, large amounts of urine were collected during manual evacuation. On the eighth day after SCT and thereafter, the total daily amount decreased to less than 70 ml and when the tactile stimulation was applied to the perigenital area by a cotton swab or during movement, the animals were seen voiding in spurts. At 15 days after SCT, all three cats exhibited reduced amplitudes of spontaneous bladder contractions (11 ± 7 mmHg, down from 33 ± 2 mmHg before SCT), which began to recover at 30 days post-SCT (23 ± 7 mmHg) (figure 6). None of the spinalized cats in this study developed the full-scale bladder–sphincter dyssynergia, as evidenced by coincident reduction of EUS tone during spontaneous bladder contractions resulting in drop-by-drop voiding. In non-anesthetized cats, this spontaneous dripping of urine, which commonly occurred during the animal’s movement, prevented bladders from overfilling. After this spontaneous voiding developed at two weeks after SCT, the residual urine amount in the bladder of all cats was about 30–45 ml (about 30–45% of the total bladder volume). Hyperreflexic twitchlike contractions of the EUS of 5–10 mmHg throughout the bladder-filling phase first appeared at 30 days after SCT and persisted thereafter.
Figure 6.
Average amplitudes of spontaneous bladder contractions in three animals with complete SCT at the T12–T13 level. Error bars indicate standard deviations. The asterisk indicates significant difference (p < 0.05) from the pre-SCT value based on one-way repeated-measures ANOVA followed by Bonferroni’s multiple comparison post-hoc test.
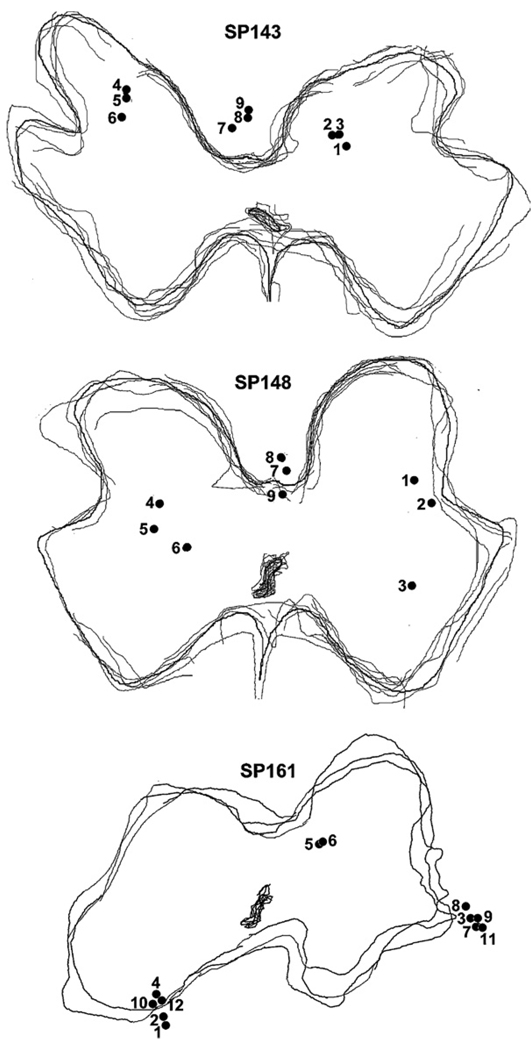
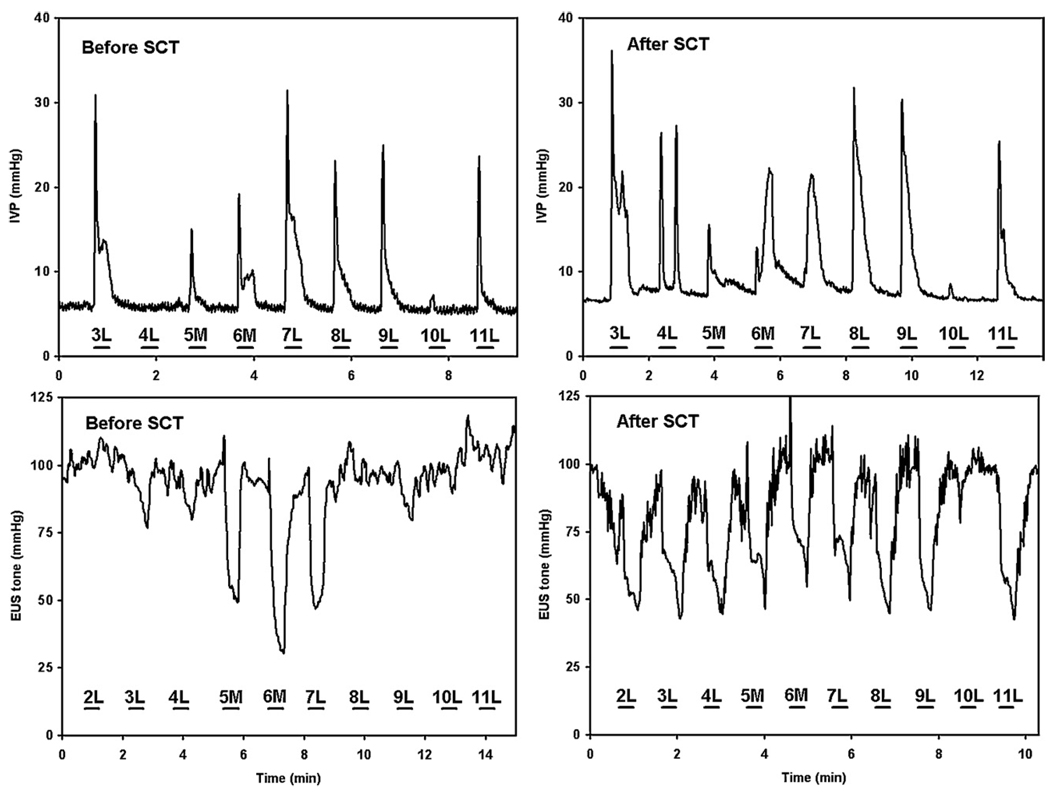
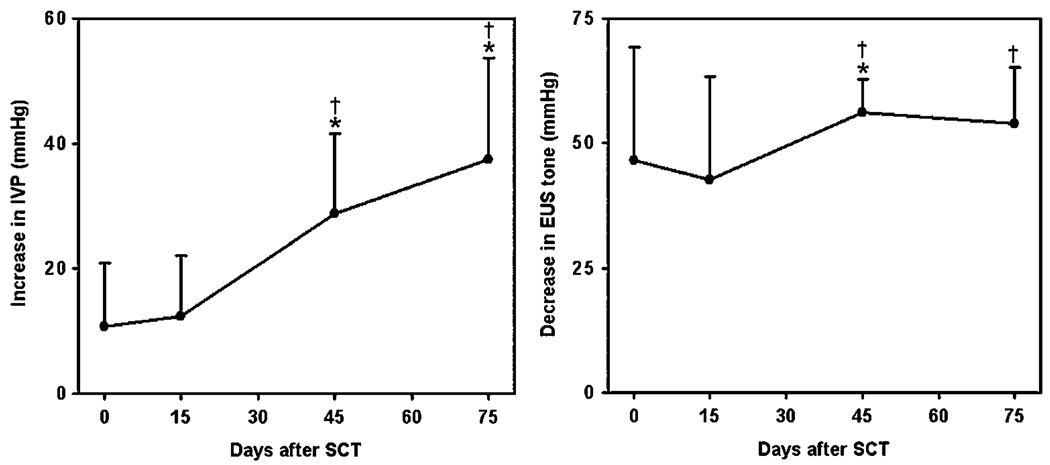
The locations of the electrode tips in the lumbosacral cord of SCT animals are shown in figure 7. Figure 8 and Figure 9 show the correlation between the tip sites and bladder and EUS responses to intraspinal stimulation with individual microelectrodes, before and after SCT. In the animal SP161, at 15 days post-SCT, bladder responses were similar to those before SCT (like those in figure 1(A)), but beginning at 45 days post-SCT, the intraspinal stimulation produced a larger increase in IVP (figure 8). Similarly, the EUS responses to stimulation increased after SCT (figure 8). Averaged longitudinal changes in the performance of effective electrodes in three SCT animals are shown in figure 9. Stimulation with the effective microelectrodes, in addition to inhibiting the EUS tone, inhibited the hyperreflexic twitch-like contractions of the EUS.
Figure 7.
Locations of the electrode tips in the lumbosacral cord of three SCT animals. Multiple gray matter outlines were traced from tissue sections through the electrode locations. The numbers next to electrode tips indicate the electrode numbers within the array.
Figure 8.
Intravesical pressure (IVP) and EUS tone responses to stimulation of individual lateral (L) and medial (M) electrodes in animal SP161 before and 45 days after the SCT.
Figure 9.
Average increase in intravesical pressure (IVP) (left panel) and decrease in EUS pressure (EUSP) (right panel) in response to stimulation with individual electrodes in three SCT animals. IVP data were compiled from all nine electrodes in SP143, three electrodes in SP148 (7, 8, 9) and three electrodes in SP161 (3, 7, 8). EUS data were compiled from all nine electrodes in SP143, all nine electrodes in SP148 and nine electrodes in SP161 (2–9, 11). Error bars indicate standard deviations. Asterisks and daggers (†) indicate significant differences (p < 0.05) from values before SCT and 15 days post-SCT, correspondingly, based on one-way repeated-measures ANOVA followed by Bonferroni’s multiple comparison post-hoc test.
After removal of the catheter, voiding was induced by stimulation with the electrodes that produced an increase in bladder pressure and a decrease in EUS tone. Voiding volume and completeness increased after SCT, in some cases producing near-complete emptying of the bladder (table 2). Since voiding was evaluated only once at each time-point, the data in table 2 are considered preliminary and no statistical analysis of it was attempted.
Table 2.
Voiding efficiency, calculated as a ratio of the voided urine to the total bladder volume, in three cats before and at 30 days after the SCT. Effective electrodes were pulsed in the interleaved fashion. Each value is based on one measurement.
| Voided volume (ml) |
Residual volume (ml) |
Voiding efficiency (%) |
||||||
|---|---|---|---|---|---|---|---|---|
| Animal | Spinal level | Pulsed electrodes | pre SCT | post SCT | pre SCT | post SCT | pre SCT | post SCT |
| SP143 | Middle S1 | 4,7,8 | 7 | 55 | 28 | 7 | 20 | 89 |
| SP148 | Caudal S1 | 4,6,7,8,9 | 1 | 9 | 27 | 50 | 4 | 15 |
| SP161 | Rostral S2 | 3,5,6,7,8,9 | 2 | 48 | 35 | 12 | 5 | 80 |
| Average ± SD | 3 ± 3 | 37 ± 25 | 30 ± 4 | 23 ± 24 | 10 ± 9 | 61 ± 40 | ||
Discussion
In this study, we have performed a comprehensive evaluation of the micturition-related responses under light anesthesia during intraspinal stimulation with two types of microelectrode arrays chronically implanted into the sacral spinal cord of cats. Stimulation with the electrodes located immediately dorsal to the DGC at the S1 level evoked strong sustained elevations of bladder pressure of about 40 mmHg and sustained inhibition of the EUS tone. Stimulating in the SPN and in adjacent white matter of the lateral funiculi at the S2 level usually produced a smaller increase in bladder pressure and inhibition of EUS tone. Twenty-six per cent of the medially located electrodes at the S1 level produced contraction of the bladder and relaxation of the EUS and also induced voiding. Altogether, some micturition (usually drop-by-drop) was achieved by stimulating with at least one of the electrodes in 15 out of 22 cats (see table 1). We attribute this low success rate to the small size and low electrical excitability (high current-distance constant) of the neuronal pools and interconnecting axonal bundles mediating micturition and to insufficient spatial density of electrodes to reliably achieve accurate targeting of these neuronal populations. This study provided an initial demonstration of neuroprosthetic micturition in the feline model of spinal cord injury and serves to inform the design of future microelectrode arrays that will require a higher density of stimulation sites in the transverse plane. The findings from this and our earlier study (McCreery et al 2004) indicate that the inter-electrode distance should be no greater than 0.3 mm, in the dorsal–ventral and the medial–lateral directions. The spatial density of stimulation sites in the arrays used in the present study would be insufficient to reliably target the stimulus in order to produce bladder emptying without adverse effect due to the current spread (e.g, uncomfortable sensations in patients with incomplete SCI). We expect that the multisite silicon probes we are developing will be able to overcome this limitation.
Our results concur with and extend the previously published results derived from an acute mapping study in the cat (Grill et al 1999), which examined the bladder and passive EUS pressure (rather than infusion pressure within the EUS as in the present study) using microelectrodes from our laboratory and stimulus parameters similar to those used in our study. However, Grill et al used short stimulus trains, 1 s in duration and, thus could not evaluate the sustainability of the pressure changes. A successful intraspinal neuroprosthesis would need to maintain contraction of the bladder and relaxation of the EUS in order to allow near-complete voiding. In our study the electrodes were implanted chronically which allows the encapsulation of the array and formation of a stable electrode-tissue interface. In addition, the chronic implant makes it possible to use very light anesthesia during urodynamic testing, allowing the animals to respond to strong tactile stimulation and with less anesthetic effect on micturition reflexes. Propofol anesthesia, according to the literature, does not inhibit the parasympathetic bladder contraction reflex, only slightly suppresses the sympathetic bladder filling reflex (Mills et al 2000), and does not affect spino-cortical axonal conduction (Liu et al 2005). The possible suppression of the sympathetic bladder filling reflex in this study may have resulted in a higher voiding threshold pressure, as compared to the unanesthetized state, but was unlikely to have affected the maximal voiding pressure or voiding efficiency.
Bladder voiding is coordinated via a supraspinal descending tract that terminates in the DGC at the S1–S2 levels and inhibits the EUS motoneurons (Blok et al 1997, 1998). The same area receives heavy innervation from primary bladder afferents (Ueyama et al 1984, Thor et al 1989). In our study, most of the electrode sites that produced activation of the bladder and suppression of the EUS tone were located in the same area and in adjacent dorsal columns. Taking into account that the effect of stimulation in the dorsal columns persisted after spinal cord transection, we speculate that predominantly fibers of spinal rather than supraspinal origin were activated, even in the animals with intact spinal cords.
In the cats with intact spinal cords, when the bladder pressure was low (20–25 mmHg), many microelectrodes produced an increase in bladder pressure, but when the bladder pressure was high enough to induce the spontaneous bladder contractions (more than 25 mmHg), the same microelectrodes produced inhibition of spontaneous contractions. This suggests a phase-dependent modality of bladder control in the sacral spinal cord, which was also reported in an acute study conduced in cats (Grill et al 1999). The phase-dependent effect of the stimulation on the bladder accompanied by the inhibition of the EUS tone suggests that the bladder and EUS motoneurons were activated indirectly via interneurons rather than directly. In this respect, placing the electrodes in the intermediate zone of the spinal cord provides unique benefits because this area mediates integration of the sensory inputs from the bladder as well as initiation of the coordination between the bladder and the EUS and thus may provide sensory information about the state of the bladder. The clinical device for micturition control in spinal cord injured patients could implement a closed-loop initiation of micturition based on bladder sensory information recorded by the same array of microelectrodes, and so initiate the stimulation before the bladder fills to the degree that electrical stimulation is less effective. In addition, activation of motoneurons indirectly via premotor interneurons provides a more natural (small to large) and more fatigue-resistant recruitment of motoneurons (Henneman et al 1965, Collins et al 2001).
It was not possible in the present study to determine whether the observed effects on the bladder and EUS were due to stimulation of cell bodies, axons or dendrites, or some combination thereof. Computer modeling studies indicate that the symmetrical cathodic-first pulses used in this study might preferentially excite presynaptic terminals of axons rather than cell bodies (McIntyre and Grill 2000, 2002) and animal studies provide empirical support for this notion (Gustafsson and Jankowska 1976, Gaunt et al 2006).
At one to two weeks after the SCT, the animals regained the ability to void spontaneously or in response to gentle tactile stimulation in the perigenital area, similarly to the results of de Groat (2006), who suggested that this recovery represents re-emergence of the neonatal reflexive voiding. In addition, the possibility of passive urine leakage due to acute weakness in EUS tone cannot be excluded. Recovery of ‘automatic’ voiding after SCT has been documented in dogs (Walter et al 1989) and even humans (de Groat 2006). In the urodynamic setting, all three cats exhibited a temporary reduction in the amplitude of spontaneous bladder contractions at 15 days post-SCT which then recovered to near-normal amplitudes after 30 days or more.
None of the chronically spinalized cats in this study developed full-scale bladder–sphincter dyssynergia preventing animals from urinating on their own. While no other studies directly evaluated the bladder and EUS pressures in SCT cats, a lack of bladder–anal-sphincter dyssynergia was seen in chronically spinalized cats when the sphincter pressure was measured directly (Walter et al 2003) as opposed to indirect evaluation using EMG electrodes implanted into the muscles of the pelvic floor (Galeano et al 1986). EMG measurements of EUS activity suffer from confounding influences of other urethral activities, such as peristaltic-like propagation of urine and urethral emptying reflex at the end of voiding (Walter et al 1997, 2003). This lack of obstructive bladder–sphincter dyssynergia is a shortcoming of animal models of SCT, because nearly all humans with complete spinal cord injury do develop complete urine retention due to this dyssynergia (Weld et al 2000, Walter et al 2003). We speculate that this difference in EUS responses after SCT is due to a competition of two types of plastic changes occurring in the sacral spinal cord (Little et al 1999, Pikov 2004). The adaptive plastic changes are due to the formation of stronger connections between bladder afferents and an integrative interneuronal pool(s) mediating coordinated micturition (such as central pattern generator for locomotion). On the other hand, maladaptive changes are due to the formation of inappropriate connections between bladder afferents and ventrally located motoneurons and pre-motoneurons in Onuf’s nucleus, involved in EUS activation.
Stimulation with implanted microelectrodes at sub-acute (15 days) and chronic (45 days or more) times after SCT revealed the development of a supra-normal bladder and EUS response to the stimulation, which may be a result of plasticity of bladder afferents or intraspinal interneurons (Vizzard 2006, Zinck and Downie 2005, Yu et al 2003). An alternative hypothesis, explaining the observed improvement in the effectiveness of the intraspinal stimulation after SCT, is a migration of electrode tips in the neural tissue. While we cannot rule out the possibility of such movements, it is very unlikely that such movements would improve the effectiveness of nearly all of the electrodes, as seen in the SCT cats.
Two methodological considerations have emerged from this study. First, the level of maximal cord dorsum potential in response to perigenital skin stimulation has been a reliable physiological marker of S1–S2 junction, allowing intraoperative localization of optimal rostrocaudal location for the array implantation. Second, near-complete bladder voiding was achieved only in a few animals, indicating the need for further improvement in the design of intraspinal arrays, such as increasing the number and density of stimulation sites.
In conclusion, this study demonstrated the feasibility of achieving effective voiding in intact and spinally injured animals by stimulating with microelectrodes chronically implanted into the sacral spinal cord. The long-term goal of this study is to apply intraspinal electrical stimulation to regain voluntary control of the bladder in people with spinal cord injuries above low lumbar segments, for which neuronal circuitry, essential to voiding, is not significantly damaged by the injury.
Acknowledgments
This work was supported by contracts N01-NS-8-2399 and N01-1-NS-1-2399 from the NINDS of the National Institutes of Health (USA). The multi-site silicon probes were fabricated by Jamille F Hetke at the University of Michigan. The authors express their appreciation to Yelena Smirnova and Nijole Kuleviciute for fabricating the microelectrode arrays, Edna Smith for assistance with the surgical procedures, Dr Albert Lossinsky for animal perfusion and autopsy, and Clarence Graham and Jesus Chavez for their histological and immunohistochemical work.
References
- Blok BF, de Weerd H, Holstege G. The pontine micturition center projects to sacral cord GABA immunoreactive neurons in the cat. Neurosci. Lett. 1997;233:109–112. doi: 10.1016/s0304-3940(97)00644-7. [DOI] [PubMed] [Google Scholar]
- Blok BF, van Maarseveen JT, Holstege G. Electrical stimulation of the sacral dorsal gray commissure evokes relaxation of the external urethral sphincter in the cat. Neurosci. Lett. 1998;249:68–70. doi: 10.1016/s0304-3940(98)00382-6. [DOI] [PubMed] [Google Scholar]
- Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Bladder emptying by intermittent electrical stimulation of the pudendal nerve. J. Neural. Eng. 2006;3:43–51. doi: 10.1088/1741-2560/3/1/005. [DOI] [PubMed] [Google Scholar]
- Brindley GS. The sacral anterior root stimulator as a means of managing the bladder in patients with spinal cord lesions. Baillieres. Clin. Neurol. 1995;4:1–13. [PubMed] [Google Scholar]
- Brown M, Wickham JE. The urethral pressure profile. Br. J.Urol. 1969;41:211–217. doi: 10.1111/j.1464-410x.1969.tb09925.x. [DOI] [PubMed] [Google Scholar]
- Buss RR, Shefchyk SJ. Sacral dorsal horn neurone activity during micturition in the cat. J. Physiol. 2003;551:387–396. doi: 10.1113/jphysiol.2003.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RR, McCreery DB, Woodford BJ, Bullara LA, Agnew WF. Micturition control by microstimulation of the sacral spinal cord of the cat: Acute studies. IEEE Trans. Rehabil. Eng. 1995;3:206–214. [Google Scholar]
- Collins DF, Burke D, Gandevia SC. Large involuntary forces consistent with plateau-like behavior of human motoneurons. J. Neurosci. 2001;21:4059–4065. doi: 10.1523/JNEUROSCI.21-11-04059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC. Anatomy and physiology of the lower urinary tract. Urol. Clin. North. Am. 1993;20:383–401. [PubMed] [Google Scholar]
- de Groat WC. Integrative control of the lower urinary tract: preclinical perspective. Br. J. Pharmacol. 2006;147 Suppl 2:S25–S40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egon G, Barat M, Colombel P, Visentin C, Isambert JL, Guerin J. Implantation of anterior sacral root stimulators combined with posterior sacral rhizotomy in spinal injury patients. World J. Urol. 1998;16:342–349. doi: 10.1007/s003450050078. [DOI] [PubMed] [Google Scholar]
- Galeano C, Jubelin B, Germain L, Guenette L. Micturitional reflexes in chronic spinalized cats—the underactive detrusor and detrusor-sphincter dyssynergia. Neurourol. Urodyn. 1986;5:45–63. [Google Scholar]
- Gaunt RA, Prochazka A, Mushahwar VK, Guevremont L, Ellaway PH. Intraspinal microstimulation excites multisegmental sensory afferents at lower stimulus levels than local α-motoneurons. J. Neurophysiol. 2006;96:2995–3005. doi: 10.1152/jn.00061.2006. [DOI] [PubMed] [Google Scholar]
- Grill WM, Bhadra N, Wang B. Bladder and urethral pressures evoked by microstimulation of the sacral spinal cord in cats. Brain. Res. 1999;836:19–30. doi: 10.1016/s0006-8993(99)01581-4. [DOI] [PubMed] [Google Scholar]
- Gustafsson B, Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J. Physiol. 1976;258:33–61. doi: 10.1113/jphysiol.1976.sp011405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J. Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Jonas U, Tanagho EA. Studies on the feasibility of urinary bladder evacuation by direct spinal cord stimulation: II. Poststimulus voiding: a way to overcome outflow resistance. Invest. Urol. 1975;13:151–153. [PubMed] [Google Scholar]
- Li JK, van Brummelen AG, Noordergraaf A. Fluid-filled blood pressure measurement systems. J. Appl. Physiol. 1976;40:839–843. doi: 10.1152/jappl.1976.40.5.839. [DOI] [PubMed] [Google Scholar]
- Little JW, Ditunno JF, Jr, Stiens SA, Harris RM. Incomplete spinal cord injury: neuronal mechanisms of motor recovery and hyperreflexia. Arch. Phys. Med. Rehabil. 1999;80:587–599. doi: 10.1016/s0003-9993(99)90204-6. [DOI] [PubMed] [Google Scholar]
- Liu EH, Wong HK, Chia CP, Lim HJ, Chen ZY, Lee TL. Effects of isoflurane and propofol on cortical somatosensory evoked potentials during comparable depth of anaesthesia as guided by bispectral index. Br. J. Anaesth. 2005;94:193–197. doi: 10.1093/bja/aei003. [DOI] [PubMed] [Google Scholar]
- McCreery D, Pikov V, Lossinsky A, Bullara L, Agnew W. Arrays for chronic functional microstimulation of the lumbosacral spinal cord. IEEE. Trans. Neural Syst. Rehabil. Eng. 2004;12:195–207. doi: 10.1109/TNSRE.2004.827223. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Grill WM. Selective microstimulation of central nervous system neurons. Ann. Biomed. Eng. 2000;28:219–233. doi: 10.1114/1.262. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Grill WM. Extracellular stimulation of central neurons: influence of stimulus waveform and frequency on neuronal output. J. Neurophysiol. 2002;88:1592–1604. doi: 10.1152/jn.2002.88.4.1592. [DOI] [PubMed] [Google Scholar]
- Mills IW, Drake MJ, Greenland JE, Noble JG, Brading AF. The contribution of cholinergic detrusor excitation in a pig model of bladder hypocompliance. BJU. Int. 2000;86:538–543. doi: 10.1046/j.1464-410x.2000.00768.x. [DOI] [PubMed] [Google Scholar]
- Nashold BS, Jr, Friedman H, Boyarsky S. Electrical activation of micturition by spinal cord stimulation. J. Surg. Res. 1971;11:144–147. doi: 10.1016/0022-4804(71)90039-4. [DOI] [PubMed] [Google Scholar]
- Nashold BS, Jr, Friedman H, Glenn JF, Grimes JH, Barry WF, Avery R. Electromicturition in paraplegia. Implantation of a spinal neuroprosthesis. Arch. Surg. 1972;104:195–202. doi: 10.1001/archsurg.1972.04180020075015. [DOI] [PubMed] [Google Scholar]
- Pikov V. Spinal Plasticity. In: Horch KW, Dhillon GS, editors. Neuroprosthetics: Theory and Practice. Singapore: World Scientific; 2004. [Google Scholar]
- Rowland SA, Shalaby SW, Latour RA, Jr, von Recum AF. Effectiveness of cleaning surgical implants: quantitative analysis of contaminant removal. J. Appl. Biomater. 1995;6:1–7. doi: 10.1002/jab.770060102. [DOI] [PubMed] [Google Scholar]
- Schmidt RA. Advances in genitourinary neurostimulation. Neurosurgery. 1986;18:1041–1052. doi: 10.1227/00006123-198612000-00026. [DOI] [PubMed] [Google Scholar]
- Sie JA, Blok BF, de Weerd H, Holstege G. Ultrastructural evidence for direct projections from the pontine micturition center to glycine-immunoreactive neurons in the sacral dorsal gray commissure in the cat. J. Comp. Neurol. 2001;429:631–637. doi: 10.1002/1096-9861(20010122)429:4<631::aid-cne9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Tai C, Booth AM, de Groat WC, Roppolo JR. Bladder and urethral sphincter responses evoked by microstimulation of S2 sacral spinal cord in spinal cord intact and chronic spinal cord injured cats. Exp. Neurol. 2004;190:171–183. doi: 10.1016/j.expneurol.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Thor KB, Morgan C, Nadelhaft I, Houston M, de Groat WC. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J. Comp. Neurol. 1989;288:263–279. doi: 10.1002/cne.902880206. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Mizuno N, Nomura S, Konishi A, Itoh K, Arakawa H. Central distribution of afferent and efferent components of the pudendal nerve in cat. J. Comp. Neurol. 1984;222:38–46. doi: 10.1002/cne.902220104. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Neurochemical plasticity and the role of neurotrophic factors in bladder reflex pathways after spinal cord injury. Prog. Brain. Res. 2006;152:97–115. doi: 10.1016/S0079-6123(05)52007-7. [DOI] [PubMed] [Google Scholar]
- Walter JS, Wheeler JS, Cai WY, Wurster RD. Direct bladder stimulation with suture electrodes promotes voiding in a spinal animal model: a technical report. J. Rehabil. Res. Dev. 1997;34:72–81. [PubMed] [Google Scholar]
- Walter JS, Wheeler JS, Robinson CJ, Khan T, Wurster RD. Urethral responses to sacral stimulation in chronic spinal dog. Am. J. Physiol. 1989;257:R284–R291. doi: 10.1152/ajpregu.1989.257.2.R284. [DOI] [PubMed] [Google Scholar]
- Walter JS, Wheeler JS, Wurster RD, Sacks J, Dunn R. Preliminary observations of a synergistic bladder-sphincter relationship following spinal cord injury in a quadruped animal. J. Spinal Cord. Med. 2003;26:372–379. doi: 10.1080/10790268.2003.11753708. [DOI] [PubMed] [Google Scholar]
- Wang AC, Chen MC. A comparison of urethral pressure profilometry using microtip and double-lumen perfusion catheters in women with genuine stress incontinence. BJOG. 2002;109:322–326. doi: 10.1111/j.1471-0528.2002.01001.x. [DOI] [PubMed] [Google Scholar]
- Weld KJ, Graney MJ, Dmochowski RR. Clinical significance of detrusor sphincter dyssynergia type in patients with post-traumatic spinal cord injury. Urology. 2000;56:565–568. doi: 10.1016/s0090-4295(00)00761-5. [DOI] [PubMed] [Google Scholar]
- Yu X, Xu L, Zhang XD, Cui FZ. Effect of spinal cord injury on urinary bladder spinal neural pathway: a retrograde transneuronal tracing study with pseudorabies virus. Urology. 2003;62:755–759. doi: 10.1016/s0090-4295(03)00486-2. [DOI] [PubMed] [Google Scholar]
- Zinck ND, Downie JW. Plasticity in the injured spinal cord: can we use it to advantage to reestablish effective bladder voiding and continence? Prog. Brain. Res. 2005;152:147–162. doi: 10.1016/S0079-6123(05)52010-7. [DOI] [PubMed] [Google Scholar]