Abstract
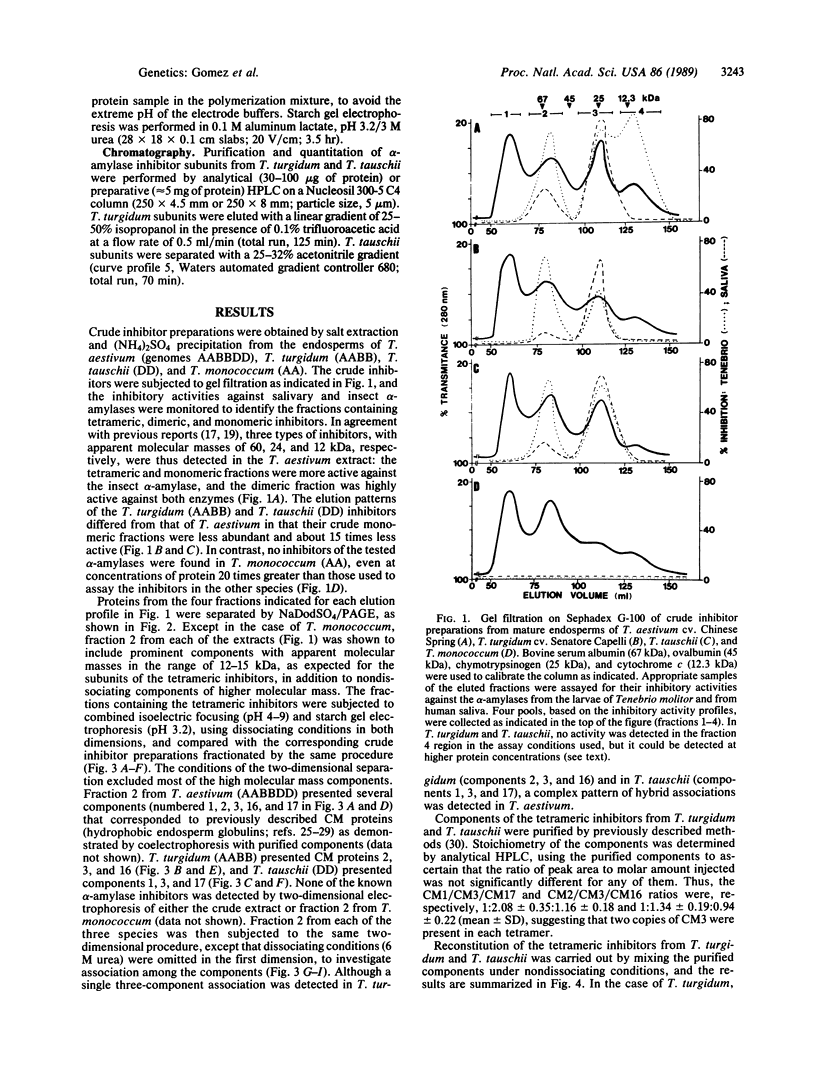
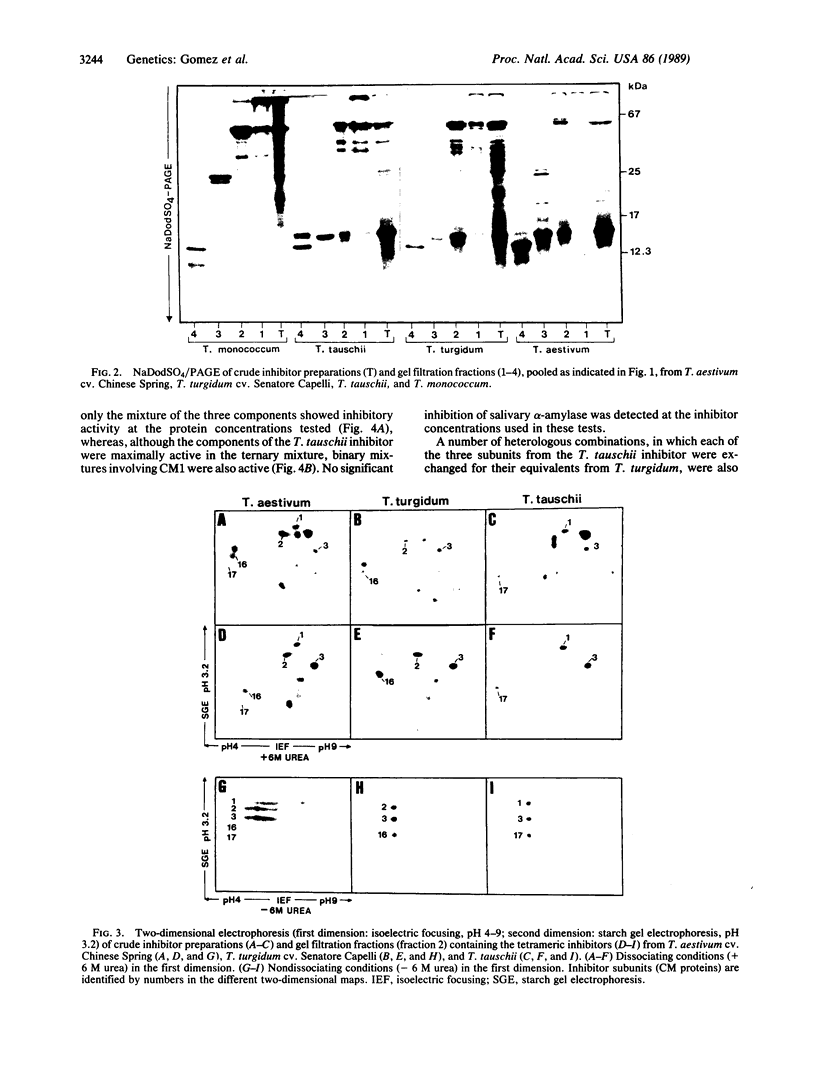
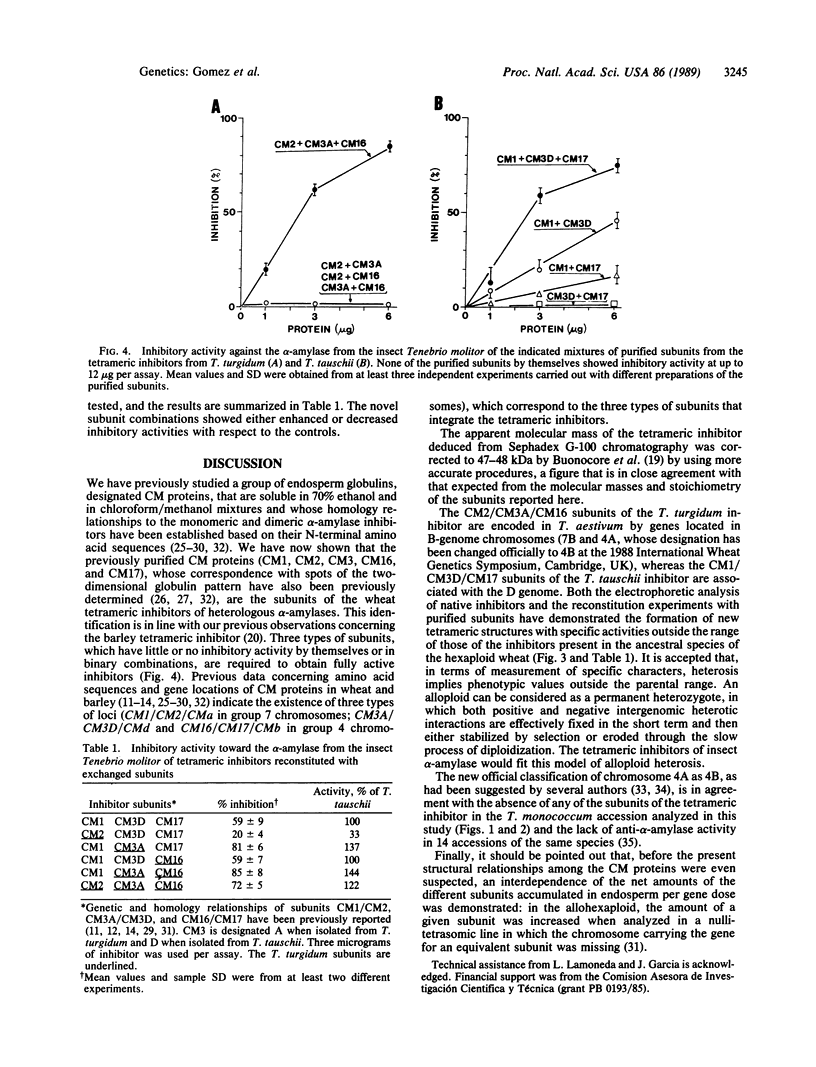
Tetrameric inhibitors of heterologous α-amylases have been characterized in allohexaploid wheat, Triticum aestivum (genomes AABBDD), as well as in Triticum turgidum (AABB) and Triticum tauschii (DD). Their subunits have been identified as the previously described CM proteins. Single oligomeric species were observed in T. Turgidum (subunits CM2, CM3A, and CM16) and in T. tauschii (CM1, CM3D, and CM17) by a two-dimensional electrophoretic method that does not dissociate the inhibitors in the first dimension. Multiple tetrameric species, resulting from different combinations of the subunits contributed by the two ancestral species, are observed by the same procedure in T. aestivum. The three types of subunits were required for significant activity when the inhibitor of T. turgidum was reconstituted from the purified subunits, whereas, in the case of T. tauschii, binary mixtures involving subunit CM1 also had some activity. Additional combinations of the subunits present in these two species, which occur in the allohexaploid T. aestivum, were also reconstituted, and their inhibitory activities ranged from 144% to 33% the activity of the reconstituted inhibitor from T. tauschii. The activity of these inhibitors toward the α-amylase (1,4-α-D-glucan glucanohydrolase, EC 3.2.1.1) of the insect Tenebrio molitor is much greater than that against the salivary enzyme. These observations, together with the previously established chromosomal locations of genes encoding CM proteins, fit a model of alloploid heterosis at the molecular level.
Keywords: Triticum aestivum, Triticum monococcum, Triticum tauschii, inhibitor reconstitution, genome interaction
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aragoncillo C., Rodríguez-Loperena M. A., Salcedo G., Carbonero P., García-Olmedo F. Influence of homoeologous chromosomes on gene-dosage effects in allohexaploid wheat (Triticum aestivum L.). Proc Natl Acad Sci U S A. 1978 Mar;75(3):1446–1450. doi: 10.1073/pnas.75.3.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber D., Sanchez-Monge R., Mendez E., Lazaro A., Garcia-Olmedo F., Salcedo G. New alpha-amylase and trypsin inhibitors among the CM-proteins of barley (Hordeum vulgare). Biochim Biophys Acta. 1986 Jan 17;869(1):115–118. doi: 10.1016/0167-4838(86)90318-3. [DOI] [PubMed] [Google Scholar]
- Bedetti C., Bozzini A., Silano V., Vittozzi L. Amylase protein inhibitors and the role of Aegilops species in polyploid wheat speciation. Biochim Biophys Acta. 1974 Sep 5;362(2):299–307. doi: 10.1016/0304-4165(74)90222-0. [DOI] [PubMed] [Google Scholar]
- Buonocore V., Silano V. Biochemical, nutritional and toxicological aspects of alpha-amylase inhibitors from plant foods. Adv Exp Med Biol. 1986;199:483–507. doi: 10.1007/978-1-4757-0022-0_28. [DOI] [PubMed] [Google Scholar]
- Carbonero P., García-Olmedo F. Purothionins in Aegilops-Triticum spp. Experientia. 1969 Oct 15;25(10):1110–1111. doi: 10.1007/BF01901464. [DOI] [PubMed] [Google Scholar]
- Ferris S. D., Whitt G. S. Evolution of the differential regulation of duplicate genes after polyploidization. J Mol Evol. 1979 Apr 12;12(4):267–317. doi: 10.1007/BF01732026. [DOI] [PubMed] [Google Scholar]
- Ferris S. D., Whitt G. S. Loss of duplicate gene expression after polyploidisation. Nature. 1977 Jan 20;265(5591):258–260. doi: 10.1038/265258a0. [DOI] [PubMed] [Google Scholar]
- García-Olmedo F. Genetics of synthesis of beta-sitosterol esters in wheat and related species. Nature. 1968 Dec 14;220(5172):1144–1145. doi: 10.1038/2201144a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lázaro A., Barber D., Salcedo G., Mendez E., García-Olmedo F. Differential effects of high-lysine mutations on the accumulation of individual members of a group of proteins encoded by a disperse multigene family in the endosperm of barley (Hordeum vulgare L.). Eur J Biochem. 1985 Jun 18;149(3):617–623. doi: 10.1111/j.1432-1033.1985.tb08969.x. [DOI] [PubMed] [Google Scholar]
- O'Connor C. M., McGeeney K. F. Isolation and characterization of four inhibitors from wheat flour which display differential inhibition specificities for human salivary and human pancreatic alpha-amylases. Biochim Biophys Acta. 1981 Apr 14;658(2):387–396. doi: 10.1016/0005-2744(81)90309-0. [DOI] [PubMed] [Google Scholar]
- Oliver J. L., Martinez-Zapater J. M., Pascual L., Enriquez A. M., Ruiz-Rejón C., Ruiz-Rejón M. Different genome amplification mechanisms and duplicate gene expression in Liliaceae. Isozymes Curr Top Biol Med Res. 1983;10:341–363. [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Wazuddin M., Driscoll C. J. Chromosome constitution of polyploid wheats: Introduction of diploid wheat chromosome 4. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3870–3874. doi: 10.1073/pnas.83.11.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]





