Abstract
Intrinsic cell death is mediated by interaction between pro-apoptotic and pro-survival proteins of the B-cell lymphoma-2 (Bcl-2) family. Members of this family are either intrinsically disordered or contain intrinsically disordered regions/domains that are critical to their function. Alternate splicing and post-translational modifications can determine the extent of these disordered regions and are critical for regulating Bcl-2 proteins. Conformational plasticity and structural transitions characterize the interactions within the Bcl-2 family, with conserved sequence motifs on both binding partners required for their molecular recognition.
Keywords: apoptosis, Bcl-2, BH3-only, intrinsically disordered protein, protein structure
1. Introduction
In response to intra-cellular stress signals cells initiate a programmed cell suicide known as apoptosis. Members of the Bcl-2 family of proteins are critical to the regulation of ‘intrinsic’ or mitochondrial initiated cell death [1]. The Bcl-2 family consists of members that either promote apoptosis, the pro-apoptotic proteins, or those that inhibit this action, the pro-survival proteins. Protein-protein interactions between these opposing factions mediate the life/death switch and it is thought the balance between the pro-survival and pro-apoptotic proteins decides cell fate [2]. As might be expected, their role as caretakers of cell fate ensures that the Bcl-2 family is highly regulated by a plethora of transcriptional, translational and post-translational mechanisms.
The sequence identity shared between members of the Bcl-2 family ranges between 3 and 40% but is typically <20%. Bcl-2, Bcl-xL and Bcl-w are the most similar with Bcl-2/Bcl-xL having 39.9% sequence identity. Among the BH3-only proteins Bim and Bmf have 20.4% shared identity, while the multi-motif pro-apoptotic protein Bax and BH3-only protein Hrk share only 3.2% identity. Although the sequence identity shared in the Bcl-2 family is low they all bear conserved sequence regions known as Bcl-2 homology (BH) domains or motifs and harbor up to four of these regions, BH1-BH4. The BH motifs are functionally important and underpin the interaction between Bcl-2 proteins [3]. The pro-apoptotic proteins either bear a single BH domain, the BH3-motif, and are known as the BH3-only proteins (members of this group include Bim, Bad, Bmf, Bid, Bik, Puma, Noxa and Hrk) or are structurally homologous to the pro-survival proteins Bcl-2, Bcl-xL, Bcl-w, Bcl-B, Bfl-1, and Mcl-1 and and are known as the multi-motif Bax-like proteins (Bax, Bak and Bok) [3] (Figures 1 and 2). In addition to bearing BH domains, Bcl-2 family proteins frequently have hydrophobic residues at their C-termini, identified as the ‘trans-membrane’ (TM) motif, which are important for localization to intra-cellular membranes such as the mitochondrial outer membrane (MOM), endoplasmic reticulum and the nuclear envelope [4].
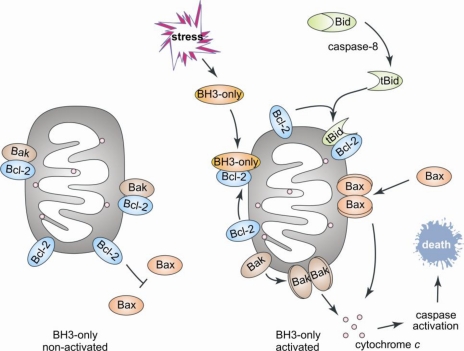
Figure 1.
General scheme showing the role of the Bcl-2 family in ‘intrinsic’ or mitochondrial activated apoptosis. The BH3-only protein Bid links cell death receptor (‘extrinsic’) signaled apoptosis to intrinsic apoptosis. Bid is activated by proteolytic cleavage by caspase-8 in its IDR.
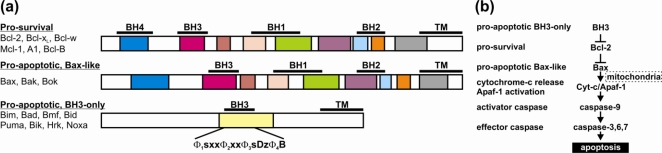
Figure 2.
The Bcl-2 family of proteins and their mode of action. (a) The Bcl-2 family is split by structure and sequence into two groups: the multi-motif proteins that includes both pro-survival (Bcl-2, Bcl-xL, Bcl-w, Mcl-1, A1, Bcl-B) and pro-apoptotic members (Bax, Bak, Bok) that have an all helical Bcl-2 fold with IDRs and the IDP BH3-only proteins that only have a single BH3 motif (Bim, Bad, Bmf, Bid, Puma, Bik, Noxa, Hrk). The colored bars indicate the extent of helices and the location of BH motifs is indicated by black bars (b) Scheme showing the mode of Bcl-2 family action. The BH3-only proteins inhibit the pro-survival action allowing the Bax-like proteins to permeablize the mitochondrial outer membrane releasing cytochrome c to activate the caspase cascade that destroys the cell.
In mammals, the Bcl-2 proteins control the integrity of the MOM by a still poorly characterized and controversial mechanism [5]. The BH3-only proteins act as the cellular sentinels that are activated in response to an apoptotic stimulus and they initiate the apoptotic process by binding in a hydrophobic groove located on the surface of the pro-survival proteins. This interaction liberates the pro-apoptotic Bax-like proteins, which effect apoptosis by releasing factors such as cytochrome c from the mitochondrial inter-membrane space into the cytosol (Figure 1). Gene knockout studies have shown that intrinsic apoptosis is dependent on the presence of Bax-like proteins and deletion of bax and bak render cells resistant to apoptotic stimuli [6]. Once released, cytochrome c interacts with cytosolic scaffold proteins promoting dimerization and activation of caspases, cysteine aspartyl proteases. Activated caspases execute a proteolytic cascade and destroy the cell from within, leading to its death, breakdown and phagocytosis [1].
Aberrant regulation of apoptosis has been directly linked to many diseases and is one of the hallmarks of cancer [7]. The Bcl-2 family proteins key role in determining cell fate has led to an intensive effort to understand their mode of action with the aim of therapeutic intervention [8]. These studies have uncovered important roles for unstructured motifs with the action of the Bcl-2 family as well as many of their up- and down-stream effectors, depending upon residues that do not have well-defined conformation. In addition covalent modification of BH3-peptides is being used to conformationally restrict these peptides to improve their in vivo proteolytic stability and the potency against tumors to explore their utility in cancer treatments [9]. Here we review the roles of intrinsically disordered proteins (IDPs) and intrinsically disordered regions (IDRs) and their interactions in the Bcl-2 family.
2. Bcl-2 Proteins Are IDPs, or Contain IDRs
The first structures in the Bcl-2 family became available over a decade ago [10–12] and a number of Bcl-2 structures and their complexes have since been solved. These have recently been incorporated into a sequence-structure database, BCL2DB [13]. The solution structure of C-terminally truncated Bcl-xL [10] revealed a helical bundle structure with a long inter-helical loop of ∼60 residues that connects helices α1 and α2. The Bcl-xL structure, as well as structures of other Bcl-2 proteins, demonstrated that both the pro-survival and Bax-like proteins share this helical fold, now described as the Bcl-2 fold. The Bcl-2 fold consists of a helical bundle where 7 amphipathic helices (α1-α4, α6-α8) pack against a central solvent inaccessible hydrophobic helix (α5) [3] (Figure 3). In addition Bax [14] and Bcl-w [15,16], that were obtained as soluble proteins without requiring such extensive truncation of their C-terminal residues, bear a ninth helix (α9), containing the TM region, that lies in a hydrophobic groove created by helices α2-α5 and α8. Biochemical data suggested that the TM region blocks access to the binding site in other pro-survival proteins [16]. Disordered residues are found in the long α1-α2 inter-helical loop of Bcl-2 and Bcl-xL, other multi-motif Bcl-2 proteins have a short mobile loop of approximately 12 residues in the corresponding position. Uniquely, Mcl-1 has an extended N-terminal region predicted to be unstructured and this region of ∼160 residues [17] contains regulatory elements such as two PEST sequences [18], (sequences enriched in proline [P], glutamic acid [E], serine [S] and threonine [T] [19]), which are in part responsible for regulating its degradation [20].
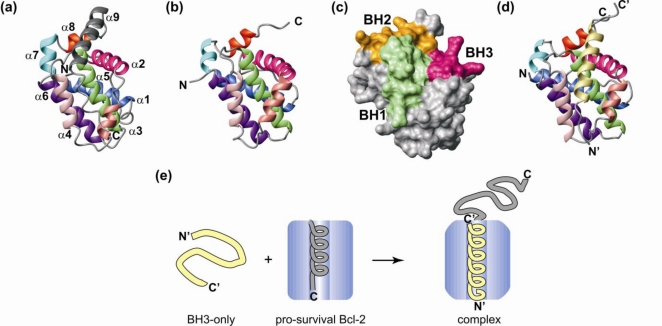
Figure 3.
Structures of pro-survival proteins and their BH3-only complexes. (a) Ribbon diagram of Bcl-w (PDB: 1O0L) [16]. The helices are labelled α1-α9. (b). Ribbon diagram of unliganded C-terminally truncated Mcl-1 (1WSX) showing the binding groove [17]. (c) The BH1, BH2 and BH3 motifs are brought into close proximity on Mcl-1 by the Bcl-2 fold of the pro-survival proteins to provide a surface exposed hydrophobic groove that binds the BH3 motif of a BH3-only protein as an α-helix. (d) The IDP Noxa bound in the groove of Mcl-1 (2ROD) [27]. (e) Schematic of BH3-only protein binding a pro-survival protein. The BH3-only proteins are IDPs that displace a structured C-terminal helix to bind and fold in a hydrophobic groove of the pro-survival protein. This displacement generates an IDR at the C-terminus of the pro-survival protein.
In contrast to their pro-survival and Bax-like relatives the BH3-only proteins lack long-range structure [21]. Sequence analysis, circular dichroism (CD), nuclear magnetic resonance (NMR) spectroscopic studies and biochemical evidence such as proteolytic susceptibility demonstrate that the BH3-only proteins are IDPs and even in the presence of their binding partners only the short BH3-motif has a defined structure [21,22]. Bid differs from other BH3-only proteins and has a Bcl-2 like fold structure with an IDR of 43 residues connecting helices α1 and α2 [23,24]. However, Bid is activated by proteolytic cleavage at a conserved site within the α1-α2 loop to generate a p7 and p15 fragment of 7 and 15 kDa respectively. The C-terminal p15 fragment of Bid is known as truncated Bid, or tBid and contains the BH3 motif. tBid has partial α-helical secondary structure, but it is in dynamic conformational exchange consistent with a molten globule state [25]. tBid is thus an example of an IDP that can be generated by post-translational modification of a folded protein.
3. Structural Transitions Characterize Bcl-2 Interactions
One of the central features underlying interactions in the Bcl-2 family is their conformational mobility [3]. The BH3-motif of IDPs like Bim, Bad, Bmf and tBid folds into an α-helix on binding in a hydrophobic groove formed from residues in helices α2-α5 and α8 on the pro-survival protein [11] (Figure 3). The exact intermolecular contacts and changes in geometry of the partners upon interaction are dependent on the specific components of the complex [17,26]. In the uncomplexed state, the groove of Bcl-xL is in a closed state with α3 and α4 well-formed helices that are parallel and closely packed [22]. On binding Bim, helix α4 of Bcl-xL rotates to accommodate the ligand and the α3 residues no longer form a regular α-helix [22]. In contrast, Mcl-1 has a binding site that is similar in the presence and absence of the ligand [27,28].
Although the exact interactions between BH3-only proteins and their pro-survival binding partners vary between complexes, a number of common features are apparent due to the presence of the 13 residue motif, Φ1sxxΦ2xxΦ3sDzΦ4B in all ligands. The conserved leucine at position Φ2 and aspartic acid residue at position D defines the BH3 motif [27]. The four hydrophobic residues, Φ1-Φ4, form a hydrophobic surface on the amphipathic BH3-helix. The other positions are: x is any residue, s small residues (G, A or S), z is normally an acidic residue and B a hydrogen bond acceptor. This short peptide motif binds and folds as an amphipathic helix in the hydrophobic groove of the pro-survival protein burying the hydrophobic residues Φ1–Φ4 in the interface. The conserved aspartic acid, D, forms a salt bridge with a conserved arginine in the BH1 motif of the pro-survival protein. Analysis of Bim and tBid binding to Bcl-w [21] and Bcl-xL [25] show that helical structure is only induced over the BH3 motif and outside this region the sequence remains poorly ordered.
Using a conformationally restricted Bim peptide, that maintained a higher helical propensity and affinity than the unmodified BH3-peptide, it was discovered that Bim binds Bax through its hydrophobic surface [29]. Curiously, although Bax is structurally homologous to the pro-survival proteins [14] the Bim binding site was not mapped to the equivalent binding groove, but rather to the opposite face. The Bim binding surface on Bax was provided by helices α1 and α6 and there was little change in the Bax conformation on binding Bim. The surface provided by α1 and α6 on Bax does not rely on interaction with highly conserved residues like the groove and demonstrates the potential of multi-motif proteins to provide alternate binding surfaces and the BH3-only protein to adapt to these surfaces. Bax is normally cytosolic but localizes to mitochondria on initiation of apoptosis [30]. Bim binding Bax may provide the trigger that activates this translocation [29].
The C-terminal TM helix of Bcl-w, α9, like that of full-length Bax [14] lies in the hydrophobic groove used by the pro-survival proteins to bind their pro-apoptotic counterparts [15,16]. Binding a BH3-only protein displaces the C-terminal residues from the groove of Bcl-w and they become unstructured in solution [15,16] forming a new IDR (Figure 3). The ligand induced order-disorder transition of the TM residues appears to be driven by a disorder-order transition in the BH3 motif of the ligand and may be the initial step that leads to membrane integration of the pro-survival protein. The TM residues are necessary for correct localization to the membrane on BH3-only protein binding [31] and studies suggest correct localization is critical as Bcl-w is not fully protective in their absence, even though binding to BH3-only proteins is retained [16].
4. Bcl-2 Protein Structural Plasticity and Multiple Binding Partners
BH3-only proteins have a striking ability to interact with the binding grooves of multiple pro-survival proteins [32–34]. Like other protein-protein interactions those between an IDP and structured protein are entropy-enthalpy compensated [27] indicating that solvation plays an important role in binding [35]. While some BH3-only proteins bind all pro-survival proteins with approximately equal (high) affinity, others bind only selected pro-survival proteins and their capacity to promote apoptosis is limited [34]. The binding affinities can be remarkably tight, with dissociation constants typically in the nanomolar range [26,27,34]. Coupled folding and binding of IDPs provides a mechanism that fine tunes binding specificity to multiple targets. Conformational mobility permits local conformational adaptation for binding [36,37] and the ability of BH3-only proteins to engage multiple binding partners is in part due to their significant structural plasticity [17,26]. Bid, Puma and Bak BH3-peptides bind A1 with dissociation constants, Kd < 1–3 nM, while Bmf has an approximately 100 fold lower affinity with a Kd of 180 nM [26]. The majority of the intermolecular contacts for these four A1 complexes are identical. The highly conserved BH3 leucine and invariant aspartate of the BH3 motif make key contacts. The leucine is buried in a hydrophobic pocket provided by a cluster of highly conserved hydrophobic residues, while the aspartate makes a salt bridge with the conserved arginine in the BH1 motif. In the case of the low affinity A1:Bmf interaction the Bmf-BH3-motif packs in the binding groove tightly, but strikingly, the inter-molecular salt-bridge with the conserved aspartate is absent, instead it forms an intra-molecular salt bridge with a nearby arginine [26]. The importance of the salt bridge for high affinity interactions was also demonstrated for interaction of Bak BH3-peptide with Bcl-xL, where mutation of the aspartate in Bak BH3 lead to significantly weaker binding [11]. Therefore, although hydrophobic contacts are important, charged and hydrogen bonding also play an important role in BH3 interactions.
Several studies have indicated the importance of the conformational mobility of the pro-survival protein binding groove and pro-survival proteins are tolerant of sequence changes in the BH3-ligand. For example, Mcl-1 tolerates a wide range of residues at the Φ1 position of the BH3-motif while Bcl-xL does not [27,38]. Novel peptide sequences selective for Mcl-1 were identified using phage display that showed Mcl-1 could tolerate some degree of change from the canonical BH3-sequence [39]. The interaction of Mcl-1 with Noxa illustrates that the sequence composition of the groove also contributes to selective BH3 binding. Mouse Noxa has two BH3-motifs differing at the N-terminal Φ1 residue, the C-terminal BH3 (NoxaB) has a glutamic acid residue instead of the usual hydrophobic residue. NoxaB, binds Mcl-1 but not A1, while the N-terminal Noxa-BH3 motif (NoxaA) has the usual hydrophobic residue at Φ1 and binds both A1 and Mcl-1 [27]. The Mcl-1 has a conserved basic patch in the α3-α4 loop that can compliment the negatively charged acidic residue E74, while in A1 at the structurally equivalent position has acidic residues (Figure 4). On binding ligand similar structural changes are induced in Mcl-1 that are almost independent of the particular BH3 peptide ligand bound [27] and mutant Bim BH3 ligands bound tightly to Mcl-1 with only minor structural changes to Mcl-1 [40]. Point mutations to groove residues on the BH3-binding binding surface of Mcl-1 had little effect on Bim binding [17]. Each of the pro-survival proteins appears to have subtly different binding grooves and the combination of the dynamics of the unstructured ligand and those of the receptor pro-survival protein determine their selectivity and affinity. The ability of IDPs and their receptors to form alternative inter-molecular contacts is an important characteristic of their interactions.
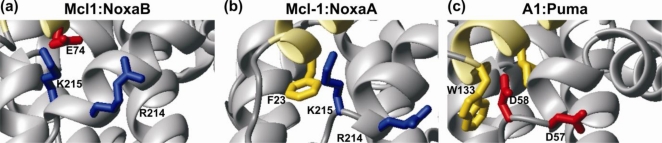
Figure 4.
Close-up view of differences between the A1 and Mcl-1 grooves. (a) The presence of a basic patch provided by residues R214 and K215 in the α3-α4 loop in Mcl-1 accommodates the presence of an acidic residue (E74) in the BH3 domain of mouse NoxaB (PDB: 2NLA) [27]. (b) The same view of the Mcl-1:NoxaA complex (PDB: 2ROC) where a hydrophobic residue (F23) occupies a similar position to E74 in (a). (c) An acidic patch formed by residues D57 and D58 occupies the structurally equivalent position in A1 (PDB: 2VOF) to the basic patch in Mcl-1. NoxaB does not bind A1 [26]. The ribbon of the BH3 ligand is colored yellow. Basic residues are shown in blue and acidic residues in red.
5. Alternate Splicing Leads to Structural Diversity
Alternate splicing is an important regulation mechanism in the Bcl-2 family and not only regulates Bcl-2 protein levels but also their interactions. New molecular species are generated by alternate splicing that have modified structures and biological behavior. The presence of multiple splice variants for most Bcl-2 family members [41] increases the complexity of apoptosis signaling. The pro-apoptotic signal of the BH3-only proteins is contained within the short ∼16 residue BH3-motif [42] and exists on a single exon [43]. Most, if not all, BH3-only proteins like Bim [44–46], Bid [47], Bad [48,49], Bmf [50,51], Puma [52] and Noxa [53] have multiple splice variants. Regions flanking the BH3 motif in BH3-only proteins do not appear necessary for either pro-survival protein binding or specificity as the BH3 segment has essentially the full binding affinity for the pro-survival target [31]. Notably, peptides that span the BH3 motif bind with affinities and specificities [34] that predict the biology [54].
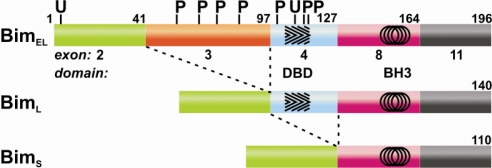
Three major splice variants of Bim have been observed; BimS, BimL and BimEL, the short, long and extra long isoforms respectively and all are capable of initiating apoptosis [44,45] (Figure 5). Sequence analysis indicates all are IDPs [21] and all three isoforms bear the BH3-motif that is encoded by exon 8, however they have different activities depending on the other exons translated. BimL and BimEL, for instance, have the binding site for the dynein motor complex protein dynein light chain 1 (DLC1) and are regulated by interaction with this protein [55]. Bmf also associates with a motor complex through binding a dynein light chain [56], but associates with the myosin V motor complex through binding DLC2 [57]. This interaction occurs through a short motif, (K/R)XTQT (X is any residue) [58] located on exon 4 that forms a complementary anti-parallel β-strand extending the β-sheet of the DLC protein. Association of Bmf and Bim with their respective DLC proteins compartmentalizes them [57] and like BimS the exon that encompasses the DLC binding motif in BmfS is not translated [51]. In addition to the three main isoforms of Bim, there are numerous other mRNA transcripts some of which are missing key exons but it is unclear if they are all translated to proteins [59]. At least three splice variants of Bid are known, and all would be expected to be IDPs like tBid, [47], yet they differ in their expression patterns, localization, regulation and apoptotic initiation. Unlike their structured counterparts IDPs are not reliant on a tertiary structure for their functionality and could be considered molecular ‘cut and paste’ frameworks. The unstructured nature of BH3-only proteins means that the splice variants can retain selected functionality and their prevalence may regulate specific apoptotic pathways. The development of unstructured regions through splice variants allows structure-independent changes in function that can modulate these molecules and impact on their ability to initiate apoptosis.
Figure 5.
Splice variants and post-translational modification sites in Bim. The BH3 motif in the BH3-only proteins occurs on a single exon while the same region is shared with other motifs in the multi-motif proteins. The three main splice variants, short, long and extra long of Bim differ in exons 3 and 4. The corresponding sequence position is given above the bar and the exons are indicated. Marked on exon 4 is the DLC binding domain (DBD, hatched) and on exon 8 circles indicate the extent of the binding region of the BH3 motif. The potential phosphorylation sites are indicated with P and ubiquitylation sites with U.
The BH1-BH3 motifs, or equivalent regions, in the pro-survival Bcl-2 proteins constitute the BH3-ligand binding surface and are required for their pro-survival activity [3]. Alternate splice variants of the multi-motif Bcl-2 proteins Bcl-2, Bcl-xL, Mcl-1, Bfl-1, Bax and Bok have been reported [41]. The BH3 motif is located on a single exon for Bax, Bak and Mcl-1, potentially making them targets of exon shuffling [43] while, in the case of Bok, it is split between two exons. In other multi-motif proteins either the BH1 or BH4 motif is found on the same exon as the BH3 motif. Many splice variants of these proteins lack the BH1 and BH2 motifs, but retain the BH3 motif. These proteins are therefore likely to lack a tertiary structure and may be pro-apoptotic by virtue of their exposed BH3 motifs. For example, Mcl-1S, a ‘short’ splice variant of Mcl-1, retains the N-terminal unstructured and BH3 motifs, but lacks the BH1, BH2 and TM motifs [60]. In contrast, the ‘extra short’ variant, Mcl-1ES retains BH1, BH2, BH3, the N-terminal residues and hydrophobic C-terminal region, but lacks the unstructured PEST sequences [61]. Mcl-1S and Mcl-1ES have been reported to have pro-apoptotic activity [39,60]. Alternate splicing thus provides an attractive mechanism for exposure of the BH3 motif and generation of an IDP with an altered apoptotic function.
In the case of Bax, a splice variant, Baxβ, has an extended C-terminal sequence that not only couples Baxβ to proteasomal degradation pathways but also renders it a more potent killer [62]. Splicing sites are generally more prevalent within IDRs as a splicing event within an IDR will minimize its effect on structured regions but broadens the functional and regulatory diversity [63]. However, not all splice sites fall in IDRs and a recent analysis indicates that many are located in structured regions and this may be a means of producing isoforms with new functional roles [64].
6. Generating New IDRs and Reactivity through Post-translational Modification
IDP levels are tightly controlled in the cell and kept at low levels by a combination of low protein synthesis and rapid degradation [65]. The control of protein activity and stability by post-translational modification is an important regulatory mechanism in apoptosis [66]. Characterized modifications in the Bcl-2 family include phosphorylation, ubiquitylation, caspase cleavage and deamidation [67]. These modification sites are largely confined to the IDRs, indicating the importance of these regions in regulating protein function.
Bim and Bad illustrate the complexity of post-translational control of BH3-only proteins and indicates their ability to interact with multiple post-translational modifying proteins. In a healthy cell Bad is inactivated by phosphorylation at multiple sites (see [67] for a summary) and sequestered to cytoskeletal 14-3-3 proteins that inhibit its action [68]. Many kinases (such as PKA, Akt/PKB or RSK) can phosphorylate and inactivate Bad and conversely, phosphatases (such as calcineurin), can dephosphorylate and reactivate its pro-apoptotic activity [69,70]. Phosphorylation by JNK at specific serines activates Bad by inhibiting its interaction with 14-3-3 proteins [71,72], but phosphorylation at other serines lowers its pro-apoptotic activity although it retains the ability to interact with Bcl-xL [73]. Like Bad, post-translational modification at multiple sites modulates Bim activity (Figure 5) and each isoform may be differentially modified [74,75]. The proline directed kinase ERK1/2 phosphorylates BimEL at multiple sites on exon 3 (Figure 5) targeting it for proteasomal degradation [76] while the absence of the same exon in BimS and BimL renders these isoforms less sensitive to ERK1/2 phosphorylation [74,75,77]. The kinase Akt also phosphorylates BimEL on exon 3, but at a site separate from that of ERK1/2, reflecting its different specificity [78]. Akt phosphorylated-Bim can bind 14-3-3 proteins and this ablates its pro-apoptotic activity [78]. Therefore depending on the cellular context, multiple post-translational events can regulate the activity of Bim, Bad and other BH3-only proteins requiring their interaction with multiple proteins.
Activation of Bid occurs by proteolytic cleavage at conserved sites in its α1-α2 IDR by proteases such as caspase-8 and Granzyme B, the latter used in the immune response by killer T-cells to activate intrinsic apoptosis to eliminate infected cells [79]. The proteolytic cleavage generates an IDP capable of neutralizing the activity of pro-survival proteins. In addition to exposing its BH3 motif for interaction with pro-survival Bcl-2 partners [25], caspase cleavage of Bid generates a potential myristoylation site at the newly generated N-terminal glycine residue of tBid that may trigger its mitochondrial translocation [80] and ultimately the permeabilization of the MOM [81]. In contrast, phosphorylation of other sites in the IDR of Bid by both casein kinases I and II has been shown to prevent cleavage by caspase-8 and protect against death receptor induced apoptosis [82].
Although characterized by their Bcl-2 helical bundle, the multi-motif Bcl-2 proteins also have IDRs. These unstructured regions present sites for conserved post-translational modifications that may either inhibit or promote apoptosis [67]. Regulatory functions have been ascribed to the α1-α2 IDR of Bcl-xL and Bcl-2 [83] and phosphorylation [84,85], deamidation [86] and proteolyic cleavage [87,88] in this region have all been associated with down regulation of pro-survival activity. Similarly, modification of residues in the IDR of Mcl-1 by proteolytic cleavage, phosphorylation or ubquitylation transforms its apoptotic activity [89]. Apart from the first 20 residues of Mcl-1 its IDR is variable [20], but the PEST sequences in the IDR are conserved and enhance its degradation contributing to its short half-life (1–5 h) [20,90]. In comparison, Bcl-2 has a half-life of ∼20 h [91]. In response to apoptotic stimuli Mcl-1 is rapidly upregulated and post-translational mechanisms are in part responsible for temporal control of protein levels [92]. Ubiquitin proteasome-mediated degradation also regulates the activity of Bcl-2, Bcl-xL and Mcl-1 and ubiquitin modified lysines in Mcl-1 have been mapped to the N-terminal IDR and α1-α2 disordered loop [67,93].
The examples given above demonstrate the complexity of Bcl-2 family regulation through post-translational events. All members of the Bcl-2 family bear conserved sites for post-translational modification in unstructured regions that regulate their activity. These post-translational modifications connect apoptosis to other signaling pathways such as those signaled through MAP kinase [94] and also have the capability to interact with multiple other modifying proteins. Post-translational modification generates new molecular species with different interaction profiles and modified biological activity. Most post-translational modifications are still poorly characterized with neither the modification sites, temporal control nor the physiological context in which specific mechanisms prevail is clear. However, the crucial role of these modifications in regulating intrinsic apoptosis is unambiguous.
7. Conclusions
Unstructured regions play important roles in every aspect of Bcl-2 regulated apoptosis. All Bcl-2 family members either contain IDRs, like Bcl-2, Bcl-xL and Mcl-1, or are IDPs, such as Bim, Bad and Bmf. The unstructured regions of Bcl-2 proteins are targets for post-translational regulation through interaction with enzymes that regulate their levels and capacity to interact with other members of the Bcl-2 family. Although the significance of many aspects of the post-translational modifications have yet to be established, collectively the multiplicity of regulatory processes that act through the unstructured regions of the Bcl-2 family attests to their importance in controlling Bcl-2 activity. The unstructured regions allow recognition by other proteins that is highly specific, but of low affinity. A key interaction regulating apoptosis that has been well investigated is that between pro-survival proteins and their BH3-only counterparts which fold and bind tightly as a helix within the groove of a pro-survival protein. Although it is thought hydrophobic interactions are overriding for IDPs [95], polar interactions make significant contributions to BH3-only binding [27]. The unstructured nature of the BH3-only proteins and their tight regulation is consistent with their position as initiators in apoptotic pathways and many have the ability to act promiscuously. This promiscuity coupled with protein compartmentalization, splice variation, post-translational modifications and degradation increases the complexity in cell death signaling and is likely to reduce the chance of inadvertently initiating a fatal signaling event [65]. Together, the interplay between the unstructured and structured components of the Bcl-2 family is crucial for determining cell fate.
Acknowledgments
Our research is supported by the Cancer Council Victoria (grant 575549) and the Leukemia and Lymphoma Society (Specialized Center for Research 7015-02). We thank Bronwyn Carlisle for assistance with figure preparation.
References and Notes
- 1.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell. Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 2.Willis SN, Adams JM. Life in the balance: How BH3-only proteins induce apoptosis. Curr. Opin. Cell Biol. 2005;17:617–625. doi: 10.1016/j.ceb.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinds MG, Day CL. Regulation of apoptosis: Uncovering the binding determinants. Curr. Opin. Struct. Biol. 2005;15:690–699. doi: 10.1016/j.sbi.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Schinzel A, Kaufmann T, Borner C. Bcl-2 family members: Integrators of survival and death signals in physiology and pathology. Biochim. Biophys. Acta. 2004;1644:95–105. doi: 10.1016/j.bbamcr.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Antignani A, Youle RJ. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr. Opin. Cell Biol. 2006;18:685–689. doi: 10.1016/j.ceb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 8.Fesik SW. Promoting apoptosis as a strategy for cancer drug discovery. Nat. Rev. Cancer. 2005;5:876–885. doi: 10.1038/nrc1736. [DOI] [PubMed] [Google Scholar]
- 9.Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL, Ng SL, Fesik SW. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 11.Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW. Structure of Bcl-xL-Bak peptide complex: Recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 12.Aritomi M, Kunishima N, Inohara N, Ishibashi Y, Ohta S, Morikawa K. Crystal structure of rat Bcl-xL. Implications for the function of the Bcl-2 protein family. J. Biol. Chem. 1997;272:27886–27892. doi: 10.1074/jbc.272.44.27886. [DOI] [PubMed] [Google Scholar]
- 13.Blaineau SV, Aouacheria A. BCL2DB: Moving ‘helix-bundled’ BCL-2 family members to their database. Apoptosis. 2009;14:923–925. doi: 10.1007/s10495-009-0376-0. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki M, Youle RJ, Tjandra N. Structure of Bax: Coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 15.Denisov AY, Madiraju MS, Chen G, Khadir A, Beauparlant P, Attardo G, Shore GC, Gehring K. Solution structure of human BCL-w: Modulation of ligand binding by the C-terminal helix. J. Biol. Chem. 2003;278:21124–21128. doi: 10.1074/jbc.M301798200. [DOI] [PubMed] [Google Scholar]
- 16.Hinds MG, Lackmann M, Skea GL, Harrison PJ, Huang DC, Day CL. The structure of Bcl-w reveals a role for the C-terminal residues in modulating biological activity. EMBO J. 2003;22:1497–1507. doi: 10.1093/emboj/cdg144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day CL, Chen L, Richardson SJ, Harrison PJ, Huang DC, Hinds MG. Solution Structure of Prosurvival Mcl-1 and Characterization of Its Binding by Proapoptotic BH3-only Ligands. J. Biol. Chem. 2005;280:4738–4744. doi: 10.1074/jbc.M411434200. [DOI] [PubMed] [Google Scholar]
- 18.Germain M, Duronio V. The N terminus of the anti-apoptotic BCL-2 homologue MCL-1 regulates its localization and function. J. Biol. Chem. 2007;282:32233–32242. doi: 10.1074/jbc.M706408200. [DOI] [PubMed] [Google Scholar]
- 19.Rogers S, Wells R, Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: The PEST hypothesis. Science. 1986;234:364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- 20.De Biasio A, Vrana JA, Zhou P, Qian L, Bieszczad CK, Braley KE, Domina AM, Weintraub SJ, Neveu JM, Lane WS, Craig RW. N-terminal truncation of antiapoptotic MCL1, but not G2/M-induced phosphorylation, is associated with stabilization and abundant expression in tumor cells. J. Biol. Chem. 2007;282:23919–23936. doi: 10.1074/jbc.M700938200. [DOI] [PubMed] [Google Scholar]
- 21.Hinds MG, Smits C, Fredericks-Short R, Risk JM, Bailey M, Huang DC, Day CL. Bim, Bad and Bmf: Intrinsically unstructured BH3-only proteins that undergo a localized conformational change upon binding to prosurvival Bcl-2 targets. Cell Death Differ. 2007;14:128–136. doi: 10.1038/sj.cdd.4401934. [DOI] [PubMed] [Google Scholar]
- 22.Liu X, Dai S, Zhu Y, Marrack P, Kappler JW. The structure of a Bcl-xL/Bim fragment complex: Implications for Bim function. Immunity. 2003;19:341–352. doi: 10.1016/s1074-7613(03)00234-6. [DOI] [PubMed] [Google Scholar]
- 23.McDonnell JM, Fushman D, Milliman CL, Korsmeyer SJ, Cowburn D. Solution structure of the proapoptotic molecule BID: A structural basis for apoptotic agonists and antagonists. Cell. 1999;96:625–634. doi: 10.1016/s0092-8674(00)80573-5. [DOI] [PubMed] [Google Scholar]
- 24.Chou JJ, Li H, Salvesen GS, Yuan J, Wagner G. Solution structure of BID, an intracellular amplifier of apoptotic signaling. Cell. 1999;96:615–624. doi: 10.1016/s0092-8674(00)80572-3. [DOI] [PubMed] [Google Scholar]
- 25.Yao Y, Bobkov AA, Plesniak LA, Marassi FM. Mapping the interaction of pro-apoptotic tBID with pro-survival BCL-xL. Biochemistry. 2009;48:8704–8711. doi: 10.1021/bi901171n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smits C, Czabotar PE, Hinds MG, Day CL. Structural plasticity underpins promiscuous binding of the pro-survival protein A1. Structure. 2008;16:818–829. doi: 10.1016/j.str.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Day CL, Smits C, Fan FC, Lee EF, Fairlie WD, Hinds MG. Structure of the BH3 domains from the p53-inducible BH3-only proteins Noxa and Puma in complex with Mcl-1. J. Mol. Biol. 2008;380:958–971. doi: 10.1016/j.jmb.2008.05.071. [DOI] [PubMed] [Google Scholar]
- 28.Lee EF, Czabotar PE, Yang H, Sleebs BE, Lessene G, Colman PM, Smith BJ, Fairlie WD. Conformational changes in Bcl-2 pro-survival proteins determine their capacity to bind ligands. J. Biol. Chem. 2009;284:30508–30517. doi: 10.1074/jbc.M109.040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, Walensky LD. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-xL during apoptosis. Proc. Natl. Acad. Sci. USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson-Annan J, O’Reilly LA, Crawford SA, Hausmann G, Beaumont JG, Parma LP, Chen L, Lackmann M, Lithgow T, Hinds MG, Day CL, Adams JM, Huang DC. Proapoptotic BH3-only proteins trigger membrane integration of prosurvival Bcl-w and neutralize its activity. J. Cell Biol. 2003;162:877–887. doi: 10.1083/jcb.200302144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 33.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential Targeting of Prosurvival Bcl-2 Proteins by Their BH3-Only Ligands Allows Complementary Apoptotic Function. Mol. Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 35.Leung DH, Bergman RG, Raymond KN. Enthalpy-entropy compensation reveals solvent reorganization as a driving force for supramolecular encapsulation in water. J. Am. Chem. Soc. 2008;130:2798–2805. doi: 10.1021/ja075975z. [DOI] [PubMed] [Google Scholar]
- 36.Dyson HJ, Wright PE. Coupling of folding and binding for unstructured proteins. Curr. Opin. Struct. Biol. 2002;12:54–60. doi: 10.1016/s0959-440x(02)00289-0. [DOI] [PubMed] [Google Scholar]
- 37.Wright PE, Dyson HJ. Linking folding and binding. Curr. Opin. Struct. Biol. 2009;19:31–38. doi: 10.1016/j.sbi.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee EF, Czabotar PE, Smith BJ, Deshayes K, Zobel K, Colman PM, Fairlie WD. Crystal structure of ABT-737 complexed with Bcl-xL: Implications for selectivity of antagonists of the Bcl-2 family. Cell Death Differ. 2007;14:1711–1713. doi: 10.1038/sj.cdd.4402178. [DOI] [PubMed] [Google Scholar]
- 39.Lee EF, Fedorova A, Zobel K, Boyle MJ, Yang H, Perugini MA, Colman PM, Huang DC, Deshayes K, Fairlie WD. Novel BCL-2 homology BH3 domain-like sequences identified from screening randomized peptide libraries for inhibitors of the pro-survival BCL 2 proteins. J. Biol. Chem. 2009;284:31315–31326. doi: 10.1074/jbc.M109.048009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fire E, Gulla SV, Grant RA, Keating AE. Mcl-1-Bim complexes accommodate surprising point mutations via minor structural changes. Protein Sci. 2010;19:507–519. doi: 10.1002/pro.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akgul C, Moulding DA, Edwards SW. Alternative splicing of Bcl-2-related genes: Functional consequences and potential therapeutic applications. Cell Mol. Life. Sci. 2004;61:2189–2199. doi: 10.1007/s00018-004-4001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang DC, Strasser A. BH3-Only proteins-essential initiators of apoptotic cell death. Cell. 2000;103:839–842. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 43.Aouacheria A, Brunet F, Gouy M. Phylogenomics of life-or-death switches in multicellular animals: Bcl-2, BH3-Only, and BNip families of apoptotic regulators. Mol. Biol. Evol. 2005;22:2395–2416. doi: 10.1093/molbev/msi234. [DOI] [PubMed] [Google Scholar]
- 44.O’Connor L, Strasser A, O’Reilly LA, Hausmann G, Adams JM, Cory S, Huang DC. Bim: A novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouillet P, Zhang LC, Huang DC, Webb GC, Bottema CD, Shore P, Eyre HJ, Sutherland GR, Adams JM. Gene structure alternative splicing, and chromosomal localization of pro-apoptotic Bcl-2 relative Bim. Mamm. Genome. 2001;12:163–168. doi: 10.1007/s003350010242. [DOI] [PubMed] [Google Scholar]
- 46.Marani M, Tenev T, Hancock D, Downward J, Lemoine NR. Identification of novel isoforms of the BH3 domain protein Bim which directly activate Bax to trigger apoptosis. Mol. Cell Biol. 2002;22:3577–3589. doi: 10.1128/MCB.22.11.3577-3589.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renshaw SA, Dempsey CE, Barnes FA, Bagstaff SM, Dower SK, Bingle CD, Whyte MK. Three novel Bid proteins generated by alternative splicing of the human Bid gene. J. Biol. Chem. 2004;279:2846–2855. doi: 10.1074/jbc.M309769200. [DOI] [PubMed] [Google Scholar]
- 48.Hamner S, Arumae U, Li-Ying Y, Sun YF, Saarma M, Lindholm D. Functional characterization of two splice variants of rat bad and their interaction with Bcl-w in sympathetic neurons. Mol. Cell Neurosci. 2001;17:97–106. doi: 10.1006/mcne.2000.0905. [DOI] [PubMed] [Google Scholar]
- 49.Seo SY, Chen YB, Ivanovska I, Ranger AM, Hong SJ, Dawson VL, Korsmeyer SJ, Bellows DS, Fannjiang Y, Hardwick JM. BAD is a pro-survival factor prior to activation of its pro-apoptotic function. J. Biol. Chem. 2004;279:42240–42249. doi: 10.1074/jbc.M406775200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morales AA, Olsson A, Celsing F, Osterborg A, Jondal M, Osorio LM. Expression and transcriptional regulation of functionally distinct Bmf isoforms in B-chronic lymphocytic leukemia cells. Leukemia. 2004;18:41–47. doi: 10.1038/sj.leu.2403183. [DOI] [PubMed] [Google Scholar]
- 51.Gomez-Esquer F, Palomar MA, Rivas I, Delcan J, Linares R, Diaz-Gil G. Characterization of the BH3 protein Bmf in Gallus gallus: Identification of a novel chicken-specific isoform. Gene. 2008;407:21–29. doi: 10.1016/j.gene.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 52.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol. Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 53.Wang Z, Sun Y. Identification and characterization of two splicing variants of human Noxa. Anticancer Res. 2008;28:1667–1674. [PubMed] [Google Scholar]
- 54.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Puthalakath H, Huang DC, O’Reilly LA, King SM, Strasser A. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell. 1999;3:287–296. doi: 10.1016/s1097-2765(00)80456-6. [DOI] [PubMed] [Google Scholar]
- 56.Puthalakath H, Villunger A, O’Reilly LA, Beaumont JG, Coultas L, Cheney RE, Huang DC, Strasser A. Bmf: A proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science. 2001;293:1829–1832. doi: 10.1126/science.1062257. [DOI] [PubMed] [Google Scholar]
- 57.Day CL, Puthalakath H, Skea G, Strasser A, Barsukov I, Lian LY, Huang DC, Hinds MG. Localization of dynein light chains 1 and 2 and their pro-apoptotic ligands. Biochem. J. 2004;377:597–605. doi: 10.1042/BJ20031251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fan J, Zhang Q, Tochio H, Li M, Zhang M. Structural basis of diverse sequence-dependent target recognition by the 8 kDa dynein light chain. J. Mol. Biol. 2001;306:97–108. doi: 10.1006/jmbi.2000.4374. [DOI] [PubMed] [Google Scholar]
- 59.Adachi M, Zhao X, Imai K. Nomenclature of dynein light chain-linked BH3-only protein Bim isoforms. Cell Death Differ. 2005;12:192–193. doi: 10.1038/sj.cdd.4401529. [DOI] [PubMed] [Google Scholar]
- 60.Bae J, Leo CP, Hsu SY, Hsueh AJ. MCL-1S, a splicing variant of the antiapoptotic BCL-2 family member MCL-1, encodes a proapoptotic protein possessing only the BH3 domain. J. Biol. Chem. 2000;275:25255–25261. doi: 10.1074/jbc.M909826199. [DOI] [PubMed] [Google Scholar]
- 61.Kim JH, Sim SH, Ha HJ, Ko JJ, Lee K, Bae J. MCL-1ES, a novel variant of MCL-1, associates with MCL-1L and induces mitochondrial cell death. FEBS Lett. 2009;583:2758–2764. doi: 10.1016/j.febslet.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 62.Fu NY, Sukumaran SK, Kerk SY, Yu VC. Baxbeta: A constitutively active human Bax isoform that is under tight regulatory control by the proteasomal degradation mechanism. Mol. Cell. 2009;33:15–29. doi: 10.1016/j.molcel.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 63.Romero PR, Zaidi S, Fang YY, Uversky VN, Radivojac P, Oldfield CJ, Cortese MS, Sickmeier M, LeGall T, Obradovic Z, Dunker AK. Alternative splicing in concert with protein intrinsic disorder enables increased functional diversity in multicellular organisms. Proc. Natl. Acad. Sci. USA. 2006;103:8390–8395. doi: 10.1073/pnas.0507916103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Birzele F, Csaba G, Zimmer R. Alternative splicing and protein structure evolution. Nucleic Acids Res. 2008;36:550–558. doi: 10.1093/nar/gkm1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gsponer J, Futschik ME, Teichmann SA, Babu MM. Tight regulation of unstructured proteins: From transcript synthesis to protein degradation. Science. 2008;322:1365–1368. doi: 10.1126/science.1163581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Puthalakath H, Strasser A. Keeping killers on a tight leash: Transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9:505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- 67.Kutuk O, Letai A. Regulation of Bcl-2 family proteins by posttranslational modifications. Curr. Mol. Med. 2008;8:102–118. doi: 10.2174/156652408783769599. [DOI] [PubMed] [Google Scholar]
- 68.Danial NN. BAD: Undertaker by night, candyman by day. Oncogene. 2008;27(Suppl. 1):S53–S70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- 69.Chiang CW, Kanies C, Kim KW, Fang WB, Parkhurst C, Xie M, Henry T, Yang E. Protein phosphatase 2A dephosphorylation of phosphoserine 112 plays the gatekeeper role for BAD-mediated apoptosis. Mol. Cell Biol. 2003;23:6350–6362. doi: 10.1128/MCB.23.18.6350-6362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klumpp S, Selke D, Krieglstein J. Protein phosphatase type 2C dephosphorylates BAD. Neurochem. Int. 2003;42:555–560. doi: 10.1016/s0197-0186(02)00174-2. [DOI] [PubMed] [Google Scholar]
- 71.Donovan N, Becker EB, Konishi Y, Bonni A. JNK phosphorylation and activation of BAD couples the stress-activated signaling pathway to the cell death machinery. J. Biol. Chem. 2002;277:40944–40949. doi: 10.1074/jbc.M206113200. [DOI] [PubMed] [Google Scholar]
- 72.Konishi Y, Lehtinen M, Donovan N, Bonni A. Cdc2 phosphorylation of BAD links the cell cycle to the cell death machinery. Mol. Cell. 2002;9:1005–1016. doi: 10.1016/s1097-2765(02)00524-5. [DOI] [PubMed] [Google Scholar]
- 73.Dramsi S, Scheid MP, Maiti A, Hojabrpour P, Chen X, Schubert K, Goodlett DR, Aebersold R, Duronio V. Identification of a novel phosphorylation site, Ser-170, as a regulator of bad pro-apoptotic activity. J. Biol. Chem. 2002;277:6399–6405. doi: 10.1074/jbc.M109990200. [DOI] [PubMed] [Google Scholar]
- 74.Luciano F, Jacquel A, Colosetti P, Herrant M, Cagnol S, Pages G, Auberger P. Phosphorylation of BimEL by Erk1/2 on serine 69 promotes its degradation via the proteasome pathway and regulates its proapoptotic function. Oncogene. 2003;22:6785–6793. doi: 10.1038/sj.onc.1206792. [DOI] [PubMed] [Google Scholar]
- 75.Ley R, Ewings KE, Hadfield K, Howes E, Balmanno K, Cook SJ. Extracellular signal-regulated kinases 1/2 are serum-stimulated “BimEL kinases” that bind to the BH3-only protein BimEL causing its phosphorylation and turnover. J. Biol. Chem. 2004;279:8837–8847. doi: 10.1074/jbc.M311578200. [DOI] [PubMed] [Google Scholar]
- 76.Ewings KE, Hadfield-Moorhouse K, Wiggins CM, Wickenden JA, Balmanno K, Gilley R, Degenhardt K, White E, Cook SJ. ERK1/2-dependent phosphorylation of BimEL promotes its rapid dissociation from Mcl-1 and Bcl-xL. EMBO J. 2007;26:2856–2867. doi: 10.1038/sj.emboj.7601723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ley R, Balmanno K, Hadfield K, Weston C, Cook SJ. Activation of the ERK1/2 signaling pathway promotes phosphorylation and proteasome-dependent degradation of the BH3-only protein, Bim. J. Biol. Chem. 2003;278:18811–18816. doi: 10.1074/jbc.M301010200. [DOI] [PubMed] [Google Scholar]
- 78.Qi XJ, Wildey GM, Howe PH. Evidence that Ser87 of BimEL is phosphorylated by Akt and regulates BimEL apoptotic function. J. Biol. Chem. 2006;281:813–823. doi: 10.1074/jbc.M505546200. [DOI] [PubMed] [Google Scholar]
- 79.Lord SJ, Rajotte RV, Korbutt GS, Bleackley RC. Granzyme B: A natural born killer. Immunol. Rev. 2003;193:31–38. doi: 10.1034/j.1600-065x.2003.00044.x. [DOI] [PubMed] [Google Scholar]
- 80.Zha J, Weiler S, Oh KJ, Wei MC, Korsmeyer SJ. Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science. 2000;290:1761–1765. doi: 10.1126/science.290.5497.1761. [DOI] [PubMed] [Google Scholar]
- 81.Garcia-Saez AJ, Ries J, Orzaez M, Perez-Paya E, Schwille P. Membrane promotes tBID interaction with BCL-xL. Nat. Struct. Mol. Biol. 2009;16:1178–1185. doi: 10.1038/nsmb.1671. [DOI] [PubMed] [Google Scholar]
- 82.Desagher S, Osen-Sand A, Montessuit S, Magnenat E, Vilbois F, Hochmann A, Journot L, Antonsson B, Martinou JC. Phosphorylation of bid by casein kinases I and II regulates its cleavage by caspase 8. Mol. Cell. 2001;8:601–611. doi: 10.1016/s1097-2765(01)00335-5. [DOI] [PubMed] [Google Scholar]
- 83.Chang BS, Minn AJ, Muchmore SW, Fesik SW, Thompson CB. Identification of a novel regulatory domain in Bcl-xL and Bcl-2. EMBO J. 1997;16:968–977. doi: 10.1093/emboj/16.5.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haldar S, Jena N, Croce CM. Inactivation of Bcl-2 by phosphorylation. Proc. Natl. Acad. Sci. USA. 1995;92:4507–4511. doi: 10.1073/pnas.92.10.4507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol. Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Deverman BE, Cook BL, Manson SR, Niederhoff RA, Langer EM, Rosova I, Kulans LA, Fu X, Weinberg JS, Heinecke JW, Roth KA, Weintraub SJ. Bcl-xL deamidation is a critical switch in the regulation of the response to DNA damage. Cell. 2002;111:51–62. doi: 10.1016/s0092-8674(02)00972-8. [DOI] [PubMed] [Google Scholar]
- 87.Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, Ueno K, Hardwick JM. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 88.Grandgirard D, Studer E, Monney L, Belser T, Fellay I, Borner C, Michel MR. Alphaviruses induce apoptosis in Bcl-2-overexpressing cells: Evidence for a caspase-mediated, proteolytic inactivation of Bcl-2. EMBO J. 1998;17:1268–1278. doi: 10.1093/emboj/17.5.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang-Yen HF. Mcl-1: A highly regulated cell death and survival controller. J. Biomed. Sci. 2006;13:201–204. doi: 10.1007/s11373-005-9064-4. [DOI] [PubMed] [Google Scholar]
- 90.Moulding DA, Akgul C, Derouet M, White MR, Edwards SW. BCL-2 family expression in human neutrophils during delayed and accelerated apoptosis. J. Leukoc. Biol. 2001;70:783–792. [PubMed] [Google Scholar]
- 91.Blagosklonny MV, Alvarez M, Fojo A, Neckers LM. bcl-2 protein downregulation is not required for differentiation of multidrug resistant HL60 leukemia cells. Leuk. Res. 1996;20:101–107. doi: 10.1016/0145-2126(95)00103-4. [DOI] [PubMed] [Google Scholar]
- 92.Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc. Natl. Acad. Sci. USA. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhong Q, Gao W, Du F, Wang X. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell. 2005;121:1085–1095. doi: 10.1016/j.cell.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 94.Deng X, Kornblau SM, Ruvolo PP, May WS., Jr Regulation of Bcl-2 phosphorylation and potential significance for leukemic cell chemoresistance. J. Natl. Cancer Inst. Monogr. 2001;28:30–37. doi: 10.1093/oxfordjournals.jncimonographs.a024254. [DOI] [PubMed] [Google Scholar]
- 95.Meszaros B, Tompa P, Simon I, Dosztanyi Z. Molecular principles of the interactions of disordered proteins. J. Mol. Biol. 2007;372:549–561. doi: 10.1016/j.jmb.2007.07.004. [DOI] [PubMed] [Google Scholar]