Summary
This study challenges the concept that herpes simplex virus type 1 (HSV-1) latency represents a silent infection that is ignored by the host immune system, and suggests antigen-directed retention of memory CD8+ T cells. CD8+ T cells specific for the immunodominant gB498-505 HSV-1 epitope are selectively retained in the ophthalmic branch of the latently infected trigeminal ganglion, where they acquire and maintain an activation phenotype and the capacity to produce IFN-γ. Some CD8+ T cells showed TCR polarization to junctions with neurons. A gB498-505 peptide-specific CD8+ T cell clone can block HSV-1 reactivation from latency in ex vivo trigeminal ganglion cultures. We conclude that CD8+ T cells provide active surveillance of HSV-1 gene expression in latently infected sensory neurons.
Introduction
Following primary infection of mouse corneas, herpes simplex virus type-1 (HSV-1) invades sensory neurons and establishes a latent infection in neuronal nuclei located within the ophthalmic branch of the ipsilateral trigeminal ganglion (TG). Although there is a consensus that latently infected neurons harbor a functional viral genome but fail to produce virions, the definition of latency at the molecular level remains contentious. The prevailing view that no HSV-1 proteins are produced during latency is now yielding to recent observations of viral transcripts derived from immediate early genes (α), early (β), and even late (γ1) viral genes in latently infected mouse neurons (Feldman et al., 2002; Chen et al., 1997, 2002; Kramer and Coen, 1995). Moreover, HSV-1 immediate early and early proteins have been detected in latently infected TG in vivo (Chen et al., 1997) and in latently infected neurons in ex vivo TG cultures (Liu et al., 2000). Thus, the concept that the virus is able to “hide” from the immune system during latency now appears less tenable.
In humans and rabbits, HSV-1 sporadically reactivates from latency in response to poorly defined stimuli and travels by anterograde axonal transport to peripheral sites. However, “spontaneous” reactivation, as defined by detection of virus at the periphery, has not been observed in mice despite the presence of multiple latently infected neurons in sensory ganglia. Thus, mice provide a useful model for investigating the mechanisms governing the establishment and maintenance of HSV-1 latency. Observations from several laboratories suggest that the immune system provides active surveillance of HSV-1 latently infected neurons in mice (Cantin et al., 1995; Chen et al., 2000; Liu et al., 1996; Halford et al., 1996). For instance, in A/J mice CD8+ T cells invade the TG around 7 days after HSV-1 corneal infection, reach maximum density around 14 days postinfection (p.i.), and are then retained in significant numbers seemingly for the life of the animal (Liu et al., 1996). Since the bulk of virus replication in the TG subsides by 7 days p.i., and a latent HSV-1 infection in neurons is uniformly established by 7–10 days p.i., the pattern of infiltration and retention of CD8+ T cells in the TG is consistent with a primary role for CD8+ T cells in maintaining the virus in a latent state. Moreover, IFN-γ appears to be continuously produced in the latently infected TG, suggesting active immune surveillance (Halford et al., 1996; Cantin et al., 1995; Liu et al., 1996; Chen et al., 2000).
Consistent with these results is our recent observation that CD8+ T cells present in the latently infected TG can suppress full HSV-1 reactivation from latency in ex vivo TG cultures (Liu et al., 2000). Importantly, CD8+ T cells block HSV-1 reactivation from latency without destroying the latently infected neurons, at least in part through an IFN-γ-dependent mechanism (Liu et al., 2001). The latter study suggested that IFN-γ acted directly on neurons to block an as yet undefined step following initiation of lytic protein expression, but prior to virion production.
Some investigators have argued that CD8+ T cells are incapable of providing immune surveillance against HSV-1 reactivation from latency in humans because (1) neurons do not express the major histocompatibility complex class I (MHC I) molecules that are required to present antigenic peptides to CD8+ T cells, and (2) the HSV ICP47 protein binds efficiently to the human transporter of antigenic peptides (TAP) and blocks peptide transport into the endoplasmic reticulum for loading on MHC I molecules (Favoreel et al., 2000). However, a variety of observations mitigate these concerns. In mice, neurons do express MHC I molecules during HSV-1 lytic infection (Pereira et al., 1994; Pereira and Simmons, 1999). If viral and MHC I genes are coregulated, and some viral gene expression occurs in latently infected neurons, then concurrent low-level expression of MHC I might also occur, permitting direct surveillance of viral gene expression by CD8+ T cells. Moreover, the strict sequence of viral gene expression that occurs during lytic infection may not be adhered to during reactivation of HSV-1 from latency resulting in the early production of HSV-1 β and even γ1 gene products (Tal-Singer et al., 1997). Such gene products could be degraded to peptides and loaded on MHC I in neurons prior to the accumulation of α gene products such as ICP47. In addition, the ICP47 effect is not absolute and can be overcome by IFN-γ (York et al., 1994), which appears to be produced continually in the latently infected mouse TG (Chen et al., 2000; Cantin et al., 1995). Given the low epitope density required to activate T cells (Valitutti et al., 1995; Labrecque et al., 2001), it remains quite possible, and based on our current findings even likely, that CD8+ T cells can monitor viral gene expression in human and mouse neurons.
Results
CD8+ T Cells Are Present in HSV-1 Latently Infected TG of C57BL/6 Mice
Using flow cytometric analysis, we quantified CD8+ T cells and CD45+ cells (bone marrow-derived infiltrating cells) in ipsilateral (infected) and contralateral (uninfected) TG single-cell suspensions obtained at various times after uniocular HSV-1 corneal infection of C57BL/6 mice. The highest density of CD8+ T cells was present in the ipsilateral ganglion 8 days p.i., the population declined through day 34 p.i., and then a constant pool of CD8+ T cells was maintained at least through day 84 p.i (Figure 1). In contrast, fewer than 100 CD8+ T cells were detected in the contralateral (uninfected) TG (not shown). Moreover, within the ipsilateral TG, CD8+ T cells were concentrated within the ophthalmic branch containing neurons that innervate the cornea (Figure 2). HSV-1 uniformly established a latent infection in the TG neurons by 14 days p.i. as demonstrated by a lack of replicating virus in extracts of freshly isolated TG obtained 14 and 34 days p.i. (data not shown). Thus, CD8+ T cells are selectively retained in infected regions of the TG long after viral latency is established.
Figure 1. Infiltration of CD8+ T Cells in the TG after HSV-1 Corneal Infection.
Single-cell suspensions of TG obtained from mice 8, 14, 34, and 84 days after HSV-1 corneal infection were simultaneously stained for CD45 and CD8. For each reaction the equivalent number of cells from two TGs were stained and a total of 5 × 105 events were collected (approximately 80% of the sample). Forward and side scatter gates were set to encompass the CD45 population, and the frequency of CD8+ T cells was expressed as a percentage of CD45+ cells (% CD8). These data are representative of two to four independent experiments.
Figure 2. Selective Retention of CD8+ T Cells in Latently Infected Tissue.
TG were excised 34 days after HSV-1 corneal infection, and frozen sections were stained for CD8. Representative fields from the ophthalmic (A) and maxillary (B) branches of the TG are shown. Superimposed fluorescence and DIC images show preferential accumulation of CD8+ T cells (red) among the neuronal cell bodies in the ophthalmic branch of the latently infected TG.
The TG CD8+ T Cell Population Exhibits a Shift to a More Activated Phenotype during the Course of Latency
Single-cell suspensions of TG obtained from C57BL/6 mice at various times after uniocular HSV-1 corneal infection were simultaneously stained for CD45, CD8, and for either the CD69 (early activation) or CD44 (activation/memory) markers (Figure 3). In the ipsilateral TG, CD44 was consistently expressed on CD8+ T cells from day 8 through day 84 p.i. (Figure 3A). In contrast, the percentage of CD8+ T cells that expressed CD69 was very low at 8 days p.i., increased through day 34 p.i., and then remained constant through day 84 p.i. (Figure 3B). The CD8+ T cells in the TG also showed a shift to elevated expression of CD8 between day 8 and day 34 p.i. (Figure 3C). These findings demonstrate that CD8+ T cells were activated in the TG after viral latency was established.
Figure 3. Activation Phenotype of CD8+ T Cells Present in the TG.
Single cell suspensions of TG were analyzed for expression of (A) CD44, (B) CD69, (C) CD8 (dashed lines), or isotype control (dark line). Following flow cytometric analysis, the forward angle and side scatter gates were set on the CD45+ population. Backgating on the CD8+ population determined the proportion of CD8+ T cells that expressed the activation markers. These data are representative of two to four independent experiments.
Antigen Specificity of CD8+ T Cells in the Latently Infected TG
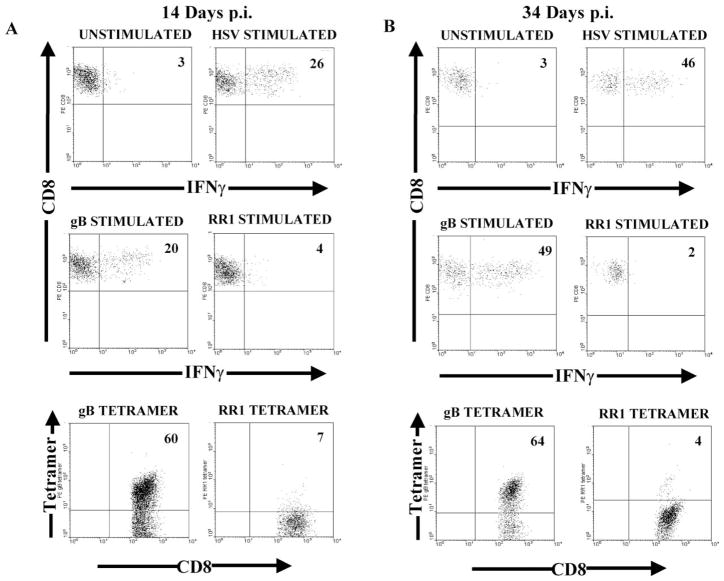
The antigen specificity of the CD8+ T cells in the HSV-1 latently infected C57BL/6 TG was determined by stimulating the TG-derived lymphocytes for 6 hr with HSV-1 infected or gB498-505 transfected stimulator cells and then staining simultaneously for CD45, CD8, and intracellular IFN-γ. Alternatively, antigen specificity was determined by staining TG-derived lymphocytes with tetramers containing the immunodominant gB498-505 or subdominant ribonucleotide reductase 1 (RR1822-829) epitopes. In TG obtained 14 days p.i., a similar percentage of CD8+ T cells produced IFN-γ when stimulated with HSV-1 infected (21.5 ± 3.3%) or gB498-505 peptide-pulsed (20.0 ± 0%) stimulator cells (Figure 4A). A much higher percentage of CD8+ T cells reacted with the gB tetramer (58.0 ± 2.0%). CD8+ T cells reactive to a subdominant epitope on RR1 were low to undetectable by both tetramer staining and IFN-γ production (Figure 4A). Thus, it appears that most HSV-specific CD8+ T cells in the TG 14 days p.i. are specific for the immunodominant gB498-505 peptide, but the majority of these are incapable of producing IFN-γ when stimulated.
Figure 4. CD8+ T Cells Retained in the TG during Latency Are Specific for HSV-1 Protein gB and Produce IFNγ Directly Ex Vivo.
Single cell suspensions of TG obtained (A) 14 days or (B) 34 days pi were incubated with the indicated stimulator cells for 6 hr in the presence of GolgiPlug and stained for intracellular IFNγ. The stimulator cells were HSV infected (HSV stimulated), gB498-505 peptide pulsed (gB stimulated), RR1 pulsed (RR1 stimulated), or uninfected (unstimulated). Alternatively, single cell suspensions of TGs were stained with an anti-CD8 mAb, anti-CD45 mAb, and either gB498-505/Kb or RR1822-829/Kb tetramers. A total of 5 × 105 events were collected. The dot plots represent the CD8 gated population. These data are representative of two to four experiments.
At 34 days p.i. (Figure 4B), there was still a similar proportion of CD8+ T cells in the TG that produced IFN-γ in response to HSV-1 infected (43.0 ± 3.5%) and gB498-505 peptide-pulsed (47.0 ± 3.0%) stimulator cells. However, the proportion of CD8+ T cells that produced IFN-γ in response to either stimulation was significantly higher at 34 days than at 14 days p.i. (compare Figures 4A and 4B). The proportion of CD8+ T cells in the day 34 TG that recognized the gB498-505 tetramer (62.0 ± 2.0%) was similar to that observed in day 14 TG. Thus, in the latently infected TG, there is selective enrichment of gB-specific CD8+ T cells capable of producing IFN-γ.
Simultaneous in situ tetramer and immunofluorescence staining of whole TG obtained 34 days after HSV-1 corneal infection demonstrated selective localization of gB498-505 tetramer positive CD8+ T cells within the ophthalmic branch of the TG (Figure 5). The majority of the CD8+ T cells were observed to be gB498-505 tetramer positive, and these cells were localized to the area of the ophthalmic branch containing neuron cell bodies. No RR1 tetramer positive cells were observed (Figure 5), which agreed with the flow data, and served as a negative control for tetramer staining. In most gB498-505 tetramer positive cells, tetramer staining was polarized and appeared patchy or ring-shaped, suggestive of immunologic synapse formation. The ring-shaped staining pattern might reflect an immature synapse, or the fact that the tetramer can only bind to TCR that are not engaged by the epitope on the target cell or that can be competed off the cell-bound epitope by the tetramer. Thus, tetramer competition for TCR binding in the central region of the synapse might be more demanding than that at the periphery of the synapse, resulting in a ring of staining around the synapse. In many of the gB498-505 tetramer positive CD8+ T cells, the TCR was polarized to the area of the cell that appeared to be in direct contact with a neuron (Figure 5), consistent with the notion that gB498-505 tetramer positive CD8+ T cells can directly monitor viral gene activity in latently infected neurons.
Figure 5. In Situ Tetramer Strain of Latently Infected TG.

TG were excised and gB498-505-specific CD8+ T cells were identified by simultaneously staining the whole tissue with MHC class I tetramers containing the gB498-505 epitope (green) and CD8 (red). The tissues were examined by confocal microscopy and presented as a merged image of a Z series. Areas of overlap between the CD8 and tetramer-bound TCR appear yellow. (A–C) The majority of CD8+ T cells that localized to the area of neuron cell bodies in the ophthalmic branch of the TG were tetramer positive. (D–F) Most of the CD8+ T cells exhibited ring- or patchy-foci of TCR polarization. (G) Grayscale image showing CD8+ T cells (arrows) in direct apposition to neurons (arrowheads); taking advantage of the intrinsic autofluorescence of neurons when excited with a argon laser and collected with a 500 longpass filter. RR1 tetramer was used as negative control. (H–J) Note the lack of RR1 tetramer staining on CD8 T cells in the ganglion. See supplemental data at http://www.immunity.com/cgi/content/full/18/5/593/DC1 for 3-D reconstruction.
A CD8+ T Cell Clone Specific for an HSV-1 gB Epitope Can Block HSV-1 Reactivation in Latently Infected Sensory Neurons
The above findings invited the hypothesis that CD8+ T cells specific for a single immunodominant epitope of the HSV-1 γ1 gene product gB can block HSV-1 reactivation from latency. To investigate this point, cultures were prepared with cell suspensions of C57BL/6 or BALB/c TG that were excised 34 days after HSV-1 corneal infection. To these cultures were added (1) varying numbers of 2D5 cells, an HSV gB498-505 peptide-specific CD8+ T cell clone derived from C57BL/6 mice; (2) naive CD8+ T cells isolated from the lymph nodes of uninfected C57BL/6 mice; or (3) no CD8+ T cells. The cultures were then monitored for HSV-1 reactivation as described in Experimental Procedures. All cultures that received 1 × 105 naive CD8+ T cells exhibited HSV-1 reactivation with similar kinetics to that observed in cultures to which no CD8+ T cells were added (Figure 6A). At the highest input (1 × 105 cells/culture), 2D5 cells did not significantly (p = 0.45) alter the course of HSV-1 reactivation from latency in the MHC incompatible BALB/c TG cultures (Figure 6A). In contrast, the addition of 2D5 cells to MHC compatible C57BL/6 TG cultures suppressed HSV-1 reactivation in a dose-dependent manner (Figure 6A). At inputs of 1 × 105 and 5 × 104, 2D5 cells significantly (p < 0.0001 and p < 0.001, respectively) suppressed HSV-1 reactivation from latency in C57BL/6 TG cultures throughout the 10 day culture period.
Figure 6. A CD8+ T Cell Clone Specific for the gB498-505 Epitope (2D5) Can Block HSV-1 Reactivation from Latency in TG Cultures.
(A) TG were excised from C57BL/6 mice 34 days after corneal infection and TG cells (0.5 TG equivalent/culture) were cultured with 1 × 105 (open rectangles) 5 × 104 (hatched rectangles), or 1 × 104 (gray rectangles) 2D5 cells (n = 10), or with 1 × 105 naive CD8+ T cells (filled rectangles) (n = 5). BALB/c TG cultures were similarly prepared and incubated with 1 × 105 2D5 cells (rectangle with line) (n = 8).
(B) TG were excised from C57BL/6 mice, depleted of endogenous CD8+ T cells, and cultured with 1 × 105 (n = 15) (open rectangles) or 1 × 104 (n = 20) (gray rectangles) 2D5 cells, or with 1 × 105 naive CD8+ T cells (n = 16) (filled rectangles). At the indicated times, cultures were examined and HSV-1 reactivation was monitored as described in Experimental Procedures. A Survival Curve Analysis determined the significance of differences in reactivation frequency (compared to cultures receiving naive CD8+ T cells). (*** p < 0.0001), (**p < 0.001). The reactivation frequency in cultures that received naive CD8+ T cells was not significantly different (p = 0.5485) from that in cultures to which no CD8+ T cells were added (not shown).
(C) Ten days after culture initiation, total RNA was extracted from CD8+ T cell-depleted TG cultures that received 1 × 105 2D5 cells and was analyzed for HSV-1 gH transcripts by RT-PCR as described in Experimental Procedures. Each sample is comprised of pooled RNA from two cultures. Lanes 1–4 represent cultures that did not receive anti-CD8 mAb and showed no viral CPE or infectious virus. Lanes 5–8 represent cultures that received anti-CD8 mAb on day 6 of culture and were positive for viral CPE and infectious virus. Note that the cultures represented in columns 7 and 8 were nearly destroyed by the virus, resulting in greatly reduced total RNA and gH transcripts. RNA extracted from a freshly excised TG obtained 5 days after corneal infection (during lytic virus infection) served as a positive control (lanes 9 and 10). Transcripts for the housekeeping gene HPRT were similarly amplified to demonstrate equal loading of RNA from each sample.
Since the TG cultures in the above experiment contained host CD8+ T cells that were present in the TG at the time of excision, the relative contribution of the endogenous CD8+ T cells and added 2D5 cells could not be determined. To address this issue, TG were excised 34 days after HSV-1 corneal infection of C57BL/6 mice and depleted of CD8+ T cells by immunomagnetic separation. Either 2D5 cells or naive syngeneic CD8+ T cells were then added to the CD8-depleted TG cultures, and the cultures were monitored for HSV-1 reactivation. Again, naive CD8+ T cells failed to influence HSV-1 reactivation from latency, while 2D5 cells inhibited HSV-1 reactivation from latency in a dose-dependent manner (Figures 6B and 6C).
The 2D5 cells are cytotoxic and produce IFN-γ when stimulated with the gB498-505 epitope. However, treating TG cultures with anti-CD8 mAb 6 days after culture initiation blocked the 2D5 protective function and allowed the previously latent virus to reactivate as indicated by the cytopathic effect (CPE) of the virus (not shown), and by expression of the γ2 gene, gH in TG cultures (Figure 6C). Thus, 2D5 cells were able to block HSV-1 reactivation in at least some of the neurons by a noncytolytic mechanism.
We previously showed that IFN-γ can block HSV-1 reactivation from latency in ex vivo TG cultures (Liu et al., 2001). Therefore, supernatant fluids from the cultures shown in Figure 6 were tested for IFN-γ by ELISA (Figure 7). C57BL/6 TG cultures containing endogenous CD8+ T cells, but no added CD8+ T cells, produced significant amounts of IFN-γ, but only after HSV-1 reactivated from latency (Figures 7A and 6A, respectively). C57BL/6 TG cultures that were depleted of endogenous CD8+ T cells and did not receive 2D5 cells, or received naive CD8+ T cells, produced low to undetectable levels of IFN-γ even after HSV-1 reactivation from latency (Figures 7B and 6B, respectively), demonstrating that HSV-specific CD8+ T cells are responsible for most or all IFN-γ production in these cultures.
Figure 7. IFN-γ Production in TG Cultures.

At the indicated times, supernatant fluids from the cultures described in Figure 4 were tested for IFN-γ content by ELISA. Data are presented for undepleted (A) and CD8+ T cell depleted (B) C57BL/6 TG cultures that received 1 × 105 2D5 cells or 1 × 105 naive CD8+ T cells.
Interestingly, the cultures that received a protective dose of 2D5 cells (1 × 105/culture) showed early production of IFN-γ on days 4 and 6 of culture, and the IFN-γ levels declined thereafter. These data suggest that the addition of exogenous gB-specific CD8+ T cells to the TG cultures allows more rapid interaction with reactivating neurons, thus permitting a protective CD8+ T cell response within the time frame necessary to block HSV-1 reactivation from latency. Together these findings demonstrate that CD8+ T cells can block HSV-1 reactivation from latency in TG cultures by a gB-specific, dose-dependent, MHC-restricted, noncytolytic mechanism that is at least partially IFN-γ dependent.
Discussion
Our current findings challenge the notion that HSV-1 latent infection in sensory ganglia represents a quiescent state wherein a lack of viral gene expression conceals the virus from the host immune system. Instead, we establish that a dynamic HSV-specific CD8+ T cell response is maintained in the TG for at least 75 days after HSV-1 latency is uniformly established. Several lines of evidence support the notion that HSV-specific CD8+ T cells are persistently stimulated within the latently infected ganglion. First, at least 60% of the CD8+ T cells in the latently infected TG were HSV specific as demonstrated by binding to the gB498-505 tetramer ex vivo. Second, during the early stages of CD8+ T cell infiltration into the infected TG, a time when virus replication was subsiding and latency was being established in sensory neurons, few CD8+ T cells expressed the early activation marker CD69. However, CD69 expression on ganglion-derived CD8+ T cells gradually increased through day 34 p.i., long after latency was uniformly established in the TG. Moreover, CD69 expression was maintained for at least 84 days p.i. Third, the variable size and level of CD8 expression on the CD8+ T cell population that initially infiltrated the TG (day 8–14 p.i.) gradually gave way to a more homogeneous, large, CD8hi population during latency (day 34–84 pi), indicative of a shift to a more uniformly activated CD8+ T cell population. Finally, in situ staining of latently infected TG with the gB498-505 tetramer revealed TCR polarization on many CD8+ T cells toward the T cell-neuron junction. This latter observation is suggestive of TCR aggregation in lipid rafts, as occurs during T cell activation (Janes et al., 1999). Although there have been a few previous reports of in situ tetramer staining (McGavern et al., 2002; Skinner et al., 2000), we believe this is the first report of such staining following infection in a nontransgenic model. Together, these findings suggest that HSV gB-specific memory CD8+ T cells are maintained in an activated state through a direct interaction with latently infected neurons that persistently or episodically express a portion of the viral genome including the γ1 gene gB.
We also noted a significant increase from 14–34 days p.i. in the proportion of ganglion-derived gB498-505 tetramer positive CD8+ T cells that produced IFN-γ in response to gB498-505 peptide-pulsed target cells. Their levels then remained constant through day 84 p.i. However, it should be noted that the total number of CD8+ T cells in the TG dropped during the period of 14–34 days p.i., so that the actual number of IFN-γ +, gB498-505 peptide-specific CD8+ T cells actually appeared to remain relatively constant. Thus, the increased proportion of IFN-γ-secreting cells could reflect either selective retention of a small population of cells capable of producing IFN-γ in response to gB peptide at day 14, or expansion of IFN-γ + CD8+ T cells in the TG through cell division or a functional change. We tend to favor the theory that the number of IFN-γ-producing cells in the TG increases during latency, because we routinely detect significantly more IFN-γ production by one TG equivalent of day 34 TG than by one TG equivalent of day 14 TG in ex vivo cultures (data not shown). Other viral infection models have demonstrated a similar enrichment for IFN-γ producing CD8+ T cells at sites of infection (Bergmann et al., 1999; Hawke et al., 1998). Together these findings suggest that CD8+ T cells that are retained at a peripheral site of infection undergo an antigen-driven maturation process resulting in enhanced IFN-γ production. Since IFN-γ can block HSV-1 reactivation from latency in sensory neurons, this functional shift would appear to produce a CD8+ T cell population that is ideally suited to the function of long-term protection with minimal tissue destruction.
It was recently reported that IFN-γ and TNF-α expression persisted in TG that were latently infected with an HSV-1 mutant lacking viral thymidine kinase (Tk− HSV-1) (Chen et al., 2000). Since Tk− HSV-1 mutants are unable to replicate their DNA in neurons, these findings suggest that maintenance of cytokine production occurred in the absence of viral DNA synthesis within the ganglion. DNA synthesis is not only required for production of infectious virions but is also necessary for expression of the γ2 class of HSV-1 genes during a lytic infection. In contrast, the α, β, and γ1 genes are expressed in the absence of viral DNA synthesis. Thus, it is reasonable to speculate that HSV α, β, and γ1 genes may be expressed in latently infected neurons in the absence of viral DNA synthesis and γ2 gene expression.
Our current studies clearly establish that a gB498-505 epitope-specific CD8+ T cell clone (2D5) can block HSV-1 reactivation from latency in ex vivo TG cultures derived from syngeneic C57BL/6 mice, but not from allogeneic BALB/c mice. This result established that the protective response is MHC restricted, and thus involves TCR engagement by an MHC I/gB498-505 peptide complex. Although sensory neurons do not normally express detectable MHC, they do express MHC I during the lytic phase of HSV-1 infection (Pereira and Simmons, 1999). Thus a reactivation stimulus that overcomes repression of viral gene expression might result in concordant expression of MHC I, allowing rapid presentation of viral epitopes to CD8+ T cells.
We show that virtually 100% of HSV-1-specific CD8+ T cells in the latently infected TG at 34 days p.i. recognized the gB498-505 epitope, and that all gB498-505 tetramer positive cells were CD69+. A similar immunodominance of the gB498-505 epitope is seen during the inductive phase of the immune response to HSV-1. (Wallace et al., 1999). It is somewhat surprising that a viral late gene product would be targeted by CD8+ T cells, and that these T cells could block HSV-1 reactivation from latency. One might predict that a viral late gene product would be expressed too late in the viral life cycle to elicit a CD8+ T cell response capable of blocking virion formation. It is noteworthy, however, that although gB is a γ1 gene, CD8+ T cells specific for gB are activated very early after infection (Mueller et al., 2002), and the gB498-505 epitope appears to be expressed on cells within hours of infection (Mueller et al., 2003).
These observations suggest a “just in time” mechanism in which CD8+ T cells within the ganglion may only respond when the virus reaches the point in its life cycle where γ1 genes are expressed, immediately prior to replication of its DNA. Such a mechanism would require a rapid response by the CD8+ T cells capable of shutting down further progression of the viral life cycle, possibly by inhibiting viral DNA synthesis. Indeed, we show in ex vivo TG cultures that CD8+ T cells reactive to the γ1 gene product gB block expression of the γ2 gene gH. The capacity of CD8+ T cells to provide such exquisite regulation of HSV-1 gene expression in vivo is suggested by their juxtaposition to neuron cell bodies in the ophthalmic branch of the latently infected TG and their persistent stimulation by neurons as indicated by CD69 expression and polarization of their TCR toward the T cell-neuron junction.
Based on this and our previous studies, we propose that CD8+ T cells employ multiple mechanisms to block HSV-1 reactivation from latency. The addition of CD8+ T cells from the lymph nodes of HSV-1 infected mice (Liu et al., 2000) or the addition of the gB498-505 epitopespecific CD8+ T cell clone, 2D5 (Figure 6), at the initiation of day 34 TG cultures completely blocked HSV-1 reactivation from latency. In contrast, the addition of IFN-γ (1000 U/ml) to day 34 TG cultures had no effect on reactivation frequency, unless the cultures were first treated for 4 days with the antiherpetic drug acyclovir (Liu et al., 2001). The addition of IFN-γ to cultures within 24 hr of acyclovir removal effectively prevented HSV-1 reactivation from latency. These findings suggest that IFN-γ can block HSV-1 reactivation only when present early in the reactivation process. Apparently during TG culture preparation some neurons exceed the window of opportunity for IFN-γ protection and require some other CD8+ T cell mechanism to block reactivation. This possibility is currently being investigated.
There is growing evidence that memory CD8+ T cells may reside in nonlymphoid tissues following viral infections (Masopust et al., 2001; Hogan et al., 2001; Wiley et al., 2001). In one study, CD8+ T cells were shown to reside in multiple tissues that did not appear to harbor viral genome or proteins, leading to the suggestion that memory CD8+ T cell retention might not be an antigen-driven process (Masopust et al., 2001). However, in that study the virus was administered intravenously, so the presence of very low levels of viral protein in multiple tissues could not be formally excluded. Following HSV-1 corneal infection, CD8+ T cells were selectively retained in the ophthalmic branch of the ipisilateral TG, where the viral genome is readily detectable. Moreover, while CD44 was consistently expressed on CD8+ T cells in the latently infected TG, CD69 expression actually increased during the first 34 days p.i., and was then maintained on 90% of CD8+ T cells through 84 days p.i. Our findings are consistent with the notion that HSV gB498-505 peptide-specific CD8+ T cells are retained in the latently infected TG in an activated, memory phenotype due to persistent low-level antigenic stimulation. The concept that functional T cell memory requires persistent antigen has been proposed and supported by experimental data (Gray and Matzinger, 1991; Ochsenbein et al., 1999; Kundig et al., 1996). Although CD69 expression has been observed on memory CD8+ T cells in tissues that appear to lack antigen (Wiley et al., 2001; Marshall et al., 2001), in one study CD69 expression diminished over time (Wiley et al., 2001). Combined with the observation that gB498-505 peptide -specific CD8+ memory T cells appear to primarily reside outside of the lymphoid organs following primary HSV-1 infection (Jones et al., 2000), our findings suggest that the latently infected TG could be a site of retention of HSV-specific CD8+ effector memory T cells.
There appears to be a dynamic balance between HSV-1 latency and reactivation involving a tripartite interaction among the virus, the host neuron, and the local immune components. We propose that the capacity of latently infected neurons to repress viral and MHC gene expression may be frequently perturbed, permitting rapid processing and presentation of some viral gene products to surrounding CD8+ T cells. The more frequent reactivation of HSV-1 in human TG may reflect a less efficient CD8+ T cell response. This would be consistent with the fact that the HSV-1 ICP47 protein binds more efficiently to human than to mouse TAPs, thus inhibiting the loading of antigenic peptides on human MHC I (Tomazin et al., 1996). In neurons that express very little MHC I, ICP47 might delay the activation of resident CD8+ T cells long enough to permit some virion formation. We propose that an appropriate vaccine targeting CD8+ T cells specific for viral proteins that are expressed during latency might prove highly efficacious in preventing recurrent herpetic disease.
Experimental Procedures
HSV-1 Infection
6- to 8-wk-old female C57BL/6 (Jackson Laboratory, Bar Harbor, ME) and BALB/c (Frederick Cancer Research Center, Frederick, MD) mice were anesthetized by intramuscular injection of 2.0 mg of ketamine hydrochloride and 0.04 mg of xylazine (Pheonix Scientific; St Joseph, MO) in 0.2 ml of HBSS (Biowhittaker, Walkersville, MD). The RE strain of HSV-1 used in these studies was grown in Vero cells, and intact virions were purified on Percoll (Pharmacia LKB Biotechnology, Inc., Piscataway, NJ) gradients. Corneas of anesthetized mice were scarified 10 times in a crisscross fashion with a sterile 30-gauge needle, and the eyes were infected topically with 3 μl of RPMI (Biowhittaker, Rockland, ME) containing 105 PFU of HSV-1.
Single Cell Suspensions of TG
At various times after HSV-1 corneal infection, mice were perfused with PBS, and TGs were excised and treated with collagenase type I (3 mg/ml; Sigma Chemical Co., St Louis, MO) for 1.5 hr at 37°C. The TGs were dissociated into single cell suspension by trituration as previously described (Liu et al., 2000).
Reagents
Peptides gB498-505 (SSIEFARL) and RR1822-829 (QTFDFGRL) were purchased from Research Genetics (Invitrogen Corporation, Carlsbad, California). Peptide purity was confirmed to be >95% by reverse phase HPLC analysis. Phycoerythrin (PE)-conjugated H-2Kb tetramers complexed with gB498-505 or RR1822-829 were generated by the NIAID Tetramer Core Facility (Emory University Vaccine Center, Atlanta, GA).
PE-conjugated anti-CD8α (clone 53-6.7); fluorescein isothiocyanate-conjugated anti-CD44 (IM7), anti-CD69 (H1.2F3), and anti-IFNγ (XMG1.2); and Cy-Chrome-conjugated anti-CD45 (30-F11) were purchased from BD Pharmingen, San Diego, CA. Isotype control antibodies were purchased from BD Pharmingen, Caltag (Burlingame, CA), and Jackson ImmunoResearch (West Grove, PA).
Immunohistochemistry
Following perfusion with PBS, mice were sacrificed and TG were excised from latently infected mice and processed for frozen sectioning, as previously described (Liu et al., 1996), or for whole organ staining. Fixed, frozen sections were reacted with rat anti-mouse CD8α (53-6.7, 1 μg/ml, BD Pharmingen) overnight. The sections were then washed and fixed with 2% paraformaldehyde for 30 min at 4°C, followed by the addition of Alexa Flour 546 goat anti-rat IgG (2 μg/ml, Molecular Probes, Eugene, OR) diluted in 2% normal goat serum and PBS. The sections were incubated at 4°C for 3 hr, washed, mounted with Immu-Mount (Thermo Shandon, Pittsburgh, PA), and analyzed using a Bio-Rad 2000 confocal microscope (Bio-Rad, Richmond, VA)
Alternatively, whole TGs were stained using a modification of a previously described in situ tetramer staining procedure (Skinner et al., 2000). In brief, excised TG were washed in PBS and incubated overnight at 4°C in round-bottom 96-well plates with PE-conjugated MHC class I tetramers (2 μg/ml) and rat anti-mouse CD8α (53-6.7, 1 μg/ml, BD Pharmingen) diluted in 2% normal goat serum and PBS. Tissues were washed, fixed with 2% paraformaldehyde for 30 min at 4°C, and incubated overnight at 4°C with Alexa Flour 546 goat anti-rat IgG (2 μg/ml, Molecular Probes) and rabbit anti-PE (1 μg/ml, Biomeda, Hayword, CA) diluted in 2% normal goat serum and PBS. The tissues were then washed and incubated for 6 hr at 4°C with Alexa Flour 488 goat anti-rabbit IgG (2 μg/ml, Molecular Probes). The tissues were washed and mounted on slides using Immu-Mount. The stained whole mount tissues were analyzed by confocal microscopy.
Flow Cytometry
Single cell suspensions of TG were pooled and passed through a 40 μ filter. Aliquots of TG cells (2 TG equivalents) were added to 5 ml polystyrene round-bottom tubes (Becton Dickinson, Franklin Lakes, NJ) and stained for cell surface markers. The cells were then fixed in 1% paraformaldehyde (PFA, Electron Microscopy Sciences, Fort Washington, PA) and stored at 4°C until analyzed. The cells were analyzed on a FACSCalibur (Becton Dickinson) using WinMDI data analysis software (J. Totter, The Scripps Clinic, La Jolla, CA). Single cell suspensions of TG were first stained with the anti-CD8α antibody for 30 min at 4°C followed by gB498-505 or RR1822-829 tetramers for 20 min at room temperature. Cells were fixed with 1% PFA and analyzed immediately by flow cytometry.
Intracellular IFNγ stains were carried out using cytofix/cytoperm kit with Golgiplug (BD Pharmingen) in accordance with the manufacturer’s instructions. In brief, aliquots of pooled TG cells (2 TG equivalents) were incubated with 1 × 106 stimulator cells and Golgiplug for 6 hr at 37°C in flow tubes. Stimulator cells were the B6WT3 fibroblast cell line that were uninfected, infected with HSV-1 at an MOI of 5 for 6 hr, transfected to produce the gB498-505 peptide (B6/T-350gB) (Fu et al., 1993), or transfected to produce the RR1822-829 peptide (B6/T-350RR1) (Salvucci et al., 1995). After the 6 hr incubation, the cells were stained for cell surface molecules and intracellular cytokines followed immediately by flow cytometric analysis.
Preparation of TG Cultures
Single cell suspensions of pooled TGs were added to each well (1/2 TG/well) of a 48-well tissue culture plate, and the cells were cultured with DMEM (Biowittaker) containing 10% FCS (HyClone, Logan, UT), and 10 U/ml recombinant murine IL-2 (R&D Systems, Inc., Minneapolis, MN) as previously described (Liu et al., 2001). Where indicated, the TG cells were depleted of CD8+ T cells by immunomagnetic separation using anti-CD8-coated dynabeads (Dynal ASA, Oslo, Norway) as previously described (Liu et al., 2001). The efficiency of CD8+ T cell depletion was routinely greater than 98% as determined by flow cytometry. The CD8+ T cell clone (2D5) specific for the HSV-1 gB498-505 epitope was maintained as previously described (Brehm et al., 1999), and used after a 3 day rest period in which no stimulant was added. On the day of the experiment, the 2D5 cells were removed from the wells using versene, washed, and added to the TG cultures at various doses. Naive CD8+ T cells were obtained from the spleens of uninfected mice by magnetic activated cell sorting (MACS, Miltenyi Biotec, Auburn, CA) according to the manufacturer’s instructions. The naive CD8+ T cells were washed and added to the TG cultures.
IFNγ Titration in TG Cultures
At various times after culture initiation, 50 μl of medium was removed from each culture and tested for IFNγ content using a standard-enzyme linked immunosorbent assay (ELISA) as described previously (Liu et al., 2001).
Monitoring of TG Cultures for HSV-1 Reactivation from Latency
Within 3 days of TG culture initiation, the neurons could be observed on a monolayer of fibroblast-like cells. The cultures were monitored using three criteria to define HSV-1 reactivation from latency. A culture was considered positive for HSV-1 reactivation if microscopic examination revealed the presence of viral CPE, or if infectious virus was detected in serial samples of culture supernatant (50 μl/culture) using a standard viral plaque assay on monolayers of Vero cells (Liu et al., 2000). To further confirm monitoring accuracy, selected cultures that were negative for reactivation based on the above criteria were tested for the presence of HSV-1 γ2 gene (glycoprotein H) transcripts by RT-PCR. Cultures that lacked viral CPE and infectious virus were uniformly negative for gH transcripts.
Reverse Transcription PCR
Ten days after initiation of TG cultures, cells were scraped off the surface of the wells and total RNA was extracted from the cells using RNeasy columns (Quiagen, Valencia, CA) according to manufacturer’s instructions. Total RNA was treated with DNase-1 using the DNA-free kit according to manufacturer’s instructions (Ambion Inc., Austin, TX). RT-PCR was performed using the GeneAmp Gold RNA PCR core kit (Applied Biosystems, Foster City, CA). The cDNA encoding glycoprotein H and housekeeping gene hypoxanthineguanine phosphoribosyl transferase (HPRT) were amplified by using the following primer pairs through 40 cycles:
The following primers were used: gH, left (TTTATGGTTCGTGGGGGTTA); right (GGTCTTCGGGATGTAAAGCA) and HPRT, left (CTG GTGAAAAGGACCTCTCG), and right (TGAAGTACTCATTATAGTCAAGGGCA). The reaction conditions for the RT were 25°C for 10 min followed by 42°C for 12 min and for the PCR reaction were (1) initial activation of AmpliTaq gold 95°C for 4 min, (2) annealing and extension at 95°C for 30 s, 55°C for 30 s, and 72°C for 1 min, and (3) final extension at 72°C for 10 min.
Acknowledgments
The authors thank Dr. JoAnne Flynn for critically reading the manuscript and Mr. Brian Carriere for excellent technical assistance, Dr. Jayakar V. Nayak for assistance with flow cytometry, and the NIAID Tetramer Core Facility, Emory University Vaccine Center, Atlanta, Georgia, for supplying the tetramers. Support for this work was provided by NIH grants EY05945 (R.L.H.), P30 EY08098 (R.L.H.), AI49719 (R.H.B.), and EY07397 (P.R.K.), an unrestricted grant from Research to Prevent Blindness, New York, N.Y., and the Eye and Ear Foundation of Pittsburgh.
References
- Bergmann CC, Altman JD, Hinton D, Stohlman SA. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J Immunol. 1999;163:3379–3387. [PubMed] [Google Scholar]
- Brehm M, Samaniego LA, Bonneau RH, DeLuca NA, Tevethia SS. Immunogenicity of herpes simplex virus type 1 mutants containing deletions in one or more alpha-genes: ICP4, ICP27, ICP22, and ICP0. Virology. 1999;256:258–269. doi: 10.1006/viro.1999.9653. [DOI] [PubMed] [Google Scholar]
- Cantin EM, Hinton DR, Chen J, Openshaw H. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J Virol. 1995;69:4898–4905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Kramer MF, Schaffer PA, Coen DM. A viral function represses accumulation of transcripts from productive-cycle genes in mouse ganglia latently infected with herpes simplex virus. J Virol. 1997;71:5878–5884. doi: 10.1128/jvi.71.8.5878-5884.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Garber DA, Schaffer PA, Knipe DM, Coen DM. Persistent elevated expression of cytokine transcripts in ganglia latently infected with herpes simplex virus in the absence of ganglionic replication or reactivation. Virology. 2000;278:207–216. doi: 10.1006/viro.2000.0643. [DOI] [PubMed] [Google Scholar]
- Chen SH, Lee LY, Garber DA, Schaffer PA, Knipe DM, Coen DM. Neither LAT nor open reading frame P mutations increase expression of spliced or intron-containing ICP0 transcripts in mouse ganglia latently infected with herpes simplex virus. J Virol. 2002;76:4764–4772. doi: 10.1128/JVI.76.10.4764-4772.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favoreel HW, Nauwynck HJ, Pensaert MB. Immunological hiding of herpesvirus-infected cells. Arch Virol. 2000;145:1269–1290. doi: 10.1007/s007050070090. [DOI] [PubMed] [Google Scholar]
- Feldman LT, Ellison AR, Voytek CC, Yang L, Krause P, Margolis TP. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc Natl Acad Sci USA. 2002;99:978–983. doi: 10.1073/pnas.022301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu TM, Bonneau RH, Tevethia MJ, Tevethia SS. Simian virus 40 T antigen as a carrier for the expression of cytotoxic T-lymphocyte recognition epitopes. J Virol. 1993;67:6866–6871. doi: 10.1128/jvi.67.11.6866-6871.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D, Matzinger P. T cell memory is short-lived in the absence of antigen. J Exp Med. 1991;174:969–974. doi: 10.1084/jem.174.5.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford WP, Gebhardt BM, Carr DJJ. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J Immunol. 1996;157:3542–3549. [PubMed] [Google Scholar]
- Hawke S, Stevenson PG, Freeman S, Bangham CR. Long-term persistence of activated cytotoxic T lymphocytes after viral infection of the central nervous system. J Exp Med. 1998;187:1575–1582. doi: 10.1084/jem.187.10.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan RJ, Usherwood EJ, Zhong W, Roberts AA, Dutton RW, Harmsen AG, Woodland DL. Activated antigen-specific CD8(+) T cells persist in the lungs following recovery from respiratory virus infections. J Immunol. 2001;166:1813–1822. doi: 10.4049/jimmunol.166.3.1813. [DOI] [PubMed] [Google Scholar]
- Janes PW, Ley SC, Magee AI. Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Cose SC, McNally JM, Jennings SR, Heath WR, Carbone FR. Diminished secondary CTL response in draining lymph nodes on cutaneous challenge with herpes simplex virus. J Gen Virol. 2000;81:407–414. doi: 10.1099/0022-1317-81-2-407. [DOI] [PubMed] [Google Scholar]
- Kramer MF, Coen DM. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J Virol. 1995;69:1389–1399. doi: 10.1128/jvi.69.3.1389-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundig TM, Bachmann MF, Oehen S, Hoffmann UW, Simard JJ, Kalberer CP, Pircher H, Ohashi PS, Hengartner H, Zinkernagel RM. On the role of antigen in maintaining cytotoxic T-cell memory. Proc Natl Acad Sci USA. 1996;93:9716–9723. doi: 10.1073/pnas.93.18.9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrecque N, Whitfield LS, Obst R, Waltzinger C, Benoist C, Mathis D. How much TCR does a T cell need? Immunity. 2001;15:71–82. doi: 10.1016/s1074-7613(01)00170-4. [DOI] [PubMed] [Google Scholar]
- Liu T, Tang Q, Hendricks RL. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol. 1996;70:264–271. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Khanna KM, Chen X, Fink DJ, Hendricks RL. CD8(+) T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neurons. J Exp Med. 2000;191:1459–1466. doi: 10.1084/jem.191.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Khanna KM, Carriere BN, Hendricks RL. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J Virol. 2001;75:11178–11184. doi: 10.1128/JVI.75.22.11178-11184.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall DR, Turner SJ, Belz GT, Wingo S, Andreansky S, Sangster MY, Riberdy JM, Liu T, Tan M, Doherty PC. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci USA. 2001;98:6313–6318. doi: 10.1073/pnas.101132698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- McGavern DB, Christen U, Oldstone MB. Molecular anatomy of antigen-specific CD8+ T cell engagement and synapse formation in vivo. Nat Immunol. 2002;3:918–925. doi: 10.1038/ni843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Jones CM, Smith CM, Heath WR, Carbone FR. Rapid cytotoxic T lymphocyte activation occurs in the draining lymph nodes after cutaneous herpes simplex virus infection as a result of early antigen presentation and not the presence of virus. J Exp Med. 2002;195:651–656. doi: 10.1084/jem.20012023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, Jones CM, Chen W, Kawaoka Y, Castrucci MR, Heath WR, Carbone FR. The early expression of glycoprotein B from herpes simplex virus can be detected by antigen-specific CD8+ T Cells. J Virol. 2003;77:2445–2451. doi: 10.1128/JVI.77.4.2445-2451.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsenbein AF, Karrer U, Klenerman P, Althage A, Ciurea A, Shen H, Miller JF, Whitton JL, Hengartner H, Zinkernagel RM. A comparison of T cell memory against the same antigen induced by virus versus intracellular bacteria. Proc Natl Acad Sci USA. 1999;96:9293–9298. doi: 10.1073/pnas.96.16.9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira RA, Simmons A. Cell surface expression of H2 antigens on primary sensory neurons in response to acute but not latent herpes simplex virus infection in vivo. J Virol. 1999;73:6484–6489. doi: 10.1128/jvi.73.8.6484-6489.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira RA, Tscharke DC, Simmons A. Upregulation of class I major histocompatibility complex gene expression in primary sensory neurons, satellite cells, and Schwann cells of mice in response to acute but not latent herpes simplex virus infection in vivo. J Exp Med. 1994;180:841–850. doi: 10.1084/jem.180.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvucci LA, Bonneau RH, Tevethia SS. Polymorphism within the herpes simplex virus (HSV) ribonucleotide reductase large subunit (ICP6) confers type specificity for recognition by HSV type 1-specific cytotoxic T lymphocytes. J Virol. 1995;69:1122–1131. doi: 10.1128/jvi.69.2.1122-1131.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner PJ, Daniels MA, Schmidt CS, Jameson SC, Haase AT. Cutting edge: in situ tetramer staining of antigen-specific T cells in tissues. J Immunol. 2000;165:613–617. doi: 10.4049/jimmunol.165.2.613. [DOI] [PubMed] [Google Scholar]
- Tal-Singer R, Lasner TM, Podrzucki W, Skokotas A, Leary JJ, Berger SL, Fraser NW. Gene expression during reactivation of herpes simplex virus type 1 from latency in the peripheral nervous system is different from that during lytic infection of tissue cultures. J Virol. 1997;71:5268–5276. doi: 10.1128/jvi.71.7.5268-5276.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomazin R, Hill AB, Jugovic P, York I, van Endert P, Ploegh HL, Andrews DW, Johnson DC. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 1996;15:3256–3266. [PMC free article] [PubMed] [Google Scholar]
- Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- Wallace ME, Keating R, Heath WR, Carbone FR. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J Virol. 1999;73:7619–7626. doi: 10.1128/jvi.73.9.7619-7626.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JA, Hogan RJ, Woodland DL, Harmsen AG. Antigen-specific CD8(+) T cells persist in the upper respiratory tract following influenza virus infection. J Immunol. 2001;167:3293–3299. doi: 10.4049/jimmunol.167.6.3293. [DOI] [PubMed] [Google Scholar]
- York IA, Roop C, Andrews DW, Riddell SR, Graham FL, Johnson DC. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell. 1994;77:525–535. doi: 10.1016/0092-8674(94)90215-1. [DOI] [PubMed] [Google Scholar]