Abstract
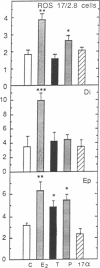
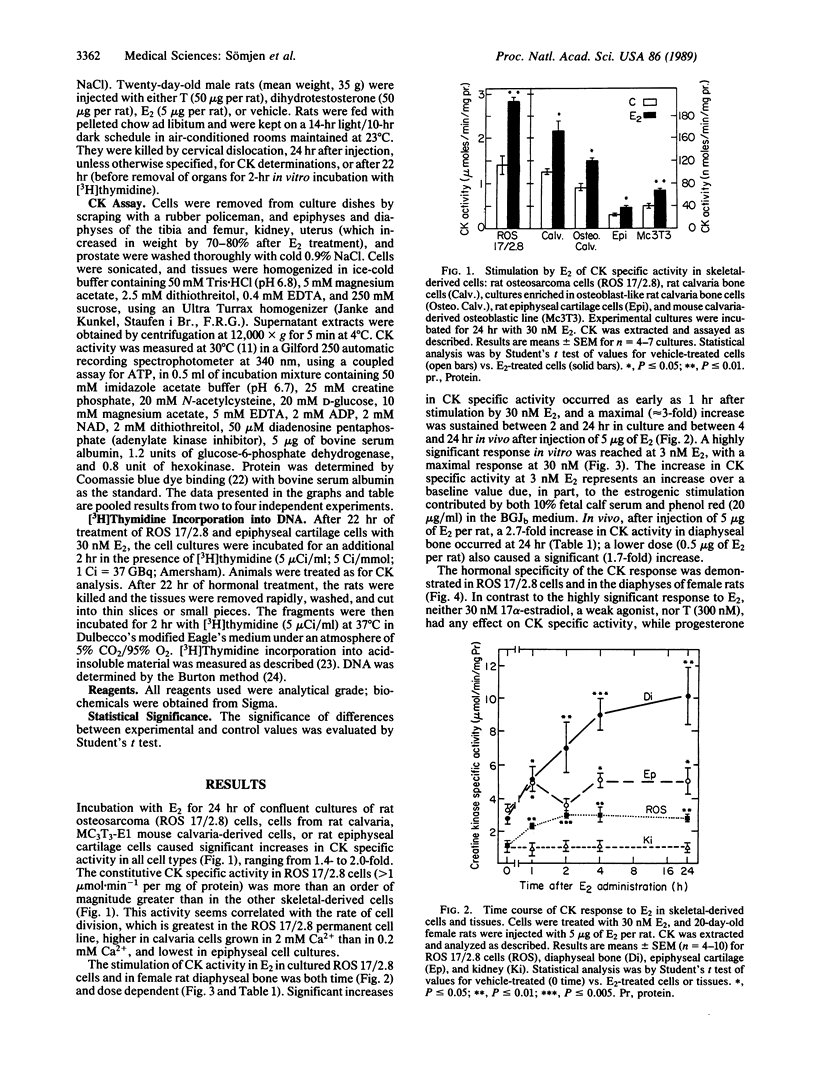
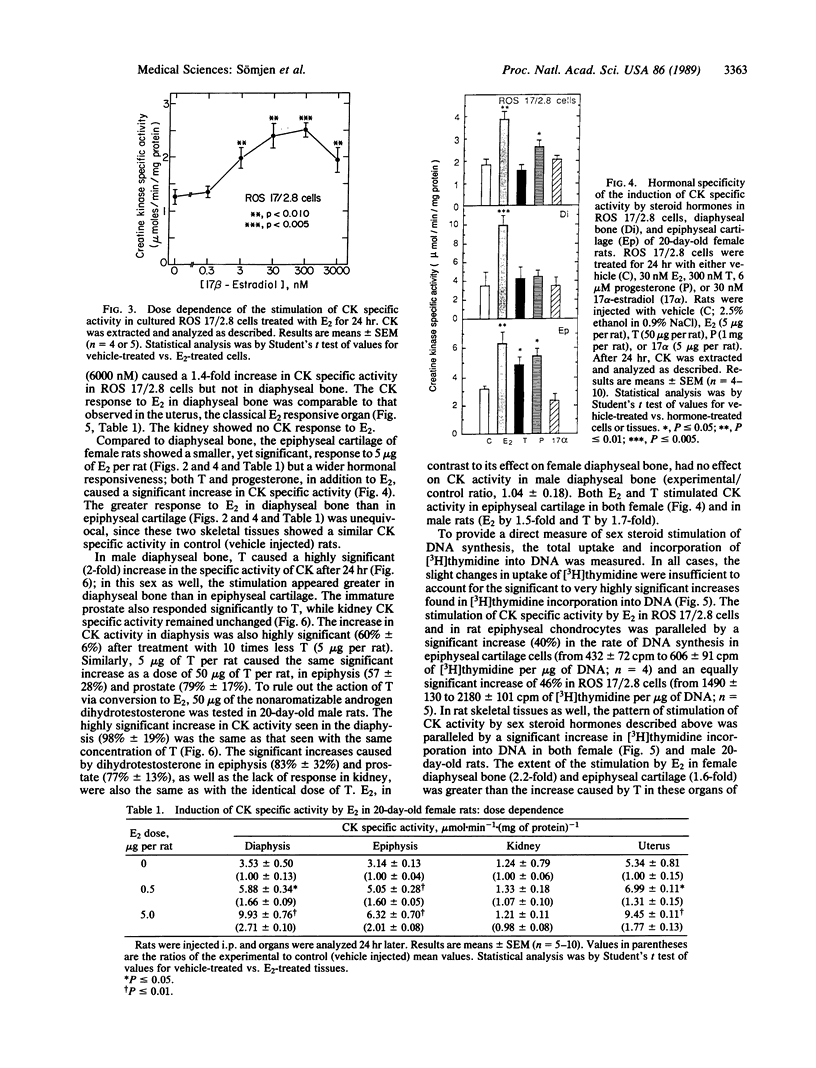
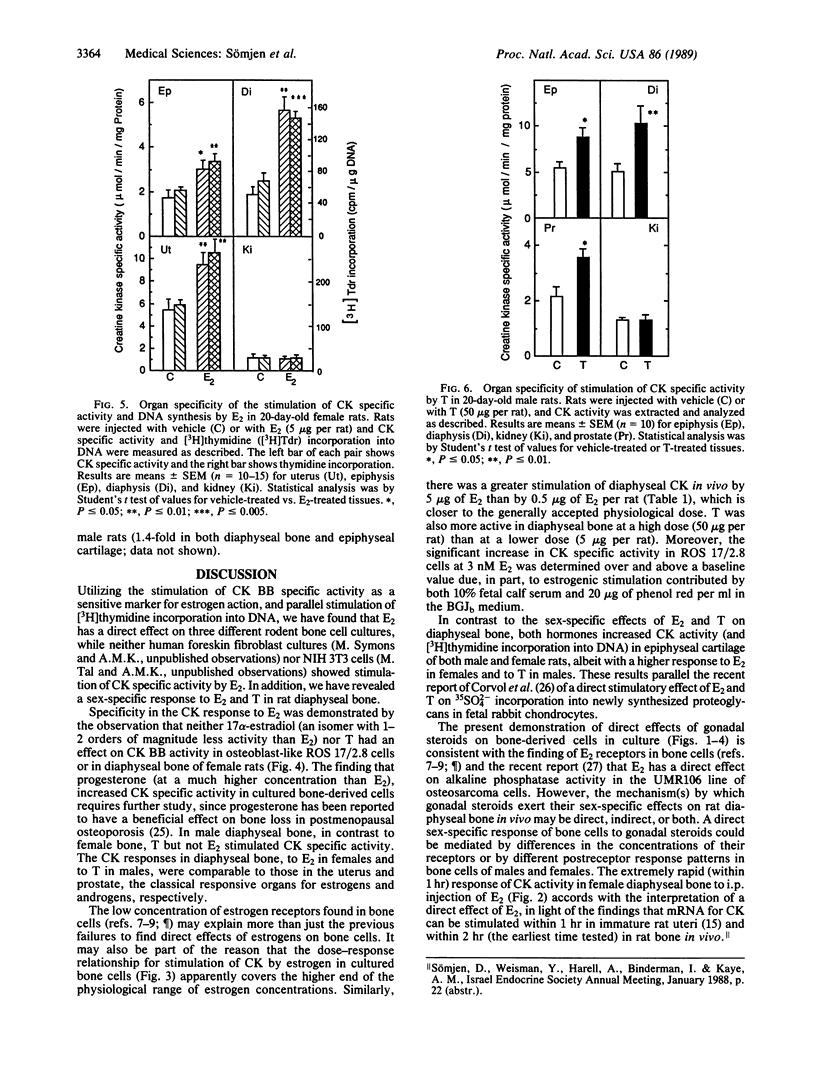
A direct in vitro effect of 17 beta-estradiol (E2) was demonstrated on bone and cartilage cell energy metabolism. Sex-specific stimulation by E2 and testosterone was shown in diaphyseal bone of weanling rats. E2 (30 nM) caused, within 24 hr, a 70-200% increase in creatine kinase (CK; ATP:creatine N-phosphotransferase, EC 2.7.3.2) specific activity in ROS 17/2.8 rat osteogenic sarcoma cells, MC3T3-E1 mouse calvaria-derived cells, and rat fetal calvaria cells, and a 40% increase in rat epiphyseal cartilage cells. Stimulation of CK activity by E2 was dose and time dependent: in ROS 17/2.8 cells, a highly significant increase was found at 3 nM E2 and a greater than 100% increase in CK activity was found 1 hr after E2 administration. In female 20-day-old Wistar-derived rats, E2 (5 micrograms per rat) increased CK activity in diaphyseal bone by 82% within 1 hr of i.p. injection, with a maximal increase of 200% after 24 hr; neither the weakly estrogenic agonist 17 alpha-estradiol, testosterone, nor progesterone showed this effect. Conversely, in male rat diaphyseal bone, testosterone or dihydrotestosterone increased CK activity after 24 hr by approximately 100%, while E2 was ineffective. In epiphyseal cartilage, both E2 and testosterone increased CK activity. Stimulation of CK activity by sex hormones was paralleled by significant increases in [3H]thymidine incorporation into DNA. Therefore, it is possible that direct sex-specific actions of gonadal steroids may contribute to stimulating bone growth and maintaining balanced bone turnover.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUDY A. M., URIST M. R., MCLEAN F. C. The effect of estrogens on the growth apparatus of the bones of immature rats. Am J Pathol. 1952 Nov-Dec;28(6):1143–1167. [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baran D. T., Bergfeld M. A., Teitelbaum S. L., Avioli L. V. Effect of testosterone therapy on bone formation in an osteoporotic hypogonadal male. Calcif Tissue Res. 1978 Dec 8;26(2):103–106. doi: 10.1007/BF02013243. [DOI] [PubMed] [Google Scholar]
- Binderman I., Duksin D., Harell A., Katzir E., Sachs L. Formation of bone tissue in culture from isolated bone cells. J Cell Biol. 1974 May;61(2):427–439. doi: 10.1083/jcb.61.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnett C. C., Reddi A. H. Influence of estrogen and progesterone on matrix-induced endochondral bone formation. Calcif Tissue Int. 1983 Jul;35(4-5):609–614. doi: 10.1007/BF02405102. [DOI] [PubMed] [Google Scholar]
- Caputo C. B., Meadows D., Raisz L. G. Failure of estrogens and androgens to inhibit bone resorption in tissue culture. Endocrinology. 1976 Apr;98(4):1065–1068. doi: 10.1210/endo-98-4-1065. [DOI] [PubMed] [Google Scholar]
- Chen T. L., Feldman D. Distinction between alpha-fetoprotein and intracellular estrogen receptors: evidence against the presence of estradiol receptors in rat bone. Endocrinology. 1978 Jan;102(1):236–244. doi: 10.1210/endo-102-1-236. [DOI] [PubMed] [Google Scholar]
- Corvol M. T., Carrascosa A., Tsagris L., Blanchard O., Rappaport R. Evidence for a direct in vitro action of sex steroids on rabbit cartilage cells during skeletal growth: influence of age and sex. Endocrinology. 1987 Apr;120(4):1422–1429. doi: 10.1210/endo-120-4-1422. [DOI] [PubMed] [Google Scholar]
- Denef C. Effect of hypophysectomy and pituitary implants at puberty on the sexual differentiation of testosterone metabolism in rat liver. Endocrinology. 1974 Jun;94(6):1577–1582. doi: 10.1210/endo-94-6-1577. [DOI] [PubMed] [Google Scholar]
- Eriksen E. F., Colvard D. S., Berg N. J., Graham M. L., Mann K. G., Spelsberg T. C., Riggs B. L. Evidence of estrogen receptors in normal human osteoblast-like cells. Science. 1988 Jul 1;241(4861):84–86. doi: 10.1126/science.3388021. [DOI] [PubMed] [Google Scholar]
- Francis R. M., Peacock M., Aaron J. E., Selby P. L., Taylor G. A., Thompson J., Marshall D. H., Horsman A. Osteoporosis in hypogonadal men: role of decreased plasma 1,25-dihydroxyvitamin D, calcium malabsorption, and low bone formation. Bone. 1986;7(4):261–268. doi: 10.1016/8756-3282(86)90205-x. [DOI] [PubMed] [Google Scholar]
- Frantz A. G., Rabkin M. T. Effects of estrogen and sex difference on secretion of human growth hormone. J Clin Endocrinol Metab. 1965 Nov;25(11):1470–1480. doi: 10.1210/jcem-25-11-1470. [DOI] [PubMed] [Google Scholar]
- Gray T. K., Flynn T. C., Gray K. M., Nabell L. M. 17 beta-estradiol acts directly on the clonal osteoblastic cell line UMR106. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6267–6271. doi: 10.1073/pnas.84.17.6267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson J. A., Stenberg A. Masculinization of rat liver enzyme activities following hypophysectomy. Endocrinology. 1974 Sep;95(3):891–896. doi: 10.1210/endo-95-3-891. [DOI] [PubMed] [Google Scholar]
- Jansson J. O., Edén S., Isaksson O. Sexual dimorphism in the control of growth hormone secretion. Endocr Rev. 1985 Spring;6(2):128–150. doi: 10.1210/edrv-6-2-128. [DOI] [PubMed] [Google Scholar]
- Kaplan F. S., Fallon M. D., Boden S. D., Schmidt R., Senior M., Haddad J. G. Estrogen receptors in bone in a patient with polyostotic fibrous dysplasia (McCune-Albright syndrome). N Engl J Med. 1988 Aug 18;319(7):421–425. doi: 10.1056/NEJM198808183190707. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Gorski J. Estrogen action in vitro. Induction of the synthesis of a specific uterine protein. J Biol Chem. 1972 Feb 25;247(4):1299–1305. [PubMed] [Google Scholar]
- Kaye A. M. Enzyme induction by estrogen. J Steroid Biochem. 1983 Jul;19(1A):33–40. [PubMed] [Google Scholar]
- Kaye A. M., Reiss N. A., Weisman Y., Binderman I., Sömjen D. Hormonal regulation of creatine kinase BB. Adv Exp Med Biol. 1986;194:83–101. doi: 10.1007/978-1-4684-5107-8_7. [DOI] [PubMed] [Google Scholar]
- Komm B. S., Terpening C. M., Benz D. J., Graeme K. A., Gallegos A., Korc M., Greene G. L., O'Malley B. W., Haussler M. R. Estrogen binding, receptor mRNA, and biologic response in osteoblast-like osteosarcoma cells. Science. 1988 Jul 1;241(4861):81–84. doi: 10.1126/science.3164526. [DOI] [PubMed] [Google Scholar]
- Lindsay R., Hart D. M., Forrest C., Baird C. Prevention of spinal osteoporosis in oophorectomised women. Lancet. 1980 Nov 29;2(8205):1151–1154. doi: 10.1016/s0140-6736(80)92592-1. [DOI] [PubMed] [Google Scholar]
- Lindsay R., Hart D. M., Purdie D., Ferguson M. M., Clark A. S., Kraszewski A. Comparative effects of oestrogen and a progestogen on bone loss in postmenopausal women. Clin Sci Mol Med. 1978 Feb;54(2):193–195. doi: 10.1042/cs0540193. [DOI] [PubMed] [Google Scholar]
- MacLusky N. J., Naftolin F. Sexual differentiation of the central nervous system. Science. 1981 Mar 20;211(4488):1294–1302. doi: 10.1126/science.6163211. [DOI] [PubMed] [Google Scholar]
- Majeska R. J., Rodan S. B., Rodan G. A. Parathyroid hormone-responsive clonal cell lines from rat osteosarcoma. Endocrinology. 1980 Nov;107(5):1494–1503. doi: 10.1210/endo-107-5-1494. [DOI] [PubMed] [Google Scholar]
- Notides A., Gorski J. Estrogen-induced synthesis of a specific uterine protein. Proc Natl Acad Sci U S A. 1966 Jul;56(1):230–235. doi: 10.1073/pnas.56.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss N. A., Kaye A. M. Identification of the major component of the estrogen-induced protein of rat uterus as the BB isozyme of creatine kinase. J Biol Chem. 1981 Jun 10;256(11):5741–5749. [PubMed] [Google Scholar]
- Riggs B. L., Jowsey J., Goldsmith R. S., Kelly P. J., Hoffman D. L., Arnaud C. D. Short- and long-term effects of estrogen and synthetic anabolic hormone in postmenopausal osteoporosis. J Clin Invest. 1972 Jul;51(7):1659–1663. doi: 10.1172/JCI106967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs B. L., Jowsey J., Kelly P. J., Jones J. D., Maher F. T. Effect of sex hormones on bone in primary osteoporosis. J Clin Invest. 1969 Jun;48(6):1065–1072. doi: 10.1172/JCI106062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman E., Melton L. J., 3rd, O'Fallon W. M., Riggs B. L. Risk factors for spinal osteoporosis in men. Am J Med. 1983 Dec;75(6):977–983. doi: 10.1016/0002-9343(83)90878-1. [DOI] [PubMed] [Google Scholar]
- Sudo H., Kodama H. A., Amagai Y., Yamamoto S., Kasai S. In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol. 1983 Jan;96(1):191–198. doi: 10.1083/jcb.96.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sömjen D., Binderman I., Weisman Y. The effects of 24R,25-dihydroxycholecalciferol and of 1 alpha,25-dihydroxycholecalciferol on ornithine decarboxylase activity and on DNA synthesis in the epiphysis and diaphysis of rat bone and in the duodenum. Biochem J. 1983 Aug 15;214(2):293–298. doi: 10.1042/bj2140293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M. D., Kaye A. M. mRNA for the rat uterine estrogen-induced protein. Translation in vitro and regulation by estrogen. J Biol Chem. 1981 Jan 10;256(1):23–26. [PubMed] [Google Scholar]
- van Paassen H. C., Poortman J., Borgart-Creutzburg I. H., Thijssen J. H., Duursma S. A. Oestrogen binding proteins in bone cell cytosol. Calcif Tissue Res. 1978 Aug 18;25(3):249–254. doi: 10.1007/BF02010778. [DOI] [PubMed] [Google Scholar]