Abstract
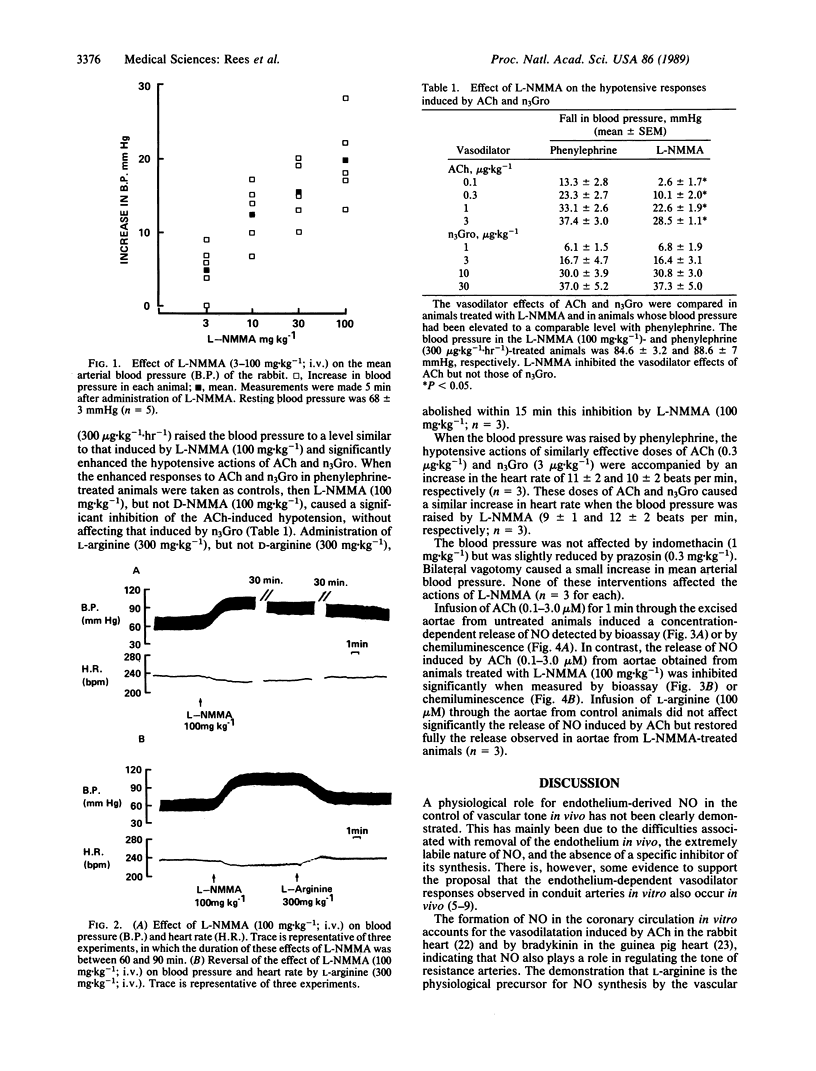
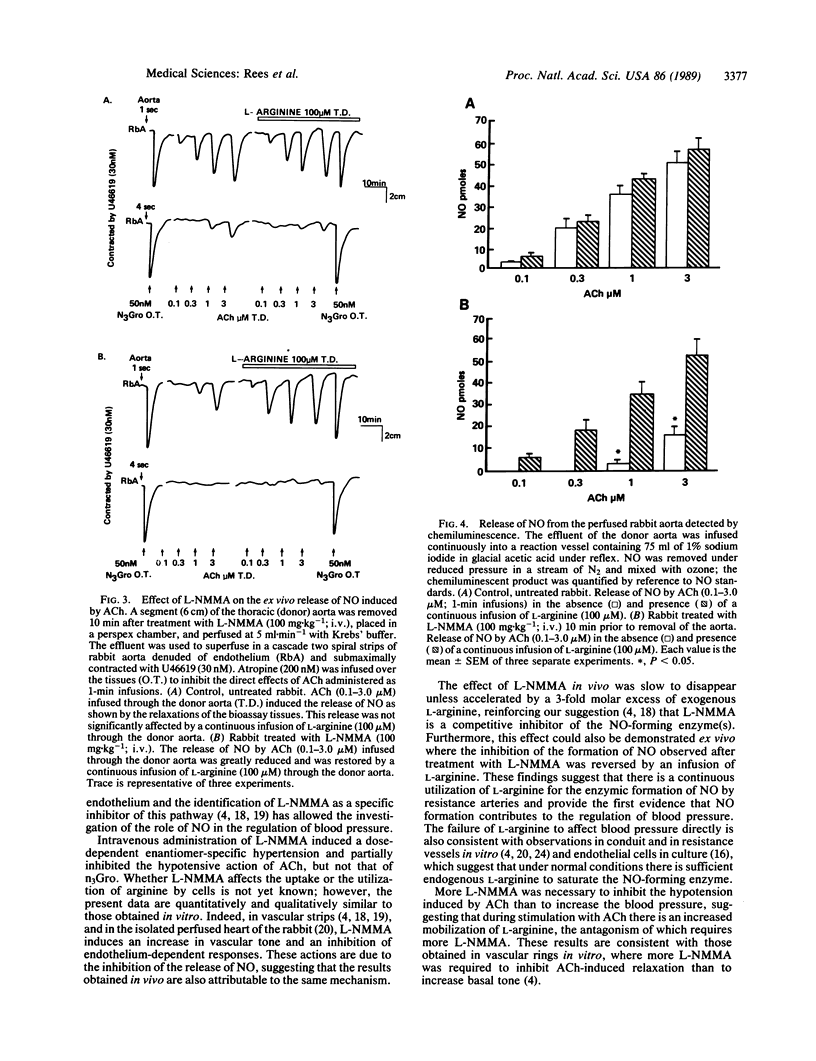
The role of endothelium-derived nitric oxide in the regulation of blood pressure in the anesthetized rabbit was studied with N omega-monomethyl-L-arginine (L-NMMA), a specific inhibitor of its formation from L-arginine. L-NMMA (3-100 mg.kg-1), but not its D-enantiomer, induced a dose-dependent long-lasting (15-90 min) increase in mean systemic arterial blood pressure. L-NMMA (100 mg.kg-1) also inhibited significantly the hypotensive action of acetylcholine, without affecting that of glyceryl trinitrate. Both these actions of L-NMMA were reversed by L-arginine (300 mg.kg-1), but not by D-arginine (300 mg.kg-1), indomethacin (1 mg.kg-1), prazosin (0.3 mg.kg-1), or by vagotomy. The effects of L-NMMA in vivo were associated with a significant inhibition of the release of nitric oxide from perfused aortic segments ex vivo. This inhibition was reversed by infusing L-arginine through the aortic segments. These results indicate that nitric oxide formation from L-arginine by the vascular endothelium plays a role in the regulation of blood pressure and in the hypotensive actions of acetylcholine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amezcua J. L., Dusting G. J., Palmer R. M., Moncada S. Acetylcholine induces vasodilatation in the rabbit isolated heart through the release of nitric oxide, the endogenous nitrovasodilator. Br J Pharmacol. 1988 Nov;95(3):830–834. doi: 10.1111/j.1476-5381.1988.tb11711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus J. A., Campbell G. R., Cocks T. M., Manderson J. A. Vasodilatation by acetylcholine is endothelium-dependent: a study by sonomicrometry in canine femoral artery in vivo. J Physiol. 1983 Nov;344:209–222. doi: 10.1113/jphysiol.1983.sp014934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudel C., Förstermann U. Gossypol attenuates selectively the blood pressure lowering effect of endothelium-dependent vasodilators in the rabbit in vivo. Eur J Pharmacol. 1988 Jan 12;145(2):217–221. doi: 10.1016/0014-2999(88)90234-8. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F. The role of endothelium in the responses of vascular smooth muscle to drugs. Annu Rev Pharmacol Toxicol. 1984;24:175–197. doi: 10.1146/annurev.pa.24.040184.001135. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H., Lewis M. J., Newby A. C., Henderson A. H. The nature of endothelium-derived vascular relaxant factor. Nature. 1984 Apr 12;308(5960):645–647. doi: 10.1038/308645a0. [DOI] [PubMed] [Google Scholar]
- Holtz J., Förstermann U., Pohl U., Giesler M., Bassenge E. Flow-dependent, endothelium-mediated dilation of epicardial coronary arteries in conscious dogs: effects of cyclooxygenase inhibition. J Cardiovasc Pharmacol. 1984 Nov-Dec;6(6):1161–1169. [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm M., Feelisch M., Spahr R., Piper H. M., Noack E., Schrader J. Quantitative and kinetic characterization of nitric oxide and EDRF released from cultured endothelial cells. Biochem Biophys Res Commun. 1988 Jul 15;154(1):236–244. doi: 10.1016/0006-291x(88)90675-4. [DOI] [PubMed] [Google Scholar]
- Koulu M., Lappalainen J., Pesonen U., Hietala J., Syvälahti E. Chronic treatment with SCH 23390, a selective dopamine D-1 receptor antagonist, decreases dopamine metabolism in rat caudate nucleus. Eur J Pharmacol. 1988 Oct 18;155(3):313–316. doi: 10.1016/0014-2999(88)90521-3. [DOI] [PubMed] [Google Scholar]
- Lüscher T. F., Diederich D., Weber E., Vanhoutte P. M., Bühler F. R. Endothelium-dependent responses in carotid and renal arteries of normotensive and hypertensive rats. Hypertension. 1988 Jun;11(6 Pt 2):573–578. doi: 10.1161/01.hyp.11.6.573. [DOI] [PubMed] [Google Scholar]
- Otsuka Y., DiPiero A., Hirt E., Brennaman B., Lockette W. Vascular relaxation and cGMP in hypertension. Am J Physiol. 1988 Jan;254(1 Pt 2):H163–H169. doi: 10.1152/ajpheart.1988.254.1.H163. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Rees D. D., Ashton D. S., Moncada S. L-arginine is the physiological precursor for the formation of nitric oxide in endothelium-dependent relaxation. Biochem Biophys Res Commun. 1988 Jun 30;153(3):1251–1256. doi: 10.1016/s0006-291x(88)81362-7. [DOI] [PubMed] [Google Scholar]
- Patthy A., Bajusz S., Patthy L. Preparation and characterization of Ng-mono-, di- and trimethylated arginines. Acta Biochim Biophys Acad Sci Hung. 1977;12(3):191–196. [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. Comparative pharmacology of endothelium-derived relaxing factor, nitric oxide and prostacyclin in platelets. Br J Pharmacol. 1987 Sep;92(1):181–187. doi: 10.1111/j.1476-5381.1987.tb11310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem Biophys Res Commun. 1987 Nov 13;148(3):1482–1489. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum W. I., Nelson G. H., Povlishock J. T. Laser-induced endothelial damage inhibits endothelium-dependent relaxation in the cerebral microcirculation of the mouse. Circ Res. 1987 Feb;60(2):169–176. doi: 10.1161/01.res.60.2.169. [DOI] [PubMed] [Google Scholar]
- Sakuma I., Stuehr D. J., Gross S. S., Nathan C., Levi R. Identification of arginine as a precursor of endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8664–8667. doi: 10.1073/pnas.85.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. H., Nau H., Wittfoht W., Gerlach J., Prescher K. E., Klein M. M., Niroomand F., Böhme E. Arginine is a physiological precursor of endothelium-derived nitric oxide. Eur J Pharmacol. 1988 Sep 13;154(2):213–216. doi: 10.1016/0014-2999(88)90101-x. [DOI] [PubMed] [Google Scholar]
- Sobey C. G., Woodman O. L., Dusting G. J. Inhibition of vasodilatation by methylene blue in large and small arteries of the dog hindlimb in vivo. Clin Exp Pharmacol Physiol. 1988 May;15(5):401–410. doi: 10.1111/j.1440-1681.1988.tb01093.x. [DOI] [PubMed] [Google Scholar]
- Thomas G., Mostaghim R., Ramwell P. W. Endothelium dependent vascular relaxation by arginine containing polypeptides. Biochem Biophys Res Commun. 1986 Dec 15;141(2):446–451. doi: 10.1016/s0006-291x(86)80193-0. [DOI] [PubMed] [Google Scholar]
- Winquist R. J., Bunting P. B., Baskin E. P., Wallace A. A. Decreased endothelium-dependent relaxation in New Zealand genetic hypertensive rats. J Hypertens. 1984 Oct;2(5):541–545. doi: 10.1097/00004872-198410000-00015. [DOI] [PubMed] [Google Scholar]


