Abstract
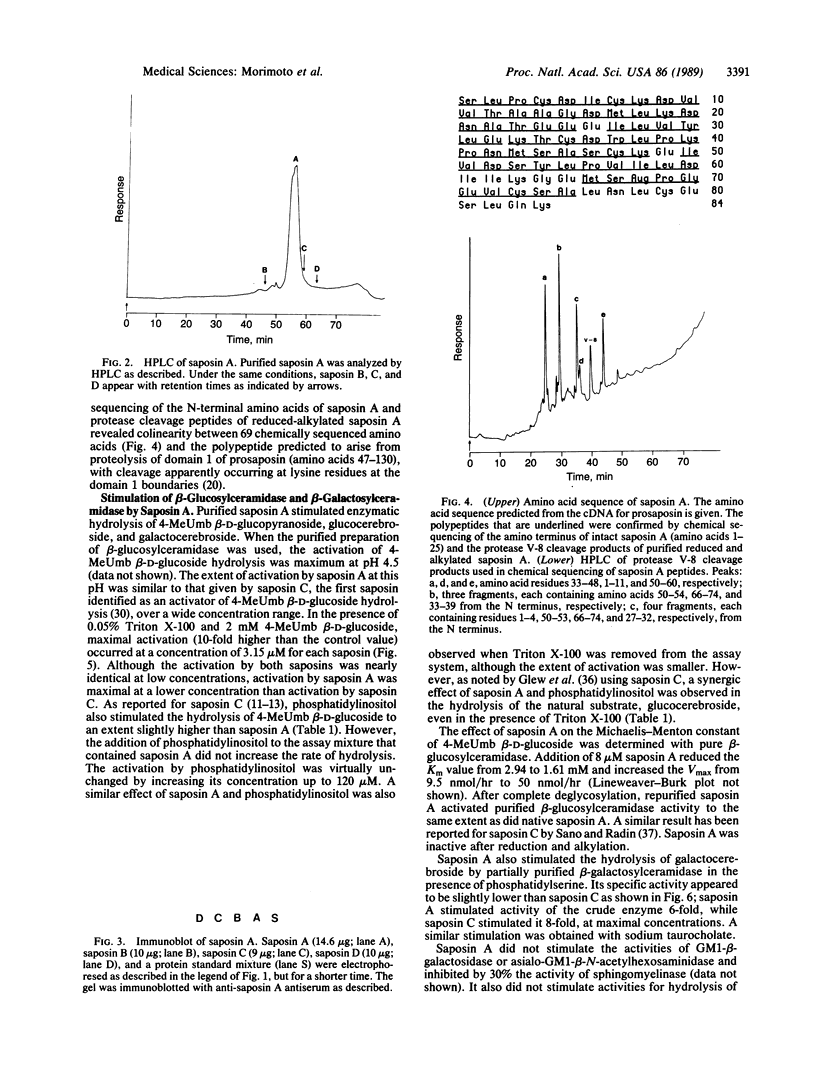
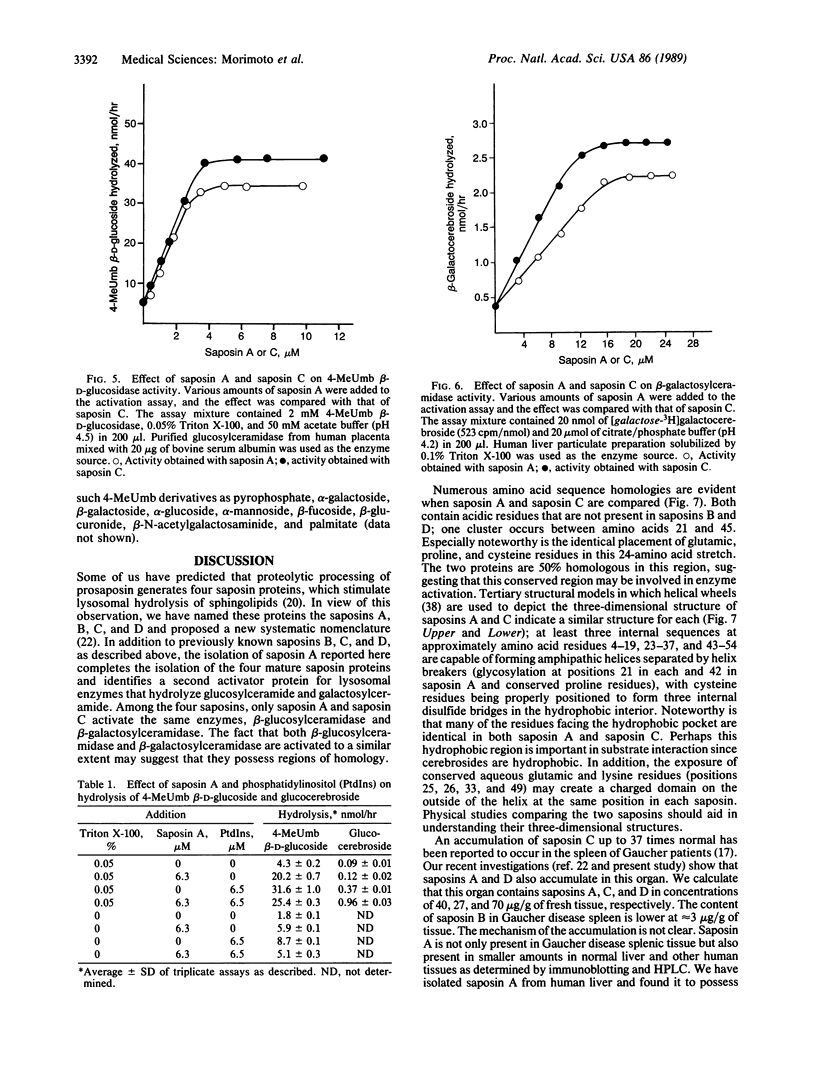
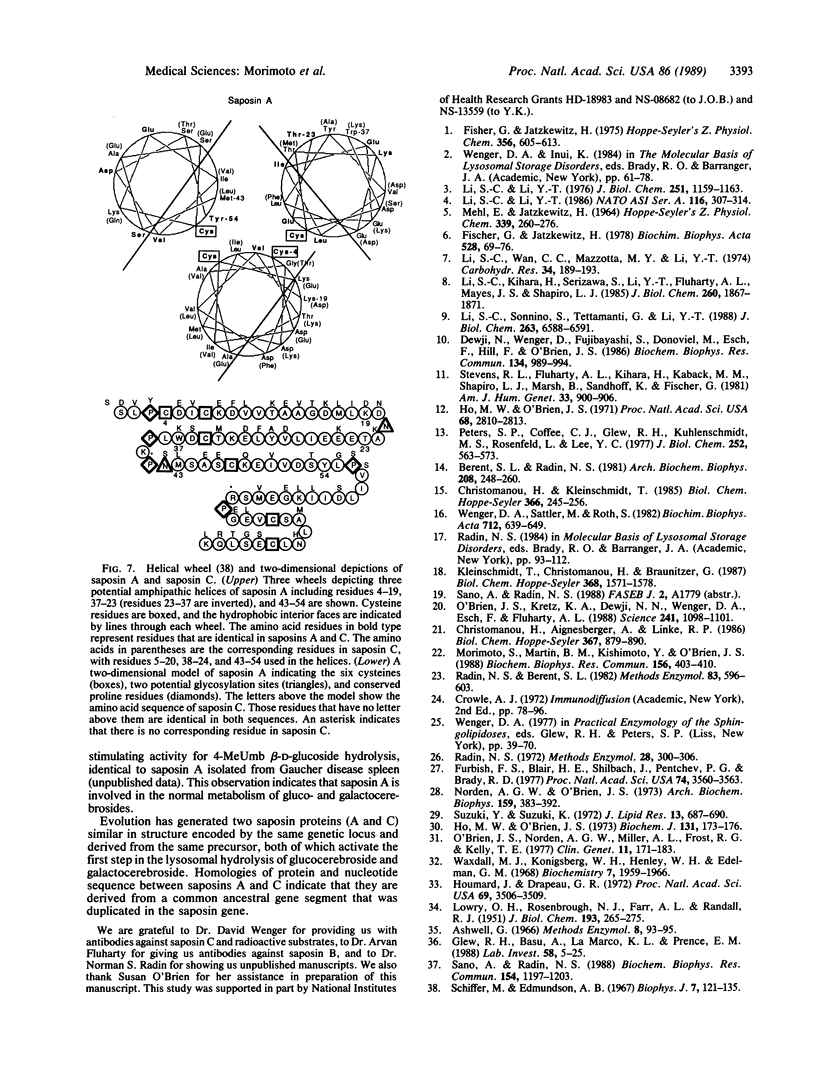
Saposin A, a heat-stable 16-kDa glycoprotein, was isolated from Gaucher disease spleen and purified to homogeneity. Chemical sequencing from its amino terminus and of peptides obtained by digestion with protease from Staphylococcus aureus strain V-8 demonstrated that saposin A is derived from proteolytic processing of domain 1 of its precursor protein, prosaposin. Processing of prosaposin (70 kDa) also generates three other previously reported saposin proteins, B, C, and D, from its second, third, and fourth domains. Similar to saposin C, saposin A stimulates the hydrolysis of 4-methylumbelliferyl beta-glucoside and glucocerebroside by beta-glucosylceramidase and of galactocerebroside by beta-galactosylceramidase, mainly by increasing the maximal velocity of both reactions. Saposin A is as active as saposin C in these reactions. Saposin A has no significant effect on other sphingolipid and 4-methylumbelliferyl glycoside hydrolases tested. Saposin A has two potential glycosylation sites that appear to be glycosylated. After deglycosylation, saposin A had a subunit molecular mass of 10 kDa and was as active as native saposin A. However, reduction and alkylation abolished the activation. A three-dimensional model comparing saposins A and C reveals significant sequence homology between them, especially preservation of conserved acidic and basic residues in their middle regions. Each appears to possess a conformationally rigid hydrophobic pocket stabilized by three internal disulfide bridges, with amphipathic helical regions interrupted by helix breakers.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berent B. L., Radin N. S. beta-Glucosidase activator protein from bovine spleen ("coglucosidase"). Arch Biochem Biophys. 1981 Apr 15;208(1):248–260. doi: 10.1016/0003-9861(81)90147-8. [DOI] [PubMed] [Google Scholar]
- Christomanou H., Aignesberger A., Linke R. P. Immunochemical characterization of two activator proteins stimulating enzymic sphingomyelin degradation in vitro. Absence of one of them in a human Gaucher disease variant. Biol Chem Hoppe Seyler. 1986 Sep;367(9):879–890. doi: 10.1515/bchm3.1986.367.2.879. [DOI] [PubMed] [Google Scholar]
- Christomanou H., Kleinschmidt T. Isolation of two forms of an activator protein for the enzymic sphingomyelin degradation from human Gaucher spleen. Biol Chem Hoppe Seyler. 1985 Mar;366(3):245–256. doi: 10.1515/bchm3.1985.366.1.245. [DOI] [PubMed] [Google Scholar]
- Dewji N., Wenger D., Fujibayashi S., Donoviel M., Esch F., Hill F., O'Brien J. S. Molecular cloning of the sphingolipid activator protein-1 (SAP-1), the sulfatide sulfatase activator. Biochem Biophys Res Commun. 1986 Jan 29;134(2):989–994. doi: 10.1016/s0006-291x(86)80518-6. [DOI] [PubMed] [Google Scholar]
- Fischer G., Jatzkewitz H. The activator of cerebroside sulphatase. Purification from human liver and identification as a protein. Hoppe Seylers Z Physiol Chem. 1975 May;356(5):605–613. doi: 10.1515/bchm2.1975.356.1.605. [DOI] [PubMed] [Google Scholar]
- Fischer G., Jatzkewitz H. The activator of cerebroside-sulphatase. A model of the activation. Biochim Biophys Acta. 1978 Jan 27;528(1):69–76. doi: 10.1016/0005-2760(78)90053-x. [DOI] [PubMed] [Google Scholar]
- Furbish F. S., Blair H. E., Shiloach J., Pentchev P. G., Brady R. O. Enzyme replacement therapy in Gaucher's disease: large-scale purification of glucocerebrosidase suitable for human administration. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3560–3563. doi: 10.1073/pnas.74.8.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glew R. H., Basu A., LaMarco K. L., Prence E. M. Mammalian glucocerebrosidase: implications for Gaucher's disease. Lab Invest. 1988 Jan;58(1):5–25. [PubMed] [Google Scholar]
- Ho M. W., O'Brien J. S. Gaucher's disease: deficiency of 'acid' -glucosidase and reconstitution of enzyme activity in vitro. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2810–2813. doi: 10.1073/pnas.68.11.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M. W., O'Brien J. S., Radin N. S., Erickson J. S. Glucocerebrosidase: reconstitution of activity from macromolecular components. Biochem J. 1973 Jan;131(1):173–176. doi: 10.1042/bj1310173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt T., Christomanou H., Braunitzer G. Complete amino-acid sequence and carbohydrate content of the naturally occurring glucosylceramide activator protein (A1 activator) absent from a new human Gaucher disease variant. Biol Chem Hoppe Seyler. 1987 Dec;368(12):1571–1578. doi: 10.1515/bchm3.1987.368.2.1571. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Li S. C., Kihara H., Serizawa S., Li Y. T., Fluharty A. L., Mayes J. S., Shapiro L. J. Activator protein required for the enzymatic hydrolysis of cerebroside sulfate. Deficiency in urine of patients affected with cerebroside sulfatase activator deficiency and identity with activators for the enzymatic hydrolysis of GM1 ganglioside and globotriaosylceramide. J Biol Chem. 1985 Feb 10;260(3):1867–1871. [PubMed] [Google Scholar]
- Li S. C., Li Y. T. An activator stimulating the enzymic hydrolysis of sphingoglycolipids. J Biol Chem. 1976 Feb 25;251(4):1159–1163. [PubMed] [Google Scholar]
- Li S. C., Sonnino S., Tettamanti G., Li Y. T. Characterization of a nonspecific activator protein for the enzymatic hydrolysis of glycolipids. J Biol Chem. 1988 May 15;263(14):6588–6591. [PubMed] [Google Scholar]
- Li S. C., Wan C. C., Mazzotta M. Y., Li Y. T. Requirement of an activator for the hydrolysis of sphingoglycolipids by glycosidases of human liver. Carbohydr Res. 1974 May;34(1):189–193. doi: 10.1016/s0008-6215(00)80383-3. [DOI] [PubMed] [Google Scholar]
- Mehl E., Jatzkewitz H. Eine Cerebrosidsulfatase aus Schweineniere. Hoppe Seylers Z Physiol Chem. 1964;339(1):260–276. [PubMed] [Google Scholar]
- Morimoto S., Martin B. M., Kishimoto Y., O'Brien J. S. Saposin D: a sphingomyelinase activator. Biochem Biophys Res Commun. 1988 Oct 14;156(1):403–410. doi: 10.1016/s0006-291x(88)80855-6. [DOI] [PubMed] [Google Scholar]
- Norden A. G., O'Brien J. S. Ganglioside GM1 beta-galactosidase: studies in human liver and brain. Arch Biochem Biophys. 1973 Nov;159(1):383–392. doi: 10.1016/0003-9861(73)90465-7. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S., Kretz K. A., Dewji N., Wenger D. A., Esch F., Fluharty A. L. Coding of two sphingolipid activator proteins (SAP-1 and SAP-2) by same genetic locus. Science. 1988 Aug 26;241(4869):1098–1101. doi: 10.1126/science.2842863. [DOI] [PubMed] [Google Scholar]
- O'Brien J. S., Norden G. W., Miller A. L., Frost R. G., Kelly T. E. Ganglioside GM2 N-acetyl-beta-D-galactosaminidase and asialo GM2 (GA2) N-acetyl-beta-D-galactosaminidase; studies in human skin fibroblasts. Clin Genet. 1977 Mar;11(3):171–183. doi: 10.1111/j.1399-0004.1977.tb01296.x. [DOI] [PubMed] [Google Scholar]
- Peters S. P., Coyle P., Coffee C. J., Glew R. H. Purification and properties of a heat-stable glucocerebrosidase activating factor from control and Gaucher spleen. J Biol Chem. 1977 Jan 25;252(2):563–573. [PubMed] [Google Scholar]
- Radin N. S., Berent S. L. Protein activator (coglucosidase) for the hydrolysis of beta-glucosides. Methods Enzymol. 1982;83:596–603. doi: 10.1016/0076-6879(82)83057-7. [DOI] [PubMed] [Google Scholar]
- Sano A., Radin N. S. The carbohydrate moiety of the activator protein for glucosylceramide beta-glucosidase. Biochem Biophys Res Commun. 1988 Aug 15;154(3):1197–1203. doi: 10.1016/0006-291x(88)90267-7. [DOI] [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R. L., Fluharty A. L., Kihara H., Kaback M. M., Shapiro L. J., Marsh B., Sandhoff K., Fischer G. Cerebroside sulfatase activator deficiency induced metachromatic leukodystrophy. Am J Hum Genet. 1981 Nov;33(6):900–906. [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y., Suzuki K. Specific radioactive labeling of terminal n-acetylgalactosamine of glycosphingolipids by the galactose oxidase-sodium borohydride method. J Lipid Res. 1972 Sep;13(5):687–690. [PubMed] [Google Scholar]
- Waxdal M. J., Konigsberg W. H., Henley W. L., Edelman G. M. The covalent structure of a human gamma G-immunoglobulin. II. Isolation and characterization of the cyanogen bromide fragments. Biochemistry. 1968 May;7(5):1959–1966. doi: 10.1021/bi00845a046. [DOI] [PubMed] [Google Scholar]
- Wenger D. A., Sattler M., Roth S. A protein activator of galactosylceramide beta-galactosidase. Biochim Biophys Acta. 1982 Sep 14;712(3):639–649. doi: 10.1016/0005-2760(82)90293-4. [DOI] [PubMed] [Google Scholar]