The role of attention in perceptual learning has been controversial. Numerous studies have reported that learning does not occur on stimulus features that are irrelevant to a subject's task [1,2] and have concluded that focused attention on a feature is necessary for a feature to be learned. In contrast, another line of studies has shown that perceptual learning occurs even on task-irrelevant features that are subthreshold, and concluded that attention on a feature is not required to learn that feature [3–5]. Here we attempt to reconcile these divergent findings by systematically exploring the relation between signal strength of the motion stimuli used during training and the resultant magnitude of perceptual learning. Our results show that performance improvements only occurred for the motion-stimuli trained at low, parathreshold, coherence levels. The results are in accord with the hypothesis that weak task-irrelevant signals fail to be ‘noticed’, and consequently to be suppressed, by the attention system and thus are learned, while stronger stimulus signals are detected, and suppressed [6], and are not learned. These results provide a parsimonious explanation of why task-irrelevant learning is found in some studies but not others, and could give an important clue to resolving a long-standing controversy.
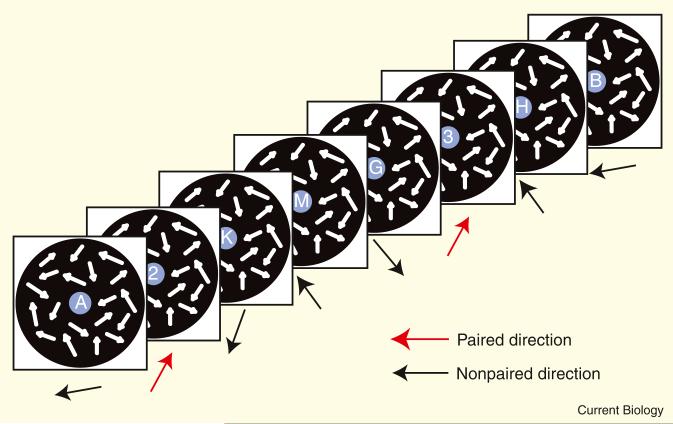
The experiment consisted of an exposure stage preceded and followed by test stages [3] (see the Supplemental data available on-line for details of the experimental procedures). In each trial of the 10-day exposure stage, a sequence of eight items (two digits and six alphabetic letters) was presented in a random order at the center of the screen, while a dynamic random-dot display of coherently moving dots (signal) and randomly moving dots (noise) was presented in the periphery (Figure 1). Subjects were instructed to focus on and report the two digits as targets while ignoring letters as distractors. The novelty is that each subject was exposed to two different target-paired motion directions, each at a different level of motion coherence. In half of the trials a motion direction, with a lower coherency (for example, 5% signal dots), was paired with targets [4], and in the other half of the trials a different motion direction, with a higher coherency (for example, 50% signal dots), was paired with targets. These two trial types were randomly interleaved and the order of presentations and choice of paired motion directions randomly determined for each subject. In the test stages, which were conducted before and after the exposure stage, subjects were asked to report the direction of coherent motion stimuli that were selected from the set of six directions (10°, 70°, 130°, 190°, 250° and 310°) that had been presented during the exposure stage (for example, two that had been paired with task-targets and four that had been paired with distractor items).
Figure 1. Exposure stage.
A display consisted of a sequence of eight items — two digits as targets and six letters as distractors — in the center and dots moving coherently or in random directions in the periphery (white arrows). Red arrows represent coherent motion directions paired with task targets. Black arrows indicate other coherent motion directions paired with task distractors.
Using similar designs, previous studies have shown that motion directions of subthreshold coherence that are paired with the task targets undergo perceptual learning, but directions paired with distractors do not [4]. Our goal was to examine the relationship between the strength of exposed task-irrelevant signals and the magnitude of perceptual learning. To do this, we exposed subjects to four coherence levels (3%, 5%, 15% and 50%), counterbalanced across four subject groups (seven subjects each). Each group had target-paired directions at two coherence levels, 3% and 15%, 3% and 50%, 5% and 15%, or 5% and 50%. We chose to use only two directions per subject as ‘learning directions’ to avoid possible interactions between different directions that might occur if too many directions were trained and angular differences between neighboring directions became too close.
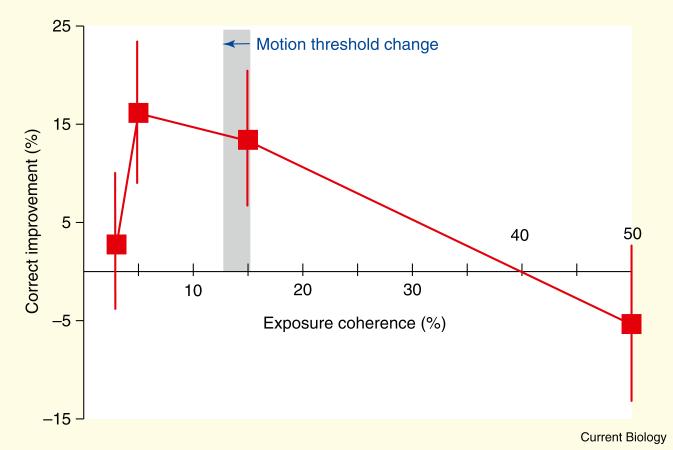
Intuitively, one might predict that higher motion coherence will lead to greater learning than lower motion coherence, because higher coherent motion signals induce stronger perceptions of motion direction [7]. However, the opposite result was observed (Figure 2): learning was found only when 5% or 15% coherent motion was exposed (paired t-test, p = 0.02 and p = 0.03, respectively). Namely, motion sensitivity was improved only for the parathreshold stimulus levels (for example, 5% and 15% coherence) but not for the suprathreshold stimuli (for example, 50% coherence) nor the weakest stimuli (for example, 3% coherence). The mean motion threshold changed from 15.3 ± 0.8% before exposure to 13.0 ± 0.8% after exposure. The performance of the RSVP task was high (93.5 ± 0.8%) and no significant difference was found between the performance of the different exposure conditions (p = 0.88, ANOVA).
Figure 2. Correct improvement as a function of the exposed coherent motion ratio.
Correct improvement (%) is defined as the subtraction of the summed performance (% correct) across coherence levels in the pre-test from that in the post-test [14] (see Supplementary Experimental Procedures in the Supplemental data for details). The horizontal blue arrow represents the mean motion threshold changed from before exposure (from 15.3 ± 0.8%) to after exposure (13.0 ± 0.8%). Error bars show standard error.
Why should such a counterintuitive result occur? A possible explanation can be found in the recent observation that the human lateral prefrontal cortex (LPFC), which usually gives inhibitory attentional control on task-irrelevant coherent motion signals [8], has a higher threshold for responding to motion-direction signals than visual area MT+, known to be specialized for motion-direction processing [6]. LPFC fails to ‘notice’ and therefore to give inhibitory control on weak task-irrelevant signals while MT+ is still activated by these weak signals [6]. In our study, task-irrelevant learning may have occurred only with the 5% and 15% coherence motion-stimuli as these weak motion signals were not ‘noticed’ during exposure, and, therefore, were not inhibited by the attentional system. However, these signals were sufficiently strong to activate MT+, which may have subserved the learning of that motion-direction. On the other hand learning may have failed for 50% coherence because this stimulus was sufficiently strong to trigger inhibitory control. Learning may have failed with the weak 3% coherent motion because this stimulus yields smaller direction selective responses in human and monkey motion processing areas compared to 5% coherent motion [7,9].
A failure of perceptual learning of a salient task-irrelevant feature [1,2,10,11] has been regarded as the evidence that attention to a feature is necessary for the feature to be learned. However, another possible explanation is that the failure of learning of strong irrelevant features is due to attentional inhibition on the feature, which prevents the feature from being learned. This is in accord with the recent finding that training of a task-relevant feature led to decrease in sensitivity to a task-irrelevant feature [12].
As mentioned, it has been highly controversial whether a feature to which attention is not directed is learned [1–4,13]. Our results indicate that task-irrelevant features are learned when task-irrelevant features are parathreshold (in this case 5% and 15% coherence) but not when they are suprathreshold. Importantly, previous studies of perceptual learning that found no task-irrelevant learning have presented suprathreshold stimuli as task-irrelevant features while studies that have shown task-irrelevant learning have presented parathreshold task-irrelevant features. Thus, we conclude that both lines of studies, which have indicated opposite conclusions as to the presence/absence of task-irrelevant learning, are correct.
Supplementary Material
Acknowledgments
We thank Sayuri Hayakawa, Amisha S. Patel and Nozomi Ito. This work was supported by NSF (BCS-0549036, BCS-PR04-137 CELEST) and NIH (R21 EY017737).
Footnotes
Supplemental data
Supplemental data are available at http://www.current-biology.com/cgi/content/full/18/12/R516/DC1
References
- 1.Ahissar M, Hochstein S. Attentional control of early perceptual learning. Proc. Natl. Acad. Sci. USA. 1993;90:5718–5722. doi: 10.1073/pnas.90.12.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiu LP, Pashler H. Improvement in line orientation discrimination is retinally local but dependent on cognitive set. Percept. Psychophys. 1992;52:582–588. doi: 10.3758/bf03206720. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe T, Nanez JE, Sasaki Y. Perceptual learning without perception. Nature. 2001;413:844–848. doi: 10.1038/35101601. [DOI] [PubMed] [Google Scholar]
- 4.Seitz AR, Watanabe T. Psychophysics: Is subliminal learning really passive? Nature. 2003;422:36. doi: 10.1038/422036a. [DOI] [PubMed] [Google Scholar]
- 5.Dinse HR, Ragert P, Pleger B, Schwenkreis P, Tegenthoff M. Pharmacological modulation of perceptual learning and associated cortical reorganization. Science. 2003;301:91–94. doi: 10.1126/science.1085423. [DOI] [PubMed] [Google Scholar]
- 6.Tsushima Y, Sasaki Y, Watanabe T. Greater disruption due to failure of inhibitory control on an ambiguous distractor. Science. 2006;314:1786–1788. doi: 10.1126/science.1133197. [DOI] [PubMed] [Google Scholar]
- 7.Britten KH, Shadlen MN, Newsome WT, Movshon JA. The analysis of visual motion: a comparison of neuronal and psychophysical performance. J. Neurosci. 1992;12:4745–4765. doi: 10.1523/JNEUROSCI.12-12-04745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knight RT, Staines WR, Swick D, Chao LL. Prefrontal cortex regulates inhibition and excitation in distributed neural networks. Acta. Psychol. 1999;101:159–178. doi: 10.1016/s0001-6918(99)00004-9. [DOI] [PubMed] [Google Scholar]
- 9.Rees G, Friston K, Koch C. A direct quantitative relationship between the functional properties of human and macaque V5. Nat. Neurosci. 2000;3:631–633. doi: 10.1038/76673. [DOI] [PubMed] [Google Scholar]
- 10.Schoups A, Vogel R, Qian N, Orban G. Practicing orientation identification improves orientation coding in V1 neurons. Nature. 2001;412:549–553. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- 11.Polley DB, Steinberg EE, Merzanich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J. Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paffen P, Verstraten F, Vidnyanszky Z. Attention-based perceptual learning increases binocular rivalry suppression of irrelevant visual features. J. Vision. 2008 doi: 10.1167/8.4.25. in press. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe T, Náñez JE, Sr, Koyama S, Mukai I, Liederman J, Sasaki Y. Greater plasticity in lower-level than higher-level visual motion processing in a passive perceptual learning task. Nat. Neurosci. 2002;5:1003–1009. doi: 10.1038/nn915. [DOI] [PubMed] [Google Scholar]
- 14.Nishina S, Seitz AR, Kawato M, Watanabe T. Effect of spatial distance to the task stimulus on task-irrelevant perceptual learning of static Gabors. J. Vision. 2007;7:1–10. doi: 10.1167/7.13.2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.