Summary
Control of parasitic protozoan infections requires the generation of efficient innate and adaptive immune responses, and in most cases both CD8 and CD4 T cells are necessary for host survival. Since intracellular protozoa remodel the vacuolar compartments in which they reside, it is not obvious how their antigens enter the MHC class I and class II pathways. Studies using genetically engineered parasites have shown that host cell targeting, intracellular compartmentalization, subcellular localization of antigen within the parasite and mechanism of invasion are important factors determining the presentation pathway utilized. The recent identification of endogenous parasite-derived CD8 T cell epitopes have helped confirm these concepts as well as provided new information on the processing pathways and the impact of parasite-stage specific antigen expression on the repertoire of responding T cells stimulated by infection. Elucidating the mechanisms governing antigen processing and presentation of intracellular protozoa may provide important insights needed for the rational design of effective vaccines.
Introduction
Intracellular protozoan parasites represent a major cause of disease and despite years of effort, no effective vaccines have been developed for routine immunization against these pathogens. To succeed as parasites, these organisms need to achieve a fine balance with their hosts in order to establish chronic infections that promote transmission. Protozoan parasites have developed numerous strategies to avoid or manipulate host immune defenses [1–3]. While the intracellular life style adopted by major parasites such as Leishmania spp, Toxoplasma gondii, Plasmodium spp (the causative agent of malaria) and Trypanosoma cruzi provides protection against humoral attack, these pathogens must at the same time evade intracellular antimicrobial mechanisms. To do so, they often remodel the host cell compartments in which they reside.
T. cruzi, T. gondii and malaria parasites actively invade mammalian cells, while in contrast, Leishmania which lack an active invasion machinery are restricted to professional phagocytes, i.e. macrophages, neutrophils and dendritic cells (DCs). After phagocytic entry, Leishmania reside in phagosomes that fuse with late endocytic compartments [4]. Although they do not significantly remodel the phagosome, Leishmania amastigotes are adapted to survive and replicate within the hostile acidic environment of the mature phagolysosome. In the case of T. cruzi, while active invasion by trypomastigotes also leads to the formation of an acidic compartment, the parasite cannot survive the low pH of a lysosome-fused parasitophorous vacuole (PV) and rapidly escapes into the host cytosol [5]. A particularly interesting scenario is provided by Toxoplasma gondii. T. gondii tachyzoites actively infect host cells by a process involving the sequential discharge of parasite secretory organelles, leading to the formation of a highly specialized PV [6]. Actively remodel of the PV membrane (PVM), renders the PV incompetent for endosome/lysosome fusion and unable to acidify [7–8]. T. gondii tachyzoites rapidly multiply within the PV until parasite egress occurs followed by host cell lysis.
Because of the diverse life styles outlined above, processing and presentation of antigens of intracellular protozoa involves a set of distinct mechanisms that provide a fascinating perspective on this critical step in the induction of the immune response. This review highlights recent progress in the area comparing three examples of protozoa (T. cruzi, T. gondii and Leishmania) that dwell in different intracellular compartments.
Antigen presentation on MHC class I
Antigen subcellular localization
T. cruzi, Leishmania and T. gondii reside in distinct intracellular compartments, nevertheless, they all induce strong CD8 T cell responses. Ag localization within the parasite may be an important factor influencing this shared immunological activity. Pioneering studies showed that host cells infected with T. cruzi expressing secretory or GPI-anchored, but not cytosolic or transmembrane OVA, process and present peptides to CD8 T cells, thus suggesting that only released proteins gain access to the class I pathway [9]. A similar requirement was observed for T. gondii when transgenic tachyzoites expressing LacZ or OVA either in the cytosol or secreted into the PV were compared [10,11]. Likewise, in the case of L. major, OVA expressed as a secreted but not cytosolic Ag, efficiently triggers CD8 T priming both in vitro and in vivo [12].
Class I presentation pathways utilized by different protozoa
As T. cruzi resides in the host cytoplasm, it is not surprising that proteins released during normal cellular infection gain direct access to the classical cytosolic MHC class I pathway (Figure 1). This mechanism is supported by studies showing that mice lacking the transporter associated with Ag processing (TAP)-1 are highly susceptible to T. cruzi infection [13]. In contrast, the presentation pathways utilized by Toxoplasma and Leishmania that are sequestered inside vacuoles, are not so obvious. Since the Ags in question are synthesized by the parasite’s own protein synthetic machinery without involving that of the host cell, we believe that it is appropriate to refer to this as “cross-presentation”, a process utilized primarily by DC. Possible cross-presentation mechanisms employed include, i) phagocytosis of bystander-infected cells, ii) uptake of dead parasites and/or soluble material, iii) injection of Ag into the cytosol at the time of infection, iv) cross-presentation of Ags derived from PVs containing live parasites (Figure 1).
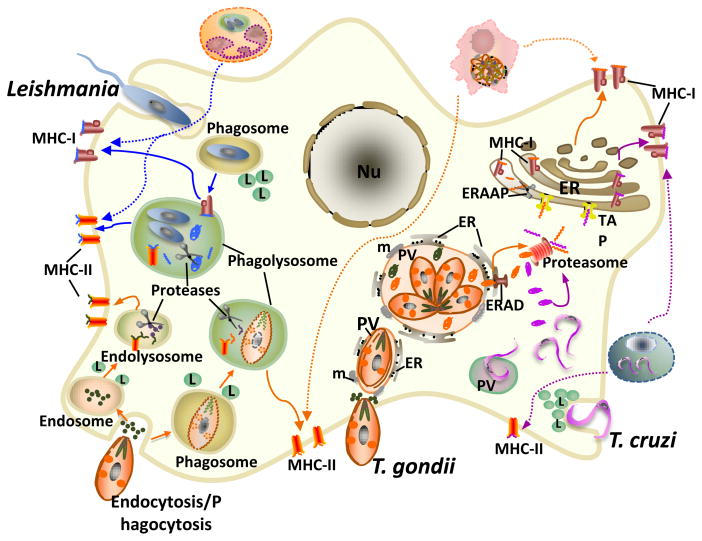
Figure 1. Working models for MHC-I and MHC-II-restricted presentation of Ags derived from Leishmania, T. gondii and T. cruzi parasites.
Leishmania (blue arrows) are taken up by phagocytosis, and replicate within a phagolysosome. Ag processing occurs within the phagolysosome where secreted/released parasite proteins are degraded by endosomal proteases and the resulting peptides loaded onto MHC-I and MHC-II molecules followed by transport of the MHC-peptide complexes from the phagolysosome to the cell surface. In the case of T. gondii (orange arrows), MHC-I processing occurs after active invasion and fusion of the resulting PV with the host ER. Parasite proteins secreted into the PV lumen are retrotranslocated by the ER-associated degradation system (ERAD) to the cytosol where they are degraded by the proteasome and the resulting peptides transported via TAP into the ER for additional proteolysis by ERAAP and loading onto MHC-I molecules. No MHC-I processing results from phagocytic uptake of live or dead parasites or from proteins injected into the cytosol during invasion. For MHC-II processing, T. gondii-derived Ags can be obtained from phagocytosis or endocytosis of whole tachyzoites or parasite-derived products and it is unclear whether proteins derived from the PV can also access the MHC-II pathway. T. cruzi (purple arrows) invasion induces the recruitment and fusion of lysosomes to the plasma membrane and results in an acidic PV where it resides transiently before escaping into the host cell cytosol. Processing of parasite-released proteins follows the cytosolic proteasome/TAP-dependent pathway. Dotted arrows indicate the potential contribution of uptake of infected cells for both MHC-I and MHC-II processing. Nu, nucleus; m, mitochondria; L, lysosome.
In the case of L.major, DCs infected with live promastigotes secreting OVA (Lm-NT-OVA) induce potent CD8 T cell responses, while DCs exposed to heat-killed (HK) L. m-NT-OVA are significantly less efficient [12]. The same study, ruled out a possible contribution of regurgitated peptides or proteolytically degraded soluble protein released by extracellular parasites or infected cells as a source of class I-binding peptides. It has been recently shown that during initial Leishmania infection, although some parasites are directly phagocytosed by DCs [14•], neutrophils are the major host cell harboring live parasites and therefore, following apoptosis could be cross-presented by DCs [15••]. Furthermore, 1–2 weeks following Leishmania inoculation the majority of parasite-containing cells at the infection site expresses a phenotype compatible with that of DCs that have phagocytosed neutrophils [16••]. This is an important cross-presentation mechanism that deserves further attention.
Studies performed with the Lm-NT-OVA system, showed that cross-presentation of L. major-derived Ags by infected DCs is TAP independent both in vitro and in vivo, and is blocked by neutralization of phagosomal pH or by endosomal proteases inhibitors but it is not affected by suppression of proteasomal activity [17•]. A dispensable role for TAP was also observed when these experiments were extended to CD8 T cells induced by natural Leishmania Ags in vivo. Taken together, these findings strongly suggest that L. major Ags are processed via an intraphagosomal pathway. In contrast, a previous report suggested that presentation of the membrane gp46/M-2 glycoprotein by L. amazonensis-infected macrophages followed a cytosolic pathway [18]. Whether these discrepancies are due to the ability of different APCs to utilize distinct Ag processing mechanisms, or to intrinsic properties of Leishmania species remains to be elucidated.
In direct contrast to Leishmania, cross-presentation of OVA transgenic T. gondii (Tg-OVA) from different parasite strains is strictly TAP and proteasome dependent [11,17•,19•]. A TAP-dependent mechanism was also demonstrated for non-transgenic T. gondii Ags in vivo [20] and for the recently identified T. gondii GRA6 epitope HF10 [21••]. Moreover, HF10 processing also requires cytosolic proteasomal degradation and additional proteolysis by the ER aminopeptidase associated with antigen processing (ERAAP) [21••].
Given that T. gondii dwells in a unique PV sequestered from the host endocytic/exocytic compartments, one could hypothesize that cross-presentation occurs as a result of uptake of infected cells, dead parasites or soluble material released in the extracellular microenvironment. Nevertheless, multiple experimental approaches indicate that active invasion is required for cross-presentation of T. gondii-derived Ags [11,19•,22••]. Indeed, killed Tg-OVA parasites fail to induce CD8 T cell priming both in vitro and in vivo [22••]. It has also been shown that DCs exposed to soluble parasites products are unable to activate CD8 T cells [19•] and that parasite replication and host cell lysis is not required [19•,22••]. Similar results were observed with polyclonal CD8 T cells derived from mice infected with non-transgenic parasite [22••]. Furthermore, phagocytosis of live and/or antibody-opsonized parasites, which are targeted to conventional phagosomes [8][23], fails to induce CD8 T cell activation ruling out a possible contribution of phagocytic uptake of live parasites during infection [22••].
During invasion T. gondii secretes proteins into the host cell cytosol (reviewed in [24]) which could directly enter the endogenous class I pathway. Nevertheless, experiments blocking cell invasion indicated that such scenario is unlikely to occur [22••]. Moreover, the use of mixed haplotype cultures suggested that phagocytosis of infected cells does not contribute to cross-presentation of T. gondii Ags [[19•] and Goldszmid et al. unpublished]. More importantly in vivo transfer of infected MHC class I deficient cells also failed to induce CD8 T cell priming [22••].
The above findings suggest a pathway in which T. gondii proteins escape from the PV and are processed in the host cytosol. A previous report indicated that a dense granule-derived protein (GRA7) could be detected on the host cell surface, consistent with its transport from the PV [25]. To address this issue an elegant experiment was performed in which T. gondii expressing a Cre recombinase secreted into the PV (Tg-secCre), was used to infect host cells harboring a silent GFP gene that is activated upon Cre-mediated deletion of the transcriptional stop signal [11]. GFP fluorescence was observed in Tg-secCre-infected cells but not in cells infected with parasites expressing a non-secreted Cre or incubated with supernatant from Tg-secCre cultures. These observations indicate that intact Cre recombinase can escape the PV and reach the host nucleus, thus supporting the hypothesis that after active infection, macromolecular parasite proteins secreted into the PV gain access to the host cytosol and enter the MHC class I pathway. Pores in the PVM have been previously described that allow exchange of small molecules < 1.3 kDa [26], and it was therefore unclear how large proteins can exit the PV. Live T. gondii is known to actively recruit host mitochondria and ER to the PV, presumably for nutrient acquisition [27]. Hence, we hypothesized that the intimate association of PVM and host ER (hER) could serve as a conduit for Ag escape into the host cytosol. Using immunogold labeling and in situ immunocytochemical staining for the luminal ER-specific marker glucose 6-phosphatase (G6Pase), an enzyme absent in T. gondii [28][29] we documented transfer of hER components into the PV indicating direct communication between the two compartments [22••]. In addition, we showed that T. gondii exploits the hER-associated degradation system to transport proteins into the cytosol, a process that has been implicated in cross-presentation of soluble and particulate-bound Ags in other systems [30–32]. Remarkably, in all the conditions assessed in which T. gondii was targeted to conventional phagosomes no ER recruitment or fusion was observed, nor was class I presentation activity detected arguing that active remodeling of the PV by the parasite is required for both processes [22••].
In vivo visualization of APC-CD8 T cell interactions
Two recently published studies using OVA-expressing fluorescent T. gondii and intravital two-photon microscopy have demonstrated that CD8 T cells engage in long lasting contacts with parasite-infected APCs within lymphoid organs [33••,34•]. Surprisingly, in both reports parasites could not be detected in a proportion of DCs associated with T cells. This observation was interpreted by Chtanova et al. [33••] as resulting from loss of fluorescence signal following parasite killing within the PV. In contrast, John at al. [34•] argued that these fluorescence-negative cells were never infected but did note a vacuolar phenotype that could be consistent with prior infection. This controversy highlights the need for further studies that more definitively determine whether APCs infection is critical for cross presentation of T. gondii Ags in vivo. In the above reports both macrophages and DCs were found to be the major APCs presenting to CD8 T cells in lymphoid tissue, and a prior study performed in the brains of chronically infected mice showed that CD8 T cells associate with infected-CD11b+myeloid cells but not with parasite-harboring astrocytes or neurons [35•].
Newly identified endogenous parasite epitopes
The sequencing of parasite genomes has provided new tools for screening and identification of natural occurring epitopes. Interestingly, in line with the data obtained with model Ags, in the case of both T. cruzi and T. gondii the newly identified immunodominant epitopes recognized by CD8 T cells correspond to secreted proteins [21••,36,37••]. Research performed on one of the recently characterized T. gondii epitopes has also provided new information on the role of ERAAP as noted above [21••], and established the importance of parasite-stage specific antigen expression in determining the repertoire of responding CD8 T cells. The latter conclusion was based on the observation that CD8 T cells specific for a GRA4 tachyzoite-derived epitope predominate during acute infection, whereas CD8 T cells recognizing ROP7 (a protein expressed on both tachyzoites and bradyzoites) are triggered primarily during chronic infection [37••].
Immunity-related GTPases (IRG) and antigen presentation
The IRG proteins are a family of interferon-induced proteins involved in resistance to intracellular pathogens [38]. IRG-mediated resistance to T. gondii depends on the recruitment of IRGs to the PVM and subsequent vacuolar disruption and parasite killing, which has been argued to involve either autophagy or necrotic cell death [39–42]. Interestingly, a member of the IRG family, Irgm3, has recently been implicated in cross-presentation of OVA-coated latex beads by DCs [43••]. Nevertheless, T. gondii-infected DCs deficient in either Irgm3 or its relative Irgm1, do not display any noticeable defects in CD8 T cells activation [[19•] and Goldszmid et al. unpublished]. The suggested role of Irgm3 in cross-presentation of bead-associated Ags stems from its negative effects on phagosomal maturation. Since T. gondii does not reside within a phagosome this could explain the difference observed in the outcome of Irgm3 deficiency in the two systems. Interestingly, it was also found that IFN-γ activated wild type but not Irgm3 deficient T. gondii-infected macrophages cross-present Ag to CD8 T cells [19•]. Whether this difference between DCs and macrophages is due to uncontrolled parasite growth and death of the infected Irgm3 deficient macrophages or reflects different roles for IRGs in macrophages versus DCs was not investigated.
Antigen presentation on MHC class II
That uptake of dead parasites or parasite-derived material leads to class II presentation seems intuitive. Nevertheless, there is evidence that infected APCs present Ag to CD4 T cells, and that in common with MHC-I presentation, Ag compartmentalization may influence this process. In the case of Leishmania, Ags released into the phagosome could be readily processed and presented on MHC-II molecules (Figure 2). In this respect, OVA expressed on the plasma membrane of L. major was presented to naïve CD4 T cells in vivo, while presentation of cytosolic OVA occurred only at high-dose infection, albeit with a significantly lower efficiency [44]. Nevertheless, it is well established that the immunodominant epitope recognized by CD4 T cells in L. major-infected BALB/c derives from the cytosolic protein LACK [45]. Interestingly, the processing of this epitope requires the presence of DM, a non-classical MHC-II molecule with peptide editor functions [46].
In the case of T. gondii, MHC-II presentation has been shown to be restricted to secreted proteins [47]. However, in contrast to MHC-I presentation, active invasion is not required and activation of CD4 T cells is more efficient after parasite phagocytosis [22••]. The lower efficiency observed with actively infected DCs may stem from down modulation of MHC-II molecules induced by T. gondii [48,49] or simply reflect the low level phagocytic uptake of tachyzoites or parasite-secreted proteins that occurs during active infection (Figure 2).
Conclusions
An important lesson from the studies described in this review is that concepts derived from experiments using inert model cargo do not necessarily translate to live intracellular pathogens such as the protozoan parasites discussed here. A further lesson is that different parasite species and/or developmental stages may employ mechanisms that are quite distinct. While much of the current information stems from studies on organisms expressing model Ags, the advent of new technologies for identifying naturally occurring T cell epitopes has now initiated a new era in which processing and presentation pathways can be studied in a more physiological setting. The information gained should be more directly applicable to candidate vaccines and the elucidation of the requirements for their efficacy. Regardless, these studies will continue to offer major insights into the basic cell biology of the host-pathogen interaction as it pertains to immune function.
Acknowledgments
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH. We apologize to those authors whose work we could not cite due to space limitations.
Footnotes
Conflict of interest
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Sacks D, Sher A. Evasion of innate immunity by parasitic protozoa. Nat Immunol. 2002;3:1041–1047. doi: 10.1038/ni1102-1041. [DOI] [PubMed] [Google Scholar]
- 2.Lang C, Gross U, Luder CG. Subversion of innate and adaptive immune responses by Toxoplasma gondii. Parasitol Res. 2007;100:191–203. doi: 10.1007/s00436-006-0306-9. [DOI] [PubMed] [Google Scholar]
- 3.Casares S, Richie TL. Immune evasion by malaria parasites: a challenge for vaccine development. Curr Opin Immunol. 2009;21:321–330. doi: 10.1016/j.coi.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 4.Antoine JC, Prina E, Courret N, Lang T. Leishmania spp.: on the interactions they establish with antigen-presenting cells of their mammalian hosts. Adv Parasitol. 2004;58:1–68. doi: 10.1016/S0065-308X(04)58001-6. [DOI] [PubMed] [Google Scholar]
- 5.Andrade LO, Andrews NW. The Trypanosoma cruzi-host-cell interplay: location, invasion, retention. Nat Rev Microbiol. 2005;3:819–823. doi: 10.1038/nrmicro1249. [DOI] [PubMed] [Google Scholar]
- 6.Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- 7.Sibley LD, Weidner E, Krahenbuhl JL. Phagosome acidification blocked by intracellular Toxoplasma gondii. Nature. 1985;315:416–419. doi: 10.1038/315416a0. [DOI] [PubMed] [Google Scholar]
- 8.Joiner KA, Fuhrman SA, Miettinen HM, Kasper LH, Mellman I. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor-transfected fibroblasts. Science. 1990;249:641–646. doi: 10.1126/science.2200126. [DOI] [PubMed] [Google Scholar]
- 9.Garg N, Nunes MP, Tarleton RL. Delivery by Trypanosoma cruzi of proteins into the MHC class I antigen processing and presentation pathway. J Immunol. 1997;158:3293–3302. [PubMed] [Google Scholar]
- 10.Kwok LY, Lutjen S, Soltek S, Soldati D, Busch D, Deckert M, Schluter D. The induction and kinetics of antigen-specific CD8 T cells are defined by the stage specificity and compartmentalization of the antigen in murine toxoplasmosis. J Immunol. 2003;170:1949–1957. doi: 10.4049/jimmunol.170.4.1949. [DOI] [PubMed] [Google Scholar]
- 11.Gubbels MJ, Striepen B, Shastri N, Turkoz M, Robey EA. Class I major histocompatibility complex presentation of antigens that escape from the parasitophorous vacuole of Toxoplasma gondii. Infect Immun. 2005;73:703–711. doi: 10.1128/IAI.73.2.703-711.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bertholet S, Debrabant A, Afrin F, Caler E, Mendez S, Tabbara KS, Belkaid Y, Sacks DL. Antigen requirements for efficient priming of CD8+ T cells by Leishmania major-infected dendritic cells. Infect Immun. 2005;73:6620–6628. doi: 10.1128/IAI.73.10.6620-6628.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar S, Tarleton RL. The relative contribution of antibody production and CD8+ T cell function to immune control of Trypanosoma cruzi. Parasite Immunol. 1998;20:207–216. doi: 10.1046/j.1365-3024.1998.00154.x. [DOI] [PubMed] [Google Scholar]
- 14 •.Ng LG, Hsu A, Mandell MA, Roediger B, Hoeller C, Mrass P, Iparraguirre A, Cavanagh LL, Triccas JA, Beverley SM, et al. Migratory dermal dendritic cells act as rapid sensors of protozoan parasites. PLoS Pathog. 2008;4:e1000222. doi: 10.1371/journal.ppat.1000222. Using intravital imaging this study shows that after intradermal injection some L. major parasites are phagocytosed by dermal DCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15••.Peters NC, Egen JG, Secundino N, Debrabant A, Kimblin N, Kamhawi S, Lawyer P, Fay MP, Germain RN, Sacks D. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science. 2008;321:970–974. doi: 10.1126/science.1159194. In this paper the authors used two photon microscopy to demonstrate that following inoculation of Leishmania into the skin by sand fly bite (the natural insect vector) neutrophils are the major cell population harbouring parasites at the inoculation site during initial infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Filipe-Santos O, Pescher P, Breart B, Lippuner C, Aebischer T, Glaichenhaus N, Spath GF, Bousso P. A dynamic map of antigen recognition by CD4 T cells at the site of Leishmania major infection. Cell Host Microbe. 2009;6:23–33. doi: 10.1016/j.chom.2009.04.014. This study examines the interaction of L. major specific CD4 T cells with infected APCs in vivo at the site of infection. [DOI] [PubMed] [Google Scholar]
- 17•.Bertholet S, Goldszmid R, Morrot A, Debrabant A, Afrin F, Collazo-Custodio C, Houde M, Desjardins M, Sher A, Sacks D. Leishmania antigens are presented to CD8+ T cells by a transporter associated with antigen processing-independent pathway in vitro and in vivo. J Immunol. 2006;177:3525–3533. doi: 10.4049/jimmunol.177.6.3525. An early reference comparing Ag presentation to CD8 T cells by DCs infected with L. major or T. gondii. [DOI] [PubMed] [Google Scholar]
- 18.Kima PE, Ruddle NH, McMahon-Pratt D. Presentation via the class I pathway by Leishmania amazonensis-infected macrophages of an endogenous leishmanial antigen to CD8+ T cells. J Immunol. 1997;159:1828–1834. [PubMed] [Google Scholar]
- 19•.Dzierszinski F, Pepper M, Stumhofer JS, LaRosa DF, Wilson EH, Turka LA, Halonen SK, Hunter CA, Roos DS. Presentation of Toxoplasma gondii antigens via the endogenous major histocompatibility complex class I pathway in nonprofessional and professional antigen-presenting cells. Infect Immun. 2007;75:5200–5209. doi: 10.1128/IAI.00954-07. This report compares the ability of professional versus non-professional APCs to cross-present T. gondii Ags and examines the role of Irgm3 in this process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldszmid RS, Bafica A, Jankovic D, Feng CG, Caspar P, Winkler-Pickett R, Trinchieri G, Sher A. TAP-1 indirectly regulates CD4+ T cell priming in Toxoplasma gondii infection by controlling NK cell IFN-gamma production. J Exp Med. 2007;204:2591–2602. doi: 10.1084/jem.20070634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanchard N, Gonzalez F, Schaeffer M, Joncker NT, Cheng T, Shastri AJ, Robey EA, Shastri N. Immunodominant, protective response to the parasite Toxoplasma gondii requires antigen processing in the endoplasmic reticulum. Nat Immunol. 2008;9:937–944. doi: 10.1038/ni.1629. Refs [21•• and 37••] describe for the first time naturally occurring T. gondii CD8 T cell epitopes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22••.Goldszmid RS, Coppens I, Lev A, Caspar P, Mellman I, Sher A. Host ER-parasitophorous vacuole interaction provides a route of entry for antigen cross-presentation in Toxoplasma gondii-infected dendritic cells. J Exp Med. 2009;206:399–410. doi: 10.1084/jem.20082108. This is a recent paper providing evidence for a role of ER-PV fusion in cross-presentation of T. gondii-derived Ag by DCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mordue DG, Sibley LD. Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. J Immunol. 1997;159:4452–4459. [PubMed] [Google Scholar]
- 24.Boothroyd JC, Dubremetz JF. Kiss and spit: the dual roles of Toxoplasma rhoptries. Nat Rev Microbiol. 2008;6:79–88. doi: 10.1038/nrmicro1800. [DOI] [PubMed] [Google Scholar]
- 25.Neudeck A, Stachelhaus S, Nischik N, Striepen B, Reichmann G, Fischer HG. Expression variance, biochemical and immunological properties of Toxoplasma gondii dense granule protein GRA7. Microbes Infect. 2002;4:581–590. doi: 10.1016/s1286-4579(02)01576-9. [DOI] [PubMed] [Google Scholar]
- 26.Schwab JC, Beckers CJ, Joiner KA. The parasitophorous vacuole membrane surrounding intracellular Toxoplasma gondii functions as a molecular sieve. Proc Natl Acad Sci U S A. 1994;91:509–513. doi: 10.1073/pnas.91.2.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinai AP, Webster P, Joiner KA. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J Cell Sci. 1997;110 ( Pt 17):2117–2128. doi: 10.1242/jcs.110.17.2117. [DOI] [PubMed] [Google Scholar]
- 28.Gajria B, Bahl A, Brestelli J, Dommer J, Fischer S, Gao X, Heiges M, Iodice J, Kissinger JC, Mackey AJ, et al. ToxoDB: an integrated Toxoplasma gondii database resource. Nucleic Acids Res. 2008;36:D553–556. doi: 10.1093/nar/gkm981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ajioka JW, Soldati A, Soldati D. Toxoplasma: Molecular and Cellular Biology. Horizon Scientific Press; Hethersett, England, UK: 2007. p. 202. [Google Scholar]
- 30.Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425:397–402. doi: 10.1038/nature01911. [DOI] [PubMed] [Google Scholar]
- 31.Houde M, Bertholet S, Gagnon E, Brunet S, Goyette G, Laplante A, Princiotta MF, Thibault P, Sacks D, Desjardins M. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425:402–406. doi: 10.1038/nature01912. [DOI] [PubMed] [Google Scholar]
- 32.Ackerman AL, Giodini A, Cresswell P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity. 2006;25:607–617. doi: 10.1016/j.immuni.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 33.Chtanova T, Han SJ, Schaeffer M, van Dooren GG, Herzmark P, Striepen B, Robey EA. Dynamics of T cell, antigen-presenting cell, and pathogen interactions during recall responses in the lymph node. Immunity. 2009;31:342–355. doi: 10.1016/j.immuni.2009.06.023. Refs. [33••, 34 • and 35•] provide important insights into the dynamics of CD8 T cell-APC interactions in lymphoyd and brain tissue in vivo during T. gondii infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.John B, Harris TH, Tait ED, Wilson EH, Gregg B, Ng LG, Mrass P, Roos DS, Dzierszinski F, Weninger W, et al. Dynamic Imaging of CD8(+) T cells and dendritic cells during infection with Toxoplasma gondii. PLoS Pathog. 2009;5:e1000505. doi: 10.1371/journal.ppat.1000505. See annotation to Ref. [33••] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Schaeffer M, Han SJ, Chtanova T, van Dooren GG, Herzmark P, Chen Y, Roysam B, Striepen B, Robey EA. Dynamic imaging of T cell-parasite interactions in the brains of mice chronically infected with Toxoplasma gondii. J Immunol. 2009;182:6379–6393. doi: 10.4049/jimmunol.0804307. See annotation to Ref. [33••] [DOI] [PubMed] [Google Scholar]
- 36.Martin DL, Weatherly DB, Laucella SA, Cabinian MA, Crim MT, Sullivan S, Heiges M, Craven SH, Rosenberg CS, Collins MH, et al. CD8+ T-Cell responses to Trypanosoma cruzi are highly focused on strain-variant trans-sialidase epitopes. PLoS Pathog. 2006;2:e77. doi: 10.1371/journal.ppat.0020077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Frickel EM, Sahoo N, Hopp J, Gubbels MJ, Craver MP, Knoll LJ, Ploegh HL, Grotenbreg GM. Parasite stage-specific recognition of endogenous Toxoplasma gondii-derived CD8+ T cell epitopes. J Infect Dis. 2008;198:1625–1633. doi: 10.1086/593019. See annotation to Ref. [21••] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor GA, Feng CG, Sher A. Control of IFN-gamma-mediated host resistance to intracellular pathogens by immunity-related GTPases (p47 GTPases) Microbes Infect. 2007;9:1644–1651. doi: 10.1016/j.micinf.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Ling YM, Shaw MH, Ayala C, Coppens I, Taylor GA, Ferguson DJ, Yap GS. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med. 2006;203:2063–2071. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao Z, Fux B, Goodwin M, Dunay IR, Strong D, Miller BC, Cadwell K, Delgado MA, Ponpuak M, Green KG, et al. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe. 2008;4:458–469. doi: 10.1016/j.chom.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao YO, Khaminets A, Hunn JP, Howard JC. Disruption of the Toxoplasma gondii parasitophorous vacuole by IFNgamma-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death. PLoS Pathog. 2009;5:e1000288. doi: 10.1371/journal.ppat.1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martens S, Parvanova I, Zerrahn J, Griffiths G, Schell G, Reichmann G, Howard JC. Disruption of Toxoplasma gondii parasitophorous vacuoles by the mouse p47-resistance GTPases. PLoS Pathog. 2005;1:e24. doi: 10.1371/journal.ppat.0010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43••.Bougneres L, Helft J, Tiwari S, Vargas P, Chang BH, Chan L, Campisi L, Lauvau G, Hugues S, Kumar P, et al. A role for lipid bodies in the cross-presentation of phagocytosed antigens by MHC class I in dendritic cells. Immunity. 2009;31:232–244. doi: 10.1016/j.immuni.2009.06.022. This recent study identifies a previously unappreciated role for Irgm3 and lipid bodies in cross-presentation of OVA coated-latex beads. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prickett S, Gray PM, Colpitts SL, Scott P, Kaye PM, Smith DF. In vivo recognition of ovalbumin expressed by transgenic Leishmania is determined by its subcellular localization. J Immunol. 2006;176:4826–4833. doi: 10.4049/jimmunol.176.8.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nat Rev Immunol. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- 46.Kamala T, Nanda NK. Protective response to Leishmania major in BALB/c mice requires antigen processing in the absence of DM. J Immunol. 2009;182:4882–4890. doi: 10.4049/jimmunol.0803956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pepper M, Dzierszinski F, Crawford A, Hunter CA, Roos D. Development of a system to study CD4+-T-cell responses to transgenic ovalbumin-expressing Toxoplasma gondii during toxoplasmosis. Infect Immun. 2004;72:7240–7246. doi: 10.1128/IAI.72.12.7240-7246.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lang C, Algner M, Beinert N, Gross U, Luder CG. Diverse mechanisms employed by Toxoplasma gondii to inhibit IFN-gamma-induced major histocompatibility complex class II gene expression. Microbes Infect. 2006;8:1994–2005. doi: 10.1016/j.micinf.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 49.McKee AS, Dzierszinski F, Boes M, Roos DS, Pearce EJ. Functional inactivation of immature dendritic cells by the intracellular parasite Toxoplasma gondii. J Immunol. 2004;173:2632–2640. doi: 10.4049/jimmunol.173.4.2632. [DOI] [PubMed] [Google Scholar]