Abstract
Polybrominated diphenyl ethers (PBDEs) are flame-retardants that upon chronic exposure enter the liver where they are biotransformed to potentially toxic metabolites. The mechanism by which PBDEs enter the liver is not known. However, due to their large molecular weights (MWs ∼485 to 1000 Da), they cannot enter hepatocytes by simple diffusion. Organic anion–transporting polypeptides (OATPs) are responsible for hepatic uptake of a variety of amphipathic compounds of MWs larger than 350 Da. Therefore, in the present study, Chinese hamster ovary cell lines expressing OATP1B1, OATP1B3, and OATP2B1 were used to test the hypothesis that OATPs expressed in human hepatocytes would be responsible for the uptake of PBDE congeners 47, 99, and 153. The results demonstrated that PBDE congeners inhibited OATP1B1- and OATP1B3-mediated uptake of estradiol-17-β-glucuronide as well as OATP2B1-mediated uptake of estrone-3-sulfate in a concentration-dependent manner. Direct uptake studies confirmed that all three PBDE congeners are substrates for the three tested hepatic OATPs. Detailed kinetic analysis revealed that OATP1B1 transported 2,2′,4,4′-tetrabromodiphenyl ether (BDE47) with the highest affinity (Km = 0.31μM) followed by 2,2′,4,4′,5-pentabromodiphenyl ether (BDE99) (Km = 0.91μM) and 2,2′,4,4′,5,5′-hexabromodiphenyl ether (BDE153) (Km = 1.91μM). For OATP1B3, the order was the same (BDE47: Km = 0.41μM; BDE99: Km = 0.70μM; BDE153: Km = 1.66μM), while OATP2B1 transported all three congeners with similar affinities (BDE47: Km = 0.81μM; BDE99: Km = 0.87μM; BDE153: Km = 0.65μM). These results clearly suggest that uptake of PBDEs via these OATPs is a mechanism responsible for liver-specific accumulation of PBDEs.
Keywords: polybrominated diphenyl ethers, organic anion–transporting polypeptides, liver, uptake assay, kinetics
Polybrominated diphenyl ethers (PBDEs) are flame-retardants used as additives in polymers incorporated into textiles, electronics, plastics, and furniture (Fig. 1). The frequent use of PBDEs is attributed to their low manufacturing cost as well as their high degree of resistance to degradation by environmental and biological systems (Darnerud et al., 2001). 2,2′,4,4′-Tetrabromodiphenyl ether (BDE47) is the predominant congener detected in human and wildlife samples, followed by 2,2′,4,4′,5-pentabromodiphenyl ether (BDE99) and 2,2′,4,4′,5,5′-hexabromodiphenyl ether (BDE153) (Lorber, 2008). The concern of rising PBDE body burdens is so significant that the penta and octa mixtures have been banned in Europe and currently are being voluntarily phased out in the United States (Birnbaum and Cohen Hubal, 2006).
FIG. 1.
Chemical structures of PBDE congeners 47, 99, and 153. Chemical structures were created by PubChem Compound (http://pubchem.ncbi.nlm.nih.gov).
Human exposure to PBDEs is chronic; however, PBDE exposure routes are not well established. Recent data suggest that diet and inhalation are the predominant routes of exposure (Allen et al., 2007, 2008; Costa et al., 2008; Schecter et al., 2004, 2005a, 2006). When compared to Europe and Japan, body burden of PBDEs in North America are one to two orders of magnitude higher and furthermore PBDE levels have doubled every 4–6 years (Hites, 2004; Petreas et al., 2003; Schecter et al., 2005b, 2007). The presence of PBDEs in human tissue is of great concern because of potential toxicological end points including carcinogenicity, neurotoxicity, reproductive toxicity, and endocrine toxicity (Darnerud et al., 2001). PBDEs have been detected in plasma and breast milk as well as liver, kidney, and adipose (Hites, 2004). In mice, after administration, BDE47 initially accumulates in the liver, followed by redistribution to other tissues, including the kidney and the adipose tissue (Staskal et al., 2005). Although the human liver burden of PBDEs is not clarified, the presence of PBDEs in human liver is particularly alarming since it has been suggested that hydroxylated metabolites may play a pivotal role in PBDE-mediated toxicity (Darnerud et al., 2007; Hamers et al., 2008; Meerts et al., 2000, 2001; Zhou et al., 2002).
An important function of the liver is to remove a variety of chemicals from the portal blood. Hepatic uptake is a prerequisite for biotransformation and subsequent elimination of various endogenous and exogenous compounds. Uptake transporters involved in this process include organic anion–transporting polypeptides (OATPs) that are a group of membrane transporters responsible for the transport of a wide array of amphipathic substrates (Hagenbuch and Gui, 2008; Kalliokoski and Niemi, 2009). As a family, OATPs are expressed in the liver, kidney, brain, and intestines, implying a critical role in drug disposition (Hagenbuch and Meier, 2003, 2004). OATP1B1 (SLCO1B1) and OATP1B3 (SLCO1B3) are two human members in the OATP1B subfamily expressed at the basolateral membrane of hepatocytes (Abe et al., 1999; Hsiang et al., 1999; Konig et al., 2000a,b). OATP2B1 (SLCO2B1) is the sole human member of the OATP2B family. Compared to the liver-specific expression pattern of OATP1B1 and OATP1B3, studies have demonstrated OATP2B1 protein to be expressed in the liver, heart, placenta, brain, and the small intestine (Bronger et al., 2005; Grube et al., 2006; Kobayashi et al., 2003; Kullak-Ublick et al., 2001; St-Pierre et al., 2002). Given that OATP substrates are, in general, amphipathic molecules with molecular weights (MWs) of more than 350 (Hagenbuch and Gui, 2008), hepatic OATP family members are promising candidate transport systems for hepatic uptake of BDE47 (MW: 485.5), BDE99 (MW: 564.7), and BDE153 (MW: 643.6)
In the current study, we evaluated the substrate specificity of OATP1B1, OATP1B3, and OATP2B1 for PBDE transport in order to test the hypothesis that the mechanism of hepatic PBDE uptake is mediated by OATPs. The results clearly showed that BDE47, BDE99, and BDE153 inhibited OATP1B1- and OATP1B3-mediated uptake of estradiol-17-β-glucuronide and OATP2B1-mediated uptake of estrone-3-sulfate. Additionally, OATP1B1, OATP1B3, and OATP2B1 are high-affinity transporters for BDE47, BDE99, and BDE153, revealed by kinetic studies.
MATERIALS AND METHODS
Chemicals.
Radiolabeled [14C]BDE47 (36.5 mCi/mmol) was a gift from Dr Kevin Crofton (U.S. Environmental Protection Agency, National Health and Environmental Effects Laboratory). Radiolabeled [14C]BDE99 (36.5 mCi/mmol) and [14C]BDE153 (27.8 mCi/mmol) were gifts from Dr Mike Sanders (National Toxicology Program at the National Institute of Environmental Health Sciences). BDE47, BDE99, and BDE153 were obtained from Cerilliant (Round Rock, TX). [3H]Estradiol-17-β-glucuronide (47.1 Ci/mmol) and [3H]estrone-3-sulfate (57.1 Ci/mmol) were purchased from Perkin-Elmer (Waltham, MA). Unlabeled estradiol-17-β-glucuronide, estrone-3-sulfate, and cell culture reagents were from Sigma-Aldrich (St Louis, MO).
Transport inhibition study in OATP1B1-, OATP1B3-, and OATP2B1-expressing Chinese hamster ovary cells.
Generation of stably transfected OATP1B1 and OATP1B3 cell lines has been described previously (Gui et al., 2008). A cell line stably expressing OATP2B1 was generated using the Flp-In System (Invitrogen, Carlsbad, CA). A six-His tag was added to the C-terminus of the open reading frame of OATP2B1*3 (Kullak-Ublick et al., 2001) using PCR. As it has been reported that the reference sequence has a higher allelic frequency and higher transport function than the OATP2B1*3 polymorphism (Nozawa et al., 2002), the construct was mutated to OATP2B1*1 using the QuikChange Site-Directed Mutagenesis Kit from Stratagene (La Jolla, CA), as per the manufacturer's instructions. The resulting construct was cloned into the pcDNA5/FRT vector, and the sequence was confirmed accurate on both strands. Flp-In Chinese hamster ovary (CHO) cells were transfected with pOG44 and OATP2B1 or the empty vector using Lipofectamine 2000 from Invitrogen. After 3 weeks of selection with 600 μg/ml Hygromycin B, single-cell clones were isolated by limited dilution and tested for OATP2B1 expression using immunofluorescence. The expression of functional OATP2B1 was confirmed by measuring uptake of estrone-3-sulfate; induction with 5mM sodium butyrate for 24 h did not increase function. Cells were maintained at 37°C in a humidified 5% CO2 atmosphere in F-12 Nutrient Mixture containing 10% fetal bovine serum, 2mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 400 μg/ml Hygromycin B. OATP1B1-, OATP1B3-, or OATP2B1-expressing or wild-type CHO cells were grown on 24-well plates at a density of 40,000 cells/well. Forty-eight hours later, medium was replaced with medium containing 5mM sodium butyrate to induce nonspecific gene expression for OATP1B1- and OATP1B3-expressing cells (Palermo et al., 1991). Estradiol-17-β-glucuronide, a model substrate of OATP1B1 and OATP1B3, and estrone-3-sulfate, a model substrate of OATP2B1, were used to test for PBDE inhibition (Abe et al., 1999; Cui et al., 2001; Gui et al., 2008; Hirano et al., 2004; Konig et al., 2000a,b; Kullak-Ublick et al., 2001; Tamai et al., 2000, 2001). Cells were washed two times with 37°C prewarmed uptake buffer (116.4mM NaCl, 5.3mM KCl, 1mM NaH2PO4, 0.8mM MgSO4, 5.5mM D-glucose, and 20mM (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), pH adjusted to 7.4 with Trizma base). Cells were incubated in the absence or presence of BDE47, BDE99, or BDE153 at concentrations of 100pM, 100nM, or 100μM for 20 s (OATP1B1 and OATP1B3) or 30 s (OATP2B1). Uptake was stopped by removing the uptake solution and washing the cells four times with ice-cold uptake buffer. Uptake rates were calculated based on net uptake after subtracting the values obtained with wild-type CHO cells and then expressed as a percentage of uptake obtained by OATP-expressing cells in the absence of PBDE congeners. Protein content was determined for each well using the Pierce BCA assay (Pierce Chemical, Rockford, IL) with bovine serum albumin (BSA) as standard.
Functional studies of BDE transport in wild-type and OATP-expressing CHO cells.
Wild-type CHO and OATP1B1- or OATP1B3-expressing cells were plated at 40,000 cells per well on 24-well plates, and 48 h later medium was replaced with medium containing 5mM sodium butyrate to induce nonspecific gene expression. After an additional 24 h in culture, the cells were used for uptake experiments. Vector-transfected and OATP2B1-expressing Flp-In-CHO cells were plated at 40,000 cells per well on 24-well plates, and 48 h later the cells were used for uptake experiments. Cells were washed two times with 37°C prewarmed uptake buffer, and for the determination of initial linear rate conditions, uptake was started by adding 200 μl of uptake buffer containing 0.3 μCi/ml of the radiolabeled substrate. Uptake was stopped at various time points by removing the uptake solution and washing the cells four times with ice-cold uptake buffer. To analyze the kinetics of OATP-mediated PBDE transport, wild-type, control, or OATP-expressing cells were incubated with [14C]BDE47, [14C]BDE99, or [14C]BDE153 at increasing substrate concentrations for a previously determined time within the initial linear uptake portion. After stopping, the cells were solubilized with 400 μl of 1% Triton X-100 and 300 μl were used for liquid scintillation counting. Protein concentration was determined using the BCA assay with BSA as a standard from the remaining samples. Uptake rates were calculated based on net uptake (in nanomoles per milligram of total protein per minute) after subtracting the values obtained with wild-type or pcDNA5/FRT-transfected CHO cells (in nanomoles per milligram of total protein per minute).
Data analysis.
Inhibition studies and uptake experiments were performed in triplicate and repeated three or four times. Data with error bars represent the means ± SE. Statistical analysis of inhibition studies between control and experimental groups were performed by one-way ANOVA followed by the Bonferroni t-test. To determine whether uptake in OATP-expressing cells was different from that of control cells, the unpaired Student's t-test was used. The p value for statistical significance was set to < 0.05. All statistical analysis was performed using SigmaStat 3.5 (Systat Software, Inc., San Jose, CA), while kinetic parameters were calculated using the nonlinear regression analysis module from SigmaPlot (Version 9.01; Systat Software, Inc., Point Richmond, CA).
RESULTS
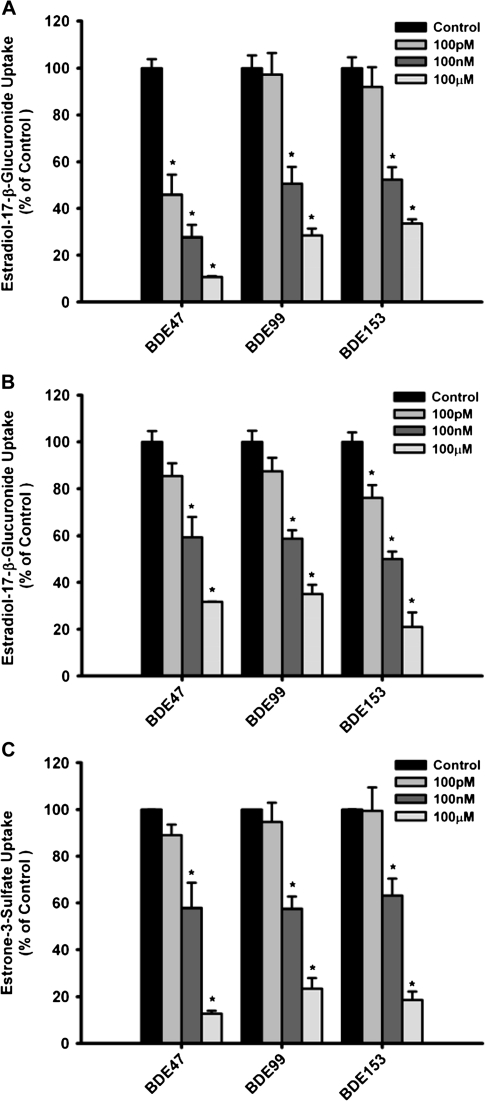
Concentration-Dependent Inhibition of OATP1B1-, OATP1B3-, and OATP2B1-Mediated Uptake by PBDE Congeners
In order to investigate whether PBDE congeners can interact with OATP-mediated transport, we determined uptake of the model substrates estradiol-17-β-glucuronide for OATP1B1 and OATP1B3 and estrone-3-sulfate for OATP2B1, in the absence or presence of BDE47, BDE99, or BDE153. All three PBDE congeners inhibited OATP1B1- and OATP1B3-mediated uptake of estradiol-17-β-glucuronide in a concentration-dependent manner (Figs 2A and 2B). For OATP1B1, BDE47 exhibited the greatest effect at all the tested concentrations (100pM, 100nM, 100μM), while BDE99 and BDE153 showed comparable levels of inhibition at all tested concentrations. Inhibition of OATP1B3-mediated uptake of estradiol-17-β-glucuronide was comparable for all three tested PBDE congeners with BDE153 exhibiting slightly greater effect. OATP2B1-mediated estrone-3-sulfate uptake was inhibited by BDE47, BDE99, and BDE153 in a similar manner but only at the 100nM and 100μM concentrations (Fig. 2C).
FIG. 2.
Effect of PBDE congeners on OATP-mediated uptake of known substrates in stably transfected CHO cells. In the absence or presence of BDE47, BDE99, or BDE153 at the indicated concentrations, uptake of 1μM [3H]estradiol-17-β-glucuronide was measured at 37°C for 20 s with (A) OATP1B1- or (B) OATP1B3-expressing and wild-type CHO cells, while uptake of 1μM [3H]estrone-3-sulfate was measured at 37°C for 30 s with (C) OATP2B1- or pcDNA5/FRT-expressing CHO cells. Values obtained with wild-type or vector-transfected CHO cells were subtracted from values obtained with OATP-expressing CHO cells and are given as percent of the control. Means ± SE of triplicate determinations are given. Differences were considered significant at p < 0.05.
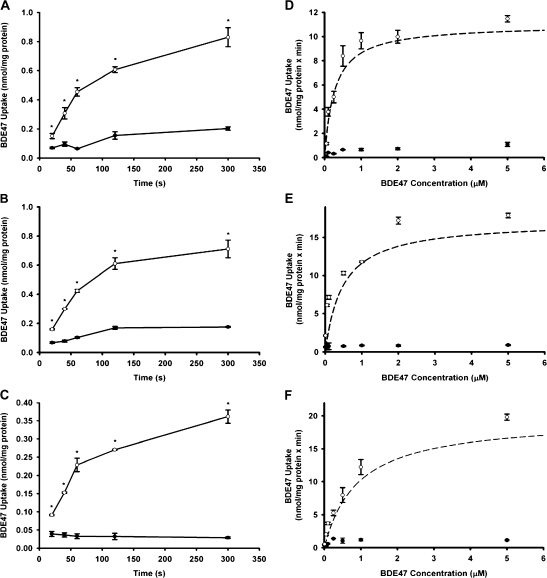
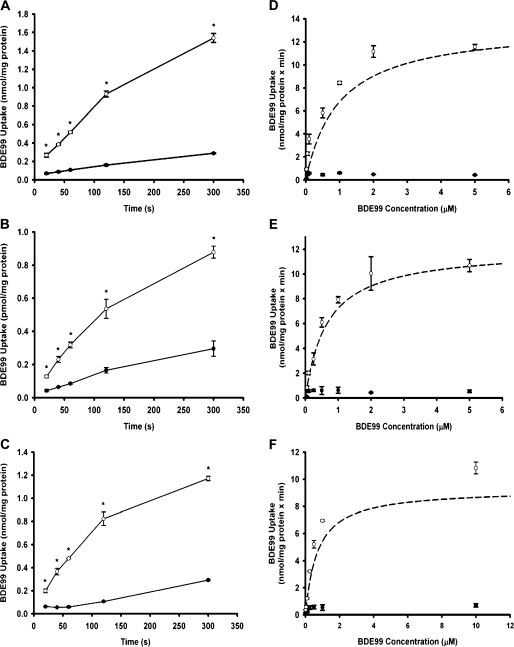
Time-Dependent BDE47, BDE99, and BDE153 Uptake by OATP1B1, OATP1B3, and OATP2B1
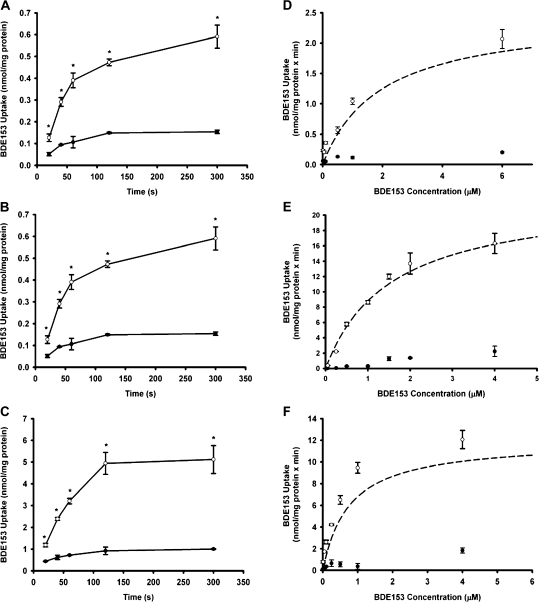
To test whether these PBDE congeners indeed are OATP substrates, uptake of [14C]BDE47, [14C]BDE99, and [14C]BDE153 by OATP1B1, OATP1B3, and OATP2B1 was measured in a time-dependent manner. BDE47 (Figs 3A and 3B), BDE99 (Figs 4A and 4B), and BDE153 (Figs 5A and 5B) were clearly transported to a greater extent into OATP1B1- and OATP1B3-expressing cells when compared to the wild-type control cells. Similarly, uptake of BDE47 (Fig. 3C), BDE99 (Fig. 4C), and BDE153 (Fig. 5C) into OATP2B1-expressing cells was significantly higher than uptake into the vector-transfected control cells. Furthermore, for all three congeners uptake was linear for at least 1 min. Thus, subsequent concentration-dependent kinetic studies were performed at 30 s.
FIG. 3.
Time- and concentration-dependent uptake of BDE47 by OATP1B1, OATP1B3, and OATP2B1. (A) OATP1B1-, (B) OATP1B3-, or (C) OATP2B1-mediated uptake of [14C]BDE47 at 37°C at the indicated time points. Filled circles (•) represent control (wild-type CHO; pcDNA5/FRT CHO) uptake, while open circles (○) represent OATP1B1, OATP1B3, or OATP2B1 uptake. Determination of kinetic parameters was performed in CHO cells expressing OATP1B1, OATP1B3, or OATP2B1. Uptake of increasing concentrations of [14C]BDE47 was measured at 37°C for 30 s. After subtracting the values of the control (•) from OATP-expressing cells (○), net (D) OATP1B1-, (E) OATP1B3-, or (F) OATP2B1-mediated uptake data were fitted by nonlinear regression analysis to the Michaelis-Menten equation and plotted as a dashed line (- - -). Means ± SE of triplicate determinations are given. The unpaired Student’s t-test was performed to determine statistical significance. Differences were considered significant at p < 0.05.
FIG. 4.
Time- and concentration-dependent uptake of BDE99 by OATP1B1, OATP1B3, and OATP2B1. (A) OATP1B1-, (B) OATP1B3-, and (C) OATP2B1-mediated uptake of [14C]BDE99 at 37°C at the indicated time points. Filled circles (•) represent control (wild-type CHO; pcDNA5/FRT CHO) uptake, while open circles (○) represent OATP1B1, OATP1B3, or OATP2B1 uptake. Determination of kinetic parameters was performed in CHO cells expressing OATP1B1, OATP1B3, or OATP2B1. Uptake of increasing concentrations of [14C]BDE99 was measured at 37°C for 30 s. After subtracting the values of the control (•) from OATP-expressing cells (○), net (D) OATP1B1-, (E) OATP1B3-, or (F) OATP2B1-mediated uptake data were fitted by nonlinear regression analysis to the Michaelis-Menten equation and plotted as a dashed line (- - -). Means ± SE of triplicate determinations are given. The unpaired Student’s t-test was performed to determine statistical significance. Differences were considered significant at p < 0.05.
FIG. 5.
Time- and concentration-dependent uptake of BDE153 by OATP1B1, OATP1B3, and OATP2B1. (A) OATP1B1-, (B) OATP1B3-, and (C) OATP2B1-mediated uptake of [14C]BDE153 at 37°C at the indicated time points. Filled circles (•) represent control (wild-type CHO; pcDNA5/FRT CHO) uptake, while open circles (○) represent OATP1B1, OATP1B3, or OATP2B1 uptake. Determination of kinetic parameters was performed in CHO cells expressing OATP1B1, OATP1B3, or OATP2B1. Uptake of increasing concentrations of [14C]BDE153 was measured at 37°C for 30 s. After subtracting the values of the control (•) from OATP-expressing cells (○), net (D) OATP1B1-, (E) OATP1B3-, or (F) OATP2B1-mediated uptake data were fitted by nonlinear regression analysis to the Michaelis-Menten equation and plotted as a dashed line (- - -). Means ± SE of triplicate determinations are given. The unpaired Student’s t-test was performed to determine statistical significance. Differences were considered significant at p < 0.05.
Determination of Kinetic Parameters for OATP1B1-, OATP1B3-, and OATP2B1-Mediated PBDE Congener Uptake
To further characterize OATP-mediated PBDE transport, we performed kinetic experiments. Uptake of all three PBDE congeners by all three OATPs was saturable, and the kinetic parameters are summarized in Table 1. BDE47 exhibited the highest affinity for both OATP1B1 (0.31 ± 0.02μM) and OATP1B3 (0.34 ± 0.02μM), respectively (Figs 3D and 3E). As shown in Figures 4D and 4E, this was followed by BDE99 (0.80 ± 0.07μM for OATP1B1; 0.72 ± 0.12μM for OATP1B3). BDE153 (1.9 ± 0.17μM for OATP1B1; 1.7 ± 0.48μM for OATP1B3) demonstrated the least affinity (Figs 5D and 5E). OATP1B1 transported BDE153 with a 10-fold lower capacity than BDE47 and BDE99, while the maximal transport rates were comparable for OATP1B3-mediated transport of all three PBDE congeners. Overall transport efficiency, characterized by Vmax/Km, followed a similar trend for both OATP1B1 and OATP1B3, with BDE47 being transported with the greatest efficiency followed by BDE99 and then BDE153.
TABLE 1.
Kinetic Parameters of OATP1B1-, OATP1B3-, and OATP2B1-Mediated Uptake of PBDEs in CHO Cells
| Transporter | Substrate | Km (μM) | Vmax (nmol/mg protein × min) | Vmax/Km |
| OATP1B1 | BDE47 | 0.31 ± 0.01 | 13.5 ± 1.33 | 44.0 ± 2.8 |
| BDE99 | 0.91 ± 0.02 | 13.9 ± 1.70 | 15.4 ± 2.2 | |
| BDE153 | 1.91 ± 0.17 | 2.4 ± 0.19 | 1.2 ± 0.02 | |
| OATP1B3 | BDE47 | 0.41 ± 0.06 | 15.7 ± 1.71 | 38.0 ± 1.1 |
| BDE99 | 0.70 ± 0.03 | 12.8 ± 0.76 | 18.3 ± 0.1 | |
| BDE153 | 1.66 ± 0.48 | 22.6 ± 0.58 | 1.5 ± 5.3 | |
| OATP2B1 | BDE47 | 0.81 ± 0.06 | 17.6 ± 1.1 | 22.6 ± 1.1 |
| BDE99 | 0.87 ± 0.22 | 8.9 ± 0.9 | 10.5 ± 1.6 | |
| BDE153 | 0.65 ± 0.07 | 14.6 ± 3.1 | 28.1 ± 8.1 |
Note. Transport rates at increasing concentrations were determined at 37°C in OATP-expressing and wild-type CHO or vector-transfected cells. Transport values obtained from OATP-expressing cells were corrected with values obtained from wild-type cells, and the resulting net carrier-mediated uptake values were fitted by nonlinear regression analysis to the Michaelis-Menten equation. Means ± SE are given for three to four experiments.
Saturation kinetics for OATP2B1-mediated transport for BDE47, BDE99, and BDE153 is shown in Figures 3F, 4F, and 5F, respectively. OATP2B1-mediated uptake exhibited similar transport affinities for the uptake of BDE47 (Km: 0.81 ± 0.03μM), BDE99 (Km: 0.87 ± 0.22μM), and BDE153 (Km: 0.65 ± 0.07μM). BDE153 was transported with a higher affinity by OATP2B1 than by the members of the OATP1B subfamily. The capacity of OATP2B1 transport was approximately twofold higher for BDE47 and BDE153 than for BDE99, which resulted in an about twofold higher efficiency (Vmax/Km values) as compared to BDE99.
DISCUSSION
The present study provides direct evidence that human hepatic OATPs (OATP1B1, OATP1B3, and OATP2B1) represent BDE47, BDE99, and BDE153 uptake systems in human liver. Additionally, we have demonstrated that OATP1B1 and OATP1B3 transport BDE47 with the highest affinity, while OATP2B1 transported all three congeners with similar affinities.
The predominant PBDE congeners detected in human liver are BDE47, BDE99, and BDE153 (Meironyte Guvenius et al., 2001; Schecter et al., 2007). Because of the anatomy of the hepatic circulation, any drug or chemical that is absorbed from the gastrointestinal tract into the portal vein must pass through the liver before reaching the systemic circulation. During this first pass effect, the potential for presystemic elimination is dependent upon the efficiency of the hepatic extraction process. PBDE concentration in the portal blood supply may be influenced by the efficiency of the hepatic extraction process. In human hepatocytes, OATP1B1, OATP1B3, and OATP2B1 function to mediate the portal clearance of large (MW > 350) amphipathic molecules (Hagenbuch and Gui, 2008). The BDE congeners with the highest serum concentration are BDE47 (∼4.2nM), BDE99 (∼1.0nM), and BDE153 (∼0.9nM) (Sjodin et al., 2008). At these concentrations, which are below the Km values of the individual BDE congeners determined in our study (Table 1), hepatic uptake, according to Michaelis-Menton kinetics, becomes first order and therefore depends on affinity for the transporters as well as blood flow. Thus, PBDEs are readily cleared from the portal blood supply and transported into the liver where they are subject to metabolic biotransformation. Importantly, it has been suggested that hydroxylated PBDES (OH-PBDEs) may have increased toxicological relevance (Hamers et al., 2008; Meerts et al., 2000, 2001; Zhou et al., 2002). Disposition studies in rodents administered PBDEs by oral gavage suggest that BDE47 and BDE99 are preferentially transported into the liver when compared to BDE153 (Chen et al., 2006; Darnerud et al., 2007; Sanders et al., 2006a,b). The results from our study are in agreement with the published in vivo rodent studies. Transport efficiency for OATP1B1- and OATP1B3-mediated uptake, characterized by Vmax/Km, was greatest for BDE47 followed by BDE99 and then BDE153 (Table 1). These data suggest that OATP1B1 and OATP1B3 preferentially transport BDE47 and BDE99 compared to BDE153, which provides an explanation for the PBDE congener profile identified in human liver.
In addition, we investigated the role of OATP2B1 for the uptake of BDE47, BDE99, and BDE153. The results suggest that OATP2B1 does not preferentially transport any PBDE congeners since affinity and efficiency were similar for BDE47, BDE99, and BDE153 (Table 1). It has been suggested that OATP2B1 may play a limited role in hepatic uptake since the pH of portal blood is unlikely acidic (Hagenbuch, 2010). Low extracellular pH has been shown to stimulate transport activity of OATP2B1 localized at the apical membrane of human intestinal epithelial cells (Kobayashi et al., 2003; Nozawa et al., 2004; Sai et al., 2006). The importance of histidine residues has been demonstrated for several pH-sensitive transporters (Ganapathy et al., 1987; Grillo and Aronson, 1986; Kato et al., 1989; Miles, 1977). Recent work has identified specific His residues that may explain the apparent pH dependency shown by OATP2B1. Specifically, in silico structural modeling of OATP2B1 revealed a His residue at position 579 in the 10th transmembrane domain that is exposed to the extracellular medium and thus susceptible protonation changes applied by the extracellular pH (Meier-Abt et al., 2005). Additionally, stimulation of transport activity at a low extracellular pH (pH 6.5), shown for several OATPs including OATP2B1, was demonstrated to be dependent upon a His residue in the third transmembrane domain (Leuthold et al., 2009). Furthermore, OATP1C1, which lacks this His residues, did not exhibit the pH-dependent transport seen with other OATPs. PBDE congeners have been shown to be absorbed from the gastrointestinal tract in rodents. Specifically, gastrointestinal absorption has been estimated to be 80–90% for BDE47, 60–90% for BDE99, while 70% for BDE153 (Chen et al., 2006; Darnerud and Risberg, 2006; Hakk et al., 2002; Sanders et al., 2006a,b; Staskal et al., 2005). Given the expression of OATP2B1 at the apical membrane of human intestinal epithelial cells together with an increase in transport activity at a lower pH, it may be that OATP2B1 might play a greater role in uptake of PBDEs from the gastrointestinal tract.
Furthermore, the results of this study clarify why the congener patterns identified in humans do not reflect the composition of the commercial Penta product. For example, BDE99, which is the predominant congener in the mixture, is found to a lesser degree than BDE47 in human samples including blood and liver (Costa et al., 2008; Covaci et al., 2008; Hites, 2004; Mazdai et al., 2003; Meironyte Guvenius et al., 2001; Schecter et al., 2007; Sjodin et al., 2008). The difference between the congeners profile found in the commercial mixture to that of human tissue, in particular the liver, can partly be explained by the higher affinity and greater overall transport efficiency for BDE47 by OATP1B1 and OATP1B3 compared to that for BDE99 (Table 1). Oxidation of many aromatic xenobiotic contaminants in the liver occurs through the catalytic action of the P450 enzymes of the hepatic mixed function oxidase system. OH-PBDE metabolites have been detected in human blood samples (Athanasiadou et al., 2008; Qiu et al., 2009; Sandanger et al., 2007). Furthermore, it was demonstrated that BDE99 is metabolized to the greatest extent followed by BDE47. Recently, two independent studies have identified oxidative metabolism of BDE47 and BDE99 through the use of human liver microsomes and cryogenically preserved human hepatocytes (Lupton et al., 2009; Stapleton et al., 2009). Again, BDE99 was shown to have a greater potential for metabolism followed by BDE47. Interestingly, BDE153 was shown to be resistant to oxidative metabolism. The authors explain this by the lack of unsubstituted adjacent carbons, which has been shown to be pivotal for the formation of the arene oxide intermediate during P450-mediated metabolism.
The greater abundance of BDE47 in human liver samples compared to that of BDE99 can be attributed to a greater uptake efficiency as well as lower rate of metabolism. Furthermore, although BDE99 is the primary component of the commercial penta mixture, its greater rate of metabolism by hepatic P450s seems to play a more important role than hepatic uptake regarding its decreased bioaccumulation. Hepatic uptake of BDE153 by OATP1B1 and OATP1B3 occurred in a low-affinity, low-capacity manner when compared to BDE47 and BDE99. However, OATP2B1-mediated transport occurs at a much higher affinity for BDE153, which is similar to Km values for OATP1B1- and OATP1B3-mediated transport of BDE99. Additionally, BDE153 has been shown to be relatively resistant to metabolism (Lupton et al., 2009; Sanders et al., 2006b). Furthermore, in addition to DE-71, BDE153 is found in the octaBDE mixture (Darnerud et al., 2001). Taken together, high-affinity transport by OATP2B1 along with minimal metabolism can explain the greater bioaccumulation of BDE153 when compared to BDE47 and BDE99.
In conclusion, we have identified PBDE congeners BDE47, BDE99, and BDE153 as substrates of OATP1B1, OATP1B3, and OATP2B1. This has provided evidence for a transporter-mediated mechanism for the hepatic accumulation of the predominant PBDE congeners. In addition, differential uptake efficiency and metabolism of BDE47, BDE99, and BDE153 together provides an explanation for the inconstancy found between ratios of PBDE concentrations in human samples to that of the commercial mixture.
FUNDING
National Institute of Health (RR021940, DK081343 to G.L.G., GM077336 to B.H.).
References
- Abe T, Kakyo M, Tokui T, Nakagomi R, Nishio T, Nakai D, Nomura H, Unno M, Suzuki M, Naitoh T, et al. Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J. Biol. Chem. 1999;274:17159–17163. doi: 10.1074/jbc.274.24.17159. [DOI] [PubMed] [Google Scholar]
- Allen JG, McClean MD, Stapleton HM, Nelson JW, Webster TF. Personal exposure to polybrominated diphenyl ethers (PBDEs) in residential indoor air. Environ. Sci. Technol. 2007;41:4574–4579. doi: 10.1021/es0703170. [DOI] [PubMed] [Google Scholar]
- Allen JG, McClean MD, Stapleton HM, Webster TF. Critical factors in assessing exposure to PBDEs via house dust. Environ. Int. 2008;34:1085–1091. doi: 10.1016/j.envint.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Athanasiadou M, Cuadra SN, Marsh G, Bergman A, Jakobsson K. Polybrominated diphenyl ethers (PBDEs) and bioaccumulative hydroxylated PBDE metabolites in young humans from Managua, Nicaragua. Environ. Health Perspect. 2008;116:400–408. doi: 10.1289/ehp.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum LS, Cohen Hubal EA. Polybrominated diphenyl ethers: a case study for using biomonitoring data to address risk assessment questions. Environ. Health Perspect. 2006;114:1770–1775. doi: 10.1289/ehp.9061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronger H, Konig J, Kopplow K, Steiner HH, Ahmadi R, Herold-Mende C, Keppler D, Nies AT. ABCC drug efflux pumps and organic anion uptake transporters in human gliomas and the blood-tumor barrier. Cancer Res. 2005;65:11419–11428. doi: 10.1158/0008-5472.CAN-05-1271. [DOI] [PubMed] [Google Scholar]
- Chen LJ, Lebetkin EH, Sanders JM, Burka LT. Metabolism and disposition of 2,2′,4,4′,5-pentabromodiphenyl ether (BDE99) following a single or repeated administration to rats or mice. Xenobiotica. 2006;36:515–534. doi: 10.1080/00498250600674477. [DOI] [PubMed] [Google Scholar]
- Costa LG, Giordano G, Tagliaferri S, Caglieri A, Mutti A. Polybrominated diphenyl ether (PBDE) flame retardants: environmental contamination, human body burden and potential adverse health effects. Acta Biomed. 2008;79:172–183. [PubMed] [Google Scholar]
- Covaci A, Voorspoels S, Roosens L, Jacobs W, Blust R, Neels H. Polybrominated diphenyl ethers (PBDEs) and polychlorinated biphenyls (PCBs) in human liver and adipose tissue samples from Belgium. Chemosphere. 2008;73:170–175. doi: 10.1016/j.chemosphere.2008.02.059. [DOI] [PubMed] [Google Scholar]
- Cui Y, Konig J, Leier I, Buchholz U, Keppler D. Hepatic uptake of bilirubin and its conjugates by the human organic anion transporter SLC21A6. J. Biol. Chem. 2001;276:9626–9630. doi: 10.1074/jbc.M004968200. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Aune M, Larsson L, Hallgren S. Plasma PBDE and thyroxine levels in rats exposed to Bromkal or BDE-47. Chemosphere. 2007;67:S386–S392. doi: 10.1016/j.chemosphere.2006.05.133. [DOI] [PubMed] [Google Scholar]
- Darnerud PO, Eriksen GS, Johannesson T, Larsen PB, Viluksela M. Polybrominated diphenyl ethers: occurrence, dietary exposure, and toxicology. Environ. Health Perspect. 2001;109(Suppl. 1):49–68. doi: 10.1289/ehp.01109s149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnerud PO, Risberg S. Tissue localisation of tetra- and pentabromodiphenyl ether congeners (BDE-47, -85 and -99) in perinatal and adult C57BL mice. Chemosphere. 2006;62:485–493. doi: 10.1016/j.chemosphere.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Balkovetz DF, Ganapathy ME, Mahesh VB, Devoe LD, Leibach FH. Evidence for histidyl and carboxy groups at the active site of the human placental Na+-H+ exchanger. Biochem. J. 1987;245:473–477. doi: 10.1042/bj2450473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo FG, Aronson PS. Inactivation of the renal microvillus membrane Na+-H+ exchanger by histidine-specific reagents. J. Biol. Chem. 1986;261:1120–1125. [PubMed] [Google Scholar]
- Grube M, Kock K, Oswald S, Draber K, Meissner K, Eckel L, Bohm M, Felix SB, Vogelgesang S, Jedlitschky G, et al. Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin. Pharmacol. Ther. 2006;80:607–620. doi: 10.1016/j.clpt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- Gui C, Miao Y, Thompson L, Wahlgren B, Mock M, Stieger B, Hagenbuch B. Effect of pregnane X receptor ligands on transport mediated by human OATP1B1 and OATP1B3. Eur. J. Pharmacol. 2008;584:57–65. doi: 10.1016/j.ejphar.2008.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B. Drug uptake systems in liver and kidney: a historic perspective. Clin. Pharmacol. Ther. 2010;87:39–47. doi: 10.1038/clpt.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Gui C. Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica. 2008;38:778–801. doi: 10.1080/00498250801986951. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim. Biophys. Acta. 2003;1609:1–18. doi: 10.1016/s0005-2736(02)00633-8. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447:653–665. doi: 10.1007/s00424-003-1168-y. [DOI] [PubMed] [Google Scholar]
- Hakk H, Larsen G, Klasson-Wehler E. Tissue disposition, excretion and metabolism of 2,2′,4,4′,5-pentabromodiphenyl ether (BDE-99) in the male Sprague-Dawley rat. Xenobiotica. 2002;32:369–382. doi: 10.1080/00498250110119117. [DOI] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Visser TJ, Van Velzen MJ, Brouwer A, Bergman A. Biotransformation of brominated flame retardants into potentially endocrine-disrupting metabolites, with special attention to 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) Mol. Nutr. Food Res. 2008;52:284–298. doi: 10.1002/mnfr.200700104. [DOI] [PubMed] [Google Scholar]
- Hirano M, Maeda K, Shitara Y, Sugiyama Y. Contribution of OATP2 (OATP1B1) and OATP8 (OATP1B3) to the hepatic uptake of pitavastatin in humans. J. Pharmacol. Exp. Ther. 2004;311:139–146. doi: 10.1124/jpet.104.068056. [DOI] [PubMed] [Google Scholar]
- Hites RA. Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations. Environ. Sci. Technol. 2004;38:945–956. doi: 10.1021/es035082g. [DOI] [PubMed] [Google Scholar]
- Hsiang B, Zhu Y, Wang Z, Wu Y, Sasseville V, Yang WP, Kirchgessner TG. A novel human hepatic organic anion transporting polypeptide (OATP2). Identification of a liver-specific human organic anion transporting polypeptide and identification of rat and human hydroxymethylglutaryl-CoA reductase inhibitor transporters. J. Biol. Chem. 1999;274:37161–37168. doi: 10.1074/jbc.274.52.37161. [DOI] [PubMed] [Google Scholar]
- Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br. J. Pharmacol. 2009;158:693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Maegawa H, Okano T, Inui K, Hori R. Effect of various chemical modifiers on H+ coupled transport of cephradine via dipeptide carriers in rabbit intestinal brush-border membranes: role of histidine residues. J. Pharmacol. Exp. Ther. 1989;251:745–749. [PubMed] [Google Scholar]
- Kobayashi D, Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Involvement of human organic anion transporting polypeptide OATP-B (SLC21A9) in pH-dependent transport across intestinal apical membrane. J. Pharmacol. Exp. Ther. 2003;306:703–708. doi: 10.1124/jpet.103.051300. [DOI] [PubMed] [Google Scholar]
- Konig J, Cui Y, Nies AT, Keppler D. A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am. J. Physiol. Gastrointest. Liver Physiol. 2000a;278:G156–G164. doi: 10.1152/ajpgi.2000.278.1.G156. [DOI] [PubMed] [Google Scholar]
- Konig J, Cui Y, Nies AT, Keppler D. Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J. Biol. Chem. 2000b;275:23161–23168. doi: 10.1074/jbc.M001448200. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Ismair MG, Stieger B, Landmann L, Huber R, Pizzagalli F, Fattinger K, Meier PJ, Hagenbuch B. Organic anion-transporting polypeptide B (OATP-B) and its functional comparison with three other OATPs of human liver. Gastroenterology. 2001;120:525–533. doi: 10.1053/gast.2001.21176. [DOI] [PubMed] [Google Scholar]
- Leuthold S, Hagenbuch B, Mohebbi N, Wagner CA, Meier PJ, Stieger B. Mechanisms of pH-gradient driven transport mediated by organic anion polypeptide transporters. Am. J. Physiol. Cell Physiol. 2009;296 doi: 10.1152/ajpcell.00436.2008. C570-582. [DOI] [PubMed] [Google Scholar]
- Lorber M. Exposure of Americans to polybrominated diphenyl ethers. J. Expo. Sci. Environ. Epidemiol. 2008;18:2–19. doi: 10.1038/sj.jes.7500572. [DOI] [PubMed] [Google Scholar]
- Lupton SJ, McGarrigle BP, Olson JR, Wood TD, Aga DS. Human liver microsome-mediated metabolism of brominated diphenyl ethers 47, 99, and 153 and identification of their major metabolites. Chem. Res. Toxicol. 2009;22:1802–1809. doi: 10.1021/tx900215u. [DOI] [PubMed] [Google Scholar]
- Mazdai A, Dodder NG, Abernathy MP, Hites RA, Bigsby RM. Polybrominated diphenyl ethers in maternal and fetal blood samples. Environ. Health Perspect. 2003;111:1249–1252. doi: 10.1289/ehp.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts IA, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, van der Burg B, Brouwer A. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds. Environ. Health Perspect. 2001;109:399–407. doi: 10.1289/ehp.01109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts IA, van Zanden JJ, Luijks EA, van Leeuwen-Bol I, Marsh G, Jakobsson E, Bergman A, Brouwer A. Potent competitive interactions of some brominated flame retardants and related compounds with human transthyretin in vitro. Toxicol. Sci. 2000;56:95–104. doi: 10.1093/toxsci/56.1.95. [DOI] [PubMed] [Google Scholar]
- Meier-Abt F, Mokrab Y, Mizuguchi K. Organic anion transporting polypeptides of the OATP/SLCO superfamily: identification of new members in nonmammalian species, comparative modeling and a potential transport mode. J. Membr. Biol. 2005;208:213–227. doi: 10.1007/s00232-005-7004-x. [DOI] [PubMed] [Google Scholar]
- Meironyte Guvenius D, Bergman A, Noren K. Polybrominated diphenyl ethers in Swedish human liver and adipose tissue. Arch. Environ. Contam. Toxicol. 2001;40:564–570. doi: 10.1007/s002440010211. [DOI] [PubMed] [Google Scholar]
- Miles EW. Modification of histidyl residues in proteins by diethylpyrocarbonate. Methods Enzymol. 1977;47:431–442. doi: 10.1016/0076-6879(77)47043-5. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Imai K, Nezu J, Tsuji A, Tamai I. Functional characterization of pH-sensitive organic anion transporting polypeptide OATP-B in human. J. Pharmacol. Exp. Ther. 2004;308:438–445. doi: 10.1124/jpet.103.060194. [DOI] [PubMed] [Google Scholar]
- Nozawa T, Nakajima M, Tamai I, Noda K, Nezu J, Sai Y, Tsuji A, Yokoi T. Genetic polymorphisms of human organic anion transporters OATP-C (SLC21A6) and OATP-B (SLC21A9): allele frequencies in the Japanese population and functional analysis. J. Pharmacol. Exp. Ther. 2002;302:804–813. doi: 10.1124/jpet.302.2.804. [DOI] [PubMed] [Google Scholar]
- Palermo DP, DeGraaf ME, Marotti KR, Rehberg E, Post LE. Production of analytical quantities of recombinant proteins in Chinese hamster ovary cells using sodium butyrate to elevate gene expression. J. Biotechnol. 1991;19:35–47. doi: 10.1016/0168-1656(91)90073-5. [DOI] [PubMed] [Google Scholar]
- Petreas M, She J, Brown FR, Winkler J, Windham G, Rogers E, Zhao G, Bhatia R, Charles MJ. High body burdens of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) in California women. Environ. Health Perspect. 2003;111:1175–1179. doi: 10.1289/ehp.6220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Bigsby RM, Hites RA. Hydroxylated metabolites of polybrominated diphenyl ethers in human blood samples from the United States. Environ. Health Perspect. 2009;117:93–98. doi: 10.1289/ehp.11660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sai Y, Kaneko Y, Ito S, Mitsuoka K, Kato Y, Tamai I, Artursson P, Tsuji A. Predominant contribution of organic anion transporting polypeptide OATP-B (OATP2B1) to apical uptake of estrone-3-sulfate by human intestinal Caco-2 cells. Drug Metab. Dispos. 2006;34:1423–1431. doi: 10.1124/dmd.106.009530. [DOI] [PubMed] [Google Scholar]
- Sandanger TM, Sinotte M, Dumas P, Marchand M, Sandau CD, Pereg D, Berube S, Brisson J, Ayotte P. Plasma concentrations of selected organobromine compounds and polychlorinated biphenyls in postmenopausal women of Quebec, Canada. Environ. Health Perspect. 2007;115:1429–1434. doi: 10.1289/ehp.10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JM, Chen LJ, Lebetkin EH, Burka LT. Metabolism and disposition of 2,2′,4,4′- tetrabromodiphenyl ether following administration of single or multiple doses to rats and mice. Xenobiotica. 2006a;36:103–117. doi: 10.1080/00498250500485107. [DOI] [PubMed] [Google Scholar]
- Sanders JM, Lebetkin EH, Chen LJ, Burka LT. Disposition of 2,2′,4,4′,5,5′-hexabromodiphenyl ether (BDE153) and its interaction with other polybrominated diphenyl ethers (PBDEs) in rodents. Xenobiotica. 2006b;36:824–837. doi: 10.1080/00498250600815906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Johnson-Welch S, Tung KC, Harris TR, Papke O, Rosen R. Polybrominated diphenyl ether (PBDE) levels in livers of U.S. human fetuses and newborns. J. Toxicol. Environ. Health A. 2007;70:1–6. doi: 10.1080/15287390600748369. [DOI] [PubMed] [Google Scholar]
- Schecter A, Papke O, Harris TR, Tung KC, Musumba A, Olson J, Birnbaum L. Polybrominated diphenyl ether (PBDE) levels in an expanded market basket survey of U.S. food and estimated PBDE dietary intake by age and sex. Environ. Health Perspect. 2006;114:1515–1520. doi: 10.1289/ehp.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecter A, Papke O, Joseph JE, Tung KC. Polybrominated diphenyl ethers (PBDEs) in U.S. computers and domestic carpet vacuuming: possible sources of human exposure. J. Toxicol. Environ. Health A. 2005a;68:501–513. doi: 10.1080/15287390590909715. [DOI] [PubMed] [Google Scholar]
- Schecter A, Papke O, Tung KC, Joseph J, Harris TR, Dahlgren J. Polybrominated diphenyl ether flame retardants in the U.S. population: current levels, temporal trends, and comparison with dioxins, dibenzofurans, and polychlorinated biphenyls. J. Occup. Environ. Med. 2005b;47:199–211. doi: 10.1097/01.jom.0000158704.27536.d2. [DOI] [PubMed] [Google Scholar]
- Schecter A, Papke O, Tung KC, Staskal D, Birnbaum L. Polybrominated diphenyl ethers contamination of United States food. Environ. Sci. Technol. 2004;38:5306–5311. doi: 10.1021/es0490830. [DOI] [PubMed] [Google Scholar]
- Sjodin A, Wong LY, Jones RS, Park A, Zhang Y, Hodge C, Dipietro E, McClure C, Turner W, Needham LL, et al. Serum concentrations of polybrominated diphenyl ethers (PBDEs) and polybrominated biphenyl (PBB) in the United States population: 2003–2004. Environ. Sci. Technol. 2008;42:1377–1384. doi: 10.1021/es702451p. [DOI] [PubMed] [Google Scholar]
- Stapleton HM, Kelly SM, Pei R, Letcher RJ, Gunsch C. Metabolism of polybrominated diphenyl ethers (PBDEs) by human hepatocytes in vitro. Environ. Health Perspect. 2009;117:197–202. doi: 10.1289/ehp.11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staskal DF, Diliberto JJ, DeVito MJ, Birnbaum LS. Toxicokinetics of BDE 47 in female mice: effect of dose, route of exposure, and time. Toxicol. Sci. 2005;83:215–223. doi: 10.1093/toxsci/kfi018. [DOI] [PubMed] [Google Scholar]
- St-Pierre MV, Hagenbuch B, Ugele B, Meier PJ, Stallmach T. Characterization of an organic anion-transporting polypeptide (OATP-B) in human placenta. J. Clin. Endocrinol. Metab. 2002;87:1856–1863. doi: 10.1210/jcem.87.4.8431. [DOI] [PubMed] [Google Scholar]
- Tamai I, Nezu J, Uchino H, Sai Y, Oku A, Shimane M, Tsuji A. Molecular identification and characterization of novel members of the human organic anion transporter (OATP) family. Biochem. Biophys. Res. Commun. 2000;273:251–260. doi: 10.1006/bbrc.2000.2922. [DOI] [PubMed] [Google Scholar]
- Tamai I, Nozawa T, Koshida M, Nezu J, Sai Y, Tsuji A. Functional characterization of human organic anion transporting polypeptide B (OATP-B) in comparison with liver-specific OATP-C. Pharm. Res. 2001;18:1262–1269. doi: 10.1023/a:1013077609227. [DOI] [PubMed] [Google Scholar]
- Zhou T, Taylor MM, DeVito MJ, Crofton KM. Developmental exposure to brominated diphenyl ethers results in thyroid hormone disruption. Toxicol. Sci. 2002;66:105–116. doi: 10.1093/toxsci/66.1.105. [DOI] [PubMed] [Google Scholar]