Abstract
Background
Effective treatment for stress urinary incontinence (SUI) is lacking. This study investigates whether transplantation of adipose tissue-derived stem cells (ADSCs) can treat SUI in a rat model.
Methods
Rats were induced to develop SUI by postpartum vaginal balloon dilation and bilateral ovariectomy. ADSCs were isolated from the peri-ovary fat, examined for stem cell properties, and labeled with thymidine analog BrdU or EdU. Ten rats received urethral injection of saline as control. Twelve rats received urethral injection of EdU-labeled ADSCs and 6 rats received intravenous injection of BrdU-labeled ADSCs through tail vein. Four weeks later, urinary voiding function was assessed by 4-channel conscious cystometry. The rats were then sacrificed and their urethras harvested for tracking of ADSCs and for quantification of elastin, collagen, and smooth muscle contents.
Results
Cystometric analysis showed that 8 out 10 rats in the control group had abnormal voiding whereas 4 of 12 (33.3%) and 2 of 6 (33.3%) rats in the Urethra-ADSC group and the Tail Vein-ADSC group, respectively, had abnormal voiding. Histological analysis showed that the ADSC-treated groups had significantly higher elastin content than the control group, and, within the ADSC-treated groups, rats with normal voiding pattern also had significantly higher elastin content than rats with voiding dysfunction. ADSC-treated, normal voiding rats had significantly higher smooth muscle content than control or ADSC-treated rats with voiding dysfunction.
Discussion
Transplantation of ADSCs via urethral or intravenous injection was effective in the treatment and/or prevention of SUI in a preclinical setting.
Keywords: Adipose tissue-derived stem cells, urethra, stress urinary incontinence, conscious cystometry, transplantation
Introduction
Urinary incontinence (UI) afflicts more than 200 million people worldwide [1] and costs nearly 20 billion dollars in the United States alone in 2000 [2]. UI is 2 to 3 times more prevalent in women than in men up to age 80, after which it affects the sexes equally [3]. In a recent National Health and Nutrition Examination Survey of 4,229 women older than 20 years, 49.6% of these women reported UI symptoms. Of those reporting UI symptoms, 49.8% reported stress urinary incontinence (SUI), 15.9% urge urinary incontinence (UUI), and 34.3% mixed urinary incontinence [4].
Current therapies for SUI do not treat the pathophysiologic causes and often involve the introduction of foreign materials [5, 6]. As such, new treatment modalities have been sought after, and in the past 8 years, one such treatment modality has progressed from animal models to clinical trials. It involves the transurethral or periurethral injection of autologous skeletal muscle-derived stem cells (MDSCs). A 1-year follow-up found improvement in five of eight women, with one achieving total continence while none having serious adverse events [7]. However, due to the limited size of the muscle biopsy and the scarcity of MDSCs, a period of 3 to 7 weeks of culturing is required to expand the primarily isolated cells to a sufficient number for treatment [8]. While cell culturing itself often requires the use of reagents that may not meet the stringent standards for human therapy, the long period of culturing is undoubtedly a large window of opportunity for microbial contamination, cell type alteration, and human errors. As such, a more abundant stem cell source should be explored - preferably one that yields sufficient amount of cells with minimal or no requirement for culturing at all.
Since first reported in 2000, adipose tissue-derived stem cells (ADSCs) have been consistently shown to possess differentiation potency at the pluripotent or multipotent level. Importantly, both skeletal and smooth muscle cells, which constitute the contractile structure of the urethral sphincter, have been shown to be among the wide array of cell types into which ADSCs are capable of differentiating [9–11]. In addition, the clinical applicability of ADSCs has been demonstrated in several clinical trials, including cranial repair [12], prevention of graft-versus-host disease [13], plastic reconstruction [14], and treatment of perianal fistula [15]. Unlike muscle biopsies, which can only be obtained in the gram range, the adipose tissue can be harvested by the hundreds of grams and is an abundant source of multipotent stem cells [16]. In fact, due to their abundance and ease of isolation, ADSCs can be isolated and injected back into the same patients for breast augmentation in approximately 4 hours [14]. Considering that the urethral sphincter is much smaller than the breasts, it is reasonable to expect that sufficient numbers of ADSCs can be isolated from most patients for SUI treatment without the need for cell culturing. If so, it is also reasonable to expect that the entire treatment procedure can be completed in a single patient visit.
As demonstrated in the MDSC clinical trial [7], urethral injection is an obvious choice for the delivery of stem cells to SUI patients. However, intravenous injection is easier to perform and has been shown to be a valid method for the delivery of stem cells including ADSCs [17]. As such, in the present study we tested both urethral and intravenous injection of ADSCs in a SUI rat model that we have previously established [18–21]. The results suggest that the two delivery methods may be equally effective in treating SUI.
Materials and methods
Animals and overview
All experimental protocols were approved by the Institutional Animal Care and Use Committee at our institution. Twenty-eight 2-month-old primiparous Sprague-Dawley rats at gestational day 16 were obtained from Charles River Laboratories (Wilmington, MA). They were subjected to development of voiding dysfunction by a previously described procedure [18–21]. Immediately after delivery, the rats underwent vaginal balloon dilation to simulate prolonged labor. One week later, the rats were anesthetized, a midline incision made in the abdomen, and both ovaries were excised. Fat pads of the excised ovaries were collected for ADSC isolation. One week later, the rats were subjected to ADSC transplantation. Four more weeks later, the rats’ urinary function was assessed and their urethras harvested.
Flow cytometry
ADSCs were isolated and cultured as previously described [10, 22]. Analysis of ADSCs by flow cytometry has also been described [22]. Briefly, cells were incubated with primary antibody in 50 µl wash buffer (PBS containing 1% FBS and 0.1% Na3N) for 30 min on ice, followed by incubation with FITC-conjugated secondary antibody. The cells were then rinsed twice with wash buffer, fixed with 1% paraformaldehyde in PBS, and analyzed by a fluorescence-activated cell sorter (FACSVantage SE System, BD Biosciences, San Jose, CA). The raw data were further analyzed with FlowJo software (Tree Star, Inc., Ashland, OR).
Transplantation of ADSCs
ADSCs were labeled with 5 µM of 5-bromo-2-deoxyuridine (BrdU, Sigma-Aldrich) or 10 µM of 5-ethynyl-2-deoxyuridine (EdU, Invitrogen, Carlsbad, CA) for 12 h prior to transplantation. Rats that were balloon-injured and ovariectomized were randomized into a Control group (n=10), a Urethra-ADSC group (n=12), and a Tail Vein-ADSC group (n=6). The Control group received injection of 400 µl of PBS in the urethra in the 5 and 7 o’clock positions at the bladder-urethra junction. The Urethra-ADSC group received injection of 1×106 EdU-labeled ADSCs (in 400 µl of PBS) in the urethra. The Tail Vein-ADSC group received injection of 1×106 BrdU-labeled ADSCs (in 400 µl of PBS) in the tail vein.
Conscious cystometry
Twenty-four h before testing, the rat was anesthetized with isoflurane. A polyethylene-90 tube was inserted into the bladder dome and secured with a purse-string suture. A second polyethylene-90 tube attached to a latex balloon was placed in the intraabdominal space to measure abdominal pressure so as to permit calculation of true vesical pressure via the cystotomy tube. Both tubes were passed through the abdominal wall muscle and tunneled subcutaneously to emerge at the dorsum of the neck. Cystometry was initiated with the rat conscious but restrained in a tunnel attached to a metabolic cage grid. The bladder was filled via the cystotomy tube at a rate of 0.1 ml/min using an infusion pump. Intravesical pressure changes were recorded by a computer with LabView 6.0 software (National Instruments, Austin, TX) at a rate of 10 samples/sec. The voided urine was recorded by an electronic scale connected to the LabView software. After stabilization of the micturition cycle for 10 min, the bladder was emptied by aspiration and micturition cycles were recorded for 40 min. Multiple cystometric variables were recorded by obtaining mean values of 4 voiding cycles. The voiding function was classified as “abnormal” if bladder filling was accompanied by frequent, low-pressure bladder contractions with urine leakage. Upon completion of cystometry the animals were euthanized by intraperitoneal injection of sodium pentobarbital and bilateral thoracotomy.
Immunofluorescence and tracking of EdU-labeled ADSCs
Cryosections of urethral tissue samples were prepared as previously described [20]. Immunofluorescence was performed as previously described [22] using anti-α-smooth muscle actin (α-SMA) antibody (Abcam Inc., Cambridge, MA). After washing with PBS, the slides were incubated with Click-iT reaction cocktail (Invitrogen), which contained Alexa-fluor 594, for 30 min followed by staining with 4',6-diamidino-2-phenylindole (DAPI, for nuclear staining, 1 µg/ml, Sigma-Aldrich). For image analysis, five randomly selected fields per tissue per animal for each treatment group were photographed and recorded using a Retiga Q Image digital still camera and ACT-1 software (Nikon Instruments Inc., Melville, NY).
Immunohistochemistry and tracking of BrdU-labeled ADSCs
For BrdU staining, urethral tissue sections were treated with 10% HCl for 30 min at 4°C followed by hydrogen peroxide/methanol to quench endogenous peroxidase activity. For other staining, the HCl step was omitted. Immunohistochemistry was then performed as previously described [22] using anti-BrdU (Santa Cruz Biotechnology, Santa Cruz, CA), anti-SDF-1α (Abcam), or anti-α-SMA antibody. Image analysis was done as above and quantified using Image-Pro Plus image software (Media Cybernetics, Silver Spring, MD).
Masson’s trichrome stain
Urethral tissue sections were prepared as above, immersed in warm (58 °C) Bouin solution for 15 min, rinsed, stained with Weigert Hematoxylin for 10 min, and then rinsed until only nuclei remained stained. The sections were then stained with Biebrich Scarlet - Acid Fuchsin for 3 min, rinsed, and immersed in phosphomolybdic acid for 45 min. Next, the sections was stained with Aniline Blue for 3 min, rinsed in distilled water for 2 min, immersed in 1% acetic acid for 2 min, and rinsed in distilled water for 2 min twice. Finally, the sections were dehydrated through increasing concentrations of ethanol, left to air dry, and mounted. To prevent variations in staining, all samples were stained simultaneously using this procedure. Image analysis was done as above and quantified using Image-Pro Plus image software.
Hart’s elastin stain
Urethral tissue sections were prepared as above, immersed in 0.25% potassium permanganate solution for 5 min, cleared in 5% oxalic acid, and soaked in resorcin-fuchsin solution overnight. After being washed in water, sections were counterstained with Van Gieson solution for 1 min, and then dehydrated in ethanol, cleared in Histo-Clear and mounted with Histomount. To prevent variations in staining, all samples were stained simultaneously using this procedure. Image analysis was done as above and quantified using Image-Pro Plus image software.
Statistical analysis
Data were analyzed with Prism 4 (GraphPad Software, Inc., San Diego, CA). T-test was used to determine significance of difference (P < 0.05) between rats with normal and abnormal voiding patterns. One-way ANOVA was used to determine significance of difference (P < 0.05) between Control (PBS-treated) and ADSC-treated rats.
Results
Pre-transplantation characterization of ADSCs
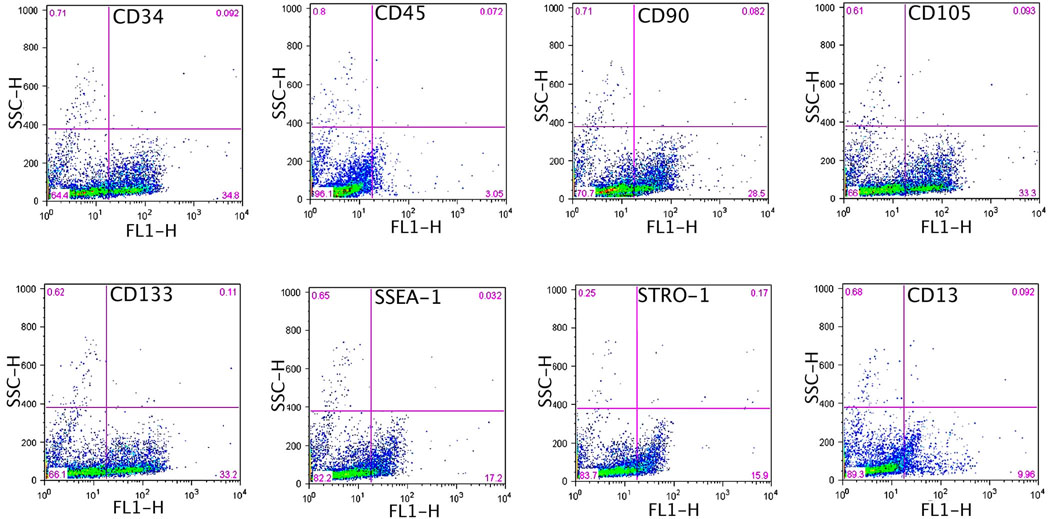
Freshly isolated ADSCs from peri-ovary fat pads were of insufficient number and thus required expansion through one passage (P1) of culturing. To ensure consistency, a small portion of each P1 cell preparation was examined by flow cytometry. The results indicated the expression of cell surface antigens in a pattern (Fig. 1) similar to most previous studies [16, 23, 24]. In addition, one cell preparation each for the intra-urethra and for the tail vein injection was examined for the ability to differentiate into endothelial, smooth muscle, and neuron-like cells. The results (not shown) were similar to our previous reports [10, 25].
Fig. 1. Cytometric analysis of rat ADSCs.
ADSCs were isolated from peri-ovary fat pads, allowed to attach to plastic dishes, expanded through one passage, and then harvested for flow cytometric analysis for the indicated markers. Percent of positive cells is shown in the right lower corner of each plot.
Pre-transplantation characterization of rat model
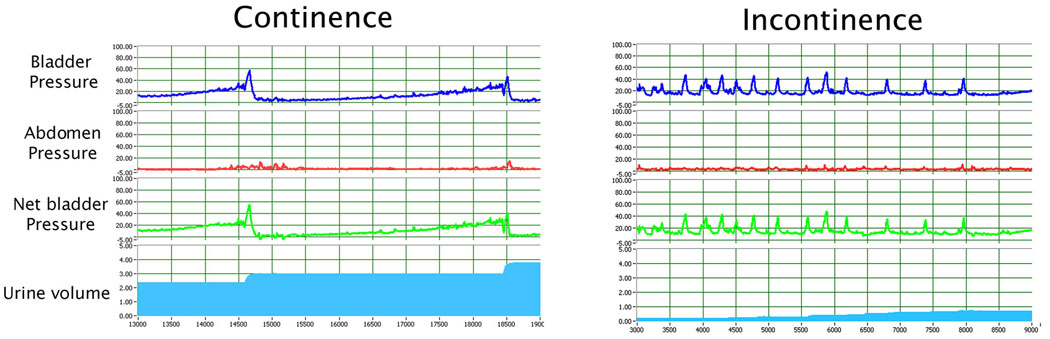
Sham-operated rats had a voiding frequency of 2 to 3 times per 10 min while the balloon-injured, ovariectomized rats had urine leakage continuously. During each voiding episode, the net baseline bladder pressure of sham-operated rats increased gradually from 0 to approximately 20 cmH2O before peaking at between 40 and 60 cmH2O and a discrete amount of around 0.5 ml of voided saline was recorded with each bladder contraction. On the other hand, balloon-injured, ovariectomized rats had bladder pressures increased to 20 cmH2O with random small peaks of around 40 cmH2O, and urine (saline) leaked continuously without a discrete voiding. Representative cystometric graphs of rats displaying such normal and abnormal voiding patterns are shown in Fig. 2.
Fig. 2. Determination of urinary continence by 4-channel conscious cystometric analysis.
(A) Normal micturition patterns were defined as pressure increases resulting in voiding with a frequency of ≤ 4 times in 10 min. (B) Abnormal voiding patterns were defined as continuous or intermittent urine leakage at low bladder pressure during the filling phase.
Post-transplantation characterization of rat model
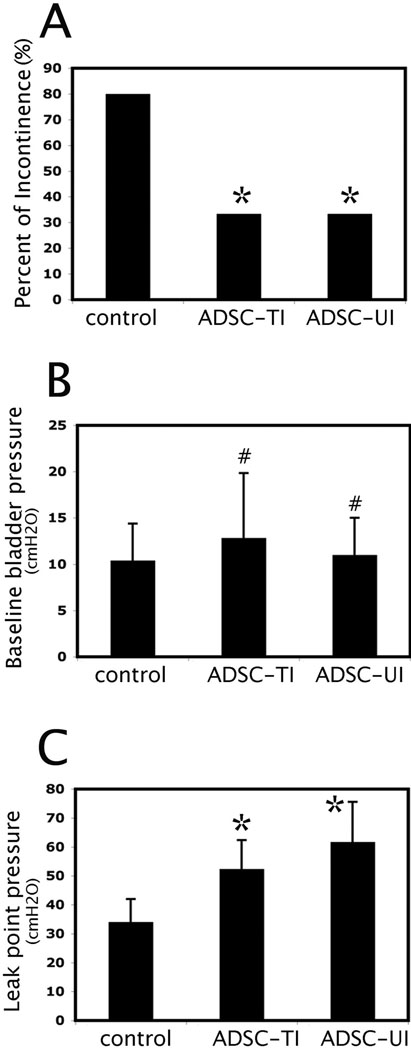
Twenty-eight rats that were subjected to vaginal balloon dilation and ovariectomy were randomized into 3 groups. The Control group (n=10) received injection of PBS in the urethra; the Urethra-ADSC group (n=12) received injection of ADSCs in the urethra; the Tail Vein-ADSC group (n=6) received injection of ADSCs through the tail vein. Four weeks later, all rats were examined for general appearance and behavior, which showed no noticeable differences among the groups. Body weight measurement also showed no statistical difference. The rats were then examined by 4-channel conscious cystometry. The results showed that 8 out 10 rats in the Control group had abnormal voiding function whereas 4 of 12 (33.3%) and 2 of 6 (33.3%) rats in the Urethra-ADSC group and the Tail Vein-ADSC group respectively had abnormal voiding function (Fig. 3A). Specifically, while there were no statistical differences in the baseline bladder pressure among the three groups (Fig. 3B), significant differences were identified in the Leak Point Pressure between the ADSC-treated groups and the Control group (Fig. 3C), suggesting improved sphincter function with ADSC treatment.
Fig. 3. Cystometric analysis results.
“Control” indicates rats treated with PBS. “ADSC-TI” and “ADSC-UI” are rats injected with ADSCs in the tail vein and urethra, respectively. (A) Comparison of percent of rats with abnormal voiding patterns. * indicates significant difference when compared to control (P<0.05). (B) Comparison of baseline bladder pressure. # indicates insignificant difference when compared to control (P>0.05). (C) Comparison of leak point pressure. * indicates significant difference when compared to control (P<0.05).
Post-transplantation tracking of ADSCs
After the completion of functional studies, the urethra was harvested from each rat for histological studies. In the Tail Vein-ADSC group, the BrdU-labeled cells were detectable in the connective tissue (Fig. 4A), demonstrating their survival for at least 4 weeks post-transplantation and suggesting the existence of a homing mechanism through which ADSCs migrated to the injured urethra. To test this possibility we examined the expression of two well-known homing factors SDF-1 and MCP-3 [26, 27]. While repeated efforts failed to obtain interpretable data for MCP-3 due to high levels of nonspecific staining, expression of SDF-1 was clearly identified in the urethras of rats with SUI (Fig. 4B). In the Urethra-ADSC group, the EdU-labeled cells were detected in the connective tissue along and underneath urothelium (Fig. 4C). A few EdU-positive nuclei appeared to localize within cells that stained positive for α-SMA (Fig. 4D), suggestive of rare but possible smooth muscle differentiation.
Fig. 4. Tracking of transplanted ADSCs and identification of SDF-1 expression in the urethra.
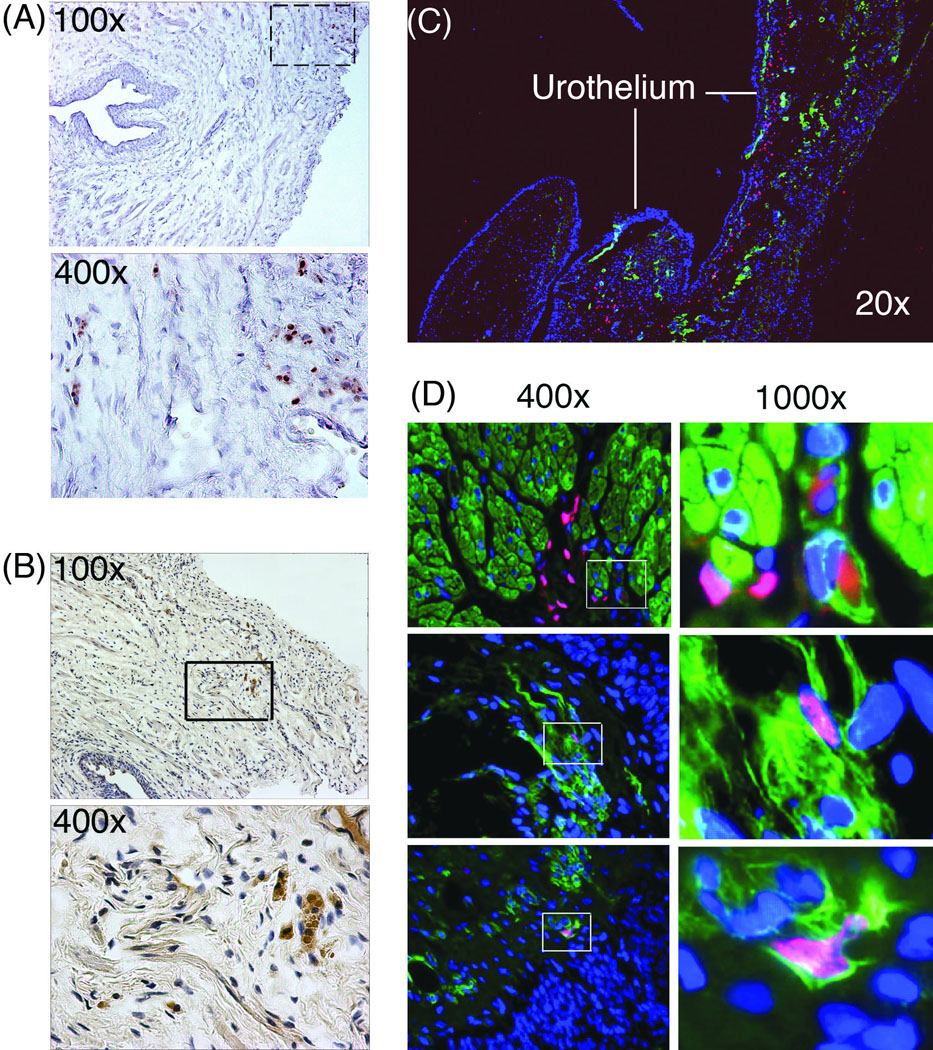
(A) ADSCs were labeled with BrdU and autologously injected into the tail vein. Four weeks later, the urethra was harvested and stained for BrdU (Brown). Boxed area in the top photograph (×100) is enlarged and shown in the bottom photograph (×400). (B) The same urethra tissue samples were also stained for SDF-1 (Brown). Boxed area in the top photograph (×100) is enlarged and shown in the bottom photograph (×400). (C) ADSCs were labeled with EdU and autologously injected into the bladder neck, which was then harvested at 4w and stained with Alexa-594 (red fluorescence), DAPI (blue fluorescence), and anti-α-SMA antibody (green fluorescence). The stained images were digitally merged. (D) ADSCs were labeled and transplanted as in panel C. Boxed areas in the 400× graphs are shown in the corresponding 1000× graphs.
Post-transplantation assessment of urethral histology
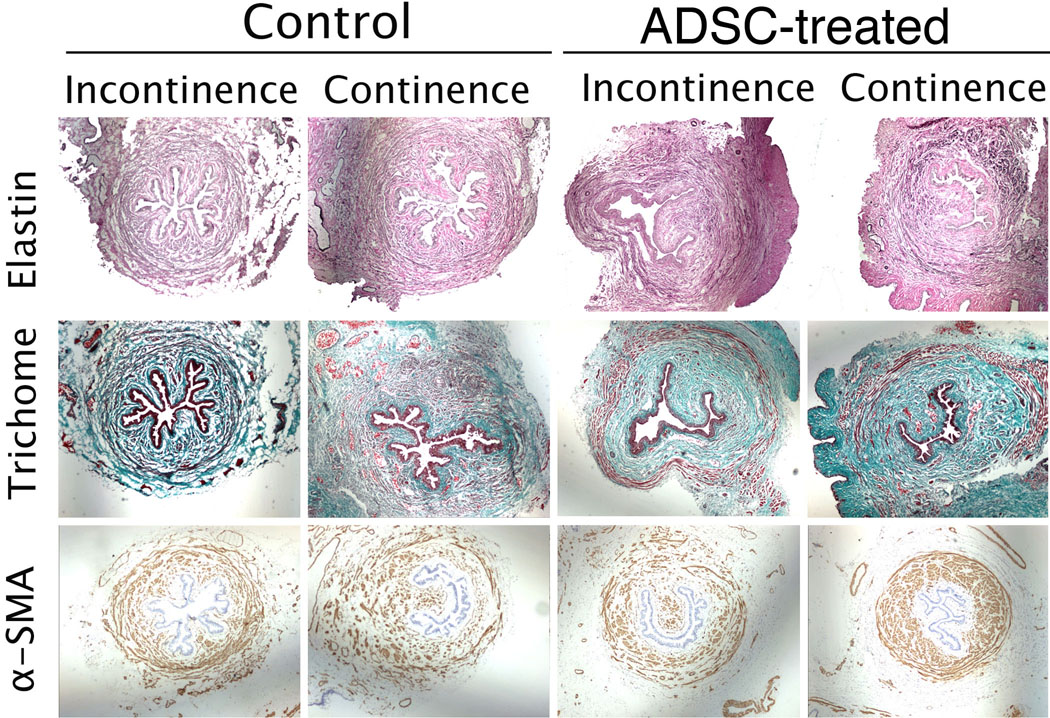
The urethras of all 28 rats were examined for elastin and smooth muscle content as well as muscle/collagen ratio. Because both the Control and ADSC-treated groups had rats with normal and abnormal voiding patterns as indicated by cystometry (see above), the histological data were categorized according to the animal’s treatment as well as their voiding status (Fig. 5 and Table 1). Tissues from the ADSC-treated groups had significantly higher elastin content than similar tissues from the Control group. Within the ADSC-treated groups, the normal voiding rats also had significantly higher elastin content than the abnormal voiding rats. However, there was no statistical difference in elastin content between normal voiding and abnormal voiding rats within the Control group. Normal voiding rats from the ADSC-treated group had significantly higher smooth muscle content than the Control group or ADSC-treated abnormal voiding rats. Within the Control group, there was no statistical difference in smooth muscle content between normal and abnormal voiding rats. These smooth muscle statistics largely repeated themselves in the muscle/collagen ratio analysis, as there were no statistical differences in the collagen content between treatment groups or between normal and abnormal voiding rats.
Fig. 5. Assessment of muscle, collagen, and elastin contents in the urethra.
“Control” indicates rats treated with PBS. “ADSC-treated” indicates rats injected with ADSCs in the tail vein or urethra. “Normal” and “Abnormal” indicate micturition patterns identified by cystometric analysis. The urethras of all 28 rats were stained for elastin and α-SMA. They were also stained by the trichrome method to determine the muscle/collagen ratio (Table 1). Representative photographs shown here were originally taken at 20× magnification.
Table 1.
Comparison of elastin, smooth muscle, and collagen content in the urethra
| Control | ADSC-treated | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| Normal | Abnormal | P | Normal | Abnormal | P | P | |
| Elastin content | 13352±587 | 12759±942 | 0.4314 | 29198±2947 | 19983±5999 | 0.0044 | <0.001 |
| α-SMA content | 72798±5417 | 70210±24391 | 0.8898 | 101829±39612 | 43302±3200 | 0.0164 | 0.026 |
| Muscle/Collagen (%) | 24.4±8.97 | 21.99±0.89 | 0.3797 | 39.48±6.21 | 17.2±5.01 | 0.0001 | <0.001 |
Content is the average number of pixels SD of areas positively stained for elastin or α-SMA. Muscle/Collagen ratio was determined from Trichrome-stained tissues.
Discussion
While numerous studies have demonstrated the potential of ADSCs for the treatment of various diseases, the present study appears to be the first to investigate the feasibility of ADSCs for the treatment of SUI. In this effort, we first examined the general properties of ADSCs and the results showed that ADSCs isolated from rats with voiding dysfunction had similar cell surface expression profile and differentiation potential as those from normal rats. However, it should be pointed out that, as an effort to minimize injury and discomfort to the animals, ADSCs used in this study were isolated from the fat pads surrounding the ovaries that were excised for the purpose of simulating menopause. This tissue source of course is different from our envisioned clinical setting, in which subcutaneous fat will be the most likely tissue source for ADSCs. Additionally, we believe that in most clinical situations, the subcutaneous fat will be much more abundant than the peri-ovary fat and therefore ex vivo expansion of the primarily isolated ADSCs will not be necessary.
We first reported the prototype of our current SUI rat model in 1998 [28] and have since used it in several studies with increasing success rates of creating the abnormal voiding condition [18–21, 29, 30]. More importantly, we have changed the cystometric analysis from an anesthetized to a conscious module, thus producing data that are physiologically more relevant [21]. In the present study we further refined the cystometry from single channel to 4-channel. Based on this improved cystometric analysis, we identified 80% of the control rats had voiding dysfunction whereas only 33% of the ADSC-treated rats had voiding dysfunction 4 weeks after PBS and ADSC injection, respectively. Histological analysis showed that the ADSC-treated rats had significantly higher elastin and smooth muscle content than the PBS-treated (control) rats. Furthermore, within the ADSC-treated groups, normal voiding rats also had significantly higher elastin and smooth muscle contents than abnormal voiding rats. On the other hand, within the control group, the small number of normal voiding rats (n=2) did not allow the derivation of valid statistics in regard to whether a correlation existed in elastin (or smooth muscle) content and voiding function.
While the cystometric and histological data suggest the therapeutic potential of ADSCs, it remains unknown how the transplanted ADSCs improved urethral function. In the Tail Vein-ADSC group the transplanted cells migrated to the injured urethra possibly on cue of homing factors such as SDF-1, as ADSCs have been shown to express the SDF-1 receptor CXCR4 and migrate to SDF-1 in cell migration assays [31]. Thus, we consider the migration of ADSCs toward the injured urethra as one of the steps through which voiding dysfunction was mitigated. Once in the urethra, the tail vein-injected ADSCs were likely able to perform functions similar to ADSCs transplanted directly in the urethra. As detected by EdU-staining, the urethrally injected ADSCs were largely localized in the submucosa (Fig. 4C) and a few of the EdU-positive nuclei appeared to reside within cells expressing α-SMA (Fig. 4D). Thus, it appears that a small fraction of the transplanted ADSCs might have differentiated into smooth muscle cells. However, since the great majority of the transplanted ADSCs appeared to remain undifferentiated, their therapeutic effects were likely mediated by trophic factors that promote host tissue regeneration. This trophic mechanism has been proposed for ADSCs [32] and has been discussed in reviews of mesenchymal stem cells in general [33, 34]. Thus, we consider that urethral or intravenous injection of ADSCs can mitigate SUI by a trophic factor mechanism that modifies both cellular and extracellular elements of the urethra.
Acknowledgements
This work was supported by grants from the Arthur Rock Foundation and the National Institutes of Health (P50-DK64538).
Footnotes
Disclosure of interest: None.
References
- 1.Norton P, Brubaker L. Urinary incontinence in women. Lancet. 2006;367:57–67. doi: 10.1016/S0140-6736(06)67925-7. [DOI] [PubMed] [Google Scholar]
- 2.Hu TW, Wagner TH, Bentkover JD, Leblanc K, Zhou SZ, Hunt T. Costs of urinary incontinence and overactive bladder in the United States: a comparative study. Urology. 2004;63:461–465. doi: 10.1016/j.urology.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 3.Gibbs CF, Johnson TM, 2nd, Ouslander JG. Office management of geriatric urinary incontinence. Am J Med. 2007;120:211–220. doi: 10.1016/j.amjmed.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 4.Dooley Y, Kenton K, Cao G, Luke A, Durazo-Arvizu R, Kramer H, et al. Urinary Incontinence Prevalence: Results From the National Health and Nutrition Examination Survey. J Urol. 2007 doi: 10.1016/j.juro.2007.09.081. [DOI] [PubMed] [Google Scholar]
- 5.Albo ME, Richter HE, Brubaker L, Norton P, Kraus SR, Zimmern PE, et al. Burch colposuspension versus fascial sling to reduce urinary stress incontinence. N Engl J Med. 2007;356:2143–2155. doi: 10.1056/NEJMoa070416. [DOI] [PubMed] [Google Scholar]
- 6.Deng DY, Rutman M, Raz S, Rodriguez LV. Presentation and management of major complications of midurethral slings: Are complications under-reported? Neurourol Urodyn. 2007;26:46–52. doi: 10.1002/nau.20357. [DOI] [PubMed] [Google Scholar]
- 7.Carr LK, Steele D, Steele S, Wagner D, Pruchnic R, Jankowski R, et al. 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2008 doi: 10.1007/s00192-007-0553-z. [DOI] [PubMed] [Google Scholar]
- 8.Furuta A, Carr LK, Yoshimura N, Chancellor MB. Advances in the understanding of sress urinary incontinence and the promise of stem-cell therapy. Rev Urol. 2007;9:106–112. [PMC free article] [PubMed] [Google Scholar]
- 9.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 10.Ning H, Lin G, Lue TF, Lin CS. Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation. 2006;74:510–518. doi: 10.1111/j.1432-0436.2006.00081.x. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci U S A. 2006;103:12167–12172. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lendeckel S, Jodicke A, Christophis P, Heidinger K, Wolff J, Fraser JK, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004;32:370–373. doi: 10.1016/j.jcms.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 13.Fang B, Song YP, Liao LM, Han Q, Zhao RC. Treatment of severe therapy-resistant acute graft-versus-host disease with human adipose tissue-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;38:389–390. doi: 10.1038/sj.bmt.1705457. [DOI] [PubMed] [Google Scholar]
- 14.Yoshimura K, Sato K, Aoi N, Kurita M, Hirohi T, Harii K. Cell-Assisted Lipotransfer for Cosmetic Breast Augmentation: Supportive Use of Adipose-Derived Stem/Stromal Cells. Aesthetic Plast Surg. 2007 doi: 10.1007/s00266-020-01819-7. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79–86. doi: 10.1007/DCR.0b013e3181973487. [DOI] [PubMed] [Google Scholar]
- 16.Strem BM, Hicok KC, Zhu M, Wulur I, Alfonso Z, Schreiber RE, et al. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–141. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 17.Kim YM, Choi YS, Choi JW, Park YH, Koo BS, Roh HJ, et al. Effects of systemic transplantation of adipose tissue-derived stem cells on olfactory epithelium regeneration. Laryngoscope. 2009 doi: 10.1002/lary.20187. [DOI] [PubMed] [Google Scholar]
- 18.Sievert KD, Bakircioglu ME, Tsai T, Dahms SE, Nunes L, Lue TF. The effect of simulated birth trauma and/or ovariectomy on rodent continence mechanism. Part I: functional and structural change. J Urol. 2001;166:311–317. [PubMed] [Google Scholar]
- 19.Resplande J, Gholami SS, Graziottin TM, Rogers R, Lin CS, Leng W, et al. Long-term effect of ovariectomy and simulated birth trauma on the lower urinary tract of female rats. J Urol. 2002;168:323–330. [PubMed] [Google Scholar]
- 20.Hayashi N, Bella AJ, Wang G, Lin G, Deng DY, Nunes L, et al. Effect of extended-term estrogen on voiding in a postpartum ovariectomized rat model. Can Urol Assoc J. 2007;1:256–263. doi: 10.5489/cuaj.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tantiwongse K, Fandel TM, Wang G, Breyer BN, Walsh TJ, Bella AJ, et al. The potential of hormones and selective oestrogen receptor modulators in preventing voiding dysfunction in rats. BJU Int. 2008;102:242–246. doi: 10.1111/j.1464-410X.2008.07582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, et al. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaffler A, Buchler C. Concise review: adipose tissue-derived stromal cells--basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 25.Ning H, Liu G, Lin G, Yang R, Lue TF, Lin CS. Fibroblast growth factor 2 promotes endothelial differentiation of adipose tissue-derived stem cells. J Sex Med. 2009;6:967–979. doi: 10.1111/j.1743-6109.2008.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 27.Schenk S, Mal N, Finan A, Zhang M, Kiedrowski M, Popovic Z, et al. Monocyte chemotactic protein-3 is a myocardial mesenchymal stem cell homing factor. Stem Cells. 2007;25:245–251. doi: 10.1634/stemcells.2006-0293. [DOI] [PubMed] [Google Scholar]
- 28.Lin AS, Carrier S, Morgan DM, Lue TF. Effect of simulated birth trauma on the urinary continence mechanism in the rat. Urology. 1998;52:143–151. doi: 10.1016/s0090-4295(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 29.Banie L, Lin G, Ning H, Wang G, Lue TF, Lin CS. Effects of estrogen, raloxifene and levormeloxifene on alpha1A-adrenergic receptor expression. J Urol. 2008;180:2241–2246. doi: 10.1016/j.juro.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 30.Lin G, Shindel AW, Banie L, Deng D, Wang G, Hayashi N, et al. Molecular Mechanisms Related to Parturition-Induced Stress Urinary Incontinence. Eur Urol. 2008;55:1213–1223. doi: 10.1016/j.eururo.2008.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JH, Lee MR, Kim JH, Jee MK, Kang SK. IFATS collection: Selenium induces improvement of stem cell behaviors in human adipose-tissue stromal cells via SAPK/JNK and stemness acting signals. Stem Cells. 2008;26:2724–2734. doi: 10.1634/stemcells.2008-0184. [DOI] [PubMed] [Google Scholar]
- 32.Sadat S, Gehmert S, Song YH, Yen Y, Bai X, Gaiser S, et al. The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochem Biophys Res Commun. 2007;363:674–679. doi: 10.1016/j.bbrc.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Castro J, Trigueros C, Madrenas J, Perez-Simon JA, Rodriguez R, Menendez P. Mesenchymal stem cells and their use as cell replacement therapy and disease modelling tool. J Cell Mol Med. 2008;12:2552–2565. doi: 10.1111/j.1582-4934.2008.00516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caplan AI. Why are MSCs therapeutic? New data: new insight. J Pathol. 2009;217:318–324. doi: 10.1002/path.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]