Abstract
Using previously isolated Bacillus brevis strains that secrete large amounts of proteins but little protease into the medium, we have developed a host-vector system for very efficient synthesis and secretion of heterologous proteins. The multiple promoters and the signal-peptide-coding region of the MWP gene, a structural gene for one of the major cell wall proteins of B. brevis strain 47, were used to construct expression-secretion vectors. With this system, a synthetic gene for human epidermal growth factor (hEGF) was expressed efficiently and a large amount (0.24 g per liter of culture) of mature hEGF was secreted into the medium. hEGF purified from the culture supernatant had the same NH2-terminal amino acid sequence, COOH-terminal amino acid, and amino acid composition as natural hEGF, and it was fully active in biological assays. These results, in combination with previous results, showed that mammalian proteins can be produced in active form 10-100 times more efficiently in B. brevis than has been reported in other systems.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Yamagata H., Tsukagoshi N., Udaka S. Multiple and tandemly arranged promoters of the cell wall protein gene operon in Bacillus brevis 47. J Bacteriol. 1989 Feb;171(2):1010–1016. doi: 10.1128/jb.171.2.1010-1016.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brake A. J., Merryweather J. P., Coit D. G., Heberlein U. A., Masiarz F. R., Mullenbach G. T., Urdea M. S., Valenzuela P., Barr P. J. Alpha-factor-directed synthesis and secretion of mature foreign proteins in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4642–4646. doi: 10.1073/pnas.81.15.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buell G., Schulz M. F., Selzer G., Chollet A., Movva N. R., Semon D., Escanez S., Kawashima E. Optimizing the expression in E. coli of a synthetic gene encoding somatomedin-C (IGF-I). Nucleic Acids Res. 1985 Mar 25;13(6):1923–1938. doi: 10.1093/nar/13.6.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Devos R., Plaetinck G., Cheroutre H., Simons G., Degrave W., Tavernier J., Remaut E., Fiers W. Molecular cloning of human interleukin 2 cDNA and its expression in E. coli. Nucleic Acids Res. 1983 Jul 11;11(13):4307–4323. doi: 10.1093/nar/11.13.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Baldridge J. S., McKeown K. S., Heyneker H. L., Chang C. N. Periplasmic production of correctly processed human growth hormone in Escherichia coli: natural and bacterial signal sequences are interchangeable. Gene. 1985;39(2-3):247–254. doi: 10.1016/0378-1119(85)90319-1. [DOI] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa T., Toibana A., Marumoto R., Nakahama K., Kikuchi M., Fujimoto K., Ikehara M. Expression of human lysozyme in an insoluble form in yeast. Gene. 1987;56(1):53–59. doi: 10.1016/0378-1119(87)90157-0. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi S., Weisblum B. Nucleotide sequence and functional map of pE194, a plasmid that specifies inducible resistance to macrolide, lincosamide, and streptogramin type B antibodies. J Bacteriol. 1982 May;150(2):804–814. doi: 10.1128/jb.150.2.804-814.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara M., Ohtsuka E., Tokunaga T., Taniyama Y., Iwai S., Kitano K., Miyamoto S., Ohgi T., Sakuragawa Y., Fujiyama K. Synthesis of a gene for human growth hormone and its expression in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5956–5960. doi: 10.1073/pnas.81.19.5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamata N., Chida K., Rikimaru K., Horikoshi M., Enomoto S., Kuroki T. Growth-inhibitory effects of epidermal growth factor and overexpression of its receptors on human squamous cell carcinomas in culture. Cancer Res. 1986 Apr;46(4 Pt 1):1648–1653. [PubMed] [Google Scholar]
- Kawamura F., Doi R. H. Construction of a Bacillus subtilis double mutant deficient in extracellular alkaline and neutral proteases. J Bacteriol. 1984 Oct;160(1):442–444. doi: 10.1128/jb.160.1.442-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Sato T., Emi M., Miyanohara A., Nishide T., Matsubara K. Expression of human salivary alpha-amylase gene in Saccharomyces cerevisiae and its secretion using the mammalian signal sequence. Gene. 1986;50(1-3):239–245. doi: 10.1016/0378-1119(86)90328-8. [DOI] [PubMed] [Google Scholar]
- Narita K., Murakami H., Ikenaka T. Reinvestigation on the amino acid composition and C-terminal group of Taka-amylase A. J Biochem. 1966 Feb;59(2):170–175. doi: 10.1093/oxfordjournals.jbchem.a128278. [DOI] [PubMed] [Google Scholar]
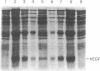
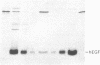
- Ohmizu H., Sasaki T., Tsukagoshi N., Udaka S., Kaneda N., Yagi K. Major proteins released by a protein-producing bacterium, Bacillus brevis 47, are derived from cell wall protein. J Biochem. 1983 Oct;94(4):1077–1084. doi: 10.1093/oxfordjournals.jbchem.a134450. [DOI] [PubMed] [Google Scholar]
- Oka T., Sakamoto S., Miyoshi K., Fuwa T., Yoda K., Yamasaki M., Tamura G., Miyake T. Synthesis and secretion of human epidermal growth factor by Escherichia coli. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7212–7216. doi: 10.1073/pnas.82.21.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennica D., Holmes W. E., Kohr W. J., Harkins R. N., Vehar G. A., Ward C. A., Bennett W. F., Yelverton E., Seeburg P. H., Heyneker H. L. Cloning and expression of human tissue-type plasminogen activator cDNA in E. coli. Nature. 1983 Jan 20;301(5897):214–221. doi: 10.1038/301214a0. [DOI] [PubMed] [Google Scholar]
- Ratzkin B., Lee S. G., Schrenk W. J., Roychoudhury R., Chen M., Hamilton T. A., Hung P. P. Expression in Escherichia coli of biologically active enzyme by a DNA sequence coding for the human plasminogen activator urokinase. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3313–3317. doi: 10.1073/pnas.78.6.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S. P., Pruss R. M., Herschman H. R. Initiation of 3T3 fibroblast cell division by epidermal growth factor. J Cell Physiol. 1975 Dec;86 (Suppl 2)(3 Pt 2):593–598. doi: 10.1002/jcp.1040860504. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Grimm E. A., McGrogan M., Doyle M., Kawasaki E., Koths K., Mark D. F. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984 Mar 30;223(4643):1412–1414. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- Saunders C. W., Schmidt B. J., Mallonee R. L., Guyer M. S. Secretion of human serum albumin from Bacillus subtilis. J Bacteriol. 1987 Jul;169(7):2917–2925. doi: 10.1128/jb.169.7.2917-2925.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroza T., Nakazawa K., Tashiro N., Yamane K., Yanagi K., Yamasaki M., Tamura G., Saito H., Kawade Y., Taniguchi T. Synthesis and secretion of biologically active mouse interferon-beta using a Bacillus subtilis alpha-amylase secretion vector. Gene. 1985;34(1):1–8. doi: 10.1016/0378-1119(85)90288-4. [DOI] [PubMed] [Google Scholar]
- Simons G., Remaut E., Allet B., Devos R., Fiers W. High-level expression of human interferon gamma in Escherichia coli under control of the pL promoter of bacteriophage lambda. Gene. 1984 Apr;28(1):55–64. doi: 10.1016/0378-1119(84)90087-8. [DOI] [PubMed] [Google Scholar]
- Smith R. A., Duncan M. J., Moir D. T. Heterologous protein secretion from yeast. Science. 1985 Sep 20;229(4719):1219–1224. doi: 10.1126/science.3939723. [DOI] [PubMed] [Google Scholar]
- Strauch K. L., Beckwith J. An Escherichia coli mutation preventing degradation of abnormal periplasmic proteins. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1576–1580. doi: 10.1073/pnas.85.5.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi W., Yamagata H., Yamaguchi K., Tsukagoshi N., Udaka S. Genetic transformation of Bacillus brevis 47, a protein-secreting bacterium, by plasmid DNA. J Bacteriol. 1983 Dec;156(3):1130–1134. doi: 10.1128/jb.156.3.1130-1134.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi A., Uchihi R., Adachi T., Sasaki T., Hayakawa S., Yamagata H., Tsukagoshi N., Udaka S. Characterization of the genes for the hexagonally arranged surface layer proteins in protein-producing Bacillus brevis 47: complete nucleotide sequence of the middle wall protein gene. J Bacteriol. 1988 Feb;170(2):935–945. doi: 10.1128/jb.170.2.935-945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukagoshi N., Ando Y., Tomita Y., Uchida R., Takemura T., Sasaki T., Yamagata H., Udaka S., Ichihara Y., Takahashi K. Nucleotide sequence and expression in Escherichia coli of cDNA of swine pepsinogen: involvement of the amino-terminal portion of the activation peptide segment in restoration of the functional protein. Gene. 1988 May 30;65(2):285–292. doi: 10.1016/0378-1119(88)90465-9. [DOI] [PubMed] [Google Scholar]
- Tsukagoshi N., Iritani S., Sasaki T., Takemura T., Ihara H., Idota Y., Yamagata H., Udaka S. Efficient synthesis and secretion of a thermophilic alpha-amylase by protein-producing Bacillus brevis 47 carrying the Bacillus stearothermophilus amylase gene. J Bacteriol. 1985 Dec;164(3):1182–1187. doi: 10.1128/jb.164.3.1182-1187.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Tsukagoshi N., Udaka S. Morphological alterations of cell wall concomitant with protein release in a protein-producing bacterium, Bacillus brevis 47. J Bacteriol. 1981 Oct;148(1):322–332. doi: 10.1128/jb.148.1.322-332.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Adachi T., Tsuboi A., Takao M., Sasaki T., Tsukagoshi N., Udaka S. Cloning and characterization of the 5' region of the cell wall protein gene operon in Bacillus brevis 47. J Bacteriol. 1987 Mar;169(3):1239–1245. doi: 10.1128/jb.169.3.1239-1245.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Nakagawa K., Tsukagoshi N., Udaka S. A stable plasmid vector and control of its copy number in Bacillus brevis 47, a protein-producing bacterium. Appl Environ Microbiol. 1985 May;49(5):1076–1079. doi: 10.1128/aem.49.5.1076-1079.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K., Toibana A., Kikuchi K., Kobayashi M., Hayakawa T., Nakahama K., Kikuchi M., Ikehara M. Differences between Saccharomyces cerevisiae and Bacillus subtilis in secretion of human lysozyme. Biochem Biophys Res Commun. 1987 Jun 15;145(2):712–718. doi: 10.1016/0006-291x(87)91023-0. [DOI] [PubMed] [Google Scholar]
- Zemel-Dreasen O., Zamir A. Secretion and processing of an immunoglobulin light chain in Escherichia coli. Gene. 1984 Mar;27(3):315–322. doi: 10.1016/0378-1119(84)90076-3. [DOI] [PubMed] [Google Scholar]
- Zsebo K. M., Lu H. S., Fieschko J. C., Goldstein L., Davis J., Duker K., Suggs S. V., Lai P. H., Bitter G. A. Protein secretion from Saccharomyces cerevisiae directed by the prepro-alpha-factor leader region. J Biol Chem. 1986 May 5;261(13):5858–5865. [PubMed] [Google Scholar]