Abstract
The design principles and specific proteins of the dynein–tubulin motor, which powers the flagella and cilia of eukaryotes, have been conserved throughout the evolution of life from algae to humans. Cilia and flagella can support both motile and sensory functions independently, or sometimes in parallel to each other. In this paper we show that this dual sensory–motile role of eukaryotic cilia is preserved in the most sensitive of all invertebrate hearing organs, the Johnston's organ of the mosquito. The Johnston's organ displays spontaneous oscillations, which have been identified as being a characteristic of amplification in the ears of mosquitoes and Drosophila. In the auditory organs of Drosophila and vertebrates, the molecular basis of amplification has been attributed to the gating and adaptation of the mechanoelectrical transducer channels themselves. On the basis of their temperature-dependence and sensitivity to colchicine, we attribute the molecular basis of spontaneous oscillations by the Johnston's organ of the mosquito Culex quinquefasciatus, to the dynein–tubulin motor of the ciliated sensillae. If, as has been claimed for insect and vertebrate hearing organs, spontaneous oscillations epitomize amplification, then in the mosquito ear, this process is independent of mechanotransduction.
Keywords: mosquito, hearing, dynein–tubulin motor, Johnston's organ, spontaneous activity, amplification
1. Introduction
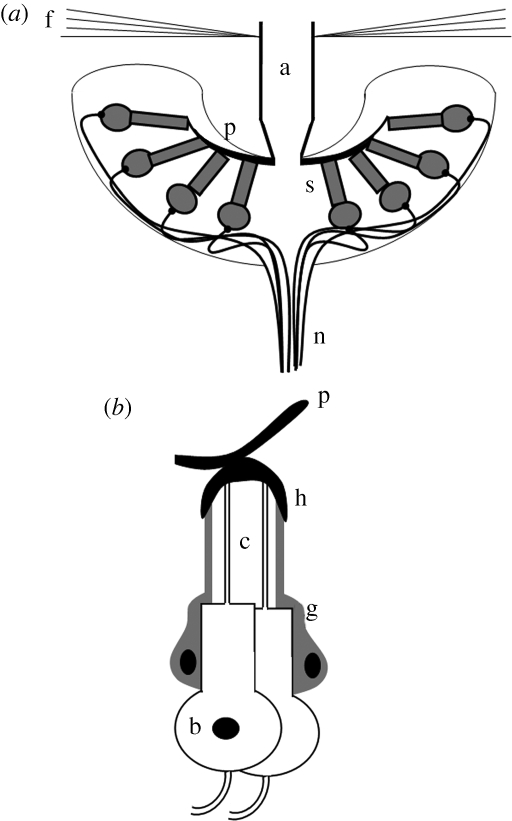
The mosquito hearing organ consists of a plumose antennal flagellum, which responds to movement of the air particles during sound propagation, and a large chordotonal organ, the Johnston's organ (JO), located in the pedicel at the base of the flagellum (Mayer 1874) (figure 1a). The flagellum tapers at its base into approximately 70 prongs, which resemble the spokes of an upturned umbrella. Attached to the prongs via their dendritic cilia are approximately 15 000 primary mechanosensory neurons (figure 1b) of the male JO that detect nanometre displacements of the flagellum and transduce them into receptor currents that generate neural impulses. The sensory neurons have a single distal dendrite, the outer dendritic segment being a modified cilium (Clements 1999) with a 9 × 2 + 0 microtubule structure (Boo 1981). The mechanosensory neurons are arranged into specialized sensory units, the scolopidia, each of which consists of one to four bipolar sensory neurons and usually three types of supporting cells. These neurons are the only non-cuticular attachments to the flagellum extension and are thought to underlie its nonlinear responses to sound stimulation (Göpfert & Robert 2001).
Figure 1.
Schematic diagram of the mosquito hearing organ. (a) Cross section through the base of the antenna (a, flagellum; p, prong; s, scolopidium; n, Johnston's organ nerve; f, fibrillae). (b) Schematized cross section through a scolopidium of the Johnston's organ (c, cilium; b, sensory cell body; g, supporting cell; h, cuticular cap).
Responses of the JO are thought to be powered by an active feedback mechanism, which selectively amplifies low-level responses of the antennal receptor to sound (Göpfert & Robert 2001; Göpfert et al. 2005). In the vertebrate inner ear, a functionally similar amplification mechanism associated with strong feedback leads, occasionally, to loss of system stability and generation of self-sustained oscillations. These autonomous oscillations manifest themselves, for example, as oscillations of the stereociliary bundles of the inner ear receptor hair cells (Crawford & Fettiplace 1985). These oscillations have been demonstrated to enhance frequency-selective gain and sensitivity of the system in noisy environments (Martin & Hudspeth 2001; Nadrowski et al. 2004), although they have also been considered as signatures of system instability caused by excessive gain (Camalet et al. 2000). Spontaneous oscillations (SO) of the antennal flagellum have also been documented for some dipteran species when their physiological state was compromised (Göpfert & Robert 2001, 2003). They have also been recorded after prolonged repetitive stimulation (Göpfert & Robert 2001) and are thought to be powered by the sensory neurons through the same mechanism that provides power amplification of the response of the flagellum to sound (Göpfert & Robert 2001). Therefore, through identifying the origin of the spontaneous oscillations, the mechanism of the power amplification of responses in the insect hearing organ might also be revealed.
Two mechanisms have been suggested to amplify the mechanical response of the flagellum to sound. The first mechanism is associated with the mechanosensitive channels responsible for mechanoelectrical transduction and is essentially the same as that described for the vertebrate, hair bundle-based, amplifier (Hudspeth 1997; Fettiplace et al. 2001). In the hair bundle model, active forces and spontaneous oscillations are produced by the hair bundle through the interplay between the gating kinetics of the transducer channels and their adaptation machinery (Hudspeth 1997). The transduction apparatus contributes significantly towards the mechanical properties of the Drosophila acoustic receiver (Albert et al. 2007; Nadrowski et al. 2008), hence it is possible that the transducer machinery of the mosquito JO is also harnessed to power active force production and oscillations in the mosquito flagellum. An alternative idea is that this motility is conferred by motor molecules separately from the transduction machinery. This suggestion was first proposed by Gray & Pumphrey (1958) and Thurm (1965) based on the morphology of the cilia of the sensory cell, where transduction takes place. The cytoskeletal components of the dendritic cilia, namely the dynein–tubulin system, match the functional components known to power motile cilia and flagella. Thus, it has been proposed that the cilia of insect sensory cells can produce a form of ciliary beating using a mechanism found and conserved in all eukaryotic branches (Wiederhold 1976; Mitchell 2007). Through synchronizing their beats, the cilia of the sensory cells in the JO could cause the flagellum to oscillate.
Here we present evidence that the flagellum of Culex quinquefasciatus actively oscillates when animals are in normal physiological conditions. To identify the molecular machinery underlying the SOs, we have studied the temperature-dependence of the SOs and their sensitivity to colchicine, which is known to depolymerize the microtubules in the cilia of scolopidial units (Moran & Varela 1971) and would perturb the SO if it receives contributions from the active beat of sensory cilia.
2. Material and methods
(a). Animal preparation
Culex quinquefasciatus were obtained from the London School of Hygiene and Tropical Medicine and raised to adults in a humid environment at 26°C and fed on sugar water.
Mosquitoes were secured to a brass metal block 4 × 3 × 3 mm in size, using low melting point bone wax (electronic supplementary material, figure S1). The metal block was clamped with a plastic screw onto a thin layer of electrode gel (Signa gel, Parker Laboratories Inc.) atop a ground electrode (EL212, WPI) ensuring that the gel contacted the mosquito. A fine stainless steel wire was used to apply a small bead of superglue (Everbuild) between the head and thorax and between each pedicel, thereby securing the pedicel to the head.
(b). Temperature control
For temperature-controlled experiments, the metal block with attached mosquito was clamped to the bottom of a stainless steel chamber, which was attached to a Peltier element and a heat sink (see electronic supplementary material, figure S1). Temperature was controlled by a custom-made device with a negative feedback loop, which altered the current fed through the Peltier element in response to the temperature read by a thermal resistor. A 2-min stabilization period was left after a new temperature was set. The exact temperature of the mosquito was measured by fixing a thermocouple modulator (80TK, Fluke) to the brass block with bone wax. Normal physiological condition of mosquitoes was confirmed by the existence of the tarsal reflex throughout and after all experiments. All experiments were performed on a vibration isolation table (VHT3636W-OPT, Newport corp.).
(c). Sound stimulation
Sound was delivered via a Beyerdynamic DT770 headphone (total harmonic distortion 0.2%) positioned approximately 10 mm from the antennae. Acoustic stimuli were expressed in particle velocity (pV), which was measured by a near-field microphone (Knowles (NR-23158-000)). The voltage output of the microphone increases linearly with pV, but in the near-field of a sound source (within λ/6, where λ is the wavelength of a pure tone) there is a nonlinear relationship between pV and sound pressure level (SPL). However, in the far field at a distance of more then λ/6, the relationship is linear. Thus, SPL was measured in the far field in response to a 1 kHz tone at 1.5 m using a pressure microphone (Brüel and Kjaer 1/2′ type: 2669). Measured SPL was used to calculate the pV at the same microphone position because of the defined relationship between sound pressure and pV in the far field. The pressure microphone was then replaced with the Knowles pV microphone and its response was measured at the same position as that of the pressure microphone. The sensitivity of the Knowles microphone was then calculated from the known pV and microphone responses. The calibrated Knowles microphone was then used to take measurements in the near field, where the relationship between SPL and pV is nonlinear.
(d). Mechanical measurements
The displacements of the flagellum were measured using a self-mixing, displacement-sensitive laser diode interferometer (Lukashkin et al. 2005). The interferometer was positioned so that its optical axis was perpendicular to the length of the flagellum and directly opposite to the speaker. Because of the limited dynamic range of the interferometer, it was focused at the base of the flagellum to minimize measured displacements. The signal from the interferometer was amplified and low-pass filtered with a cut-off frequency of 3 kHz. The interferometer was calibrated by vibrating the piezo stack, on which it was mounted, over a known range of displacements. This calibrating vibration creates a sharp peak in spectrograms of the mechanical responses.
(e). Electrical measurements
The receptor potential of the JO was measured using an electrolytically sharpened tungsten electrode inserted into the pedicel. Another electrolytically sharpened tungsten electrode was inserted into the thorax and served as a reference electrode. Signals from the recording and reference electrodes were fed into a differential amplifier with a gain of 10 000 and band pass-filtered between 5 and 2000 Hz.
(f). Signal processing
Signals from the interferometer and the electrode amplifier were digitized at 25 kHz with a Data Translation 3010 data acquisition board. Frequency spectrums of signals were calculated using a 16384-point FFT. Data acquisition and preliminary data analysis were performed under computer control using programs written in Matlab.
(g). Perfusion of Johnston's organ
During injection experiments, the pedicel was punctured by a 50 µm glass pipette and then injected with either colchicine solution or colchicine-free control solution. About 10 mM of colchicine and 9 mM methylene blue were added to Hanks solution. Control Hanks solution contained methylene blue but no colchicine. Both solutions were seen to fill the pedicel with a dark blue colour (see electronic supplementary material, figure S2b). Cryosections revealed a distinct uniform blue staining in the pedicels of control and colchicine-injected mosquitoes.
(h). Determining mass of flagellum
Flagella weight was measured by pulling the flagellum from live mosquitoes and placing them onto the tip of a finely pulled glass rod of calibrated stiffness. Bending of the rod owing to the weight of the flagellum was measured and the mass of the 11 flagella calculated and averaged. The stiffness of the rod was calibrated in a similar way using known weights made of Blu–Tack (Bostik).
3. Results
(a). Spontaneous oscillations of the flagellum
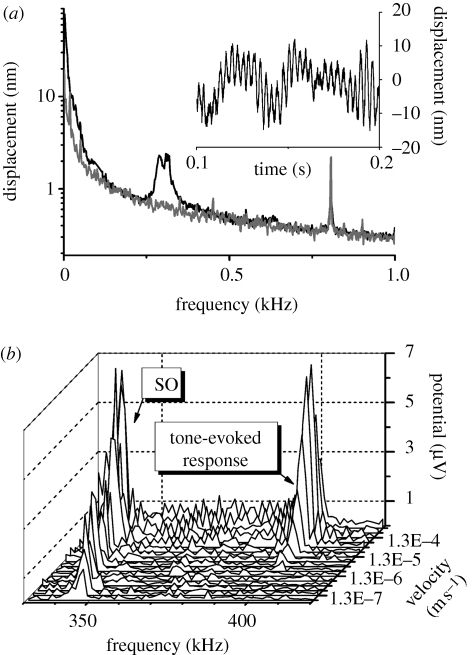
SOs of the flagellum were observed in both male and female mosquitoes in normal physiological condition (figure 2a). The physiological state of the mosquito was determined before and after altering the temperature and was confirmed as normal if a strong reflex was elicited in response to tarsal stimulation. The amplitude of the SOs at room temperature remained at almost constant amplitude and frequency throughout the experiment. In this study we present data collected from only male mosquitoes because the SO amplitude was much larger in males. SOs were observed in 31 out of 35 male mosquitoes that were believed to be in normal physiological condition. The inset in figure 2a shows a typical example of SOs of the flagellum recorded in the time domain. The SO frequency in this case was 290 Hz with a maximum amplitude of 15 nm recorded at a point 200 µm distal to the pivot of the flagellum, which corresponds to 4.3 × 10−3 degrees of angular displacement. The SOs usually appear as a single peak in the amplitude spectra of the mechanical responses, although it was not uncommon to observe two peaks corresponding to different frequencies (e.g. 289 Hz and 312 Hz in figure 2a). Similar peak-splitting has been observed in the oscillations of isolated axonemes (Kamimura & Kamiya 1989) and its origin is not known. A shift of the SO frequency during the sampling period may be responsible for the peak-splitting in the mechanical recordings reported here. The SO persisted during stimulation with a pure tone (figure 2b), its amplitude increasing with stimulus level but the frequency of the SOs decreased, thereby deviating from the frequency of the evoked response.
Figure 2.
Mechanical SOs of the flagellum. (a) Amplitude spectrum of the displacement of the flagellum without stimulation showing the frequency-specific SOs (black). The grey trace shows a spectrum recorded from the same mosquito after cooling to 5°C. The sharp peak at 800 Hz corresponds to a calibrating vibration of 2 nm in amplitude. Inset shows the waveform of the SOs in the time domain. (b) Amplitude spectrum of the electrical activity of the JO in response to a 400 Hz pure tone at increasing intensity.
The SOs of the flagellum were metabolically sensitive and disappeared post-mortem and were reversibly suppressed when the animals were cooled to 5°C (figure 2a). Similar metabolic sensitivity of the SOs has been demonstrated in other mosquito genera and in Drosophila through injection of dimethyl sulphoxide, which initially increased and then eliminated the SOs on death (Göpfert & Robert 2001, 2003).
(b). Temperature-dependence of the oscillation frequency
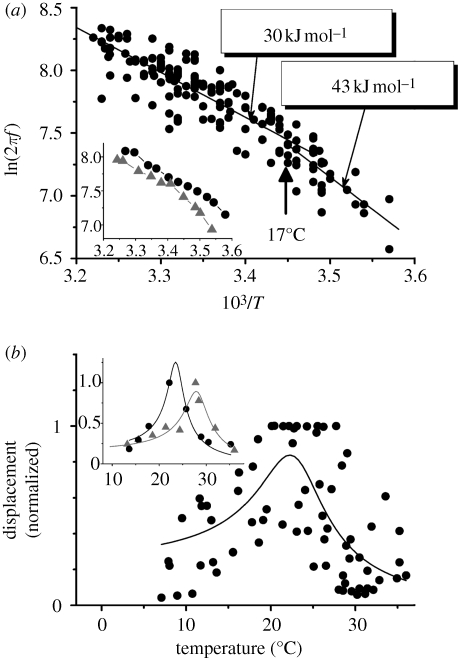
The frequency of the SOs of the flagellum depends on temperature. Measurements of this dependence were confined to between 5 and 35°C because outside this range the amplitudes of the SOs fall below the noise floor of our recording system. SO frequency versus temperature when plotted in Arrhenius coordinates (figure 3a) is not linear and has a break point at about 17°C. Break points in the temperature-dependence of the beat frequency have been documented for different types of motile cilia and flagella (Coakley & Holwill 1974; Grove et al. 2005). A break point in temperature-dependence has been shown for the activity of several motor molecules (Stein et al. 1982; Millar & Geeves 1983; Bohm et al. 2000) and is likely to be associated with conformational changes and/or phosphorylation in the motor ATPases and/or in associated molecules (Coakley & Holwill 1974; Grove et al. 2005; Salathe 2007). The dependence shown in figure 3a (pooled data from 12 mosquitoes) is fitted well by two linear regression lines above and below the breakpoint. Let us assume that the SO frequency is proportional to the rate constant of the reaction responsible for the SO. Then, the regression lines correspond to the natural logarithms of a modified Arrhenius equation
| 3.1 |
which gives the dependence of the SO frequency ω (T) = 2πf on the absolute temperature T and activation energy E of a single rate-limited reaction responsible for the SOs. In this equation, K is the pre-exponential factor and R is the gas constant. Corresponding activation energies derived from the slopes of the regression lines are 30 kJ mol−1 (correlation coefficient, −0.86; s.d., 0.14; p < 0.0001) for temperatures above 17°C and 43 kJ mol−1 (correlation coefficient, −0.71; s.d., 0.15; p < 0.0001) for temperatures below that. These energies are within the range found for the dynein and kinesin ATPase family and are significantly lower than the activation energy measured for myosin ATPases for in vivo systems (table 1 and references within).
Figure 3.
Temperature-dependence of the mechanical spontaneous oscillations of the Johnston's organ. (a) Arrhenius plot of the SO frequency (the natural logarithm of the SO frequency against the inverse absolute temperature). Two linear regression plots are fitted to data pooled from 12 mosquitoes with a break point at about 17°C. The activation energy values are indicated for both linear regression plots. Inset shows the Arrhenius plots for two individual mosquitoes. (b) Temperature-dependence of the amplitude of mechanical SOs. Pooled data for nine mosquitoes are shown. A simple harmonic oscillator model was fitted to the dependence of the normalized amplitude of the SOs with respect to temperature. Inset illustrates the same model fitted to the temperature dependences from two individual mosquitoes.
Table 1.
Activation energy values for in vivo systems operating in their natural temperature range.
| activation energy (kJ mol−1) | reference |
|---|---|
| dynein | |
| 57 | calculated from Gray (1923) |
| 31 | Holwill & Silvester (1967) |
| 44 | Holwill (1968) |
| 40 | Stephens & Levine (1970) |
| 51 | calculated from Machemer (1972) |
| 29 | Coakley & Holwill (1974) |
| 25 | Holwill & Wais (1979) |
| 20 | calculated from Auger et al. (1990) |
| 12 | calculated from Clary-Meinesz et al. (1992) |
| median 31 | |
| myosin | |
| 66 | Stein et al. (1982) |
| 53 | Bárány (1967) |
| 84 | Ranatunga (1984) |
| 50 | Anson (1992) |
| 94 | Grove et al. (2005) |
| median 66 | |
(c). Temperature-dependence of the oscillation amplitude
Pooled data (recorded from seven mosquitoes) for the dependence of the normalized amplitude, AN (ω(T)) = A(ω(T))/Amax of SOs on temperature T are shown in figure 3b. Here, A(ω(T)) and Amax are the frequency-dependent amplitude and maximal amplitude of SOs, respectively. The normalized amplitude was plotted to accommodate variations in the amplitude measurements associated with slightly different positions of the recording location along different flagella and unknown angle between direction of the SOs and the optical axis of the interferometer. The dependence AN (ω(T)) is bell-shaped and falls below the noise floor of the recording system beyond the 5–35°C temperature range, which is well outside that usually encountered by Cx. quinquefasciatus mosquitoes in their habitat. Within this range the observed amplitude maximum is scattered between 20 and 28°C (mean is 23.6 ± 2.7°C, n = 7) which, according to the regression line in figure 3a, corresponds to changes in the frequency between 311–431 Hz (the value corresponding to the mean temperature of 23.6°C is 361 Hz). Normalized amplitude data in figure 3b are fitted by the equation for the amplitude of vibrations of a harmonic oscillator
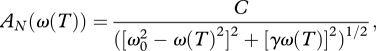 |
3.2 |
with temperature-dependent changes in the angular frequency ω (T) defined by equation (3.1). ω0 in equation (3.2) is the natural angular frequency of the flagellum. C = F/mAmax and γ = b/m are constants, with m and b being the effective mass of the flagellum and damping coefficient, respectively. F is the effective force acting on the flagellum. The fitted curve in figure 3b reveals the pooled natural frequency of the flagellum, f0 = ω0/2π = 353 ± 8 Hz, and its pooled damping coefficient, b = mγ = (1.915 ± 0.532) × 10−4 Ns m−1, taking into account that the flagella mass measured independently is m = 276 ± 58 × 10−9g (n = 11). Then the quality factor for pooled data is Q = ω0/γ = 3.2 ± 0.6. When equation (3.2) is used to fit data obtained from individual flagella (figure 3b, inset), the damping coefficient is almost two times smaller, being b = (1.054 ± 0.350) × 10−4 Ns m−1, which corresponds to sharper tuning with Q = 6.1 ± 1.3 (n = 7). The averaged natural frequency of the individual fits f0 = 354 ± 45 Hz (n = 7) is not however significantly different from the pooled natural frequency. Table 2 systematizes the parameters that characterize the mechanical properties of the flagellum, including its stiffness coefficient, K = mω20, obtained from the pooled data and individual fits.
Table 2.
Parameters characterizing the mechanical properties of the flagellum. Recordings of mechanical and electrical responses were made in the same animals. Recordings of electrical responses were made in four additional animals, where mechanical recordings were not possible.
| fit to pooled data |
individual fit |
|||
|---|---|---|---|---|
| mechanical | electrical | mechanical (n = 7) | electrical (n = 11) | |
| f0 (Hz) | 353 ± 8 | 419 ± 7 | 354 ± 45 | 413 ± 54 |
| γ (s−1) | 694 ± 126 | 643 ± 98 | 382 ± 98 | 312 ± 88 |
| b (×10−4 Ns m−1) | 1.915 ± 0.532 | 1.775 ± 0.461 | 1.054 ± 0.350 | 0.861 ± 0.303 |
| Q | 3.2 ± 0.6 | 4.1 ± 0.6 | 6.1 ± 1.3 | 8.8 ± 2.0 |
| K (N m−1) | 1.36 ± 0.29 | 1.91 ± 0.41 | 1.37 ± 0.38 | 1.86 ± 0.52 |
| m (×10−9 g) | 276 ± 58 (n = 11) | |||
(d). Temperature-dependence of the JO neural responses
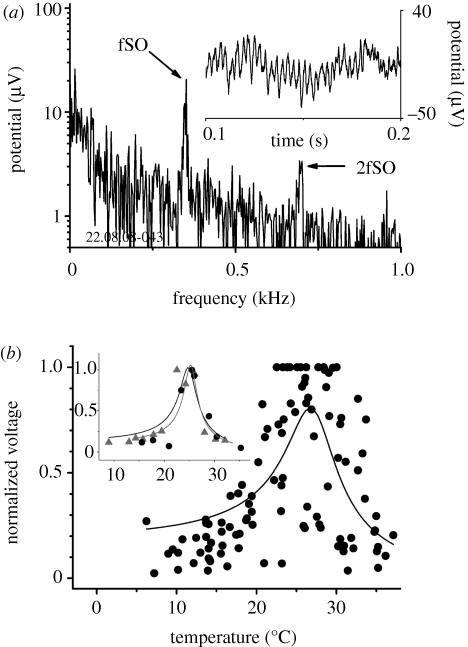
Oscillations of the compound electrical responses could be recorded from inside the JO at the frequency of the SOs of the flagellum (figure 4a). The similarity in frequency of the mechanical and electrical endogenous SOs indicates the close association between these two types of activity and, probably, that they share the same origin. We commonly recorded a component of the electrical response at twice the frequency of the SOs (2fSO; figure 4a). Frequency-doubling of the electrical responses of the JO to a pure-tone acoustic stimulus is a well-documented phenomenon for mosquitoes (Wishart et al. 1962). The magnitude of the electrical oscillations followed the same bell-shaped temperature-dependence (figure 4b) as the amplitude of the mechanical displacement of the flagellum (figure 3b). Assuming that the magnitudes of the electrical and mechanical SOs are governed by properties of the same harmonic oscillator and fitting this dependence with a combination of equations (3.1) and (3.2), we find slightly different values for the natural frequency (table 2). This difference is likely to indicate the existence of additional processing stages of the electrical responses within the JO and require modifications to the harmonic oscillator model used to fit the electrical responses. The other characteristics of the harmonic oscillator however are not significantly different from parameters obtained from the temperature-dependence of the mechanical SOs (table 2). This suggests that electrical and mechanical oscillations are tightly coupled. It is worth noting that, for the temperature-dependence of the electrical SOs (figure 4b), the fitting of equations to data obtained from individual JOs (figure 4b, inset) also reveals significantly sharper tuning (compare the two columns of parameters for electrical responses in table 2). As for the mechanical SOs, this difference in parameters between pooled and individual data reflects the scatter of the maximum amplitude of mechanical responses obtained from different mosquitoes (figure 3b).
Figure 4.
Temperature-dependence of the electrical spontaneous oscillations of the Johnston's organ. (a) Amplitude spectrum of the electrical potential of the JO in the absence of stimulation. Inset shows the waveform of the SOs in the time domain. Spectral peaks corresponding to the frequency of SO (fSO) and its second harmonic (2fSO) are indicated by arrows. (b) Temperature-dependence of the amplitude of electrical SOs. Pooled data for 11 mosquitoes are shown. A simple harmonic oscillator model was fitted to the dependence of normalized amplitude of the electrical SO recorded from the JO. Inset illustrates the same model fitted to the temperature dependences from two individual mosquitoes.
(e). Colchicine blocks the SOs
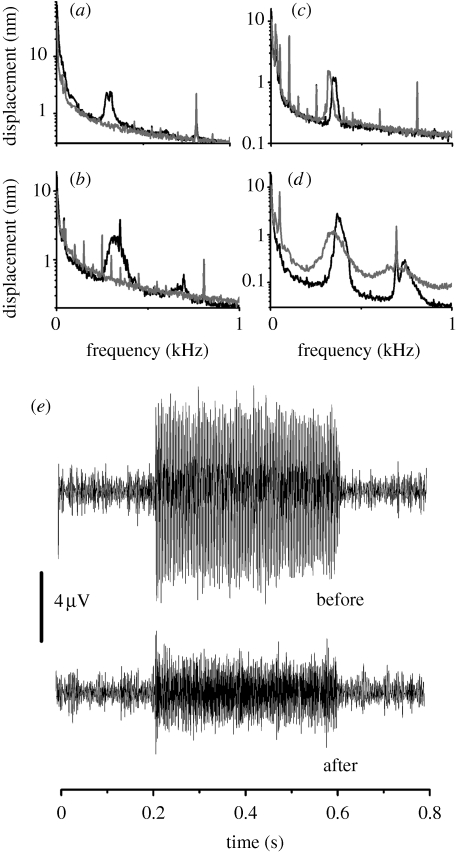
Extracellular application of the alkaloid colchicine causes disruption of microtubules in the dendritic cilia of Arthropoda mechanoreceptors (Moran & Varela 1971). To examine the involvement of the microtubular system in the SO generation, we injected insect physiological solution containing 10 mM of colchicine and 9 mM of methylene blue into the JO. Methlylene blue was used as a marker to ensure that solutions filled the JO (electronic supplementary material, figure S2). Staining of the JO with methylene blue was confirmed by inspection of cryosections of the injected JO after the experiment. Injections of colchicine-containing solution blocks SOs within 10 min (figure 5a,b). SOs did not re-appear during further observations (up to 2 h). The oscillations remained after injection of a control, colchicine-free physiological solution, which contained methylene blue only (figure 5c,d). Colchicine injection did not block mechanotransduction and acoustically evoked electrical responses, though smaller in amplitude, could still be elicited in the JO following the colchicine-associated block of the SOs (figure 5e). The decrease in amplitude of electrical responses following the injection could be owing to changes in the electrical properties of the JO or to local damage of the sensory cells in the vicinity of the pipette tip caused by the injection.
Figure 5.
Effect of colchicine on SOs and mechanoelectrical transduction. (a–d) SOs before and after injection of colchicine. Amplitude spectrum of the displacement of the flagellum before (black) and 10 min after (grey) injection of colchicine solution (a,b) and colchicine-free solution (c,d). Sharp peaks at 700 (d) or 800 Hz (a–c) corresponds to a calibrating vibration of 1 (b,c) or 2 nm (a,d) in amplitude. Other sharp peaks in these recordings are electrical noise and pickup. (e) Electrical response of the JO to a pure tone presented between 0.2 and 0.6 s at an intensity of 1 × 10−4 ms−1 at 300 Hz before and after injection of colchicine.
The colchicine experiments reveal that the mechanism responsible for the SOs is not part of the transduction apparatus because, after the application of colchicine and the loss of the SOs, receptor potentials could still be recorded from the JO. There is a possibility, however, that the transduction machinery is complex and that colchicine application disrupted only a microtubule-associated part of the transduction machinery making SOs impossible but leaving the mechanically gated transducer channels intact, thereby allowing mechanostransduction.
4. Discussion
The dendritic cilia of the sensory neurons of the JO, which are the only non-cuticular attachments to the prongs of the mosquito flagellum, have been proposed to underlie its SOs (Göpfert & Robert 2001). Most of the evidence in support of this hypothesis has come from measurements of the Drosophila antennal receiver, which has an organization and function similar to that of the mosquito JO, where it has been demonstrated that the generation of SOs is because of an active nonlinear process associated with cilia of the auditory neurons (Göpfert & Robert 2003; Göpfert et al. 2005). Mechanical responses of the antennal receiver are linear and passive and SOs are never observed in Drosophila mutants with ciliary defects (Göpfert & Robert 2003). It has been suggested (Göpfert et al. 2005) that the molecular motor driving the active amplification of responses, and hence likely to drive the SOs of the antennal receiver is analogous to the molecular machinery responsible for the SOs that have been measured in the hair bundles of vertebrate hair cells, where oscillations are generated because of interaction between the mechanoelectrical transducer gating kinetics and a myosin-based adaptation mechanism (Martin et al. 2000). While the transducer apparatus contributes significantly towards the mechanical properties of the antennal receiver (Albert et al. 2007; Nadrowski et al. 2008), the presence of a myosin–actin motor associated with transducer channels of the JO sensory neurons has not been demonstrated. In fact, data presented in this report show that the rate-limiting reaction associated with the SOs of the mosquito antennal organ is unlikely to be owing to a myosin-based motor.
Our estimates of the activation energy of the rate-limiting molecular processes powering the SOs for temperatures above 17°C, which is well within the usual temperature range of Culex mosquitoes, gives a value of 30 kJ mol−1 (figure 3a). This activation energy, and in fact the activation energy of 43 kJ mol−1 for temperatures below 17°C, is lower than for in vivo systems based on the myosin ATPase but matches that of in vivo systems powered by dynein and kinesin (see table 1 and references within). Dynein, however, is the only motor molecule distributed throughout the length of the sensory cilium (Thurm et al. 1983). This strongly suggests that the dynein–tubulin system powers the SOs. The experiment where colchicine is used to block the SOs, presumably via depolymerization of microtubules and hence depriving the dynein of its substrate, provide additional evidence for this hypothesis.
Colchicine blockade of the SOs demonstrates that microtubules are an essential part of the mechanism, which is responsible for the SO. The transduction apparatus alone is not capable of generating the SOs because the electrical responses of the JO and hence transduction were preserved after complete block of the SOs by colchicine. Neural electrical responses were consistently observed after colchicine injection and loss of all oscillations. Thus, the transduction mechanism appears not to depend on the integrity of the microtubule cytoskeleton. This also has been confirmed by Erler (1983) who recorded electrical responses in the hair receptor of the cricket after destruction of the tubular body by visblatine. Interestingly, Göpfert & Robert (2001) recorded flagellar oscillations in mosquitoes, which failed to elicit electrical responses in the JO. This provides further evidence that mechanical SOs may not depend on the transduction machinery.
Based on the morphology of the sensory cilia (Boo 1981) and mechanical responses of the antennal receptor (Nadrowski et al. 2008), the dynein–tubulin system may be involved in the generation of the SOs through two fundamentally different mechanisms. Firstly, the SOs may be generated using the same ancestral mechanism that powers motile cilia. This idea is based on the similarity in morphology of the dendritic cilia of insect scolopidial neurons and motile cilia (Wiederhold 1976). In fact, the dendritic cilia of the JO have a 9 × 2 + 0 axonemal organization that lacks the central pair of microtubules which, does not usually prevent cilia from being motile (Göpfert & Robert 2008). The sensitivity of motile cilia to mechanical stimulation has been reviewed (Wiederhold 1976). Hence, it is logical to suggest that cilia with a primary sensory function can also be motile (Wiederhold 1976), using this motility for active amplification of responses to weak stimulation. So far, active bending of cilia in scolopidial organs has been demonstrated in morphological studies only (Moran et al. 1977). Involvement of the dynein–tubulin motor in SO generation does not, however, exclude the involvement of the transducer machinery. In this second scheme, the dynein–tubulin system plays the role of an adaptation motor, analogous to the tip-link-associated myosin adaptation motor of hair cells of lower vertebrates (Kernan & Zuker 1995). Here, SOs are generated because of interplay between the gating kinetics of the transducer channels and the dynein–tubulin-based adaptation motor. It is worth noting however that cyclic movement is natural to the dynein–tubulin complex as demonstrated by in vitro experiments, when the presence of microtubules was sufficient to activate oscillatory movement of the dynein ATPase (Kamimura & Kamiya 1989; Shingyoji et al. 1998).
It has been demonstrated that autonomous oscillations can significantly improve system sensitivity and gain in noisy environments (Martin & Hudspeth 2001; Nadrowski et al. 2004). The SOs of male mosquitoes are observed within the frequency range of the female flight tone and, hence, may be functionally significant in detecting flying females when mating in swarms (Gibson & Russell 2006). It is worth noting that both the frequency of SOs at any given temperature (figure 3a) and the calculated natural frequencies of oscillations of the antennal receivers (table 2) demonstrate a significant scatter, which may be important for covering the range of the female mosquito flying tones.
This report provides the first in vivo evidence for the involvement of the dynein–tubulin system in the generation of SOs and, hence, power amplification in mosquito antennal receivers. The auditory receptor of mosquitoes has recruited a mechanism already engineered for generating motility in cilia and flagella. The molecular basis of this mechanism is distinct from that of the mechanoelectrical transduction channels in the cilia membrane and, therefore, different from that in vertebrate auditory and vestibular receptors, where motility and transduction have been attributed to the transduction apparatus.
Acknowledgements
This work was supported by grants from the BBSRC and the MRC and a BBSRC research studentship to B.W.
We thank James Hartley for his technical assistance and Shahida Begum and the London School of Hygiene and Tropical Medicine for the provision of mosquitoes.
References
- Albert J. T., Nadrowski B., Göpfert M. C.2007Mechanical signatures of transducer gating in the Drosophila ear. Curr. Biol. 17, 1000–1006 (doi:10.1016/j.cub.2007.05.004) [DOI] [PubMed] [Google Scholar]
- Anson M.1992Temperature-dependence and Arrhenius activation-energy of F-actin velocity generated in vitro by skeletal myosin. J. Mol. Biol. 224, 1029–1038 (doi:10.1016/0022-2836(92)90467-X) [DOI] [PubMed] [Google Scholar]
- Auger J., Serres C., Feneux D.1990Motion of individual human spermatozoa, both normal and lacking the outer dynein arms, during a continuous temperature rise. Cell Motil. Cytoskeleton 16, 22–32 (doi:10.1002/cm.970160105) [DOI] [PubMed] [Google Scholar]
- Bárány M.1967ATPase activity of myosin correlated with speed of muscle shortening. J. Gen. Physiol. 50, 197–218 (doi:10.1085/jgp.50.6.197) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm K. J., Stracke R., Baum M., Zieren M., Unger E.2000Effect of temperature on kinesin-driven microtubule gliding and kinesin ATPase activity. FEBS Lett. 466, 59–62 (doi:10.1016/S0014-5793(99)01757-3) [DOI] [PubMed] [Google Scholar]
- Boo K. S.1981Discontinuity between ciliary root processes and triple microtubules of distal basal body in mosquito sensory cilia. Kor. J. Entomol. 11, 5–18 [Google Scholar]
- Camalet S., Duke T., Julicher F., Prost J.2000Auditory sensitivity provided by self-tuned critical oscillations of hair cells. Proc. Natl Acad. Sci. USA 97, 3183–3188 (doi:10.1073/pnas.97.7.3183) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clary-Meinesz C. F., Cosson J., Huitorel P., Blaive B.1992Temperature effect on the ciliary beat frequency of human nasal and tracheal ciliated cells. Biol. Cell 76, 335–338 (doi:10.1016/0248-4900(92)90436-5) [DOI] [PubMed] [Google Scholar]
- Clements A. N.1999The antennae and hearing. In The biology of mosquitoes. Vol. 2: Sensory reception and behaviour, pp. 55–87 New York, NY: CABI Publishing [Google Scholar]
- Coakley C. J., Holwill M. E. J.1974Effects of pressure and temperature-changes on flagellar movement of Crithidi oncopelti. J. Exp. Biol. 60, 605–629 [DOI] [PubMed] [Google Scholar]
- Crawford A. C., Fettiplace R.1985The mechanical properties of ciliary bundles of turtle cochlear hair-cells. J. Physiol. (Lond.) 364, 359–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erler G.1983Sensitivity of an insect mechanoreceptor after destruction of dendritic microtubules by means of vinblastine. Cell Tissue Res. 229, 673–684 (doi:10.1007/BF00207705) [DOI] [PubMed] [Google Scholar]
- Fettiplace R., Ricci A. J., Hackney C. M.2001Clues to the cochlear amplifier from the turtle ear. Trends Neurosci. 24, 169–175 (doi:10.1016/S0166-2236(00)01740-9) [DOI] [PubMed] [Google Scholar]
- Gibson G., Russell I. J.2006Flying in tune: sexual recognition in mosquitoes. Curr. Biol. 16, 1311–1316 (doi:10.1016/j.cub.2006.05.053) [DOI] [PubMed] [Google Scholar]
- Göpfert M. C., Robert D.2001Active auditory mechanics in mosquitoes. Proc. R. Soc. Lond. B 268, 333–339 (doi:10.1098/rspb.2000.1376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göpfert M. C., Robert D.2003Motion generation by Drosophila mechanosensory neurons. Proc. Natl Acad. Sci. USA 100, 5514–5519 (doi:10.1073/pnas.0737564100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göpfert M. C., Robert D.2008Active processes in insect hearing. In Active processes and otoacoustic emissions (eds Manley G. A., Fay R. R., Popper A. N.), pp. 191–209 New York, NY: Springer [Google Scholar]
- Göpfert M. C., Humphris A. D. L., Albert J. T., Robert D., Hendrich O.2005Power gain exhibited by motile mechanosensory neurons in Drosophila ears. Proc. Natl Acad. Sci. USA 102, 325–330 (doi:10.1073/pnas.0405741102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J.1923The mechanism of ciliary movement. III. The effect of temperature. Proc. R. Soc. Lond. B 95, 6–15 (doi:10.1098/rspb.1923.0019) [Google Scholar]
- Gray E. G., Pumphrey R. J.1958Ultra-structure of the insect ear. Nature 181, 618 (doi:10.1038/181618a0) [DOI] [PubMed] [Google Scholar]
- Grove T. J., McFadden L. A., Chase P. B., Moerland T. S.2005Effects of temperature, ionic strength and pH on the function of skeletal muscle myosin from a eurythermal fish, Fundulus heteroclitus. J. Muscle. Res. Cell Motil. 26, 191–197 (doi:10.1007/s10974-005-9010-0) [DOI] [PubMed] [Google Scholar]
- Holwill M. E. J.1968Kinetic studies of the flagellar movement of sea-urchin spermatozoa. J. Exp. Biol. 50, 203–222 [DOI] [PubMed] [Google Scholar]
- Holwill M. E. J., Silvester N. R.1967Thermodynamic aspects of flagellar activity. J. Exp. Biol. 47, 249–265 [DOI] [PubMed] [Google Scholar]
- Holwill M. E. J., Wais J.1979Thermodynamic and hydrodynamic studies relating to the mechanochemical cycle in the flagellum of Crithidia oncopelti. J. Exp. Biol. 82, 177–195 [Google Scholar]
- Hudspeth A. J.1997Mechanical amplification of stimuli by hair cells. Curr. Opin. Neurobiol. 7, 480–486 (doi:10.1016/S0959-4388(97)80026-8) [DOI] [PubMed] [Google Scholar]
- Kamimura S., Kamiya R.1989High-frequency nanometre-scale vibration in quiescent flagellar axonemes. Nature 340, 476–447 (doi:10.1038/340476a0) [DOI] [PubMed] [Google Scholar]
- Kernan M., Zuker C.1995Genetic approaches to mechanosensory transduction. Curr. Opin. Neurobiol. 5, 443–448 (doi:10.1016/0959-4388(95)80003-4) [DOI] [PubMed] [Google Scholar]
- Lukashkin A. N., Bashtanov M. E., Russell I. J.2005A self-mixing laser-diode interferometer for measuring basilar membrane vibrations without opening the cochlea. J. Neurosci. Meth. 148, 122–129 (doi:10.1016/j.jneumeth.2005.04.014) [DOI] [PubMed] [Google Scholar]
- Machemer H.1972Temperature influences on ciliary beat and metachronal coordination in Paramecium. J. Mechanochem. Cell Motil. 1, 57–66 [Google Scholar]
- Martin P., Hudspeth A. J.2001Compressive nonlinearity in the hair bundle's active response to mechanical stimulation. Proc. Natl Acad. Sci. USA 98, 14 386–14 391 (doi:10.1073/pnas.251530498) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P., Mehta A. D., Hudspeth A. J.2000Negative hair-bundle stiffness betrays a mechanism for mechanical amplification by the hair cell. Proc. Natl Acad. Sci. USA 97, 12 026–12 031 (doi:10.1073/pnas.210389497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A. N.1874Experiments on the supposed auditory apparatus of the mosquito. Am. Nat. 8, 577–592 (doi:10.1086/271388) [Google Scholar]
- Millar N. C., Geeves M. A.1983The limiting rate of the ATP-mediated dissociation of actin from rabbit skeletal-muscle myosin subfragment-1. FEBS Lett. 160, 141–148 (doi:10.1016/0014-5793(83)80954-5) [DOI] [PubMed] [Google Scholar]
- Mitchell D. R.2007The evolution of eukaryotic cilia and flagella as motile and sensory organelles. Adv. Exp. Med. Biol. 607, 130–140 (doi:10.1007/978-0-387-74021-8_11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran D. T., Varela F. G.1971Microtubules and sensory transduction. Proc. Natl Acad. Sci. USA 68, 757–760 (doi:10.1073/pnas.68.4.757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran D. T., Varela F. J., Rowley J. C.1977Evidence for active role of cilia in sensory transduction. Proc. Natl Acad. Sci. USA 74, 793–797 (doi:10.1073/pnas.74.2.793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadrowski B., Martin P., Julicher F.2004Active hair-bundle motility harnesses noise to operate near an optimum of mechanosensitivity. Proc. Natl Acad. Sci. USA 101, 12 195–12 200 (doi:10.1073/pnas.0403020101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadrowski B., Albert J. T., Göpfert M. C.2008Transducer-based force generation explains active process in Drosophila hearing. Curr. Biol. 18, 1365–1372 (doi:10.1016/j.cub.2008.07.095) [DOI] [PubMed] [Google Scholar]
- Ranatunga K. W.1984The force–velocity relation of fast-twitch and slow-twitch muscles examined at different temperatures. J. Physiol. (Lond.) 351, 517–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salathe M.2007Regulation of mammalian ciliary beating. Annu. Rev. Physiol. 69, 401–422 (doi:10.1146/annurev.physiol.69.040705.141253) [DOI] [PubMed] [Google Scholar]
- Shingyoji C., Higuchi H., Yoshimura M., Katayama E., Yanagida T.1998Dynein arms are oscillating force generators. Nature 393, 711–714 (doi:10.1038/31520) [DOI] [PubMed] [Google Scholar]
- Stein R. B., Gordon T., Shriver J.1982Temperature-dependence of mammalian muscle contractions and ATPase activities. Biophys. J. 40, 97–107 (doi:10.1016/S0006-3495(82)84464-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. E., Levine E. E.1970Some enzymatic properties of axonemes from cilia of Pecten irradians. J. Cell. Biol. 46, 416–421 (doi:10.1083/jcb.46.2.416) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurm U.1965An insect mechanoreceptor. I. Fine structure and adequate stimulus. Cold Spring Harb. Symp. Quant. Biol. 30, 75–82 [DOI] [PubMed] [Google Scholar]
- Thurm U., Erler G., Godde J., Kastrup H., Keil T., Volker W., Vohwinkel B.1983Cilia specialized for mechanoreception. J. Submicrosc. Cytol. Pathol. 15, 151–155 [Google Scholar]
- Wiederhold M. L.1976Mechanosensory transduction in ‘sensory’ and ‘motile’ cilia. Annu. Rev. Biophys. Bioeng. 5, 39–62 (doi:10.1146/annurev.bb.05.060176.000351) [DOI] [PubMed] [Google Scholar]
- Wishart G., Sickle G. R., Riordan D. F.1962Orientation of males of Aedes aegypti (L.) (Diptera: Culicidae) to sound. Can. Entomol. 94, 613–626 (doi:10.4039/Ent94613-6) [Google Scholar]