Abstract
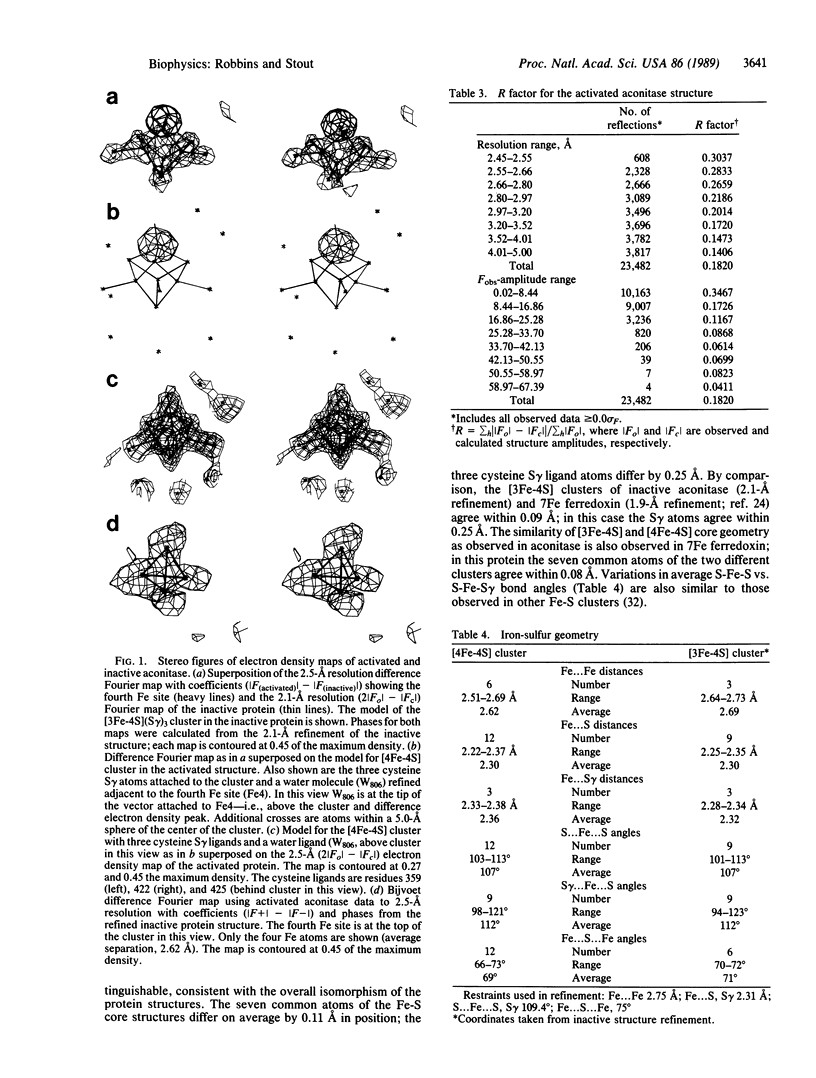
The structure of activated pig heart aconitase [citrate(isocitrate) hydro-lyase, EC 4.2.1.3] containing a [4Fe-4S] cluster has been refined at 2.5-A resolution to a crystallographic residual of 18.2%. Comparison of this structure to the recently determined 2.1-A resolution structure of the inactive enzyme containing a [3Fe-4S] cluster, by difference Fourier analysis, shows that upon activation iron is inserted into the structure isomorphously. The common atoms of the [3Fe-4S] and [4Fe-4S] cores agree within 0.1 A; the three common cysteinyl S gamma ligand atoms agree within 0.25 A. The fourth ligand of the Fe inserted into the [3Fe-4S] cluster is a water or hydroxyl from solvent, consistent with the absence of a free cysteine ligand in the enzyme active site cleft and the isomorphism of the two structures. A water molecule occupies a similar site in the crystal structure of the inactive enzyme.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonio M. R., Averill B. A., Moura I., Moura J. J., Orme-Johnson W. H., Teo B. K., Xavier A. V. Core dimensions in the 3Fe cluster of Desulfovibrio gigas ferredoxin II by extended X-ray absorption fine structure spectroscopy. J Biol Chem. 1982 Jun 25;257(12):6646–6649. [PubMed] [Google Scholar]
- Beinert H., Emptage M. H., Dreyer J. L., Scott R. A., Hahn J. E., Hodgson K. O., Thomson A. J. Iron-sulfur stoichiometry and structure of iron-sulfur clusters in three-iron proteins: evidence for [3Fe-4S] clusters. Proc Natl Acad Sci U S A. 1983 Jan;80(2):393–396. doi: 10.1073/pnas.80.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinert H., Thomson A. J. Three-iron clusters in iron-sulfur proteins. Arch Biochem Biophys. 1983 Apr 15;222(2):333–361. doi: 10.1016/0003-9861(83)90531-3. [DOI] [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Emptage M. H., Dreyers J. L., Kennedy M. C., Beinert H. Optical and EPR characterization of different species of active and inactive aconitase. J Biol Chem. 1983 Sep 25;258(18):11106–11111. [PubMed] [Google Scholar]
- Emptage M. H., Kent T. A., Kennedy M. C., Beinert H., Münck E. Mössbauer and EPR studies of activated aconitase: development of a localized valence state at a subsite of the [4Fe-4S] cluster on binding of citrate. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4674–4678. doi: 10.1073/pnas.80.15.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard A. J., Nielsen C., Xuong N. H. Software for a diffractometer with multiwire area detector. Methods Enzymol. 1985;114:452–472. doi: 10.1016/0076-6879(85)14030-9. [DOI] [PubMed] [Google Scholar]
- Kennedy M. C., Beinert H. The state of cluster SH and S2- of aconitase during cluster interconversions and removal. A convenient preparation of apoenzyme. J Biol Chem. 1988 Jun 15;263(17):8194–8198. [PubMed] [Google Scholar]
- Kennedy M. C., Emptage M. H., Beinert H. Incorporation of [35S]sulfide into the Fe-S cluster of aconitase. J Biol Chem. 1984 Mar 10;259(5):3145–3151. [PubMed] [Google Scholar]
- Kennedy M. C., Emptage M. H., Dreyer J. L., Beinert H. The role of iron in the activation-inactivation of aconitase. J Biol Chem. 1983 Sep 25;258(18):11098–11105. [PubMed] [Google Scholar]
- Kennedy M. C., Kent T. A., Emptage M., Merkle H., Beinert H., Münck E. Evidence for the formation of a linear [3Fe-4S] cluster in partially unfolded aconitase. J Biol Chem. 1984 Dec 10;259(23):14463–14471. [PubMed] [Google Scholar]
- Kennedy M. C., Werst M., Telser J., Emptage M. H., Beinert H., Hoffman B. M. Mode of substrate carboxyl binding to the [4Fe-4S]+ cluster of reduced aconitase as studied by 17O and 13C electron-nuclear double resonance spectroscopy. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8854–8858. doi: 10.1073/pnas.84.24.8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S. C., Rauner R., Gawron O. On pig heart aconitase. Biochem Biophys Res Commun. 1972 May 26;47(4):740–745. doi: 10.1016/0006-291x(72)90554-2. [DOI] [PubMed] [Google Scholar]
- Kent T. A., Dreyer J. L., Kennedy M. C., Huynh B. H., Emptage M. H., Beinert H., Münck E. Mössbauer studies of beef heart aconitase: evidence for facile interconversions of iron-sulfur clusters. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1096–1100. doi: 10.1073/pnas.79.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent T. A., Emptage M. H., Merkle H., Kennedy M. C., Beinert H., Münck E. Mössbauer studies of aconitase. Substrate and inhibitor binding, reaction intermediates, and hyperfine interactions of reduced 3Fe and 4Fe clusters. J Biol Chem. 1985 Jun 10;260(11):6871–6881. [PubMed] [Google Scholar]
- Kuo D. J., Rose I. A. Aconitase: its source of catalytic protons. Biochemistry. 1987 Dec 1;26(24):7589–7596. doi: 10.1021/bi00398a009. [DOI] [PubMed] [Google Scholar]
- Moura J. J., Moura I., Kent T. A., Lipscomb J. D., Huynh B. H., LeGall J., Xavier A. V., Münck E. Interconversions of [3Fe-3S] and [4Fe-4S] clusters. Mössbauer and electron paramagnetic resonance studies of Desulfovibrio gigas ferredoxin II. J Biol Chem. 1982 Jun 10;257(11):6259–6267. [PubMed] [Google Scholar]
- Plank D. W., Howard J. B. Identification of the reactive sulfhydryl and sequences of cysteinyl-tryptic peptides from beef heart aconitase. J Biol Chem. 1988 Jun 15;263(17):8184–8189. [PubMed] [Google Scholar]
- Ramsay R. R., Singer T. P. Molecular forms of aconitase and their interconversions. Biochem J. 1984 Jul 15;221(2):489–497. doi: 10.1042/bj2210489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins A. H., Stout C. D. Iron-sulfur cluster in aconitase. Crystallographic evidence for a three-iron center. J Biol Chem. 1985 Feb 25;260(4):2328–2333. [PubMed] [Google Scholar]
- Rose I. A., O'Connell E. L. Mechanism of aconitase action. I. The hydrogen transfer reaction. J Biol Chem. 1967 Apr 25;242(8):1870–1879. [PubMed] [Google Scholar]
- Ruzicka F. J., Beinert H. A mitochondrial iron protein with properties of a high-potential iron-sulfur protein. Biochem Biophys Res Commun. 1974 Jun 4;58(3):556–563. doi: 10.1016/s0006-291x(74)80456-0. [DOI] [PubMed] [Google Scholar]
- Ruzicka F. J., Beinert H. The soluble "high potential" type iron-sulfur protein from mitochondria is aconitase. J Biol Chem. 1978 Apr 25;253(8):2514–2517. [PubMed] [Google Scholar]
- Rydén L., Ofverstedt L. G., Beinert H., Emptage M. H., Kennedy M. C. Molecular weight of beef heart aconitase and stoichiometry of the components of its iron-sulfur cluster. J Biol Chem. 1984 Mar 10;259(5):3141–3144. [PubMed] [Google Scholar]
- Schloss J. V., Emptage M. H., Cleland W. W. pH profiles and isotope effects for aconitases from Saccharomycopsis lipolytica, beef heart, and beef liver. alpha-Methyl-cis-aconitate and threo-Ds-alpha-methylisocitrate as substrates. Biochemistry. 1984 Sep 25;23(20):4572–4580. doi: 10.1021/bi00315a010. [DOI] [PubMed] [Google Scholar]
- Stout C. D. 7-Iron ferredoxin revisited. J Biol Chem. 1988 Jul 5;263(19):9256–9260. doi: 10.2210/pdb3fd1/pdb. [DOI] [PubMed] [Google Scholar]
- Stout C. D. Refinement of the 7 Fe ferredoxin from Azotobacter vinelandii at 1.9 A resolution. J Mol Biol. 1989 Feb 5;205(3):545–555. doi: 10.1016/0022-2836(89)90225-8. [DOI] [PubMed] [Google Scholar]
- Stout G. H., Turley S., Sieker L. C., Jensen L. H. Structure of ferredoxin I from Azotobacter vinelandii. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1020–1022. doi: 10.1073/pnas.85.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strahs G., Kraut J. Low-resolution electron-density and anomalous-scattering-density maps of Chromatium high-potential iron protein. J Mol Biol. 1968 Aug 14;35(3):503–512. doi: 10.1016/s0022-2836(68)80010-5. [DOI] [PubMed] [Google Scholar]
- Telser J., Emptage M. H., Merkle H., Kennedy M. C., Beinert H., Hoffman B. M. 17O electron nuclear double resonance characterization of substrate binding to the [4Fe-4S]1+ cluster of reduced active aconitase. J Biol Chem. 1986 Apr 15;261(11):4840–4846. [PubMed] [Google Scholar]
- Villafranca J. J., Mildvan A. S. The mechanism of aconitase action. I. Preparation, physical properties of the enzyme, and activation by iron (II). J Biol Chem. 1971 Feb 10;246(3):772–779. [PubMed] [Google Scholar]


