Abstract
Growth of the human lens and the development of its internal features are examined using in vivo and in vitro observations on dimensions, weights, cell sizes, protein gradients and other properties.
In vitro studies have shown that human lens growth is biphasic, asymptotic until just after birth and linear for most of postnatal life. This generates two distinct compartments, the prenatal and the postnatal. The prenatal growth mode leads to the formation of an adult nuclear core of fixed dimensions and the postnatal, to an ever-expanding cortex. The nuclear core and the cortex have different properties and can readily be physically separated. Communication and adhesion between the compartments is poor in older lenses.
In vivo slit lamp examination reveals several zones of optical discontinuity in the lens. Different nomenclatures have been used to describe these, with the most common recognizing the embryonic, foetal, juvenile and adult nuclei as well as the cortex and outer cortex. Implicit in this nomenclature is the idea that the nuclear zones were generated at defined periods of development and growth.
This review examines the relationship between the two compartments observed in vitro and the internal structures revealed by slit lamp photography. Defining the relationship is not as simple as it might seem because of remodeling and cell compaction which take place, mostly in the first 20 years of postnatal life. In addition, different investigators use different nomenclatures when describing the same regions of the lens.
From a consideration of the dimensions, the dry mass contents and the protein distributions in the lens and in the various zones, it can be concluded that the juvenile nucleus and the layers contained within it, as well as most of the adult nucleus, were actually produced during prenatal life and the adult nucleus was completed within 3 months after birth, in the final stages of the prenatal growth mode. Further postnatal growth takes place entirely within the cortex. It can also be demonstrated that the in vitro nuclear core corresponds to the combined slit lamp nuclear zones.
In view of the information presented in this review, the use of the terms foetal, juvenile and adult nucleus seems inappropriate and should be abandoned.
Keywords: Lens, growth, nucleus, cortex, compaction, gradients, zones of discontinuity
1. Introduction
The eye lens is unique in that it grows throughout life by the addition of new cells inside the surrounding capsule. The old cells are not discarded or dismantled but, instead, are packed into the centre of the organ. This is necessary to maintain the metabolic viability of the outer cortex (and hence, that of the whole organ) and for generation of the refractive properties needed to focus images on the retina and reduce spherical aberration, but has untoward effects with advancing age, namely the development of presbyopia and cataracts.
In order to understand the processes leading to these conditions, and possibly develop strategies for their amelioration, a thorough understanding of the growth of the lens and the effects of age-related changes in its properties is required. A great deal has been learnt from animal studies, but there are substantial differences between human and non-primate lenses which make it mandatory to undertake studies on the human lens (Augusteyn, 2007, 2008). In particular, information is needed on the formation and properties of the lens nucleus to help understand its role in accommodation and in the development of presbyopia and some forms of cataract.
A substantial body of information on the internal structure and properties of the human lens has been gathered from both in vivo and in vitro studies. One would imagine that it would be relatively simple to reconcile the various observations, given that, in many cases, the locations of internal features have been carefully documented. However, this is not the case. There are significant differences in the reported dimensions and locations of internal lens features. This can be attributed, in part, to the methods used and the conditions under which the data were obtained as well as their interpretation. In vivo data may be corrected for optical or sonic distortions or uncorrected but, even when corrections are applied, they may differ between laboratories. In vitro postmortem tissue handling, prolonged storage in or out of the eye, freezing and thawing, all can alter the properties of the lens, most common being uptake (eye storage) or loss (lens freezing) of water.
In addition, major difficulties with interpreting the available data are the widely differing views of what constitutes the lens nucleus and the differing nomenclatures employed for the internal features. For example, the nucleus has been described as the lens at birth, the region involved in accommodation, all the cells inside a 350 micron zone from the outside of the lens, the tissue laid down up to age 49, the tissue comprising the central 83% of lens thickness and diameter and the central tissue with a 7 mm equatorial diameter. Some of these appear to represent the same tissues, but others do not. As noted by Duke-Elder and Wybar in 1961, as well as Brown in 1973, the anatomical definition of the nucleus is arbitrary and ambiguous and there is a need for authoritative standardization. Almost 50 years later, nothing has changed!
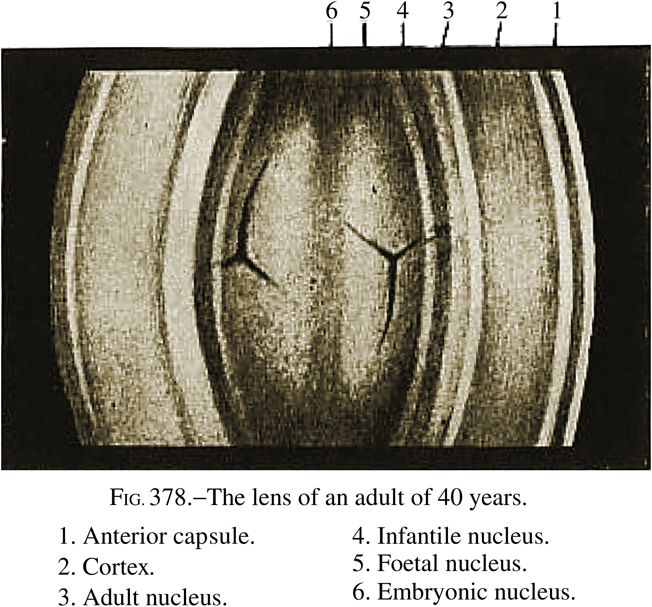
In this review, I will examine some aspects of the formation and properties of the internal structures of the human lens and attempt to reconcile in vitro and in vivo observations. Vogt’s original nomenclature, modified as described in Systems of Ophthalmology (Duke-Elder and Wybar, 1961), will be used as the primary reference since versions of this are the most frequently used. In order to avoid any misunderstandings, the Systems diagram showing this nomenclature has been reproduced in its entirety and without modification (Figure 1). Some of the ideas and information presented here have been discussed previously, in a different context (Augusteyn, 2008). It is possible that some relevant studies have been missed. It would be very much appreciated if these could be brought to my attention.
Figure 1.
Reproduction of Figure 378 from Systems of Ophthalmology (Duke-Elder and Wybar, 1961) showing the nomenclature assigned to the Optical Zones of Discontinuity. (Reproduced with permission from Elsevier Limited.) The combined nuclear layers represent 60–70% of the total lens thickness.
2. Lens growth
In order to appreciate the observations and data to be discussed, the following brief overview of human lens growth may be found useful. A more comprehensive description and analysis of lens growth in various species has been presented in a recent review by Augusteyn (2008). Lens ageing has been reviewed by Bron et al (2000).
Human lens induction occurs at around 28 days' gestation (Carnegie stage 13) with the thickening of surface ectoderm, near the optic vesicle, to generate the lens placode. This is followed by formation of a lens vesicle which, except for an anterior monolayer of epithelial cells, is filled with the linear primary fibre cells, aligned parallel to the optic axis. The vesicle is complete by around day 56 (Carnegie Stage 22) and measures ~400 µm. With the establishment of lens polarity, further growth takes place through a ubiquitous and unique mechanism in which epithelial cells in the lens germinative zone (just anterior to the equator) undergo mitosis and the daughter cells migrate to the transitional zone (posterior to the equator) where they differentiate and elongate into the secondary fibre cells. The new crescent shaped fibre cells, produced in the outer cortex (100 µm wide), are laid down in concentric shells over older cells while synthesizing large quantities of the crystallin proteins in the bow region of the lens, so called because of the arrangement of the cell nuclei. They then move into a ~25 µm wide Remodeling Zone (Lim et al, 2009) where they become disorganized and develop numerous membrane undulations. From here, they move into a region also called the Transitional Zone (not the same as that above) lose their cellular organelles and are immediately compacted to become, essentially, inert bags of concentrated protein solution. These processes continue throughout life with the older cells being packed in the centre of the organ. Since there is no loss of cells or their contents, the lens retains a record of its growth, a 3D equivalent of tree rings. This can be very useful when assessing age-related changes in the lens.
The mature secondary fibre cells span half the circumference of the lens, meeting in the centre of the anterior and posterior surfaces where their ends overlap to form the sutures. At birth, the suture pattern consists of 3 branches in a simple Y shape. However, as the lens grows larger, the sutures become more complex, forming 6 (simple star), 9 (star) and then 12 (complex star) branch structures (Kuszak, 1996). The regions in the lens containing the different suture patterns have different light scattering properties and are visible in the slit lamp as the Optical Zones of Discontinuity (Figure 1). The sutural complexity appears to be unique to primate lenses and has been suggested to generate an optically superior lens (Kuszak, 1995). Suture patterns have been used in several studies to identify regions in the lens.
As will be discussed below, the simplest and most reliable way to assess lens growth is by in vitro determination of wet weight, solids content or dimensions for lenses of different ages. Reflecting the fact that lens growth starts early in gestation, all ages used in this review will refer to the time since conception.
2.1. Lens dimensions
Many investigators have equated in vivo age-related changes in lens dimensions with growth. Such data do not necessarily reflect growth. In vivo dimensions are dependent on the interactions between the lens and other ocular components and these interactions may vary with age, optical stimulus and other influences. Thus, in vivo observations can provide information on changes in lens functional properties, rather than growth.
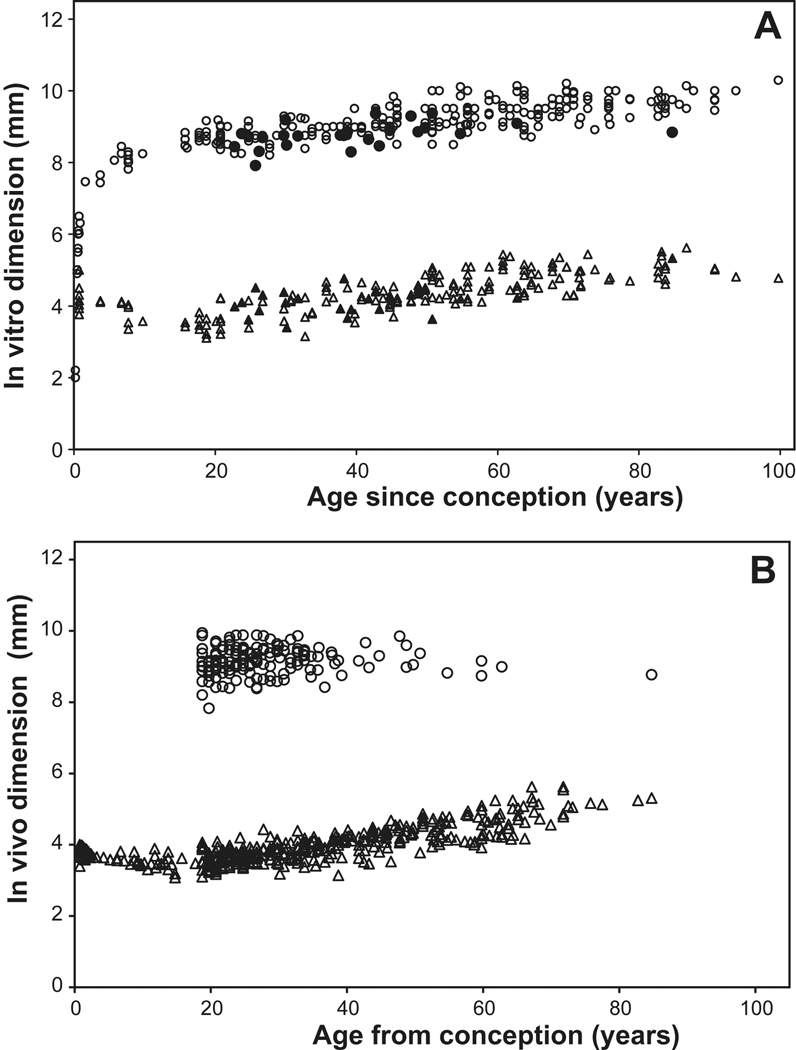
At birth, the in vitro lens measures around 6 mm in diameter and 4 mm in thickness and has a dry weight of 23–25 mg (Ehlers et al, 1968; Goldstein et al, 1998). It grows, i.e. its weight increases, through the continuous addition of new fibre cells. As would be expected from the continued growth and the way new cells are laid down, both diameter and thickness increase with age (Figure 2). However, the increases are not simple.
Figure 2.
In vitro accommodated (A) and in vivo unaccommodated (B) lens thickness ( ) and equatorial diameter (
) and equatorial diameter ( ) as a function of age. The fully accommodated in vivo dimensions of Strenk et al (1999) are included in Figure 2A as solid symbols. Data were obtained from the studies of Augusteyn (unpublished), Ehlers et al (1968), Jones et al (2007), Rosen et al (2006), Schachar 2006, Smith (1883), Strenk et al (1999), Uhlhorn et al (2008), Weekers et al (1973).
) as a function of age. The fully accommodated in vivo dimensions of Strenk et al (1999) are included in Figure 2A as solid symbols. Data were obtained from the studies of Augusteyn (unpublished), Ehlers et al (1968), Jones et al (2007), Rosen et al (2006), Schachar 2006, Smith (1883), Strenk et al (1999), Uhlhorn et al (2008), Weekers et al (1973).
During childhood, the lens is remodeled and compacted to generate the more elliptical adult shape and the refractive index gradient. Since there is no turnover of protein, the remodeling must involve redistribution of cell cytoplasm and its proteins as well as loss of water. As shown in Figure 2A, in vitro measurements indicate thickness gradually decreases from the 4 mm at birth to a minimum of around 3.3 mm in the mid to late teens, while the equatorial diameter increases. Primary fibre cell shortening is responsible for some of this decrease. When newly formed, the primary fibres are close to 400 microns long. By age 15 years, the length is 172 microns (Al-Ghoul et al, 2001). Similar changes are seen with the in vivo thickness (Figure 2B) although the data suggest that the minimum thickness may be reached some 5 years earlier (Mutti et al, 1998). More in vitro and in vivo data are needed to define the age more precisely. The thinning may be in response to zonular forces generated by growth in the axial diameter of the eye which increases from about 16 to 24 mm between birth and age 20.
The in vivo thickness has been reported to increase at linear rates varying from 0.013 to .025 mm/year in adults (Brown and Tripathi, 1974; Dubbelman et al, 2001; Jones et al, 2007; Koretz et al, 1989, 2004; Richdale et al, 2008; Strenk et al, 1999). The differences between laboratories can probably be attributed to differences in the corrections used as well as variations in the data. In vitro observations suggest adult lens thickness increases at 0.012 mm/year (Rosen et al, 2006). It is probable that the in vitro increases are asymptotic, rather than linear as concluded in the previous studies.
Once lens remodeling is complete, both thickness and diameter increase (Figure 2A), maintaining a constant (in vitro) aspect ratio of ~2.2 and a constant ratio of anterior to posterior thickness of around 0.7 (Pierscionek and Augusteyn, 1991; Rosen et al, 2006). A similar anterior/posterior thickness ratio is observed for fully accommodated lenses in vivo (Hermans et al, 2009). These two ratios are useful indicators of the condition of lenses used for in vitro measurements. Values below 2.0 for the aspect ratio generally indicate that the lens is swollen, a common occurrence with lenses left in the post-mortem eye (Augusteyn and Cake, 2005; Augusteyn et al, 2006). This is generally beyond the investigator’s control. Caution is needed when interpreting results from such lenses, given that most of the swelling occurs along the optic axis (Augusteyn et al, 2006). Data from several studies in which the aspect ratios were below 2 have been not been included in Figure 2A.
MRI measurements seem to indicate that the unaccommodated in vivo equatorial diameter does not change between the ages of 18 and 83 years, averaging 9.2–9.3 mm (Figure 2B) (Jones et al, 2007; Strenk et al, 1999). This has been interpreted as indicating there is no equatorial growth beyond age 18 (Strenk et al, 1999, 2005). However, the available data do not provide very strong support for this conclusion since most fall into a narrow age range and they vary randomly from ~8.5–9.9 mm (Figure 2B). For the same eyes, the in vivo diameter at maximum accommodation is reported to increase until about age 50, when it becomes the same as the unaccommodated diameter and stays constant thereafter (Strenk et al, 1999).
This is quite different from the in vitro observations on dimensions (Fig 2A). In vitro (fully accommodated) measurements, in several laboratories, have shown there is a definite increase in the equatorial diameter of >1.0 mm from age 20 up until age 95 (open symbols, Figure 2A) with the maximum diameter reached, close to 10mm. It is been suggested that young lenses accommodate more on removal from the eye than older lenses and that this is responsible for the smaller diameters in vitro and the apparent increase with age. Such a suggestion requires that, in vivo, young lenses cannot fully accommodate, since the maximum change on accommodation in vivo is only around 0.3 mm, well short of the observed 1 mm in vitro increase in size with age. There is no evidence that this is the case. The limited data for the fully accommodated in vivo lens have been included in Figure 2A (solid symbols). They are indistinguishable from those obtained in vitro (Fig 2A).
These various observations suggest that there may be a limit to the equatorial size which can achieved in the eye, perhaps because of constraints in the accommodative apparatus. It seems highly unlikely that the anatomical diameter does not increase, given the way new fibres are laid down in the growing lens. All must pass through the equatorial plane and would contribute to the diameter. Furthermore, zonular fibre insertion points gradually move onto the anterior lens surface with increasing age (Farnsworth and Shyne, 1979). This has to be due to the addition of tissue (i.e. anatomical growth) in the equatorial region, behind the insertion points. More data are required to overcome the variability and to determine why the in vivo accommodated lens diameter appears to have a fixed upper limit, which is substantially below the in vitro size. As pointed out earlier, in vivo measurements provide information on lens function, not on growth.
2.2 Lens weight
There have been intermittent reports, over the years, that lens weight in different species is dependent on body size, diet, environment and gender (reviewed by Augusteyn, 2008). This includes a report that men have heavier lenses than women (Harding et al, 1977). However, additional measurements and/or careful re-analysis of the published data have shown that these earlier reports are all unlikely to be correct (Augusteyn, 2007, 2008). The only significant determinant of lens weight in any species, including man, is age.
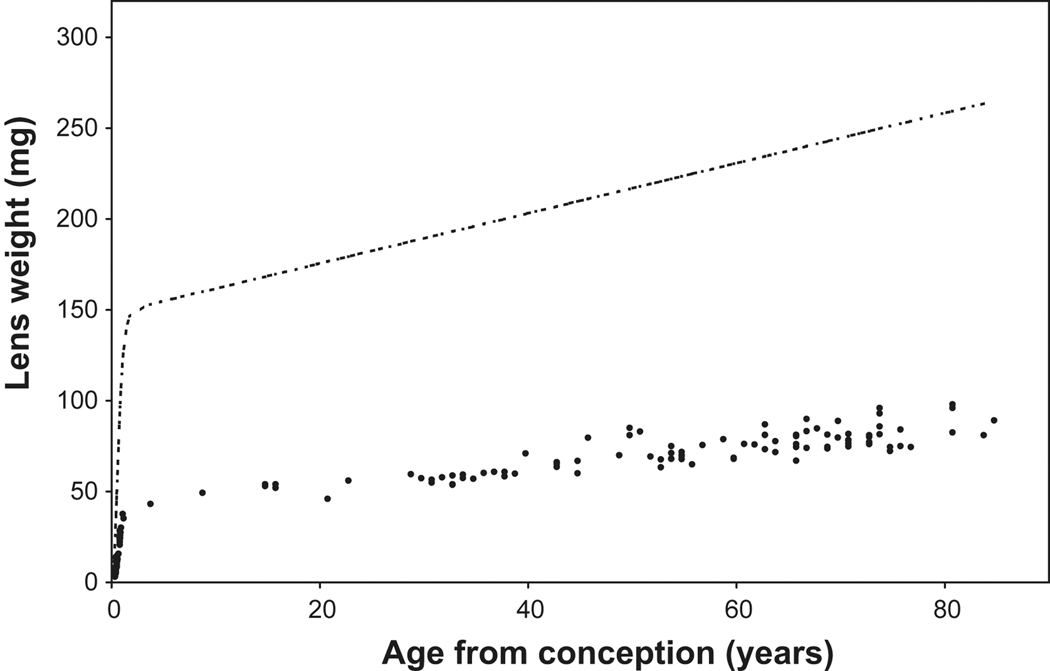
Analysis of the age-related increase in wet weight and changes in protein synthesis patterns have revealed that, unlike non-primates, human lens growth is biphasic and that different regulatory processes operate before and after birth (Augusteyn 2007, 2008). Prenatally, growth is asymptotic while, postnatally, it is linear (Figure 3). γ-crystallins are produced during the asymptotic growth but, with the exception of γS(βS)-crystallin, not in the postnatal linear growth mode (Thomson and Augusteyn, 1985) and the sizes of the newly produced fibre cells appear to be different. This generates two different compartments, the prenatal core and the postnatal cortex, which have different properties. The currently available lens wet weight data are too variable to permit determination of the precise time of the transition, but it takes place at, or soon after, birth, suggesting that the signal may be associated with birth or with the first exposure to light. However, the effects would not be seen for some time after, since, at birth, there will be a cohort of cells which were already committed to the prenatal growth mode. These will continue to undergo differentiation and maturation to form more of the prenatal lens compartment. Data on lens dry weights are presently being collected in order to pinpoint the transition time. On the basis of the limited data available so far (Figure 3), it seems the transition takes place within 3 months after birth (~1 year after conception).
Figure 3.
Lens wet ( ) and fixed dry weight (
) and fixed dry weight ( ) as a function of age since conception. The wet weight line is derived from the relationship, Lens WW = 1.38Ab+149e^[e^(1.6–3Ac)], where Ab is postnatal age in years, Ac is the time in years since conception and lens weight is in milligrams (Augusteyn 2007). The fixed dry weights are actual data available late in 2009 (Augusteyn, unpublished; Bours et al 1986; Clapp, 1913; Nordman et al 1974). The data from lenses over 20 years old fit on the straight line, Dry wt = 41 + 0.55*Ab. Inset: An expanded view of the dry weight data for the first 10 years. It should be noted that fixation increases the dry weight by 5–10% relative to the weights obtained by lyophilization or thermogravimetric analysis. Thus a lens with a dry mass of 30 mg could have a fixed dry weight of up to 33 mg.
) as a function of age since conception. The wet weight line is derived from the relationship, Lens WW = 1.38Ab+149e^[e^(1.6–3Ac)], where Ab is postnatal age in years, Ac is the time in years since conception and lens weight is in milligrams (Augusteyn 2007). The fixed dry weights are actual data available late in 2009 (Augusteyn, unpublished; Bours et al 1986; Clapp, 1913; Nordman et al 1974). The data from lenses over 20 years old fit on the straight line, Dry wt = 41 + 0.55*Ab. Inset: An expanded view of the dry weight data for the first 10 years. It should be noted that fixation increases the dry weight by 5–10% relative to the weights obtained by lyophilization or thermogravimetric analysis. Thus a lens with a dry mass of 30 mg could have a fixed dry weight of up to 33 mg.
3. Compaction
Partly because of the limitations of space and partly because of the need for a high refractive index, lens fibres are compacted. Since there is no further protein synthesis once cells have lost their organelles and entered the cortex, any increase in RI must involve a reduction in fibre cell volume due to a loss of water from the cells and extracellular space and then from the lens. Because the fibre cells must continue to span from the centre of the anterior to the centre of the posterior, this will result in a decrease in cell thickness/cross-sectional area. Although most would agree that this is the case, there are questions about when, where and how this actually takes place. In particular, while it is clear that cells are compacted as part of the maturation process when they are packed into the centre of the lens, it is not clear if there is further compaction with increasing age.
3.1 Fibre cell dimensions
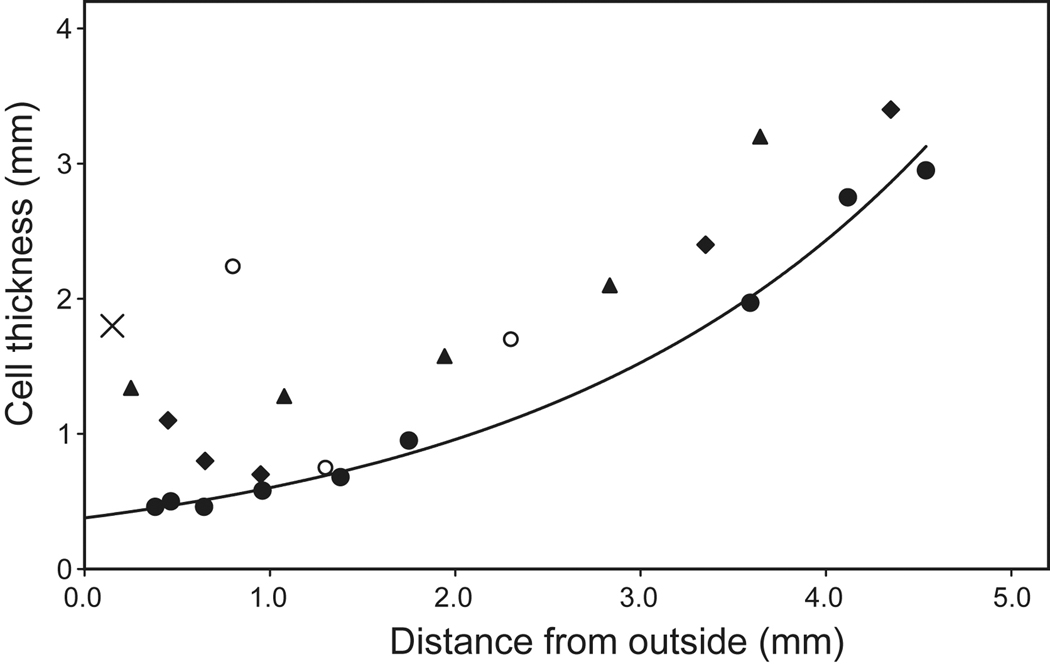
Electron microscopy has shown that fibre cells in different regions of the lens have different sizes and morphologies. New secondary fibre cells in the outer cortex of adult lenses are 1.8–2.2 µm thick (X, Figure 4) and have cross-sectional areas of ~24 µm2 (Taylor et al, 1996; Gilliland et al, 2009). The cells in the adjacent tissues are 0.46–0.95 µm thick (Gilliland et al, (2009) (Figure 4) and have cross-sectional areas of 7±2 µm2 (Taylor et al, 1996). The electron micrographs show that their membranes are extensively folded with complex interdigitations. These cells were considered to be in the adult nucleus but, from their location at 3–4.2 mm from the lens centre, are more likely to lie in the inner cortex. As can be seen in Figure 4, it would appear that they are compacted 3–4 fold immediately they leave the outer cortex. Fagerholm and Philipson (1973) observed a sharp increase in the concentration of protein at about 100 microns from the capsule along the sagittal axis. This is unrelated to the more central protein gradients which will be discussed later. Lim et al (2009) reported, using confocal microscopy, that the sharp compaction took place within 8 cell layers at the end of the Transitional Zone (0.35 mm from the capsule).
Figure 4.
Fibre cell thickness, from electron micrographs of human lenses aged 25 ( ) (Al-Ghoul et al 2001; Gilliland et al 2009), 66 (
) (Al-Ghoul et al 2001; Gilliland et al 2009), 66 ( ) (Gilliland et al 2009) and 61 years (
) (Gilliland et al 2009) and 61 years ( ) (Taylor et al, 1996) and from confocal microscopy of a 16 year old lens (
) (Taylor et al, 1996) and from confocal microscopy of a 16 year old lens ( , Lim et al 2009). The outermost point in each data set probably represents a region 0.3–0.4 mm from the capsule but may be further in. The single point at 0.15 mm (X) represents the thickness of newly formed fibre cells near the capsule of a 44 yo lens (Gilliland et al 2009). The approximate locations of the regions named the foetal (
, Lim et al 2009). The outermost point in each data set probably represents a region 0.3–0.4 mm from the capsule but may be further in. The single point at 0.15 mm (X) represents the thickness of newly formed fibre cells near the capsule of a 44 yo lens (Gilliland et al 2009). The approximate locations of the regions named the foetal ( ) and juvenile (
) and juvenile ( ) nuclei are indicated.
) nuclei are indicated.
The cells in the regions identified as the foetal and juvenile nuclei are much larger, with thicknesses ranging from 1.97–2.74 µm (Gilliland et al, (2009). Cross-sectional areas are 35 ± 22 µm2 for the foetal nuclear cells and 14 ± 5 µm2 for the juvenile nuclear cells (Taylor et al, 1996). Thus, these cells are actually larger than the newly formed fibre cells in the adult cortex, referred to above. As discussed earlier, the nuclear fibre cells and the adult cortical cells are formed through different processes - the prenatal and postnatal growth modes - and have quite different properties. The electron microscopy data indicate that there must be substantial differences in the dimensions of the secondary fibre cells produced in the different growth modes. Thus, it is not possible to make conclusions about compaction by comparing the two different cell populations.
There is an interesting aspect to the electron microscopy data, in that fibre cell thickness and cross-sectional area in the nuclear layers decrease towards the outside of the lens (Figure 4). One might have expected the opposite with continuous compaction. A similar trend can be seen in the confocal microscopy images of Lim et al (2009). Perhaps, there is an upper limit to the volume of mature fibre cells. When this is reached, cell thickness must decrease to compensate for the increasing length needed to span the greater distances, as the lens grows larger.
Comparison of the data available for 25 and 66 year old lenses (Figure 4) indicates that the cells in the older lens may be as much as 20–30% thinner, suggesting further compaction takes place with increasing age. Since the thinning of cells is found throughout the lens, it would appear that compaction is continuous, rather than intermittent. This also seems to be the case in the embryonic nucleus. In 15–25 yo lenses, primary fibre length is 172 microns and this decreases to 108 microns at age 59 and above, through formation of compaction folds along the length of the fibre (Al-Ghoul et al, 2001).
3.2. Lens gradients
Evidence for the age-related compaction of central cells has come from measurements of the RI and protein distributions within the lens and from the determination of variations in the speed of ultrasound through the lens. Unfortunately, the situation is still not clear since different techniques yield different results. Some of these data are summarized in Figure 5.
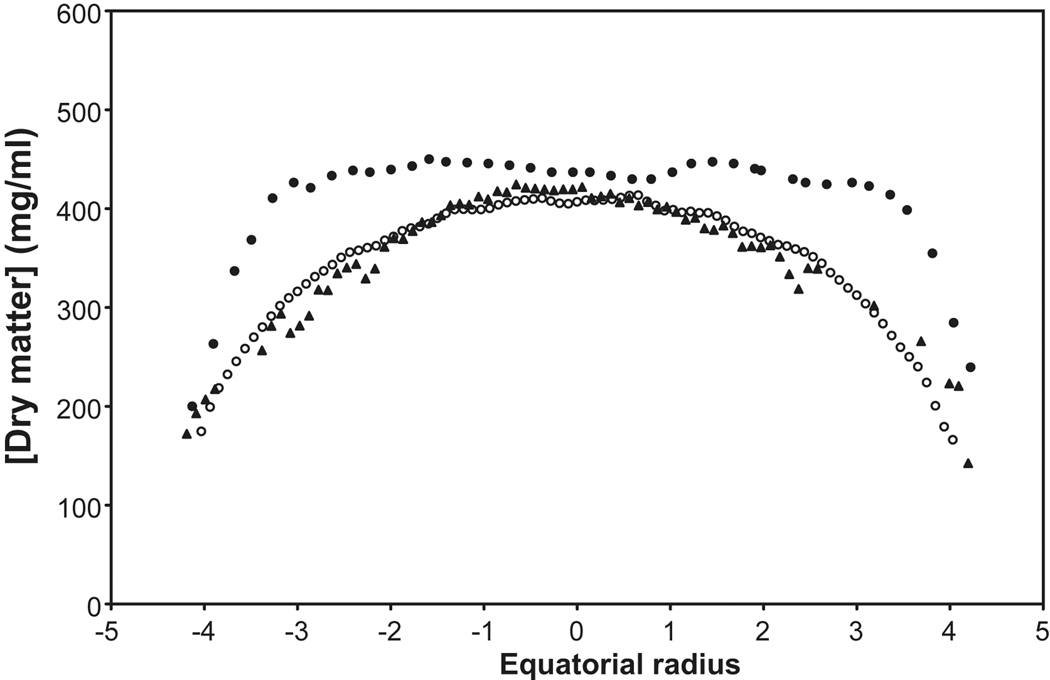
Figure 5.
The distribution of protein along the equatorial axes of human lenses measured by radiomicrography ( , 27yo; Fagerholm et al, 1981), calculated from the measured refractive index (
, 27yo; Fagerholm et al, 1981), calculated from the measured refractive index ( ; 28 yo; Augusteyn et al 2008) and from the speed of sound (
; 28 yo; Augusteyn et al 2008) and from the speed of sound ( ; 27 yo; R. Urs, 2009, personal communication). For the ultrasound calculations, a constant of 3.9 was employed, rather than the 3.2 deduced by Goss et al (1980). The microradiography distributions are sagittal data, scaled to the equatorial size. Compaction changes are more rapid in the sagittal plane than in the equatorial (Augusteyn et al 2008) so the extrapolated protein plateau size is likely to be overestimated.
; 27 yo; R. Urs, 2009, personal communication). For the ultrasound calculations, a constant of 3.9 was employed, rather than the 3.2 deduced by Goss et al (1980). The microradiography distributions are sagittal data, scaled to the equatorial size. Compaction changes are more rapid in the sagittal plane than in the equatorial (Augusteyn et al 2008) so the extrapolated protein plateau size is likely to be overestimated.
Unlike most other species, the refractive index of the human lens centre does not continuously increase with age (Pierscionek and Augusteyn, 1993). However, although it is clear that there is a RI gradient, there is still no certainty about its shape and location. In vitro MRI observations have led to the conclusion that, in young lenses (7 and 20 yo), there is a gradient of increasing RI from the outside to the centre. This can be seen in both sagittal and equatorial planes but the gradient shapes differ (Jones et al, 2005). With increasing age, the central RI increases until a maximum of 1.418 is reached. The surrounding tissues gradually follow, generating a core of constant RI which spreads outwards until around age 60 when the core reaches its final size of about 7×3 mm (Augusteyn et al, 2008). It should be noted that the young lenses from which some of these data were obtained, had substantially larger than normal thicknesses, suggesting they may have been swollen, as is often the case with lenses obtained from eye banks (Augusteyn et al, 2006). This could have altered the shape of the gradient and reduced the width of the plateau in the sagittal plane. Consistent with this, more recent in vivo MRI measurements have suggested that the equatorial plateau may be as wide as 8mm, even in young lenses, and the maximum RI my be close to 1.41 (Kasthurirangan et al, 2008).
Optical ray tracing experiments have led to somewhat different conclusions. Pierscionek and Chan (1989) reported that the maximum RI in the centre of 16 to 84 yo lenses lay between 1.40 and 1.41 and that this decreased very slowly (if at all) in the equatorial plane until 1–1.5 mm from the outside. A steep gradient down to 1.34 was located in the outer 1–1.5 mm. Thus, in contrast to the in vitro MRI observations, no equatorial RI plateau seemed to be present in lenses as young as 16. However, in a later study, using a reflectometric fibre optic sensor, it was found that lenses, aged 23, 24 and 25, had much broader gradients (Pierscionek, 1997). For the 24 yo, the gradient occupied around 70% of the equatorial diameter. This is similar to the MRI estimate of 80% (Augusteyn et al, 2008). Older lenses (>47 years) had virtually no gradient. Large differences were also observed in the edge refractive index with values of around 1.34 for some lenses and 1.38–1.39 for others, with no apparent trend with age. The low values may be due to lens swelling but the high values seem excessive compared with other estimates.
The speed of sound through biological tissues varies with protein content (type and concentration) (Goss et al, 1980). Recent studies by Urs et al (2010) have revealed that the speed of sound in the lens varies with age and with location. These speeds can be converted to protein concentrations using the relationship described by Goss et al (1980) and the acoustic constant of 3.8 determined for haemoglobin (Carstensen & Schwan, 1959). This generated protein concentrations in keeping with other estimates. As shown in Figure 5, the calculated distribution along the equatorial axis of a 27 yo lens (orange triangles) exhibits as a gradient with a 1.5–2 mm central plateau, very similar to the shape of the protein distribution calculated from the refractive index profile of a 27 yo lens (open blue circles) which has a plateau of 2 cm. In older lenses, a wider plateau develops in the ultrasound speed profile and the calculated protein concentration seems to coincide with that calculated from the RI gradients. However, data could not be obtained for the cortical gradients, making it difficult to determine the limits of the plateaus (data not shown). Further work is required to overcome problems such as this and should lead to a powerful new method for assessing lens function and properties.
Concentration plateaus and age-related widening are observed in the protein distributions determined using microradiography of lens sagittal sections (Figure 5, solid circles) (Fagerholm, et al, 1981). However, no upper limit to the size of the plateau was detected and, instead, it was concluded that the plateau represented a constant 60–70% of lens thickness from 6 months of age. This is similar to the conclusions of Pierscionek and Chan from optical measurements of the RI gradients (1989). The Fagerholm et al protein plateaus for the 1.5 and 24 year old lenses (~2 and 3 mm) were significantly wider than those observed in the 7 and 21 year old lenses using MRI (1.6 and 2.0 mm), consistent with the above suggestion that the young lenses used for MRI measurements may have been swollen.
3.3. Water content
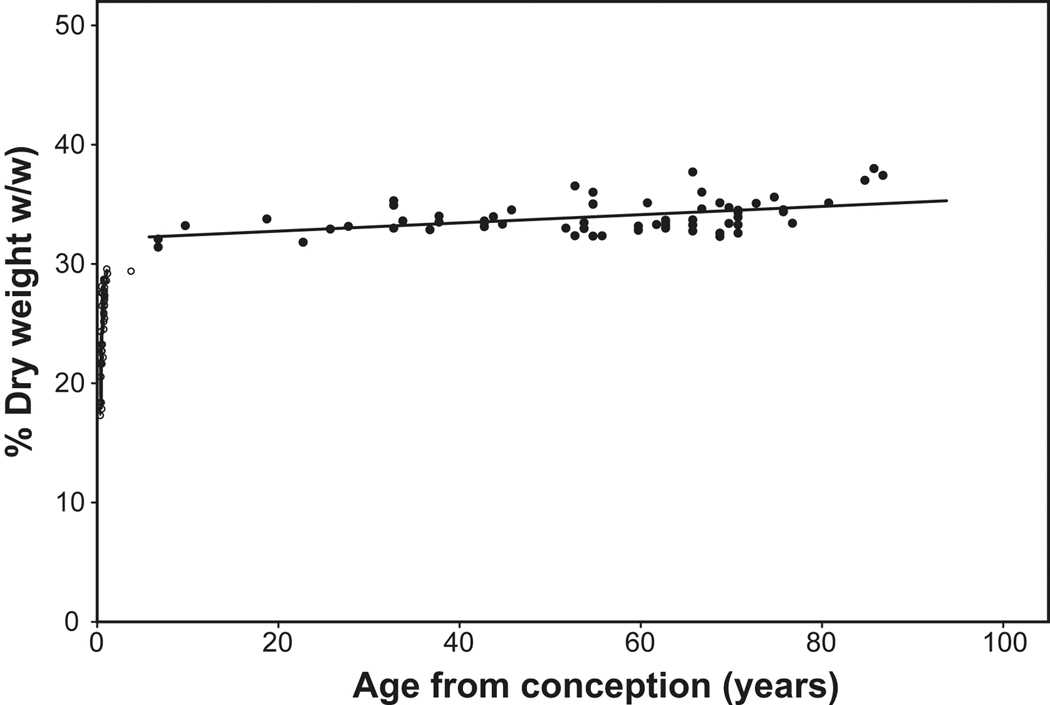
Age-related compaction is also suggested by differences in the rates of wet and dry weight accumulation (Figure 3, Figure 6). In the youngest tissues examined, dry weight represents 17% of lens wet weight. This increases rapidly so that, by 3 months after birth, it has reached 30%. Thus, the cells destined to form the adult nucleus, were largely compacted during the prenatal growth phase, but not completely. The average lens dry weight concentration increases slowly until 32% is reached around age 6 and, thereafter, appears to increase linearly by 0.034% pa to ~36% at age 93. However, the correlation coefficient for the linear fit between 6 and 93 years is low (R2=0.22) and the average for all lenses in this age range is 34 ±1.4 %. A small increase in the average would be expected if the increase in dry weight was due solely to an increase in plateau width and the remained constant. More data are required to distinguish the possibilities.
Figure 6.
Percentage fixed dry weight of lens wet weight as a function of age. The data for lenses aged 6–92 years (solid symbols) can be described by the relationship, %Fixed dry weight = 0.034*Age +32 (solid line). Percentages were calculated from data obtained in studies by Augusteyn (unpublished), Bours et al (1986), Clapp (1913) and Nordman et al (1974). (As noted previously, fixation increases dry weight so it is not possible to estimate water content from these data by simple subtraction.
The changes in dry weight/protein concentration (Figure 5 and Figure 6) indicate that water must be lost from the lens central regions until at least 60 years of age. However, this has been difficult to demonstrate. The numerous attempts to measure the water content of the nucleus have yielded values ranging from 50–85%. The lowest values have come from Raman spectroscopic studies which indicated that the water content of the nucleus increases with age (Siebinga et al, 1991), opposite to what might be expected. The Raman observations have been widely cited and used in the interpretation of other lens data. However, they are unlikely to be correct since the lenses used for the measurements had been fixed in paraformaldehyde and it has been shown that such fixation results in substantial loss and relocation of lens water (Augusteyn et al, 2006).
Van Heyningen (1972) reported that the water content of the nucleus (65% w/w) did not change with age. Fisher and Pettet (1973) also found that the water content in the lens nucleus (obtained by removing a 2 mm shell from the outside) was constant at 63.4 ± 2.8 % (w/w) for lenses aged 0–80 years. This led them to conclude that sclerosis did not occur in the ageing human lens. It is not clear how a 2mm shell could be removed from lenses under 30 years old, which have a thickness of only 4 mm or less. Furthermore, with continued growth in older lenses, the remaining tissue, taken as nucleus, would gradually increase in diameter. More recently, Heys et al (2005) obtained values of ~63–68% (w/w) for a 4 mm core from the nuclei of 13–82 year old lenses and concluded that there was no compaction in the human lens. As shown by the RI and protein distribution profiles (Fagerholm et al, 1981; Jones et al, 2005), compaction in the central 4 mm is essentially complete by middle childhood but takes place outside of this region up to about age 60. It is possible that the previous studies on the nucleus measured water content in that part of the central region which had already completed the compaction process.
Making no allowance for possible differential contributions by salts, proteins and membranes, the 1.418 RI maximum, obtained from in vitro MRI measurements, corresponds to 420mg/ml dry mass. Fagerholm et al (1981) reported 440–460 mg/ml. Both estimates are substantially higher than the 385 mg/ml which can be calculated from the van Heyningen (1972) and Heys et al (2005) nuclear water contents, the RI data of Pierscionek and Chan (1989) and the speed of sound (Urs et al 2010). In the outer cortex, the values calculated from the MRI and microradiography data are ~170 and ~200 mg/ml, respectively and ~40mg/ml from the ray tracing experiments. The reason for the large differences in the various estimates is not obvious at this time. Regardless, all sets of data show that dry mass concentration in the nucleus is 2–3 times that of the outer cortex, indicating that there has been substantial compaction of fibre cells as they are packed into the nucleus. What drives this process is not known. It appears likely that protein aggregation and modification of the specific complement of proteins present in the nucleus could play significant roles by reducing bound water (Augusteyn, 2008; Tardieu et al, 1992). However, the distribution of ions may also be involved. It has been found that the chemical potential and distribution of water in the lens are dominated by ions, particularly K+ and Cl− (A. Stevens, personal communication). Further work is required to explore this possibility.
Some of the water lost during compaction could be derived from the extracellular space which accounts for around 5% of the lens volume. Most of this would be in the outer regions of the lens, effectively reducing the concentration of dry matter. Thus, one can envisage fibre cells, which already have the maximum dry weight concentration, being more tightly packed into the centre by eliminating extracellular space. Thus, the extracellular space would move outwards gradually, maintaining a gradient in front of it and leaving a plateau behind. Such a process would be consistent with the gradual increase seen in lens stiffness, as well as the reduction in the diffusion of water and GSH into the centre (later).
What, then, can we conclude regarding age-related compaction? The in vitro MRI, cell thickness, some optical and the ultrasound speed data all indicate that the nucleus in young lenses contains a RI gradient and that compaction takes place up till about age 60 to generate a nuclear core, measuring approximately 7 × 3 mm. The slow increase in dry weight concentration, the biphasic growth mode, the formation of a diffusion barrier and the unique γ-crystallin distribution are all consistent with age-related compaction, but limited to the cells produced during the prenatal growth mode. By contrast, in vivo NMR and protein microradiography as well as some ray tracing studies indicate that the RI has already reached its maximum over the whole nuclear region very early in life and that the nucleus continues to grow larger throughout life. Measurements of water content in the lens centre are consistent with the early plateau formation. The available evidence seems to favour the view that age-related compaction occurs in the lens. However, further information is required.
3.4. Lens hardening
Lens stiffening/hardening1 has long been considered to be a major contributor to the development of presbyopia although there are dissenting views (Schachar and Pierscionek, 2007). A number of different techniques have been used in attempting to assess stiffening quantitatively but many of these suffer from drawbacks which make the data unreliable or difficult to compare (Burd et al, 2006). For example, measurements on intact lenses (Fisher, 1971; Pau and Kranz, 1991; van Alphen and Graebel, 1991; Glasser and Campbell, 1999; Weeber et al, 2007) are complicated by the influence of the capsule and provide only average properties for the whole lens. Measurements on lens fragments (Tabendah et al, 1994; Heys et al, 2005; Weeber et al, 2005) may have been affected by changes during preparation and handling of the tissue.
Recently, Heys et al (2005) and Weeber et al (2005) used Dynamic Mechanical Analysis to evaluate stiffness at different positions across the equatorial plane of bisected lenses of different ages. As would be expected, they found that the adult nucleus was stiffer than the cortex and reported that stiffness in all parts of the lens increases between ages 30 and 50 years, by up to 1000 (Heys et al, 2005) or 10,000 (Weeber et al, 2005) fold in the centre. Both groups concluded that there was also an increase in cortical stiffness, from measurements at 3–3.5 mm from the lens centre. However, as will be discussed later and can be seen in Figure 7, a point 3–3.5 mm from the centre actually lies near the border of the prenatal and postnatal tissues. Thus the observed increase in cortical stiffness probably reflects changes taking place in the outer edge of the adult nuclear core, near the interface with the cortex. In a promising development, which may eliminate the need to study fragmented lenses, Hollman et al (2007) used acoustic radiation force to move laser-produced bubbles in different regions of the lens. The movement of the bubbles was consistent with the stiffness trends reported by Heys et al and Weeber et al.
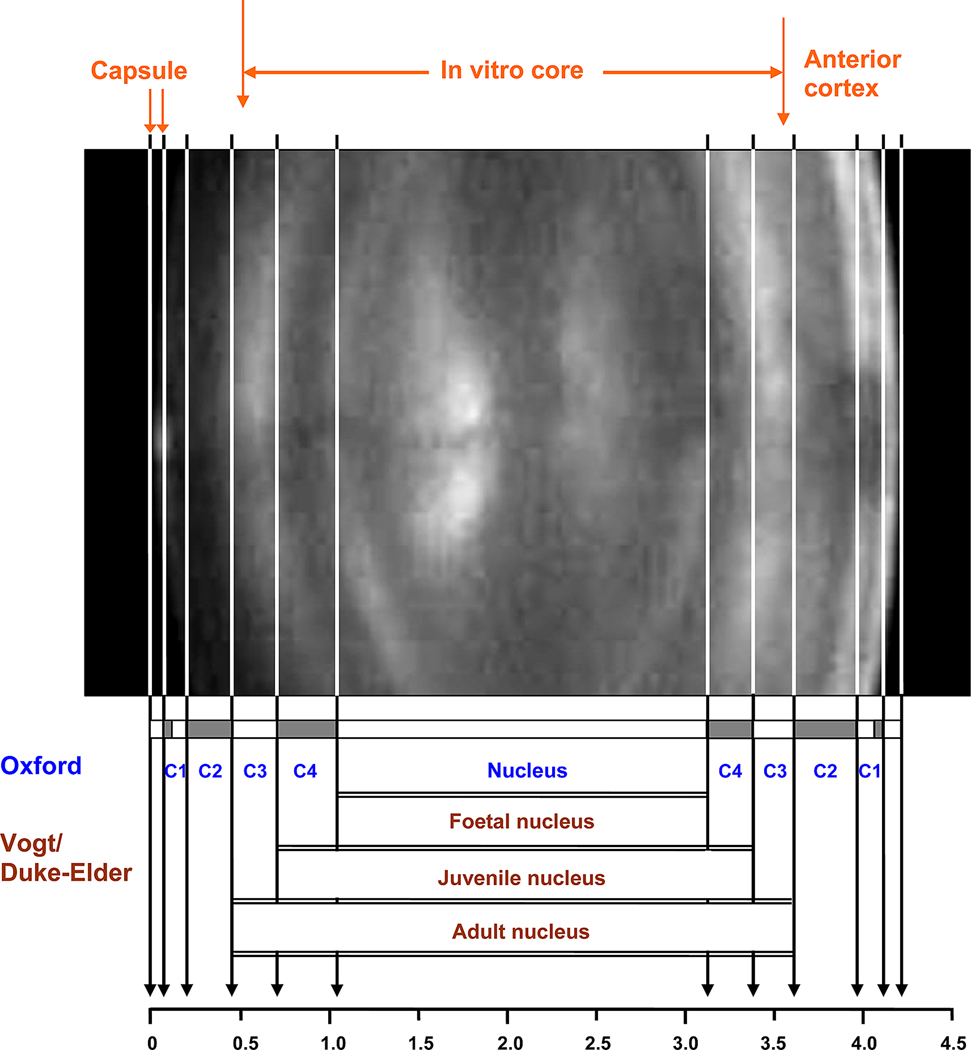
Figure 7.
Scheimpflug photograph of a 51 year-old lens with the boundaries of the Zones of Optical Discontinuity outlined. Zones which appear light in the figure are those which scatter light back to the observer while the dark zones are more transparent. The modified Vogt and Oxford nomenclatures for the various zones are shown. A scale is included to allow assessment of the sizes of the features. The slit lamp photograph was kindly supplied by Dr M Dubbelman.
There appears to be no quantitative relationship between compaction and stiffness. This may be seen by comparison of the stiffness measurement with the RI in the same position. In a 27 yo lens, the RI at 3 mm from the centre along the equatorial axis, is ~1.400 (Figure 6). In lenses > 60 yo, it is 1.418 (Jones et al, 2007). This equates to a 30% increase in protein concentration and a 2.5% increase in density. However, the stiffness at this position increases by almost 4,000%, from around 70 Pa to 2700 kPa! Furthermore, the RI is virtually constant in the lens centre after age 20 years, but stiffness increases at least 1000 fold. It must be concluded that stiffness is generated through a process other than compaction. This could involve changes in cellular interactions, as suggested by the observation that, when old human lenses are lyophilized, the nucleus remains intact, while the cortical tissues disintegrate, as do young lenses (Truscott and Augusteyn, 1977). Fisher and Petit (1973) came to a similar conclusion, that changes in lens deformability were due to an ageing change, not related to lens moisture content.
Another possibility is that the increase in stiffness is due to modification of the crystallins. It is well known that lens proteins become progressively modified with age through polypeptide shortening, racemization, deamidation, crosslinking and a variety of other processes (Wilmarth et al, 2006). Some of these modifications may be required to promote protein-protein interactions, reducing bound water and allowing the compaction of cells through elimination of the excess water. Continued modification, beyond that required for compaction, could be responsible for the increasingly larger amounts of insoluble protein, most from the nucleus, which are obtained when transparent lenses of increasing age are disrupted. Much of the insoluble protein is α-crystallin which completely disappears from the <1×106 Da molecular mass soluble protein fraction in the nucleus by around age 40, possibly as a consequence of its chaperoning activity (Roy and Spector, 1976). It has been suggested that the decreased availability of soluble α-crystallin may be involved in the stiffening of the lens (Heys et al, 2007). Support for this suggestion comes from the observation that heating pig lenses at 50°C leads to insolubilization of the lens structural proteins, loss of soluble α-crystallin and a concomitant increase in stiffness (Heys et al, 2007). Whether ‘cooking’ a pig lens is relevant to the age-related hardening of the in vivo human lens, remains to be established.
More recent observations suggest that binding of proteins to cell membranes may be involved. Friedrich and Truscott (2009) used density gradient centrifugation to examine age-related changes in the association of proteins with membranes. They found two bands of high density which appeared around age 50 in the central 4.5 mm diameter core, coincident with a sharp decrease in soluble protein. These bands contained >70% of the total membrane lipids and around half of the total protein. With increasing age, the bands gradually spread outwards into the region between 4.5 and 6 mm and their concentrations reached a maximum around age 60.
4. The internal structure of the lens
4.1 In vivo observations
In vivo slit lamp biomicroscopy of the lens (Figure 1, Figure 7) reveals a number of alternating light and dark zones which are referred to as Zones of Optical Discontinuity or simply as Zones of Discontinuity. The most commonly used descriptive names for the zones- the embryonic, foetal, juvenile (infantile) and adult nuclei and the cortex - were introduced by Vogt (Vogt 1919, 1931). As the names imply, the nuclear zones were thought to be generated during specific periods of life. Together, they represent 60–70% of adult lens sagittal thickness. (Brown and Tripathi, 1974; Dubbelman et al, 2003; Duke-Elder and Wybar, 1961).
Scheimpflug photography is used for the assessment of light scattering due to cataract and two classification systems, based on densitometric scans of the photographs, have been devised for the zones in order to facilitate the description of cataract locations. These were developed in Oxford (Sparrow et al, 1986) and Bonn (Hockwin et al, 1982). Most published studies on lens internal structure and properties have used the Oxford and/or the historical Vogt nomenclature, which will also be used in this review. The Bonn system for cataract assessment has been recently reviewed by Wegener and Laser-Junga (2009).
A recently obtained Scheimpflug photo is presented in Figure 7, with the boundaries of the various zones indicated together with the Vogt and Oxford nomenclature.
The Oxford system identifies 4 cortical zones, C1, C2, C3 and C4, as well as the nucleus (Sparrow et al, 1986; Brown and Tripathi, 1974). The outer cortex, C1, comprises the C1α and C1β regions, also named the Subcapsular Clear Zone and The Zone of Disjunction, respectively. Confocal microscopy (Lim et al, 2009) indicates that C1β corresponds to the Transitional Zone (125–350 mµ from the capsule) while the 100mm clear zone under the capsule together with the Remodeling Zone constitute C1α. It may be seen from Figure 7 that the central zones overlap with those named by Vogt. Thus, C4 corresponds to the juvenile nucleus and C3 to the adult nucleus. The Oxford nucleus is defined as the lens mass present at birth and therefore would correspond to the Vogt foetal nucleus. This includes the embryonic nucleus which is comprised of the primary fibre cells, those laid down by 56 days gestation when the lens bag was first filled with cells. It is the foetal lens which is involved in accommodation (Brown, 1973) and which is considered to become the hard coloured nucleus which can ultimately create difficulties during phacoemulsification in cataract operations.
The Zones of Discontinuity can be distinguished on the basis of changes in the complexity of their suture patterns (Kuszak, 1994). The simplest pattern, the Y suture is found in the foetal nucleus. Further out from the lens centre, the sutures become increasingly more branched and complex. In the juvenile nucleus, the suture changes to a 6-branched structure, referred to as the simple star. In the adult nucleus the 9-branch star pattern is found while in the cortex, the 12-branch complex star suture pattern is observed.
4.2 Growth of in vivo layers
A number of conclusions have been made about the growth of the lens as judged from in vivo changes in the thickness of the various layers. Caution is needed when interpreting such data from young subjects. The combined effects of remodeling and compaction can complicate interpretation of the data.
Early studies concluded that the anterior cortex of the adult lens grows by 7 µm per year (Huggert, 1946) and led Bellows (1968) to formulate the term Phakochronology to describe the technique for determining the time of events affecting the lens from the distance between the capsule and the region of interest. For example, an opacity at 21 µm from the capsule would be estimated to have formed 3 years previously. More recent observations, as well as geometrical considerations, suggest this may be an oversimplification. In order for the thickness radius to increase by a constant amount each year, it would be necessary for lens growth rate to increase with age.
In general, it is agreed that lens growth gives rise to a continual increase in the width of the in vivo cortex (Brown and Tripathi, 1974; Cook et al, 1994; Dubbelman et al, 2003; Duke-Elder and Wybar, 1961). Dubbelman et al (2003) determined the thickness of the various cortical zones from corrected Scheimpflug images. They found that C1 and C3 were of constant thickness from age 16 to age 66 whereas C2 increased during this period at 0.012 mm/yr. This is consistent with the increase in total lens thickness of 0.013–0.025 mm/year estimated from in vitro measurements (Rosen et al, 2006; Schachar, 2005). In vivo measurements on unaccommodated subjects, have suggested that growth is greater in the anterior cortex than that in the posterior (Cook et al, 1994).
The situation is not so clear with the nucleus. Brown’s slit lamp observations (1973) suggest that the thickness of the in vivo nucleus increases from around 2.7 mm at age 8 years, to a plateau of 3 mm around age 30–40. By contrast, over the same period, the equatorial diameter of the nucleus was found to decrease from 7.5 mm to around 6.5 mm, presumably as part of the remodeling process. It is probable that Brown’s nucleus represents the foetal nucleus since the data were not corrected for optical distortion by the lens. As shown by Hermans et al (2007) and Dubbelman et al (2003) these corrections are age-related and can result in large decreases in the estimated dimensions, especially in the equatorial diameter. In unaccommodated young adult lenses, the foetal nucleus has a (corrected) sagittal thickness of 2.4–2.5 mm and diameter of 5.6–6.0 mm (Hermans et al 2007). There have been reports that the thickness of the foetal nucleus both increases (Dubbelman et al, 2003) and decreases (Cook et al, 1994) continuously with age, with a maximum change of about 10% for thicknesses of around 2.2 and 2.0 mm, respectively. The differences in these various reports may be a function of the protocols employed to correct for image distortion by the ocular tissues. Examination of the latter two sets of data, which show wide variations, suggests that the difference is more likely due to the scatter of the data and that there is probably no change.
In view of the growth of the lens, a small decrease might be expected in the nuclear thickness due to the compaction of the fibre cells. As shown earlier (Figure 4), electron and confocal microscopy measurements of cell sizes in the various nuclear layers indicate that cell thicknesses decrease by 20–30% between ages 15 and 59 (Al-Ghoul et al, 2001; Lim et al, 2009; Taylor et al 1996). It would be expected that this would significantly reduce the diameters of the nuclear layers. Yet, the Scheimpflug studies indicate that there is probably no (or very little) change in the size of the nuclei. From a consideration of the RI gradients in the sagittal plane (Jones et al, 2005), it may be estimated that, between ages 16 and 60, the tissue inside the nuclear core will be compressed by a maximum of ~8%. If uniformly distributed, this would equate to a 2.5% reduction in the dimensions of the core, i.e. <0.1 mm for the thickness, small enough to escape detection, given the variability commonly observed in slit lamp data.
It was reported by Koretz et al (1994) that, with increasing age, the inner boundaries of all the outer Zones of Discontinuity remained at fixed distances from the centre of the lens, indicating that there was no growth in these zones (Figure 4). This is not consistent with other reports in the literature, including one from the same laboratory (Cook et al, 1994). Close examination of the data presented by Koretz et al suggests that, as found in other studies, there may actually be an increase in their Zone 3, i.e. C2, which is masked by some outliers.
4.3. In vitro observations
As described earlier, human lens growth is biphasic and generates two distinct lens compartments, the prenatal and the postnatal. Several in vitro observations also indicate that the prenatal growth mode leads to the formation of a nuclear core of fixed dimensions and properties in the adult lens and the postnatal growth produces an ever-expanding cortex.
Lyophillization of intact adult human lenses (normal and cataractous) reproducibly leaves a flakey residue from the cortex and an intact dry pellet from the nucleus (Truscott and Augusteyn, 1977). The weight of this pellet, at 31 mg, is virtually identical to that calculated for the dry weight content of the core. Both weights are close to the asymptotic 35 mg, determined from preliminary logistic analysis of the prenatal dry mass accumulation (Figure 3 and Augusteyn, unpublished).
Hydrodissection (Ayaki et al, 1993; Gullapilli et al, 1995) or, more simply, gentle stirring of decapsulated lenses in buffer (Augusteyn, unpublished) readily removes the outer cortical layer from adult lenses, leaving a relatively stable core measuring 6.5–7 × 3 mm. These and the above observations suggest that there are differences in the cellular adhesion properties of the nuclear core and the cortex.
Garland et al (1996) manually peeled layers (shells) of cells from a large number of fresh lenses. They observed that, in all adult lenses, ‘as successive shells were peeled, there was a point at which there was a very clear demarcation between shells’. This occurred when the remaining lens core measured 7 mm in diameter. The authors identified this, from the suture pattern, as the outer edge of the adult nucleus and noted that the size of this core was constant after age 20. Thus, it would seem that the core is already present at an early age, apparently well before the refractive index plateau has reached its maximum diameter.
By contrast, Nordmann et al (1974) had earlier reported that the size of the anatomical nucleus, obtained by “unshelling between two fingers or with a soft brush”, increased with age. Although there appeared to be substantial differences between a 25 yo and a 53 yo nucleus, no dimensions were provided so it is not possible to determine exactly what Nordmann and colleagues were examining.
The central plateau of constant RI, which develops with increasing age, reaches its maximum size around age 60 and encompasses a spheroidal compartment with a diameter of 7 mm and a thickness of 3.05 mm. This corresponds to the prenatal compartment. It was noted, earlier, that ultrasound speed measurements in the equatorial plane are consistent with such a plateau but protein distribution for the sagittal plane suggest that there is no upper limit.
Electron microscopy observations indicate that fibre cells produced during the prenatal growth phase are larger than those produced postnatally. The various observations, discussed above, suggest that the cells laid down during the prenatal growth phase have different cell-cell adhesion properties which enables them to remain connected during lyophilization, shell peeling and hydrodissection. By contrast the cortical cells do not have such adhesive properties and readily separate from each other and from the cells at the surface of the core. Similar observations were made by Fisher and Pettit (1973) from an analysis of lens responses to centrifugal strain and the ability to remove water by drying. They concluded that adhesion between nuclear fibres increased with age but not between cortical fibres.
4.4. The nuclear-cortical boundary
Consistent with the conclusions about a 2-compartment lens, is the observation that there is limited communication between the two compartments, i.e. the nuclear core and the surrounding cortex. This may be related to the weak adhesion between the two compartments.
Studies on the lenticular distribution of [35S]glutathione (metabolically generated from [35S]cysteine provided during lens organ culture) have revealed that there is a barrier to its diffusion into the centre (Sweeney and Truscott 1998). This barrier is located at the edges of a nuclear core, estimated to measure 7.2 × 2.8 mm, and starts significantly limiting the movement of GSH at around age 30. Even the diffusion of water is slowed in this region (Moffatt et al, 1999). Maximum impediment to GSH diffusion is reached some time between 50 and 60 years. Interestingly, this coincides with the age at which the RI plateau (Augusteyn et al, 2008) and the nuclear stiffness (Heys et al, 2005) reach their maxima but it is not known if these events are related.
To date, the origin of the diffusion barrier has not been identified. One possibility, recently mooted, is that the membranes channels/pores required for the movement of water and GSH, become blocked through the binding of proteins (Korlimbinis et al, 2009). It has been observed that, between ages 50 and 60 years, large amounts of proteins become associated with the membranes in the central 6 mm of the lens (Friedrich and Truscott, 2009). No information is available on the remaining outer layers of the lens, including the actual barrier region, located around 7 mm. However, it seems unlikely that protein binding is responsible for the barrier since diffusion is already significantly affected by age 30, long before any protein binding to the membrane is observed.
It has recently been suggested that the GSH barrier may be the same as the barrier to the penetration of Texas red-dextran (Lim et al, (2009) and Lucifer yellow (Grey et al, 2003). However, this latter barrier is at a fixed distance (0.35 mm) from the outside of the lens and is located in the extracellular space, whereas the GSH barrier is located at a fixed distance from the centre, and an ever-increasing distance from the capsule, and would most likely be intercellular. It seems probable that the Texas red-dextran barrier is located at the interface between the Transition (C1β) zone and the cortex (C2) and its creation coincides with the insertion of MP20 into the membrane and the degradation of fibre cell nuclei (Grey et al, 2003).
4.5. The relationship between in vivo and in vitro nuclei
Comparison of the dimensions and properties of the different zones and, in particular, their suture patterns, indicates that the nuclear core corresponds to the adult nucleus (C3) and the layers within, as defined by slit lamp photography (Figure 7).
The thickness of the adult nucleus, obtained from Scheimpflug images, varies from 2.8–3.2 mm, the same as the size range reported for the nuclear core. The nuclear core contains 30–31 mg of dry mass. As will be discussed later, calculations of the dry mass contents, (Table 1) reveals that the adult nucleus contains the same amount of dry mass as the core.
Table 1.
Estimation of the age of completion for in vivo nuclear layers in a 60 yo lens.
| Layer | Dimensions2 mm |
Calculated dry weight mg |
Postnatal Age when completed years |
|---|---|---|---|
| Foetal nucleus | 2.3 × 4.6 | 12 | −0.4 |
| Juvenile nucleus | 2.5 × 5.8 | 16 | −0.2 |
| Adult nucleus | 3.1 × 6.9 | 30 | 0.3 |
| In vitro nuclear core | 3.0 × 7.0 | 31 | 0.3 |
The dimensions used for the nuclear layers were obtained by averaging data from several in vivo slit lamp studies ((Cook et al, 1994; Dubbelman et al, 2003; Hermans et al 2007). Where no data were available for the diameter, an aspect ratio of 2.2 (Rosen et al 2006) was used to calculate diameter from the known thickness. The nuclear core dimensions were taken from Augusteyn et al (2008).
Further evidence that the core and adult nucleus are the same may be found in the experiments of Garland et al (1996). They reported that the zone with the adult star suture pattern had an equatorial diameter of 7 mm, the same as that of the nuclear core. They also showed, using 2D electrophoresis, that the protein distributions in the various nuclear layers, within the 7mm equatorial diameter, were indistinguishable, but clearly different from those in the cortex. This suggests a common growth mode for all the nuclear layers, ie. the prenatal growth mode. These observations are consistent with the demonstration that the protein expression pattern in newly differentiated fibre cells changes at, or soon after birth (Thomson and Augusteyn, 1985). The most notable changes are the cessation of γ (C and D)-crystallin synthesis and the increase in the production of γS(βS)-crystallin. As a result, γC and γD-crystallins are confined to the core of the lens while γS(βS)-crystallin is the major low molecular weight in the cortex.
There are considerably differing opinions regarding the locations and sizes of the nuclei, which make it difficult to be absolutely certain of the alignments. For example, estimates of the outer radius of the adult nucleus range from 60 to 84% of lens thickness, dependent on age. Some of the variations would be due to the continued growth of the cortex while others can be attributed to differences in the corrections used for optical distortion in slit lamp images, although these tend to be small for the thickness (Hermans et al, 2007). The highest values for the nuclear thickness were obtained by electron microscopy of fixed lenses. Even though the zone identifications were based on the same changes in suture patterns, the reported cortex sizes of 16 and 17% of lens diameter, and thickness, respectively (Taylor et al, 1996), are a little over half of the sizes that have been observed in other studies (Brown and Tripathi; 1974; Cook et al, 1994; Dubbelman et al, 2003; Duke-Elder and Wybar, 1961; Garland et al, 1996) This suggests that there may have been selective shrinkage of the cortex during fixation which could have resulted in an overestimate of the remaining tissue size. It has been shown that fixation induces a significant loss of water from the human lens (Augusteyn et al, 2006). Since no actual dimensions were reported, it is not possible to assess whether localized shrinkage had taken place.
5. How old are the various layers really?
As described earlier, the internal central structure of the lens is commonly described in terms of multiple nuclear zones, referred to as the embryonic, foetal, juvenile and adult nuclei (Vogt, 1919). Inherent in this terminology is the assertion that these layers were formed at the corresponding time of life. This concept has been retained to the present day.
Koretz et al (1994) estimated the ages at which the zones started to form by reference to the growth of anterior lens thickness as a function of age. They concluded that formation of the foetal, juvenile, and adult nuclei and the cortex originated at 4, 9, 19 and 46 years, respectively. It would appear that the authors erred in their identification of the zones. From their published dimensions, the estimate of 4 years should actually be associated with the juvenile nucleus (and so on for the other layers), making the age estimates more consistent with the historical terminology. However, the estimates were made using a simple polynomial model of growth, based on the assumption that lens thickness increases continually from birth. This is not correct. Lens thickness does not increase simply with age and the anterior thickness is not around 0.5 mm at the age of 1 year, as predicted by the authors’ model. In vivo lens thickness actually decreases from birth until the mid to late teens before it starts to increase again (Figure 2). Changes in cortical thickness cannot be used with young lenses because of the remodeling and are only useful for obtaining rough estimates of ages in the cortex of adult lenses.
What, then, are the ages of the various layers? These can be more reliably estimated from a consideration of the growth of the lens and calculations using in vitro data on lens dry weight and volumes.
The shapes of the various nuclear zones are similar to the shape of the lens (Taylor et al, 1996), so it is possible to calculate reasonably accurate volumes for the various nuclear zones from their dimensions, where known, or from the known thickness and estimated diameter, calculated using the aspect ratio of 2.2 (Rosen et al, 2006). Together with the dry mass concentration, which can be calculated from the refractive index, and the relationship between dry weight and age (Figure 3; Augusteyn, unpublished), it is then possible to estimate the age at which each nuclear layer/Zone of discontinuity was completed. The calculations for a 60 year-old lens are shown in Table 1. This age was chosen since the concentration of dry matter is constant throughout the central 7×3mm region at 385 mg/ml (Augusteyn et al, 2008; Heys et al, 2005), making the calculations a little simpler.
The calculations are very informative. For example, in the 60 year-old lens, the ‘adult’ nucleus is estimated to contain 30 mg of dry mass. This is the same as the dry mass found (Truscott and Augusteyn, 1977) and calculated (Table 1) for the in vitro nuclear core, consistent with the conclusion that the core and the adult nucleus are the same. However, it is obvious that this amount of dry matter cannot be adult in origin. The newborn lens already has a dry mass of 23–25 mg. Thus, only 5–7 mg of dry mass need to been added to complete the adult nucleus/nuclear core. Preliminary data on the dry weights of lenses (Figure 3; Augusteyn unpublished) indicate that this additional mass is laid down within the first 3 post natal months, consistent with the expectation of a brief period of continued growth in the prenatal mode, outlined earlier. More data are needed for lens dry weights in the first two years of postnatal life for a more precise estimate of when the nuclear dry mass accumulation is complete.
However, substantial and reliable data are available for prenatal dry weights (Figure 3). These show that the definition of the adult foetal nucleus as the lens at birth, is not sustainable. The 60 yo foetal nucleus contains only 12 mg of dry mass (Table 1), barely half that of the newborn lens. Even the juvenile nucleus has much less dry mass than the new born lens. Clearly, the tissues giving rise to the foetal as well as the juvenile nuclei were laid down long before birth. The conclusions from dry weight determinations are consistent with the biphasic growth of the lens and the different γ-crystallin distributions in the nucleus and cortex discussed earlier.
Thus, it is clear that the fibre cells comprising the various nuclear layers are generated during the prenatal growth mode, much earlier than the descriptions of the layers would imply. However, the optical discontinuities, which are used to distinguish these, may not form until later. Their appearance may be related to the remodeling early in life as well as the gradual compaction which takes place up to about age 60. It may be that the Zones of Discontinuity become visible when extracellular space has decreased and light scattering centres, such as interactions between fibre cells at the sutures, are brought closer together.
In view of the above considerations, it is suggested that the descriptions of foetal, juvenile and adult nuclear layers be abandoned since they have no relevance to the age of the tissue. The zones of discontinuity appear to represent regions of the lens where the suture pattern changes, rather than defined growth periods. It is further suggested that the term nucleus be restricted to that part of the lens which was generated by the prenatal growth mode, i.e. until about 3 months postnatally. This is very similar to the clinicians' definition of the nucleus (the lens at birth) and would also correspond to the nuclear core identified in vitro. Further studies on specific regions of this nuclear core, such as that involved in accommodation, should specify the location or function, not the perceived origin.
6. Conclusion
In order to understand the mechanisms underlying the development of presbyopia and cataracts and to develop possible treatments, a thorough understanding of the growth of the lens would be of enormous value. It is hoped this review will have demonstrated that, at best, our knowledge is fragmentary and concepts, long considered to be set in concrete, were actually built on very shaky foundations.
Growth of the lens is far more complex than implied by the simple model of an onion like structure comprised of concentric layers of identical fibre cells laid down in specific periods of life. Not all lenses follow the same growth pattern. In particular, the biphasic growth of human (and probably all primate) lenses is different from the continuous single mode growth in non-primates and generates an organ with quite unique properties. Consequently, data obtained from species, other than primates, are unlikely to be applicable to the human lens.
Although great progress has been made in recent years in characterizing lens properties such as hardness, cell dimensions and concentration gradients, there is still no general agreement on most properties and many questions remain unanswered. Much more needs to be done with human lenses. Given the difficulties in obtaining tissues which have not been altered by storage, it may be a long time before we can say with certainty what lens changes lead to presbyopia and cataract.
Supplementary Material
Acknowledgements
This work was supported, in part, by NIH Consortium Grant RO1EYO14225 and the Australian Federal Government CRC Scheme through the Vision Cooperative Research Centre. The author is very grateful for the helpful discussions and/or unpublished data provided by David Atchison, Tony Bron, Joe Costello, Michiel Dubbelman, Arthur Ho, Jerome Kuszak, Jean-Marie Parel, Barbara Pierscionek, Roger Truscott, Raksha Urs and the many others who helped shape my views over the years.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Although stiffness and hardness are different properties of a material, no distinction is made in the present discussions since they will have similar effects on lens functioning.
References
- Al-Ghoul KJ, Nordgren RK, Kuszak AJ, Freel CD, Costello MJ, Kuszak JR. Structural evidence of human nuclear fiber compaction as a function of ageing and cataractogenesis. Exp. Eye Res. 2001;72:199–214. doi: 10.1006/exer.2000.0937. [DOI] [PubMed] [Google Scholar]
- Atchison DA, Markwell EL, Kasthurirangan S, Pope JM, Smith G, Swann P. Age-related changes in optical and biometric characteristics of emmetropic eyes. J. Vision 8. 2008;29:1–20. doi: 10.1167/8.4.29. [DOI] [PubMed] [Google Scholar]
- Augusteyn RC. Growth of the human eye lens. Mol. Vis. 2007;13:252–257. [PMC free article] [PubMed] [Google Scholar]
- Augusteyn RC. Growth of the lens: in vitro observations. Clin. Exp. Optom. 2008;91:226–239. doi: 10.1111/j.1444-0938.2008.00255.x. [DOI] [PubMed] [Google Scholar]
- Augusteyn RC, Cake MA. Post mortem uptake of water by sheep lenses left in the eye. Mol. Vis. 2005;11:749–751. [PubMed] [Google Scholar]
- Augusteyn RC, Jones C, Pope J. Age-related development of a RI plateau in the human lens: evidence for a distinct nucleus. Clin. Exp. Optom. 2008;91:296–30. doi: 10.1111/j.1444-0938.2007.00244.x. [DOI] [PubMed] [Google Scholar]
- Augusteyn RC, Rosen AM, Borja D, Ziebarth NM, Parel JM. Biometry of Primate Lenses during Immersion in Preservation Media. Mol. Vis. 2006;12:740–747. [PubMed] [Google Scholar]
- Augusteyn RC, Vrensen GJM, Willekins B. The effect of paraformaldehyde fixation and PBS storage on the water content of the human lens. Mol. Vis. 2008;14:90–94. [PMC free article] [PubMed] [Google Scholar]
- Ayaki M, Onde H, Yokoyama N. Size of the lens nucleus separated by hydrodissection. Ophthalmic Surg. 1993;24:492–493. [PubMed] [Google Scholar]
- Bellows J. Phakochronology. The study of dating structural changes in the lens. Br. J. Ophthalmol. 1968;52:540–545. doi: 10.1136/bjo.52.7.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bours J, Fodisch HJ. Human fetal lens: wet and dry weight with increasing gestational age. Ophthalmic Res. 1986;18:363–368. doi: 10.1159/000265464. [DOI] [PubMed] [Google Scholar]
- Bron AJ, Vrensen GFJM, Koretz JF, Maraini G, Harding JJ. The ageing lens. Ophthalmologica. 2000;214:86–104. doi: 10.1159/000027475. [DOI] [PubMed] [Google Scholar]
- Brown NA. The Human Lens in Relation to Cataract, Ciba Symposium 19. Elsevier London: 1973. Lens changes with age and cataract; slit-image photography, in The human lens in relation to cataract; pp. 65–71. [Google Scholar]
- Brown NA, Tripathi R. The loss of the anterior sub-capsular clear zone of the lens; prognostic significance in cataract formation. Trans. Ophthalmol. Soc. U.K. 1974;94:29–45. [Google Scholar]
- Burd HJ, Wilde GS, Judge SJ. Can reliable values of Young’s Modulus be deduced from Fisher’s (1971) spinning lens measurements? Vis. Res. 2006;46:1346–1360. doi: 10.1016/j.visres.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Carstensen EL, Schwan HP. Acoustic properties of haemoglobin solutions. J. Acoust. Soc. Am. 1959;31:305–311. [Google Scholar]
- Clapp CA. A communication upon the weight of infants’ lenses and their solids. Arch. Ophthalmol. 1913;42:619–624. [Google Scholar]
- Coghlan SD, Augusteyn RC. Changes in the distribution of proteins in the aging human lens. Exp. Eye Res. 1977;25:603–611. doi: 10.1016/0014-4835(77)90139-7. [DOI] [PubMed] [Google Scholar]
- Cook CA, Koretz JF, Pfahnl A, Hyun J, Kaufman PL. Aging of the human crystalline lens and anterior segment. Vis. Res. 1994;34:2945–2954. doi: 10.1016/0042-6989(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Dubbelman M, van der Heijde R, Weeber HA, Vrensen GFJM. Changes in the internal structure of the human crystalline lens with age and accommodation. Vis Res. 2003;43:2363–2375. doi: 10.1016/s0042-6989(03)00428-0. [DOI] [PubMed] [Google Scholar]
- Dubbelman M, van der Heijde R, Weeber HA. The thickness of the aging human Lens obtained from corrected Scheimpflug images. Optom. Vis. Sci. 2001;78:411–416. doi: 10.1097/00006324-200106000-00013. [DOI] [PubMed] [Google Scholar]
- Duke-Elder S, Wybar KC. System of Ophthalmology. Volume II, The Anatomy of the Visual System. The CV Mosby Company,St Louis. 1961 [Google Scholar]
- Ehlers N, Matthieson ME, Andersen H. Prenatal growth of the human eye. Acta Ophthalmol. (Copenh.) 1968;46:329–349. doi: 10.1111/j.1755-3768.1968.tb02813.x. [DOI] [PubMed] [Google Scholar]
- Fagerholm PP, Philipson BT, Lindstrom B. Normal human lens- the distribution of protein. Exp. Eye Res. 1981;33:615–620. doi: 10.1016/s0014-4835(81)80101-7. [DOI] [PubMed] [Google Scholar]
- Farnsworth PN, Shyne SE. Anterior zonular shifts with age. Exp Eye Res. 28:291–297. doi: 10.1016/0014-4835(79)90091-5. [DOI] [PubMed] [Google Scholar]
- Fisher RF. The elastic constants of the human lens. J. Physiol. 1971;212:147–180. doi: 10.1113/jphysiol.1971.sp009315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RF, Pettet BE. Presbyopia and the water content of the human crystalline lens. J. Physiol. 1973;234:443–447. doi: 10.1113/jphysiol.1973.sp010353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MG, Truscott RWE. Membrane association of proteins in the aging human lens: profound changes take place in the fifth decade of life. Invest. Ophthalmol. Vis. Sci. 2009;50:4786–4793. doi: 10.1167/iovs.09-3588. [DOI] [PubMed] [Google Scholar]
- Garland DL, Douglas-Tabor Y, Jimenez-Asensio J, Datiles MB, Magno B. Nucleus of the human lens: demonstration of a highly characteristic protein pattern by two-dimensional electrophoresis and a new method of lens dissection. Exp. Eye Res. 1996;62:285–291. doi: 10.1006/exer.1996.0034. [DOI] [PubMed] [Google Scholar]
- Gilliland KO, Metlapally S, Costello MJ. Morphological Analysis of Extensive Fiber Cell Compaction in the Adult Nucleus of Aged Human Transparent Lenses. IOVS. 2009;50 ARVO E-Abstract 4388. [Google Scholar]
- Glasser A, Campbell MCW. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vis. Res. 1999;39:1991–2015. doi: 10.1016/s0042-6989(98)00283-1. [DOI] [PubMed] [Google Scholar]
- Goldstein I, Tamir A, Zimmer EZ, Itskovitz-Eldor J. Growth of the fetal orbit and lens during normal regnancies. Ultrasound Obstet. Gynecol. 1998;12:175–179. doi: 10.1046/j.1469-0705.1998.12030175.x. [DOI] [PubMed] [Google Scholar]
- Goss SA, Frizzel LA, Dunn F. Dependence of the ultrasonic properties of biological tissues on constituent proteins. J.Acoustic Soc. Am. 1980;67:1041–1044. [Google Scholar]
- Grey AC, Jacobs MD, Gonen T, Joerg Kistler J, Donaldson PJ. Insertion of MP20 into lens fibre cell plasma membranes correlates with the formation of an extracellular diffusion barrier. Exp. Eye Res. 2003;77:567–574. doi: 10.1016/s0014-4835(03)00192-1. [DOI] [PubMed] [Google Scholar]
- Gullapalli VK, Murthy PR, Murthy KR. Colour of the nucleus as a marker of nuclear hardness diameter and central thickness. Indian J. Opthalmol. 1995;44:181–184. [PubMed] [Google Scholar]
- Harding JJ, Rixon KC, Marriott FHC. Men have heavier lenses than women of the same age. Exp. Eye Res. 1977;25:651. doi: 10.1016/0014-4835(77)90143-9. [DOI] [PubMed] [Google Scholar]
- Helmholtz H. The accommodation of the eyes. Alb. Graefes Arch. Klin. Exp. Ophthalmol. 1855;1:1–70. [Google Scholar]
- Hermans E, Dubbelman M, van der Heijde R, Heethaar R. The shape of the human lens nucleus with accommodation. J. Vision 7. 2007;16:1–10. doi: 10.1167/7.10.16. doi:10.1167/7.10.16 http://journalofvision.org/7/10/16/ [DOI] [PubMed]
- Heys KR, Cram SL, Truscott RJW. Massive increase in the stiffness of the lens nucleus with age: the basis for presbyopia? Mol. Vis. 2005;10:256–263. [PubMed] [Google Scholar]
- Heys KR, Friedrich MG, Truscott RJW. Presbyopia and heat: changes associated with aging of the human lens suggest a functional role for the small heat shock protein, a-crystallin, in maintaining lens flexibility. Aging Cell. 2007;6:807–815. doi: 10.1111/j.1474-9726.2007.00342.x. [DOI] [PubMed] [Google Scholar]
- Hockwin O, Dragomirescu V, Laser H. Measurements of lens transparency or its disturbances by densitometric image analysis of Scheimpflug photographs. Albrecht Graef. Arch. Klin. Exp. Ophthalmol. 1982;219:255–262. doi: 10.1007/BF00231409. [DOI] [PubMed] [Google Scholar]
- Hollmann KW, O’Donell M, Erpelding TN. Mapping elasticity in human lenses using bubble-based acoustic radiation force. Exp. Eye Res. 2007;85:890–893. doi: 10.1016/j.exer.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huggert A. The thickness of the cortex of the crystalline lens in different ages. Acta Ophthalmol. 1946;24:43–62. [Google Scholar]
- Jones C, Atchison DA, Meder R, Pope JM. Refractive index distribution and optical properties of the isolated human lens measured using magnetic resonance imaging (MRI) Vis. Res. 2005;45:2352–2366. doi: 10.1016/j.visres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Jones C, Atchison DA, Pope JM. Changes in lens dimensions and refractive index with age and accommodation. Optom. Vis. Sci. 2007;84:990–995. doi: 10.1097/OPX.0b013e318157c6b5. [DOI] [PubMed] [Google Scholar]
- Kasthurirangan S, Markwell EL, Atchison DA, Pope JM. In vivo study of changes in refractive index distribution in the human crystalline lens with age and accommodation. Invest. Ophthalmol. Vis. Sci. 2008;49:2531–2540. doi: 10.1167/iovs.07-1443. [DOI] [PubMed] [Google Scholar]
- Koretz JF, Kaufman PL, Neider MW, Goeckner PA. Accommodation and presbyopia in the human eye: aging of the anterior segment. Vis. Res. 1989;29:1685–1692. doi: 10.1016/0042-6989(89)90150-8. [DOI] [PubMed] [Google Scholar]
- Koretz JF, Cook CA, Kuszak JR. The zones of discontinuity in the human lens: development and distribution with age. Vis. Res. 1994;34:2955–2962. doi: 10.1016/0042-6989(94)90267-4. [DOI] [PubMed] [Google Scholar]
- Koretz JF, Strenk SA, Strenk JM, Semmlow JL. Scheimpflug and high-resolution magnetic resonance imaging of the anterior segment; a comparative study. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2004;21:346–354. doi: 10.1364/josaa.21.000346. [DOI] [PubMed] [Google Scholar]
- Korlimbinis A, Berry Y, Thibault D, Schey K, Truscott RJW. Protein ageing: Truncation of aquaporin 0 in human lens regions is a continual age-dependent process. Exp. Eye Res. 2009;88:966–973. doi: 10.1016/j.exer.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuszak JR. Development of sutures in the lens. Progr. Retinal Eye Res. 1995;14:567–591. [Google Scholar]
- Larsen JS. The saggital growth of the eye. II Ultrasonic measurements of the axial diameter of the lens and the anterior segment from birth to puberty. Acta Ophthalmol. 1971;49 doi: 10.1111/j.1755-3768.1971.tb00968.x. [DOI] [PubMed] [Google Scholar]
- Lim JC, Walker KL, Sherwin T, Schey KL, Donaldson PJ. Confocal microscopy reveals zones of membrane remodelling in the outer cortex of the human lens. Invest. Ophthalmol. Vis. Sci. 2009;50:4304–4310. doi: 10.1167/iovs.09-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias RT, Rae JL, Baldo GJ. Physiological properties of the normal lens. Physiol. Rev. 1997;77:21–50. doi: 10.1152/physrev.1997.77.1.21. [DOI] [PubMed] [Google Scholar]
- Mutti DO, Zadnik K, Fusari RE, Friedman NE, Sholtz RI, Adamas AJ. Optical and Structural Development of the Crystalline Lens in Childhood. Invest. Ophthalmol. Vis. Sci. 1998;39:120–133. 1. [PubMed] [Google Scholar]
- Moffatt BA, Landman KA, Sweeney MHJ, Truscott RJW, Pope JM. Age-related changes in the kinetics of water transport in normal human lenses. Exp. Eye Res. 1999;69:663–669. doi: 10.1006/exer.1999.0747. [DOI] [PubMed] [Google Scholar]
- Nordmann J, Mack G, Mack G. Nucleus of the human lens III Its separation, its hardness. Ophthalmic Res. 1974;6:216–222. [Google Scholar]
- Nordmann J, Fink H, Hockwin O. Die wachstunkurve der menschenlichen lines. l Albrecht Graef. Arch. Klin. Exp. Ophthal. 1974;191:165–175. doi: 10.1007/BF00414943. [DOI] [PubMed] [Google Scholar]
- Pau H, Kranz J. The increasing sclerosis of the human lens with age and its relevance to accommodation and presbyopia. Graefe’s. Arch. Clin. Exp. Ohthalmol. 1991;229:294–296. doi: 10.1007/BF00167888. [DOI] [PubMed] [Google Scholar]
- Philipson BT, Fagerholm PP. CIBA Symposium 19. Amsterdam: Associated Scientific Publishers; 1973. Lens changes responsible for increased light scattering in some types of senile cataract, in The Human Lens in Relation to Cataract; pp. 45–58. [Google Scholar]
- Pierscionek BK. Refractive index contours in the human lens. Exp. Eye Res. 1997;64:887–893. doi: 10.1006/exer.1996.0252. [DOI] [PubMed] [Google Scholar]
- Pierscionek BK, Augusteyn RC. Shape and dimensions of in vitro human lenses. Clin. Exp. Optom. 1991;74:223–228. [Google Scholar]
- Pierscionek BK, Augusteyn RC. Species variations in optical parameters of the eye lens. Clin. Exp. Optom. 1993;76:22–26. [Google Scholar]
- Pierscionek BK, Chan DYC. The refractive index gradient of the human lens. Optom. Vis. Sci. 1989;66:822–829. doi: 10.1097/00006324-198912000-00004. [DOI] [PubMed] [Google Scholar]
- Richdale K, Bullimore M, Zadnik K. Lens thickness with age and accommodation by optical coherence tomography. Ophthalm. Physiol. Optics. 2008;28:441–447. doi: 10.1111/j.1475-1313.2008.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen AM, Denham DB, Fernandez V, Borja D, Ho A, Manns F, Parel JM, Augusteyn RC. In vitro dimensions and curvatures of human lenses. Vis. Res. 2006;46:1002–1009. doi: 10.1016/j.visres.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Roy D, Spector A. Absence of low molecular weight α-crystallin in nuclear region of old human lenses. Proc. Natl. Acad. Sci. USA. 1976;73:3484–3487. doi: 10.1073/pnas.73.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachar RA. Growth patterns of fresh human crystalline lenses measured by in vitro photographic biometry. J. Anat. 2005;206:575–580. doi: 10.1111/j.1469-7580.2005.00422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachar RA, Pierscionek BK. Lens hardness not related to the age-related decline of accommodative amplitude. Mol. Vis. 2007;13:1010–1011. [PMC free article] [PubMed] [Google Scholar]
- Siebinga I, Vrensen GFJM, De Mu lFFM, Greve J. Age-related changes in local water and protein content of human eye lenses measured by Raman Spectroscopy. Exp. Eye Res. 1991;153:233–239. doi: 10.1016/0014-4835(91)90079-t. [DOI] [PubMed] [Google Scholar]
- Smith P. Diseases of crystalline lens and capsule. 1. On the growth of the crystalline lens. Trans. Ophthalmol. Soc. U.K. 1883;3:79–99. [Google Scholar]
- Sparrow JM, Bron AJ, Brown NA, Ayliffe W, Hill AR. The oxford clinical cataract classification and grading system. Int. Ophthalmol. 1986;9:207–225. doi: 10.1007/BF00137534. [DOI] [PubMed] [Google Scholar]
- Strenk SA, Semmlow JL, Strenk LM, Munoz P, Gronlund-Jacob J, DeMarco K. Age-related changes in human ciliary muscle and lens; a magnetic resonance imaging study. Invest Ophthalmol Vis. Sci. 1999;40:1162–1169. [PubMed] [Google Scholar]
- Strenk SA, Strenk LM, Koretz JF. The mechanism of presbyopia. Progr. Ret. Eye Res. 2005;24:379–393. doi: 10.1016/j.preteyeres.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Sweeney MHJ, Truscott RJW. An impediment to glutathione diffusion in older normal human lenses: a possible precondition for nuclear cataract. Exp. Eye Res. 1998;67:587–595. doi: 10.1006/exer.1998.0549. [DOI] [PubMed] [Google Scholar]
- Tabendah H, Thompson GM, Heysworth P, Dorey S, Woods AJ, Lynch D. Water content, lens hardness and cataract appearance. Eye. 1994;8:125–129. doi: 10.1038/eye.1994.25. [DOI] [PubMed] [Google Scholar]
- Tardieu A, Veretout F, Krop B, Slingsby C. Protein interactions in the calf lens; interactions between beta-crystallins are repulsive whereas in gamma-crystallins they are attractive. Eur. Biophys. J. 1992;21:1–12. doi: 10.1007/BF00195438. [DOI] [PubMed] [Google Scholar]
- Taylor L, Al-Ghoul KJ, Lane CW, Davis VA, Kuszak JR, Costello MJ. Morphology of the normal human lens. Invest. Ophthalmol. Vis Sci. 1996;37:1396–1410. [PubMed] [Google Scholar]
- Thomson JA, Augusteyn RC. Ontogeny of human lens crystallins. Exp. Eye Res. 1985;40:393–410. doi: 10.1016/0014-4835(85)90152-6. [DOI] [PubMed] [Google Scholar]
- Truscott RJW, Augusteyn RC. Changes in human lens proteins during nuclear cataract formation. Exp. Eye Res. 1977;24:159–170. doi: 10.1016/0014-4835(77)90256-1. [DOI] [PubMed] [Google Scholar]
- Uhlhorn S, Borja D, Manns F, Parel J-M. Refractive index measurement of the isolated crystalline lens using optical coherence tomography. Vis. Res. 2008;48:2732–2738. doi: 10.1016/j.visres.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urs R, Fabrice Manns F, Arrieta E, Ziebarth N, Uhlhorn S, Dunne’ S, Ho A, Augusteyn R, Parel J-M. Speed of sound gradient across the equatorial profile of the isolated human crystalline lens. IOVS 2010. 2010;51 ARVO E-Abstract to be advised. [Google Scholar]
- van Alpen GWH, Graebel WP. Elasticity of tissues involved in accommodation. Vis. Res. 1991;31:1417–1438. doi: 10.1016/0042-6989(91)90061-9. [DOI] [PubMed] [Google Scholar]
- Van Heyningen R. The human lens. 3. Some observations on the post-mortem lens. Exp. Eye. Res. 1972;13:155–160. doi: 10.1016/0014-4835(72)90028-0. [DOI] [PubMed] [Google Scholar]
- Vogt A. Das farbenschillen des hinteren linsenbildes. Klin Mbl Augen. 1919;62:582. [Google Scholar]
- Vogt A. Zweiter Teil. Berlin: Springer; 1931. Lehrbuch und atlas der spaltlampenmikroskopie des auges. [Google Scholar]
- Weeber HA, Eckert G, Soergel F, Meyer CH, Pechhold W, van der Heijde RGL. Dynamic mechanical properties of human lenses. Exp. Eye Res. 2005;80:425–434. doi: 10.1016/j.exer.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Weeber HA, Eckert G, Pechfold W, van den Heijde RGL. Stiffness gradient in the crystalline lens. Graefe’s Arhiv. Clin. Exp. Ophthalmol. 2007 doi: 10.1007/s00417-007-0537-1. Doi; 10.1007/s00417-007-0537-1. [DOI] [PubMed] [Google Scholar]
- Weekers R, Delmarcelle Y, Luyckx-Bacus J, Collignon J. CIBA Foundation Symposium 19. London: Elsevier; 1973. Morphological changes of the lens with age and cataract; pp. 25–40. [Google Scholar]
- Wegener A, Laser-Junga H. Photography of the anterior eye segment according to Scheimpflug’s principle: options and limitations – a review. Clin. Exp. Ophthalmol. 2009;37:144–154. doi: 10.1111/j.1442-9071.2009.02018.x. [DOI] [PubMed] [Google Scholar]
- Wilmarth PAS, Tanner S, Dasari SR, Nagalla SR, Riviere MA, Bafna VPA, Pevzner PA, David LL. Age-Related Changes in Human Crystallins Determined from Comparative Analysis of Post-translational Modifications in Young and Aged Lens: Does Deamidation Contribute to Crystallin Insolubility? J. Proteome Res. 2006;5:2554–2566. doi: 10.1021/pr050473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Foster PJ, Ng TP, Tielsch JM, Johnson GJ, Seah SKL. Variations in ocular biometry in an adult Chinese population in Singapore: the Tanjong Pagar study. Invest. Ophthalmol. Vis. Sci. 2001;42:73–80. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.