Abstract
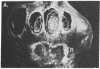
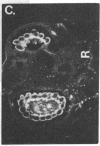
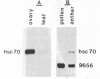
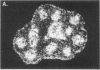
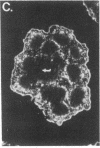
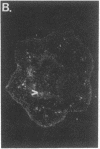
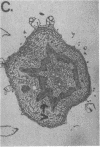
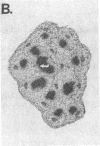
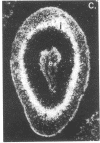
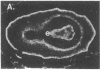
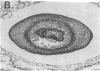
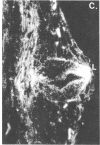
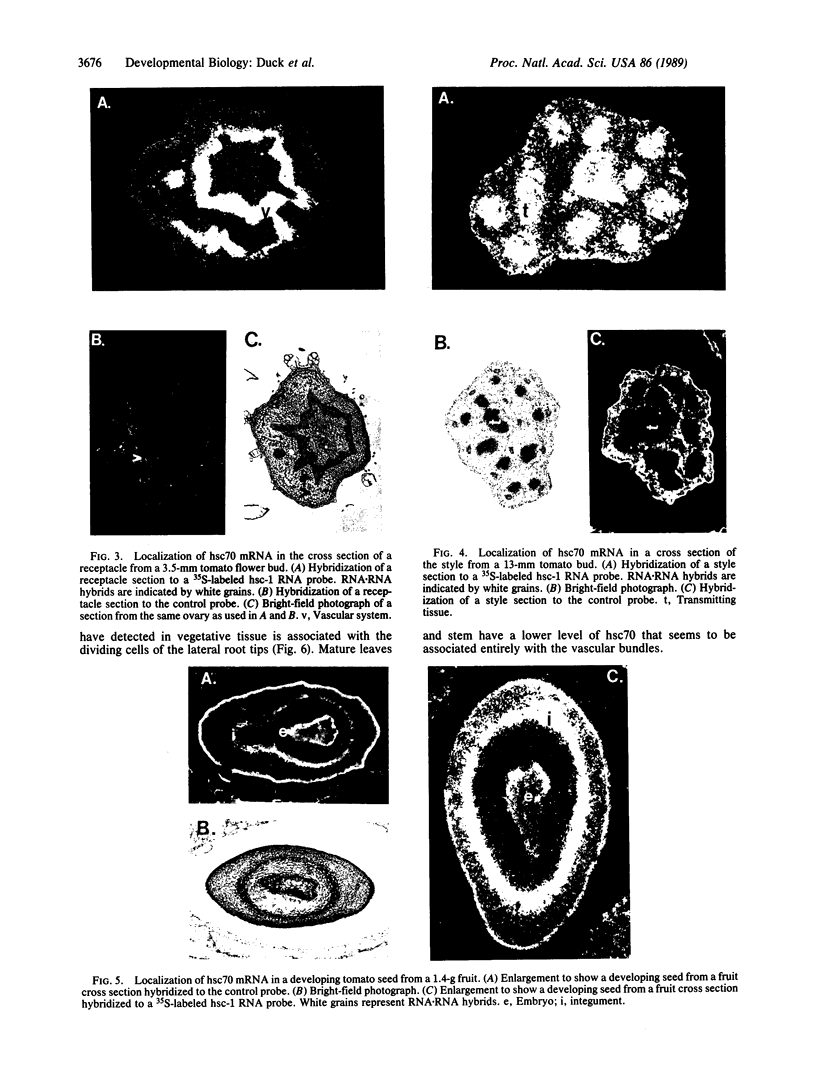
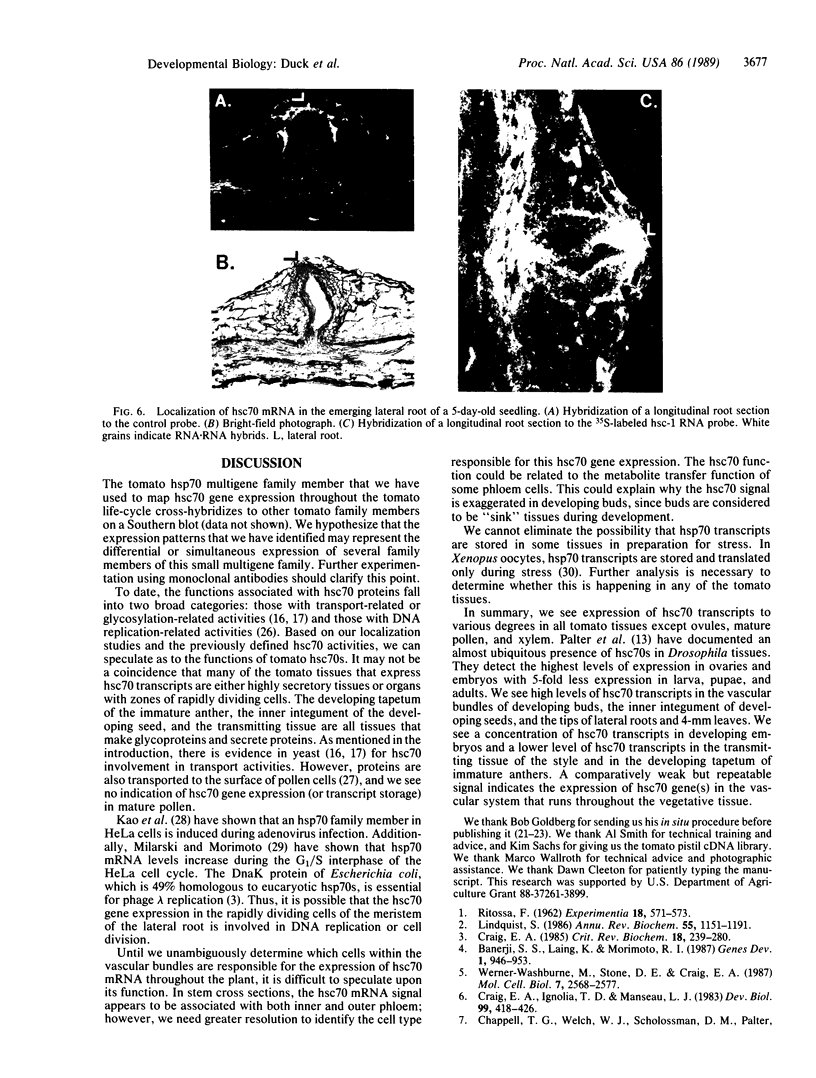
We have detected hsc70 gene expression (heat shock protein hsp70 cognate) during vegetative growth and reproductive development in tomato (Lycopersicon esculentum). Using RNA from a tomato hsc70 cDNA as a probe in in situ hybridizations, we have determined expression patterns of hsc70 in nonstressed tomato roots, stems, leaves, flowers, and developing fruits. We have localized high levels of hsc70 transcript to the vascular system of the ovary, dividing cells of the lateral root tips, and the inner integument of developing seeds. We also see expression in the transmitting tissue, in immature anthers, and in embryos. We cannot detect expression in mature pollen, xylem, or ovules. These data indicate that the expression of at least some tomato hsp70 family members is developmentally regulated.
Keywords: tomato, development, hsc70
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji S. S., Laing K., Morimoto R. I. Erythroid lineage-specific expression and inducibility of the major heat shock protein HSP70 during avian embryogenesis. Genes Dev. 1987 Nov;1(9):946–953. doi: 10.1101/gad.1.9.946. [DOI] [PubMed] [Google Scholar]
- Barker S. J., Harada J. J., Goldberg R. B. Cellular localization of soybean storage protein mRNA in transformed tobacco seeds. Proc Natl Acad Sci U S A. 1988 Jan;85(2):458–462. doi: 10.1073/pnas.85.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensaude O., Morange M. Spontaneous high expression of heat-shock proteins in mouse embryonal carcinoma cells and ectoderm from day 8 mouse embryo. EMBO J. 1983;2(2):173–177. doi: 10.1002/j.1460-2075.1983.tb01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M., Gurdon J. B. The heat-shock response in Xenopus oocytes is controlled at the translational level. Cell. 1982 Jul;29(3):811–819. doi: 10.1016/0092-8674(82)90443-3. [DOI] [PubMed] [Google Scholar]
- Bond U., Schlesinger M. J. Heat-shock proteins and development. Adv Genet. 1987;24:1–29. doi: 10.1016/s0065-2660(08)60005-x. [DOI] [PubMed] [Google Scholar]
- Chappell T. G., Welch W. J., Schlossman D. M., Palter K. B., Schlesinger M. J., Rothman J. E. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell. 1986 Apr 11;45(1):3–13. doi: 10.1016/0092-8674(86)90532-5. [DOI] [PubMed] [Google Scholar]
- Chirico W. J., Waters M. G., Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988 Apr 28;332(6167):805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Craig E. A., Ingolia T. D., Manseau L. J. Expression of Drosophila heat-shock cognate genes during heat shock and development. Dev Biol. 1983 Oct;99(2):418–426. doi: 10.1016/0012-1606(83)90291-9. [DOI] [PubMed] [Google Scholar]
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Davis J. Q., Dansereau D., Johnstone R. M., Bennett V. Selective externalization of an ATP-binding protein structurally related to the clathrin-uncoating ATPase/heat shock protein in vesicles containing terminal transferrin receptors during reticulocyte maturation. J Biol Chem. 1986 Nov 25;261(33):15368–15371. [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Imperiale M. J., Kao H. T., Feldman L. T., Nevins J. R., Strickland S. Common control of the heat shock gene and early adenovirus genes: evidence for a cellular E1A-like activity. Mol Cell Biol. 1984 May;4(5):867–874. doi: 10.1128/mcb.4.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao H. T., Capasso O., Heintz N., Nevins J. R. Cell cycle control of the human HSP70 gene: implications for the role of a cellular E1A-like function. Mol Cell Biol. 1985 Apr;5(4):628–633. doi: 10.1128/mcb.5.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox R. B., Heslop-Harrison J. Pollen-wall proteins: localization and enzymic activity. J Cell Sci. 1970 Jan;6(1):1–27. doi: 10.1242/jcs.6.1.1a. [DOI] [PubMed] [Google Scholar]
- Kurtz S., Rossi J., Petko L., Lindquist S. An ancient developmental induction: heat-shock proteins induced in sporulation and oogenesis. Science. 1986 Mar 7;231(4742):1154–1157. doi: 10.1126/science.3511530. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Milarski K. L., Morimoto R. I. Expression of human HSP70 during the synthetic phase of the cell cycle. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9517–9521. doi: 10.1073/pnas.83.24.9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- Palter K. B., Watanabe M., Stinson L., Mahowald A. P., Craig E. A. Expression and localization of Drosophila melanogaster hsp70 cognate proteins. Mol Cell Biol. 1986 Apr;6(4):1187–1203. doi: 10.1128/mcb.6.4.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochester D. E., Winer J. A., Shah D. M. The structure and expression of maize genes encoding the major heat shock protein, hsp70. EMBO J. 1986 Mar;5(3):451–458. doi: 10.1002/j.1460-2075.1986.tb04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E. The 70-kd mammalian heat shock proteins are structurally and functionally related to the uncoating protein that releases clathrin triskelia from coated vesicles. EMBO J. 1985 Dec 16;4(13A):3385–3391. doi: 10.1002/j.1460-2075.1985.tb04094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Washburne M., Stone D. E., Craig E. A. Complex interactions among members of an essential subfamily of hsp70 genes in Saccharomyces cerevisiae. Mol Cell Biol. 1987 Jul;7(7):2568–2577. doi: 10.1128/mcb.7.7.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakeri Z. F., Wolgemuth D. J. Developmental-stage-specific expression of the hsp70 gene family during differentiation of the mammalian male germ line. Mol Cell Biol. 1987 May;7(5):1791–1796. doi: 10.1128/mcb.7.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]