Abstract
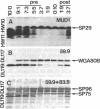
Prestalk and prespore cells form a simple pattern in the pseudoplasmodium of the cellular slime mold Dictyostelium discoideum. Prestalk cells are distinguished from prespore cells by a low level of expression of a glycoantigen on their surfaces and by reduced intercellular cohesion. We examined the possible significance of these differences, using the modB mutation, which eliminates this glycoantigen genetically, leading to reduced intercellular cohesion, modB mutant cells were allowed to develop together with normal cells to form chimeric slugs. Mutant cells labeled by feeding with fluorescent bacteria were highly enriched in the prestalk cell zone at the anterior end of the slug. In contrast, normal cells, if in a minority, were concentrated in the rear part of the prespore cell zone. Immunoblot analysis and cell-by-cell double-label immunofluorescence of these mixtures showed that mutant cells underproduced several prespore cell markers. Mutant cells tended not to form spores in chimeras unless they exceeded a threshold proportion of ca. 30%. However, mutant cells showed no tendency to produce excess prestalk cells when allowed to develop alone. These findings are most simply explained by postulating that reduced glycoantigen expression and intercellular adhesion encourage a more anterior cell localization, which in turn causes differentiation into a prestalk cell. Since normal prestalk cells also show reduced glycoantigen expression and intercellular adhesion, this suggests that a similar mechanism may contribute to pattern formation during normal development.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brookman J. J., Jermyn K. A., Kay R. R. Nature and distribution of the morphogen DIF in the Dictyostelium slug. Development. 1987 May;100(1):119–124. doi: 10.1242/dev.100.1.119. [DOI] [PubMed] [Google Scholar]
- Durston A. J. Tip formation is regulated by an inhibitory gradient in the dicytostelium discoideum slug. Nature. 1976 Sep 9;263(5573):126–129. doi: 10.1038/263126a0. [DOI] [PubMed] [Google Scholar]
- Forman D., Garrod D. R. Pattern formation in Dictyostelium discoideum. II. Differentiation and pattern formation in non-polar aggregates. J Embryol Exp Morphol. 1977 Aug;40:229–243. [PubMed] [Google Scholar]
- Freeze H. H. Modifications of lysosomal enzymes in Dictyostelium discoideum. Mol Cell Biochem. 1986 Nov-Dec;72(1-2):47–65. doi: 10.1007/BF00230635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch G., Weinhart U., Bertholdt G., Claviez M., Stadler J. Incomplete contact site A glycoprotein in HL220, a modB mutant of Dictyostelium discoideum. J Cell Sci. 1985 Feb;73:49–68. doi: 10.1242/jcs.73.1.49. [DOI] [PubMed] [Google Scholar]
- Gomer R. H., Firtel R. A. Cell-autonomous determination of cell-type choice in Dictyostelium development by cell-cycle phase. Science. 1987 Aug 14;237(4816):758–762. doi: 10.1126/science.3039657. [DOI] [PubMed] [Google Scholar]
- Grant W. N., Welker D. L., Williams K. L. A polymorphic, prespore-specific cell surface glycoprotein is present in the extracellular matrix of Dictyostelium discoideum. Mol Cell Biol. 1985 Oct;5(10):2559–2566. doi: 10.1128/mcb.5.10.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann H. P., Bozzaro S., Merkl R., Wallraff E., Yoshida M., Weinhart U., Gerisch G. Post-translational glycosylation of the contact site A protein of Dictyostelium discoideum is important for stability but not for its function in cell adhesion. EMBO J. 1987 Dec 1;6(12):3663–3671. doi: 10.1002/j.1460-2075.1987.tb02699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht D. A., Dimond R. L., Wheeler S., Loomis W. F. Antigenic determinants shared by lysosomal proteins of Dictyostelium discoideum. Characterization using monoclonal antibodies and isolation of mutations affecting the determinant. J Biol Chem. 1984 Aug 25;259(16):10633–10640. [PubMed] [Google Scholar]
- Krefft M., Voet L., Gregg J. H., Mairhofer H., Williams K. L. Evidence that positional information is used to establish the prestalk-prespore pattern in Dictyostelium discoideum aggregates. EMBO J. 1984 Jan;3(1):201–206. doi: 10.1002/j.1460-2075.1984.tb01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach C. K., Ashworth J. M., Garrod D. R. Cell sorting out during the differentiation of mixtures of metabolically distinct populations of Dictyostelium discoideum. J Embryol Exp Morphol. 1973 Jun;29(3):647–661. [PubMed] [Google Scholar]
- Loomis W. F. Regulation of cell-type-specific differentiation in Dictyostelium. Cold Spring Harb Symp Quant Biol. 1985;50:769–777. doi: 10.1101/sqb.1985.050.01.095. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. A., Springer W. R., Barondes S. H. Adhesion mutants of Dictyostelium discoideum lacking the saccharide determinant recognized by two adhesion-blocking monoclonal antibodies. Dev Biol. 1985 May;109(1):111–117. doi: 10.1016/0012-1606(85)90351-3. [DOI] [PubMed] [Google Scholar]
- MacWilliams H. K., Bonner J. T. The prestalk-prespore pattern in cellular slime molds. Differentiation. 1979;14(1-2):1–22. doi: 10.1111/j.1432-0436.1979.tb01006.x. [DOI] [PubMed] [Google Scholar]
- MacWilliams H., Blaschke A., Prause I. Two feedback loops may regulate cell-type proportions in Dictyostelium. Cold Spring Harb Symp Quant Biol. 1985;50:779–785. doi: 10.1101/sqb.1985.050.01.096. [DOI] [PubMed] [Google Scholar]
- Maeda Y., Maeda M. Heterogeneity of the cell population of the cellular slime mold Dictyostelium discoideum before aggregation, and its relation to the subsequent locations of the cells. Exp Cell Res. 1974 Mar 15;84(1):88–94. doi: 10.1016/0014-4827(74)90383-8. [DOI] [PubMed] [Google Scholar]
- Matsukuma S., Durston A. J. Chemotactic cell sorting in Dictyostelium discoideum. J Embryol Exp Morphol. 1979 Apr;50:243–251. [PubMed] [Google Scholar]
- McDonald S. A., Durston A. J. The cell cycle and sorting behaviour in Dictyostelium discoideum. J Cell Sci. 1984 Mar;66:195–204. doi: 10.1242/jcs.66.1.195. [DOI] [PubMed] [Google Scholar]
- McDonough J. P., Springer W. R., Barondes S. H. Species-specific cell cohesion in cellular slime molds. Demonstration by several quantitative assays and with multiple species. Exp Cell Res. 1980 Jan;125(1):1–14. doi: 10.1016/0014-4827(80)90182-2. [DOI] [PubMed] [Google Scholar]
- Murray B. A., Wheeler S., Jongens T., Loomis W. F. Mutations affecting a surface glycoprotein, gp80, of Dictyostelium discoideum. Mol Cell Biol. 1984 Mar;4(3):514–519. doi: 10.1128/mcb.4.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M. S. Does differential adhesion govern self-assembly processes in histogenesis? Equilibrium configurations and the emergence of a hierarchy among populations of embryonic cells. J Exp Zool. 1970 Apr;173(4):395–433. doi: 10.1002/jez.1401730406. [DOI] [PubMed] [Google Scholar]
- Sternfeld J., Bonner J. T. Cell differentiation in Dictyostelium under submerged conditions. Proc Natl Acad Sci U S A. 1977 Jan;74(1):268–271. doi: 10.1073/pnas.74.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi I., Yabuno K. Disaggregation of slime mold pseudoplasmodia using EDTA and various proteolytic enzymes. Exp Cell Res. 1970 Jul;61(1):183–190. doi: 10.1016/0014-4827(70)90272-7. [DOI] [PubMed] [Google Scholar]
- Tasaka M., Noce T., Takeuchi I. Prestalk and prespore differentiation in Dictyostelium as detected by cell type-specific monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5340–5344. doi: 10.1073/pnas.80.17.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasaka M., Takeuchi I. Role of cell sorting in pattern formation in Dictyostelium discoideum. Differentiation. 1981;18(3):191–196. doi: 10.1111/j.1432-0436.1981.tb01122.x. [DOI] [PubMed] [Google Scholar]
- Weijer C. J., Duschl G., David C. N. Dependence of cell-type proportioning and sorting on cell cycle phase in Dictyostelium discoideum. J Cell Sci. 1984 Aug;70:133–145. doi: 10.1242/jcs.70.1.133. [DOI] [PubMed] [Google Scholar]
- West C. M., Brownstein S. A. EDTA treatment alters protein glycosylation in the cellular slime mold Dictyostelium discoideum. Exp Cell Res. 1988 Mar;175(1):26–36. doi: 10.1016/0014-4827(88)90252-2. [DOI] [PubMed] [Google Scholar]
- West C. M. Current ideas on the significance of protein glycosylation. Mol Cell Biochem. 1986 Nov-Dec;72(1-2):3–20. doi: 10.1007/BF00230632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C. M., Erdos G. W., Davis R. Glycoantigen expression is regulated both temporally and spatially during development in the cellular slime molds Dictyostelium discoideum and D. mucoroides. Mol Cell Biochem. 1986 Nov-Dec;72(1-2):121–140. doi: 10.1007/BF00230640. [DOI] [PubMed] [Google Scholar]
- West C. M., Erdos G. W. The expression of glycoproteins in the extracellular matrix of the cellular slime mold Dictyostelium discoideum. Cell Differ. 1988 Mar;23(1-2):1–16. doi: 10.1016/0045-6039(88)90032-2. [DOI] [PubMed] [Google Scholar]
- West C. M., Loomis W. F. Absence of a carbohydrate modification does not affect the level or subcellular localization of three membrane glycoproteins in modB mutants of Dictyostelium discoideum. J Biol Chem. 1985 Nov 5;260(25):13803–13809. [PubMed] [Google Scholar]
- West C. M., Lubniewski A. J., Gregg J. H., Newton B. A temperature-dependent choice in cell differentiation. Differentiation. 1982;23(2):91–102. doi: 10.1111/j.1432-0436.1982.tb01271.x. [DOI] [PubMed] [Google Scholar]
- West C. M., Simon J., Hicks L. A temperature-dependent block in spore differentiation is transduced intercellularly by Dictyostelium discoideum. Cell Differ. 1983 Sep;13(1):69–76. doi: 10.1016/0045-6039(83)90079-9. [DOI] [PubMed] [Google Scholar]
- Ziska S. E., Henderson E. J. Cell surface oligosaccharides participate in cohesion during aggregation of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1988 Feb;85(3):817–821. doi: 10.1073/pnas.85.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]