Abstract
Therapeutic subunit vaccines based on tumor-associated antigens (TAAs) represent an attractive approach for the treatment of cancer. However, poor immunogenicity of TAAs requires potent adjuvants for therapeutic efficacy. We recently proposed the TNF family costimulatory ligands as potential adjuvants for therapeutic vaccines, and hence generated a soluble form of 4-1BBL chimeric with streptavidin (SA-4-1BBL) that has pleiotropic effects on cells of innate, adaptive, and regulatory immunity. We herein tested whether these effects can translate into effective cancer immunotherapy when SA-4-1BBL was also used as a vehicle to deliver TAAs in vivo to DCs constitutively expressing the 4-1BB receptor. SA-4-1BBL was internalized by DCs upon receptor binding, and immunization with biotinylated antigens conjugated to SA-4-1BBL resulted in increased antigen uptake and cross-presentation by DCs, leading to the generation of effective T cell immune responses. Conjugate vaccines containing human papilloma virus 16 (HPV-16) E7 oncoprotein or survivin as a self TAA had potent therapeutic efficacy against TC-1 cervical and 3LL lung carcinoma tumors, respectively. Therapeutic efficacy of the vaccines was associated with increased CD4+ T and CD8+ T cell effector and memory responses and higher intratumoral CD8+ T effector/CD4+CD25+Foxp3+ T regulatory cell ratio. Thus, potent pleiotropic immune functions of SA-4-1BBL combined with its ability to serve as a vehicle to increase delivery of antigens to DCs in vivo endow this molecule with the potential to serve as an effective immunomodulatory component of therapeutic vaccines against cancer and chronic infections.
Keywords: Costimulation, SA-4-1BBL, Conjugate Vaccines, Adjuvant
Introduction
Subunit vaccines based on TAAs hold great promise as therapeutic cancer vaccines because of their safety profile as well as potential efficacy in preventing recurrences due to the establishment of long-term immunological memory. However, the therapeutic potential of cancer vaccines remains to be realized despite many advances in vaccinology. The weak immunogenicity of TAAs, potential tolerance to self TAAs, and various immune evasion mechanisms employed by progressing tumors are obvious culprits for the inefficacy of therapeutic cancer vaccines (1, 2). Therefore, vaccine strategies that target both the innate and adaptive immune systems for the generation/upregulation of potent anti-tumor immune responses and simultaneously overcome tumor immune evasion mechanisms are more likely to succeed in the clinic. In this context, the development/discovery of adjuvants that not only have pleiotropic effects on a broad range of immune cells, but also can be used as a vehicle to deliver antigens to DCs in vivo will be an important step forward for improving therapeutic efficacy of cancer vaccines.
DCs play key roles in orchestrating innate, adaptive, and regulatory immune responses (3), and as such have been targeted for the development of therapeutic vaccines. Vaccination with DCs that have been manipulated ex vivo by various means to present TAAs although effective in inducing immune responses, clinical responses were achieved in only a small number of patients (4). In addition, DC-based cellular vaccines are time and labor intensive, costly, and most importantly are patient-customized, which severely limit their broad clinical application. Therefore, intense efforts have been devoted to manipulate DCs in vivo for the improvement of therapeutic efficacy of TAA-based conventional vaccines (4). Accumulated knowledge suggest that TAA-based vaccine formulations that target DCs in vivo may benefit from potent adjuvants with dual functions; as an antigen delivery vehicle and modulator of DC activation, antigen uptake, and cross presentation. Adjuvants that also directly enhance the function of immune effector cells, such as T cells and NK cells, as well as overcome various tumor-mediated immunosuppressive mechanisms, such as CD4+CD25+FoxP3+ T regulatory (Treg) cells, will have added advantages.
We recently hypothesized that the TNF family costimulatory ligands may serve as important immunomodulators for the development of therapeutic cancer vaccines (5-7) because of their critical roles in immune responses (8). We particularly focused on 4-1BBL as signaling through its receptor, 4-1BB, has pleiotropic effects on cells of innate (9, 10), adaptive (11, 12), and regulatory immunity (13). A subset of DCs constitutively expresses 4-1BB (9, 10), and triggering of 4-1BB on these cells has been shown to increase their survival (14), secretion of IL-6 and IL-12 (9, 10), and improved ability to stimulate T cells (9). In addition, 4-1BBL has been reported to be the most potent costimulatory member of the TNF family in stimulating human T cells (15), resulting in clonal expansion, survival, and the establishment and maintenance of long-term immune memory (8, 11). Importantly, 4-1BB signaling endows T effector (Teff) cells resistant to Treg cell suppression (7, 13, 16) and has been demonstrated to reverse T cell anergy (17).
Inasmuch as the natural 4-1BBL functions as a cell membrane-bound protein and has no activity in soluble form (18), we recently generated a novel form of this ligand (SA-4-1BBL) by fusing the extracellular domain of murine 4-1BBL to the C-terminus of a modified core streptavidin (SA) (16). This molecule served as a safe and more effective immunomodulatory component of therapeutic cancer vaccines than toll-like receptor (TLR) agonists CpG, LPS, and its derivative MPL as well as an agonistic antibody to 4-1BB receptor (6, 7). The immune efficacy of SA-4-1BBL was associated with its pleiotropic effects on cells of innate, adaptive, and regulatory immunity (7). In addition, the SA portion of SA-4-1BBL allows for rapid conjugation with biotinylated TAAs, taking advantage of the unsurpassed noncovalent interaction between SA and biotin (19). Since DCs constitutively express 4-1BB (9, 10), we hypothesized that SA-4-1BBL can serve as a vehicle to deliver biotinylated antigens to DCs in vivo for improved antigen uptake, cross-presentation, and initiation of effective immune responses.
We herein demonstrated that SA-4-1BBL is internalized by DCs upon receptor binding and conjugating antigens to SA-4-1BBL significantly improved vaccine efficacy in vivo through increased DC antigen uptake, cross-presentation, and activation, leading to robust T cell proliferation and effector functions. A single vaccination with SA-4-1BBL conjugated to HPV E7 (viral TAA) or survivin (bona fide self TAA) resulted in increased therapeutic efficacy over nonconjugate vaccine formulations for the eradication of established primary and metastatic TC-1 cervical and 3LL lung carcinoma tumors, respectively. Importantly, vaccine efficacy was associated with enhanced CD4+ T and CD8+ T cell effector and memory responses and increased intratumoral CD8+ Teff/Treg cell ratio. The pleiotropic immune effects of SA-4-1BBL combined with its ability to deliver TAAs to DCs in vivo presents a novel vaccine approach with implications for the treatment of cancer and chronic infections.
Materials and Methods
Mice
C57BL/6.SJL, C57BL/6, OT-I/rag-/-, and OT-II/rag-/- mice were purchased from The Jackson Laboratory, Taconic, or bred in our barrier animal facility at the University of Louisville. C57BL/6 4-1BB-/- mice were kindly provided by Dr. A.T. Vella (University of Connecticut) with permission from Dr. B.S. Kwon (University of Ulsan). All animals were cared for in accordance with institutional and NIH guidelines.
Reagents
The construction, expression, and purification of SA-4-1BBL and core streptavidin (SA) have previously been described (16), except that SA-4-1BBL was modified to include a linker between SA and 4-1BBL to improve protein expression. These proteins had undetectable endotoxin levels. Fluorochrome-conjugated Abs to various immune cell surface markers or cytokines and isotype controls were purchased from BD Bioscience, eBioscience, and BioLegend. Chicken ovalbumin (Ova) was purchased from Sigma-Aldrich, and after biotinylation was tested for endotoxin (Ova-bio = 0.045 EU/μg protein). Detailed information of the cloning, expression, and purification of HPV16 E7 (0.021 EU/μg protein) and mouse survivin (0.066 EU/μg protein) can be found in Supplementary Material and Methods.
SA-4-1BBL internalization assay
DCs were derived from wild type or 4-1BB-/- bone marrow cells (7). 1×106 cells were incubated with 10 μg/mL FITC-labeled SA-4-1BBL protein at 4°C for 1 h, washed, and incubated in complete MLR medium either kept at 4°C or incubated at 37°C for 1 h. Cells were then washed, plated on coverslips, fixed in 4% paraformaldehyde, washed, blocked FcγR with a mAb against this receptor (clone, 2.4G2; BD Pharmingen) in 1% BSA, and stained for CD11c. Confocal microscopy was used to assess the binding and internalization of SA-4-1BBL-FITC.
Flow cytometry
Phenotyping and T cell sorting were performed as recently described (6). Intracellular cytokine staining was performed on PMA (5 ng/ml) and ionomycin (500 ng/ml) stimulated cells as reported (20).
Conjugation of biotinylated proteins to SA-4-1BBL
Ova was biotinylated using the ChromaLink™ Biotin Protein Labeling kit (Solulink), whereas E7 and survivin were biotinylated using the EZ-Link Sulfo-NHS-Biotinylation kit (Pierce) following the manufacturers' protocols. SA-4-1BBL or SA was conjugated to biotinylated antigens at various ratios on ice for 45 min before testing in various assays.
In vivo antigen targeting, uptake, and cross-presentation by DCs
C57BL/6 mice were immunized s.c. with FITC-labeled Ova (10 μg) alone, conjugated SA-4-1BBL-Ova-FITC (25-10 μg), or nonconjugated SA-4-1BBL+Ova-FITC (25+10 μg). Draining LNs were harvested 24 h later and processed into a single-cell suspension. After blocking Fc-receptors, cells were stained with CD11c-PE, CD11b-PerCP-Cy5.5, B220-PE-cy7, and CD8-APC-cy7 Abs and uptake of Ova-FITC by DCs was analyzed using flow cytometry. Antigen cross-presentation was studied under similar conditions, except Ova without FITC was used for immunization. The presence of H-2Kb/SIINFEKL complexes was detected on the surface of DCs by flow cytometry with APC-conjugated 25D1.16 Ab.
In vivo cytotoxicity
For Ova or E7-specific in vivo killing assays, naïve C57BL/6 (CD45.2+) mice were immunized s.c. with either Ova (50 μg), conjugated SA-4-1BBL-Ova (25-50 μg), or nonconjugated SA-4-1BBL+Ova (25+50 μg), or E7 (10 μg), conjugated SA-4-1BBL-E7 (25-10 μg), or nonconjugated SA-4-1BBL+E7 (25+10 μg). One wk later, mice received CFSE-labeled target cells (CD45.1+) pulsed with SIINFEKL or E749-57 peptide respectively, as previously described (7).
In vivo OT-I and OT-II proliferation assays
To test if the conjugate vaccine first targets DCs for immune efficacy, C57BL/6.SJL (CD45.1+) mice were immunized s.c. with Ova either conjugated or mixed with various doses of SA-4-1BBL. After 24 or 48 h, flow-sorted OT-I CD8+ T cells (CD45.2+) were labeled with 2.5 μM CFSE and 2 × 106 of these cells were transferred i.v. into naïve C57BL/6.SJL mice (CD45.1+). OT-I T cell division in draining LNs was assessed 3 days later by analyzing the CFSE dilution of CD45.2+CD8+ T cells using flow cytometry. The direct effect of conjugate vaccine on T cell proliferation was assessed using CFSE-labeled OT-I and OT-II CD4+ T (CD45.2+) cells in an adoptive transfer model as previously described (6). The same protocol was used for the tracking of OT-I T cells in C57BL/6 and C57BL/6 4-1BB-/- mice, except OT-I T cell proliferation was assessed by analyzing the CFSE dilution of Vβ5.1/5.2+CD8+ T cells using flow cytometry.
Tumor models and vaccination
For tumor therapeutic studies, mice were challenged s.c. with 1×105 TC-1 or 3LL cells and vaccinated on day 6 post-tumor challenge. For the TC-1 lung metastasis model, 5 × 104 TC-1 cells were injected i.v. into the tail vein, vaccinated i.v. on day 6 post-tumor challenge and euthanized 21 days after tumor challenge for analysis of lung metastasis and tumor burden.
Immunohistochemistry analysis of tumor-infiltrating CD8+ T cells and CD4+Foxp3+ Treg cells
Mice bearing TC-1 tumors of ∼3-4 mm in diameter were injected s.c. with conjugated SA-4-1BBL-E7 (25-10 μg), nonconjugated SA-4-1BBL+E7 (25+10 μg), or control conjugated SA-E7 (10-10 μg). Seven days later tumors were dissected, washed, embedded in OCT, and snap-frozen in optimal cutting temperature solution. Five μm sections were analyzed for confocal staining of CD8+ T cells as previously reported (7).
For CD4+Foxp3+ Treg cells staining, tumor sections were incubated with rat anti-mouse CD4 Ab for 1 h at r.t. After washing, sections were incubated with goat anti-rat Alexa 647, counterstained with Hoechst 33342 followed by staining with anti-FoxP3 Ab-FITC and analyzed by confocal.
Statistics
Statistical analyses were performed using the Student's t test, Mann-Whitney U test, or Log-rank test using SPSS software. For each test, P values less than 0.05 and 0.001 were considered significant (*) and very significant (**), respectively.
Results
SA-4-1BBL delivers the conjugated antigen to DCs in vivo for increased antigen uptake, cross-presentation, and generation of effective T cell responses
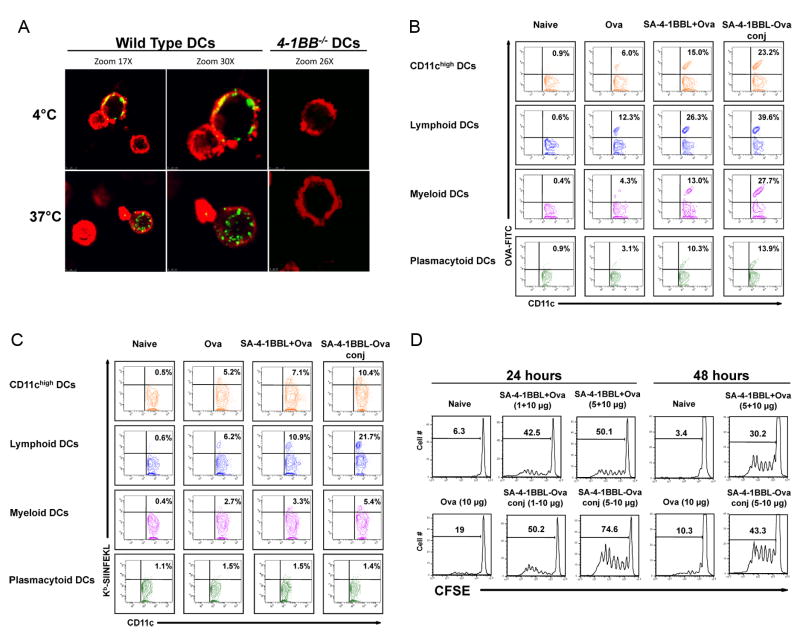
A subset of DCs constitutively expresses 4-1BB and signaling via this receptor results in DC activation (9, 10). We tested whether DCs internalize SA-4-1BBL upon receptor binding. SA-4-1BBL bound to a subpopulation of wild type, but not 4-1BB-/-, DCs in vitro and was rapidly internalized at 37°C (Fig. 1A). Therefore, we sought to test if antigen uptake and presentation by DCs could be enhanced in vivo using SA-4-1BBL conjugated Ova as a model antigen. Based on the most effective vaccine dose reported in our previous study (7), we prepared a conjugate vaccine by mixing 10 μg of biotinylated Ova with 25 μg of SA-4-1BBL. Western blot analysis (Supplementary Figure 1) showed that over 20% of Ova was conjugated to SA-4-BBL as assessed by densitometry (data not shown). Injection of mice s.c. with SA-4-1BBL conjugated to Ova-FITC resulted in amplified increased Ova accumulation in total CD11chi DCs (23.2%) in draining lymph nodes as compared to nonconjugate SA-4-1BBL+Ova vaccine (15.0%) and Ova alone (6.0%) (Fig. 1B). The conjugate vaccine enhanced antigen uptake by lymphoid DCs (CD11chiCD11b-CD8α+; 39.6% vs. 26.3% for nonconjugate vaccine and 12.3% for Ova alone), myeloid DCs (CD11chiCD11b+CD8α-; 27.7% vs. 13.0% for nonconjugate vaccine and 4.3% for Ova alone), and plasmacytoid DCs (CD11cintCD11b-B220+; 13.9% vs. 10.3% for nonconjugate vaccine and 3.1% for Ova alone) (Fig. 1B). Overall, the increase in antigen uptake by these three DC subtypes investigated was > 3.2-fold for conjugate vaccine and > 2.1-fold for nonconjugate vaccine as compared with Ova alone and confirms our previous findings (7). Notably, we did not detect Ova uptake by other APCs including B cells and macrophages (data not shown).
Figure 1.
SA-4-1BBL targets conjugated antigen in vivo to DCs for enhanced uptake and cross-presentation. A, Bone marrow-derived DCs internalize SA-4-1BBL following 4-1BB binding. DCs from wild type and 4-1BB-/- C57BL/6 mice were incubated with SA-4-1BBL-FITC at 4°C for receptor binding, washed, and then incubated for 1 h at 4°C or 37°C for internalization. CD11c (red) DCs from wild type, but not 4-1BB-/-, mice internalize SA-4-1BBL (green) at 37°C, but not 4°C. A minimum of 3 fields per slide were analyzed. B, SA-4-1BBL-Ova conjugates enhance antigen uptake by DCs in vivo. C57BL/6 mice were injected s.c. with conjugated SA-4-1BBL-Ova-FITC (25-10 μg), nonconjugated SA-4-1BBL+Ova-FITC (25+10 μg), Ova-FITC alone (10 μg), or left untreated. Draining lymph node cells were harvested 24 h later, stained with CD11c-PE, CD11b-PErcp-Cy5.5, B220-PEcy7, and CD8α-APC-cy7 Abs, and analyzed in flow cytometry. Data for Panels A and B are representative of two independent experiments. C, SA-4-1BBL-Ova conjugates increase antigen cross-presentation by DCs in vivo. C57BL/6 mice were vaccinated as in B, except Ova without FITC was used as antigen. Draining lymph node cells were harvested 24 h later and subjected to Ab staining as in B. APC-conjugated 25D1.16 Ab was used to detect Kb/SIINFEKL on the surface of DCs. D, SA-4-1BBL-Ova conjugates result in increased antigen presentation to OT-I CD8+ T cells in vivo. C57BL/6.SJL (CD45.1+) mice were immunized s.c. with Ova (10 μg) conjugated or nonconjugated with various doses (μg) of SA-4-1BBL as indicated. 2×106 sorted OT-I T cells (CD45.2+) were labeled with CFSE and injected i.v. 24 or 48 h later. Proliferation was assessed using flow cytometry 3 days later. Data are representative of a minimum of three independent experiments for Panels C and D.
The conjugate vaccine also resulted in increased Ova cross-presentation by total CD11chi DCs (10.4%) as compared to nonnconjugate vaccine (7.1%) and Ova alone (5.2%) (Fig. 1C) as assessed by a mAb (25-D1.16) recognizing the dominant CD8+ T cell epitope SIINFEKL in the context of H-2Kb. Effect of the conjugate vaccine was mostly on lymphoid DCs as > 21% of these cells scored positive for SIINFEKL/H-2Kb vs. 10.9% for nonconjugate vaccine and 6.2% for Ova alone (Fig. 1C). Conjugate vaccine also increased cross-presentation of Ova by myeloid DCs (5.4% vs. 3.3% for nonconjugate vaccine and 2.7% for Ova alone; Fig. 1C). However, there was no effect on plasmacytoid DCs (Fig. 1C). The conjugation effect on lymphoid DCs was long-lasting as a significant portion of these cells scored positive for SIINFEKL/H-2Kb on days 4 and 7 post vaccination (Supplementary Figure 2). In marked contrast, there was no detectable cross presentation by myeloid DCs on day 4 post vaccination (data not shown). The increased cross-presentation was dependent on a functional 4-1BB receptor since this effect was abolished in 4-1BB-/- mice (Supplementary Figure 3).
To further confirm that SA-4-1BBL targets DCs for immune activation and that the increased cross-presentation by DCs enhances CD8+ T cell responses in vivo, mice were immunized with conjugate SA-4-1BBL-Ova vaccine, nonconjugate SA-4-1BBL+Ova, and Ova alone either 24 h or 48 h before transfer of CFSE-labeled Ova-specific CD8+ OT-I T cells. Immunization with the conjugate vaccine resulted in greater proliferation of OT-I T cells (Fig. 1D) in a dose-dependent manner as compared with nonconjugate vaccine and Ova alone. Importantly, immunization with the conjugate vaccine also resulted in greater proliferation of CD4+ OT-II T cells (Supplementary Figure 4), suggesting that SA-4-1BBL-Ova conjugates are able to route antigens to both the MHC class I and MHC class II pathways of antigen presentation. Taken together, these data demonstrate that antigens conjugated to SA-4-1BBL via SA-biotin interaction can be targeted to DCs in vivo for enhanced uptake, cross-presentation, and generation of effective T cell responses.
SA-41BBL conjugate vaccines induce T cell proliferation and potent effector functions
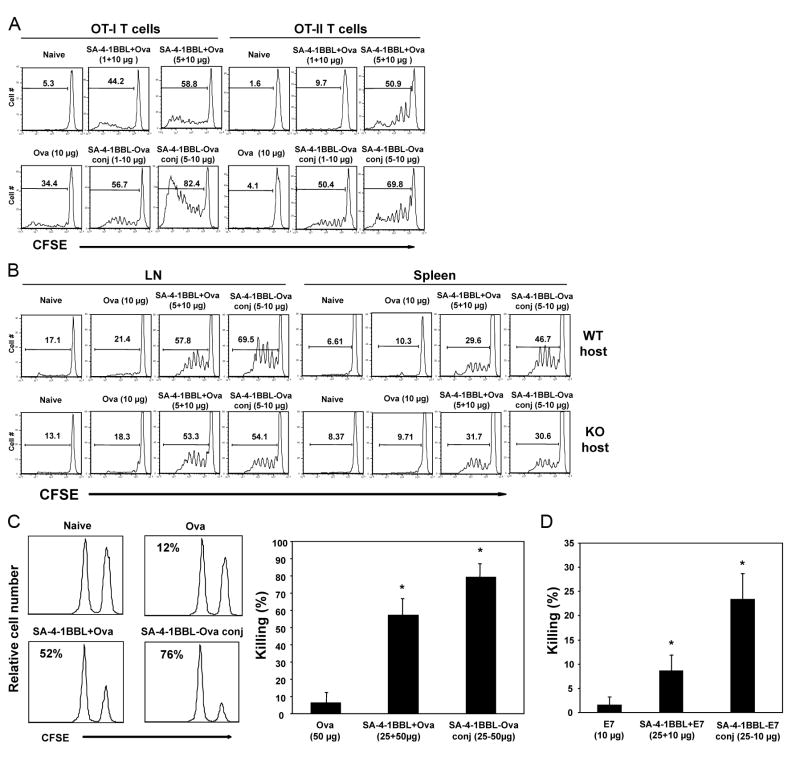
4-1BB is inducibly expressed and reaches peak expression levels within 24 h on T cells in response to antigenic challenge in vivo (8). Therefore, we anticipated that the conjugate vaccine would first target DCs constitutively expressing 4-1BB for the generation of immune responses in a naive host, and then subsequently directly work on activated T cells that have upregulated 4-1BB receptor. Consistent with this notion, i.v. vaccination with the SA-4-1BBL-Ova conjugate generated better proliferative responses in both CD8+ OT-I and CD4+ OT-II T cells than nonconjugate vaccine at all doses tested (Fig. 2A). Importantly, the effect of conjugation was abolished in 4-1BB-/- mice (Fig. 2B), further substantiating the role of DCs in the efficacy of conjugate vaccine. The enhanced efficacy of conjugate vaccine was even more pronounced in an in vivo killing assay where immunization with the SA-4-1BBL-Ova conjugate generated significantly higher endogenous CTL killing responses over nonconjugate vaccine (79.5 ± 7.6% vs. 57.4 ± 10.0% lysis; Fig. 2C). This immunostimulatory effect of the conjugate vaccine was not confined to Ova as vaccination with SA-4-1BBL conjugated with the HPV-16 E7 antigen also generated potent CTL responses in vivo (Fig. 2D).
Figure 2.
SA-4-1BBL conjugate vaccine has improved immunostimulatory activity than nonconjugate vaccine. A, in vivo OT-I and OT-II proliferation. 2×106 sorted OT-I T and OT-II T cells (CD45.2+) were labeled with CFSE and injected i.v. into naïve congenic C57BL/6.SJL (CD45.1+) mice. Twenty-four h later mice were vaccinated s.c. with Ova (10 μg) conjugated or nonconjugated to various doses of SA-4-1BBL as indicated. Proliferation was assessed 3 days after vaccination by gating on CD4+CD45.2+ and CD8+CD45.2+ cells using flow cytometry. Data is representative of four independent experiments. B, as in A, but flow sorted OT-I T cells were injected into 4-1BB-/- mice and OT-I T cell proliferation was assessed by gating on Vβ5.1/5.2+CD8+ T cells. Data is representative of two independent experiments. C, in vivo endogenous CTL killing response. C57BL/6 (CD45.2+) mice were immunized s.c. with Ova (50 μg) conjugated or nonconjugated with SA-4-1BBL (25 μg). Seven days post vaccination, mice received SIINFEKL-pulsed syngeneic splenocytes from C57BL/6.SJL (CD45.1+) and peptide-specific killing was assessed 2 days later and expressed as percent lysis for each histogram (n = 5; * P < 0.05 compared with each other and Ova control). D, as in C, but mice were immunized with a recombinant HPV-16 E7 protein (10 μg) conjugated or nonconjugated to SA-4-1BBL (25 μg). n = 5, * P < 0.05 compared with each other and E7 control.
SA-4-1BBL conjugate vaccines demonstrate enhanced efficacy in therapeutic cancer settings
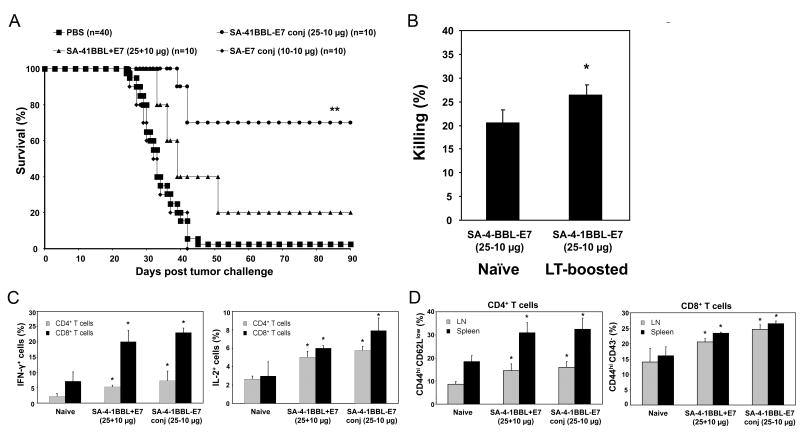
To test if the enhanced immunomodulatory activity of conjugate vaccine on DCs and T cells translates into better therapeutic efficacy, we used the TC-1 cell line expressing HPV-16 E7 oncogene as a transplantable tumor model for cervical cancer. A single s.c. injection of 10 μg of recombinant E7 protein conjugated to 25 μg of SA-4-1BBL into mice with established TC-1 tumors resulted in a 70% survival rate over a 90-day observation period (Fig. 3A). In contrast, injection with the nonconjugate vaccine resulted in only a 20% survival rate, while all mice vaccinated with 10 μg of E7 conjugated with 10 μg of SA (equimolar to 25 μg SA-4-1BBL) expired within 40 days.
Figure 3.
SA-4-1BBL conjugate vaccine has robust efficacy in TC-1 therapeutic tumor model. A, immunization with SA-4-1BBL conjugate vaccine demonstrates potent efficacy in eradicating established TC-1 tumors. C57BL/6 mice were challenged with live TC-1 cells and vaccinated once s.c. on day 6 post-tumor challenge with E7 (10 μg) conjugated or nonconjugated with SA-4-1BBL (25 μg) or an equimolar quantity of SA (10 μg). ** P < 0.001 for conjugated SA-4-1BBL-E7 vs. all other groups. Long-term surviving animals develop E7-specific CD8+ T-cell effector and memory pool (B) as determined by in vivo killing response (n = 3, * P < 0.05 vs. naïve control), (C) increased CD4+ and CD8+ T cell intracellular IFN-γ and IL-2 expression (n = 3, * P < 0.05 vs. naïve control), and (D) increased percentages of total memory CD44hiCD43-CD8+ T cells (n = 3, * P < 0.05 vs. each other and naïve control), and effector memory CD44hiCD62LlowCD4+ T cells (n = 3, * P < 0.05 vs. naïve control). Data for (B-D) are representative of two independent experiments.
Importantly, animals with eradicated tumors retained long-term immunological memory as assessed by increased E7 peptide-specific in vivo killing responses (Fig. 3B) and production of IFN-γ and IL-2 by both CD4+ and CD8+ T cells (Fig. 3C). Consistent with the general role of 4-1BB signaling in the development and maintenance of T cell memory, we observed a significant increase in CD4+CD44hiCD62Llow effector memory and CD8+CD44hiCD43- total memory T cells in tumor-free long-term mice vaccinated with the conjugate vaccines as compared with controls (Fig. 3D). The conjugate vaccine generated significantly enhanced CD8+CD44hiCD43- total memory pool as compared with nonconjugate vaccine, while this difference was not significant for the CD4+CD44hiCD62Llow effector memory pool.
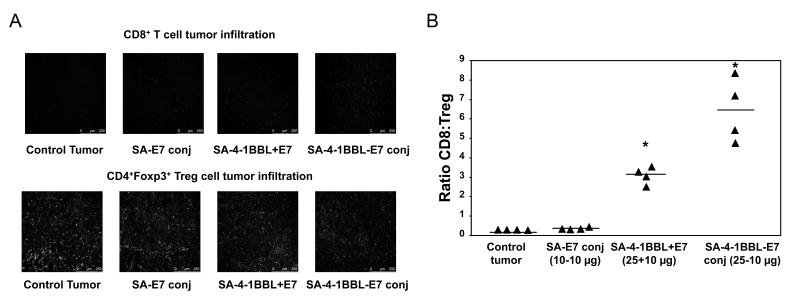
Importantly, the better therapeutic efficacy of conjugate vaccine correlated with significantly increased CD8+ T cells and decreased CD4+Foxp3+ Treg cells within the tumors as compared with nonconjugate vaccine or controls (Fig. 4A), resulting in a significantly increased intratumoral CD8+ Teff/Treg cell ratio (Fig. 4B).
Figure 4.
SA-4-1BBL conjugate vaccine increases the intratumoral CD8+ Teff/Treg ratio. A, vaccination with SA-4-1BBL-E7 conjugates increased intratumoral CD8+ T cells and decreased CD4+Foxp3+ Treg cells, as determined by confocal microscopy, resulting in increased ratio of intratumoral CD8+ Teff/Treg cells (B; n = 4, * P < 0.05 vs. each other and naïve and SA-E7 controls). A minimum of 3 fields/tumor section were analyzed and pictures were taken under 20× objective.
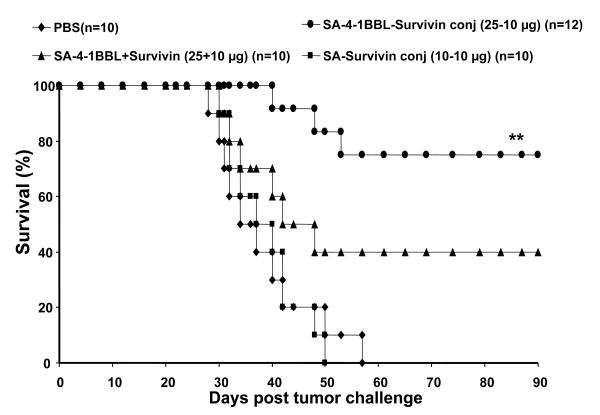
We further confirmed the enhanced therapeutic efficacy of the conjugate vaccine in the 3LL mouse lung carcinoma model using recombinant survivin protein as a self-TAA. A single s.c. injection of mice with established 3LL tumors with a conjugate vaccine consisting of 10 μg of recombinant survivin protein and 25 μg of SA-4-1BBL resulted in > 75% survival over a 90-day observation period (Fig. 5). In contrast, immunization with a nonconjugate vaccine resulted in < 40% survival rate, while all control mice immunized with survivin-SA (10+10 μg) expired within 50 days.
Figure 5.
SA-4-1BBL conjugate vaccine has robust efficacy in eradicating established 3LL tumors. C57BL/6 mice were challenged with 3LL tumor cells and vaccinated s.c. on day 6 post-tumor challenge with survivin (10 μg) conjugated or nonconjugated with SA-4-1BBL (25 μg) or an equimolar quantity of SA (10 μg). ** P < 0.001 for conjugated SA-4-1BBL-survivin vs. all other groups.
SA-4-1BBL conjugate vaccine demonstrates therapeutic efficacy in a metastatic lung tumor model
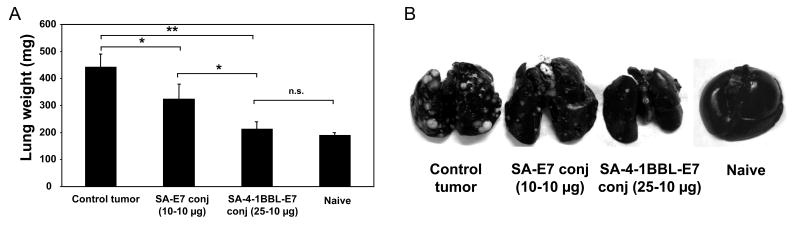
Metastasis is the cause of 90% of human deaths from cancer. Therefore, we tested whether conjugate vaccine demonstrates therapeutic efficacy in a TC-1 lung metastasis model (21). A single i.v. injection of 10 μg of recombinant E7 protein conjugated to 25 μg of SA-4-1BBL into mice with established metastatic TC-1 tumors resulted in almost complete eradication of lung-tumors as demonstrated by both lung weight (Fig. 6A) and presence of tumor nodules (Fig. 6B).
Figure 6.
SA-4-1BBL conjugates serve as an effective therapeutic vaccine in TC-1 lung metastasis model. C57BL/6 mice (n = 3 per group) were challenged with live TC-1 cells by i.v. tail injection and vaccinated i.v. on day 6 post-tumor challenge with E7 (10 μg) conjugated with SA-4-1BBL (25 μg) or an equimolar quantity of SA (10 μg). A, mice were euthanized 21 d after tumor challenge and lungs were weighed, and (B) photographed after injection with Indian ink to assess tumor nodules. ** P < 0.001 and * P < 0.05. Data are representative of a minimum of three independent experiments for each panel.
Discussion
DCs coordinate innate, adaptive, and regulatory immune responses, and as such have been the target of various therapeutic cancer vaccine strategies that aim to exploit the exceptional T cell immunostimulatory features of these cells for improved efficacy (4). Targeting antigens to DCs by various means without providing proper activation and maturation stimuli can result in T cell tolerance (22), induction of Treg cells (23), and increased expression of Foxp3, CTLA-4, TGF-β, and IL-10 by antigen specific CD4+ T cells (24). Therapeutic studies in humans demonstrated that DC maturation is required for the generation of effective immunity (25). In view of these findings, various strategies delivering antigens to DCs in vivo by targeting specialized receptors such as DEC205 (22, 26), Clec9A (27), the mannose receptor (28), and Dectin-1 (29) required TLR ligands or agonistic anti-CD40 Abs as adjuvants to mature the targeted DCs for the generation of endogenous CTL responses and tumor eradication. Although various adjuvants have shown efficacy in preclinical models, their toxic side-effects may limit their use at therapeutic doses in humans, thereby compromising vaccine efficacy (30-32). In contrast, SA-4-1BBL demonstrated superior therapeutic efficacy as a component of E7 peptide-based vaccine over various TLR ligands (LPS, MPL, and CpG) and an agonistic Ab to 4-1BB (7) without the severe toxicity associated with these adjuvants (6). Additionally, unlike these vaccine approaches that rely on an adjuvant for DC activation, we report that SA-4-1BBL not only delivers antigens to DCs, but also activates DCs for enhanced antigen uptake and presentation, and importantly further amplifies the generated immune responses in antigen-specific manner by directly targeting activated T cells for expansion, survival, and establishment of long-term memory (6, 7).
SA-4-1BBL was internalized upon binding 4-1BB receptor on DCs, and SA-4-1BBL specifically targeted the conjugated Ova to CD11chigh DCs in vivo, but not other APC types, such as B cells that can induce tolerance (33) and macrophages that can rapidly eliminate targeted antigen (34), leading to decreased immune efficacy. While all three analyzed DC subsets, lymphoid, myeloid, and plasmacytoid, showed enhanced antigen uptake, mainly lymphoid DCs exhibited enhanced and longer-duration cross-presentation of the conjugated antigen. Lymphoid DCs specialize in cross-presentation of exogenous antigens on MHC class I (35, 36), and promote Th1 responses through the production of IL-12 (37). While SA-4-1BBL greatly enhanced the presentation of SIINFEKL on MHC class I, it is presently unknown if enhanced cross-presentation is the direct effect of signaling through the 4-1BB receptor or is simply the byproduct of enhanced antigen uptake by DCs. The timing of signals required for DC maturation significantly impacts antigen cross-presentation capability of these cells, as stimuli too long before or too long after the capture of antigens may impair cross-presentation (38). Importantly, conjugate vaccines using SA-4-1BBL generated potent T cell proliferative responses and effector functions, suggesting that our vaccine approach avoids complications arising from the timing of antigen capture and delivery of maturation signals. Although our data attributes the robust therapeutic efficacy of the conjugate vaccine to DC targeting since DCs are the only APCs to constitutively express 4-1BB receptor in a naïve host, once the immune response is initiated by the vaccine or tumor itself, an immune activating microenvironment may lead to up regulation of 4-1BB on various other immune cell types, such as macrophages, NK, and NKT cells, which can then perpetuate the response. Moreover, the efficacy of conjugate vaccine does not seem to be due to the presence of higher SA-4-1BBL aggregates with improved immunostimulatory activity since both conjugate and nonconjugate vaccines had similar effects on T cell proliferation in vitro (Supplementary Figure 5).
Cross-presentation of antigens by DCs to CD8+ T cells in the absence of Th1 cell help may result in unproductive primary (39) and memory (40) CD8+ T cell responses. Importantly, vaccination with SA-4-1BBL-Ova conjugates enhanced the antigen uptake by myeloid DCs, which are perceived specialized APCs for CD4+ T cells due to their expression of relatively high levels of proteins implicated in the MHC class II presentation pathway (41). Consistent with this notion, vaccination with SA-4-1BBL-Ova conjugates generated a potent CD4+ T cell proliferative response that was partially dependent on DCs. Furthermore, immunization with conjugate vaccines, including HPV E7 or survivin as TAAs, in tumor bearing mice resulted in strong CD4+ T cell responses. In addition, SA-4-1BBL conjugates increased antigen uptake by plasmacytoid DCs, which specialize in anti-viral responses through the secretion of IFN-α and cross-present viral antigens to CD8+ T cells. This suggests that SA-4-1BBL can be used as an immunomodulator/delivery vehicle component of vaccines against viruses.
Treg cells represent a major barrier to effective cancer immunotherapy with high abundance of Treg cells in tumors associated with poor prognosis of cancer patients (42), while a high Teff/Treg cell ratio positively correlates with successful therapies (43). Vaccination of mice with established TC-1 tumors resulted in greatly increased numbers of CD8+ T cells and decreased numbers of Treg cells within the tumor, resulting in a favorable Teff/Treg cell ratio. Although the exact nature of implicated mechanisms are not known, it is possible that SA-4-1BBL treatment affects the trafficking and entry of both Teff and Treg cells into the tumor microenvironment by regulating the expression of relevant chemokine receptors on T cells and/or their respective ligands in the tumor microenvironment. SA-4-1BBL treatment might also preferentially improve the survival of CD8+ T cells over Treg cells or prevent the conversion of CD4+ Teff into Treg cells, which has recently been shown for OX40, another TNFR family member (44). Furthermore, the initial action of SA-4-1BBL on DCs and T cells may have secondary consequences to the tumor microenvironment, permitting a therapeutic window for antitumor immunity. Treatments targeting OX40 using an agonist Ab resulted in significant changes in tumor stroma, leading to decreased Treg cells, macrophages, myeloid-derived suppressor cells, and expression of TGF-β (45).
In conclusion, we herein corroborate our recent studies demonstrating the potent pleiotropic effects of SA-4-1BBL on various cells of the immune system (6, 7) and further demonstrate its use as a vehicle to target antigens in vivo to DCs for enhanced uptake, cross-presentation, and activation of both CD4+ and CD8+ T cells, their gain of effector functions, establishment of long-term memory, and therapeutic efficacy in three different tumor models. The potent immunomodulatory effects of SA-4-1BBL requires SA as the structural component of the molecule allowing SA-4-1BBL to exist as tetramers and oligomers (data not shown) that can cross-link the 4-1BB receptor for potent signal transduction, as a trimeric form of 4-1BBL has no costimulatory activity on T cells (Supplementary Figure 5) (18). Therefore, SA-4-1BBL represents a novel immunomodulator with significant potential for the development of therapeutic vaccines against cancer and chronic infections. Importantly, 4-1BB is also constitutively expressed on human DCs (46), allowing for eventual translation of our conjugate vaccine concept to human clinical application.
Supplementary Material
Acknowledgments
We thank Orlando Grimany-Nuño and Vahap Ulker for their excellent technical help with the production of recombinant proteins.
Grant support: This work was funded in parts by grants from the NIH (R43 AI071618, R41 CA121665, R44 AI071618, and R43AI074176), Kentucky Lung Cancer Research Program, W.M. Keck Foundation, and the Commonwealth of Kentucky Research Challenge Trust Fund.
Footnotes
Disclosure of Potential Conflict of Interest: The SA-4-1BBL described in this manuscript is licensed from UofL by ApoImmune, Inc., Louisville, KY, for which Haval Shirwan serves as CSO and Haval Shirwan and Esma S. Yolcu have significant equity interest in the Company. The other authors disclosed no potential conflict of interest.
Reference List
- 1.Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630–41. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 2.Ishii KJ, Akira S. Toll or toll-free adjuvant path toward the optimal vaccine development. J Clin Immunol. 2007;27:363–71. doi: 10.1007/s10875-007-9087-x. [DOI] [PubMed] [Google Scholar]
- 3.Steinman RM, Pope M. Exploiting dendritic cells to improve vaccine efficacy. J Clin Invest. 2002;109:1519–26. doi: 10.1172/JCI15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tacken PJ, de Vries IJ, Torensma R, Figdor CG. Dendritic-cell immunotherapy: from ex vivo loading to in vivo targeting. Nat Rev Immunol. 2007;7:790–802. doi: 10.1038/nri2173. [DOI] [PubMed] [Google Scholar]
- 5.Schabowsky RH, Sharma RK, Madireddi S, Srivastava A, Yolcu ES, Shirwan H. ProtEx technology for the generation of novel therapeutic cancer vaccines. Exp Mol Pathol. 2009;86:198–207. doi: 10.1016/j.yexmp.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schabowsky RH, Elpek KG, Madireddi S, et al. A novel form of 4-1BBL has better immunomodulatory activity than an agonisitc anti-4-1BB Ab without Ab associated severe toxicity. Vaccine. 2009;28:512–22. doi: 10.1016/j.vaccine.2009.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma RK, Elpek KG, Yolcu ES, et al. Costimulation as a platform for the development of vaccines: a peptide-based vaccine containing a novel form of 4-1BB ligand eradicates established tumors. Cancer Res. 2009;69:4319–26. doi: 10.1158/0008-5472.CAN-08-3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 9.Wilcox RA, Chapoval AI, Gorski KS, et al. Cutting edge: Expression of functional CD137 receptor by dendritic cells. J Immunol. 2002;168:4262–7. doi: 10.4049/jimmunol.168.9.4262. [DOI] [PubMed] [Google Scholar]
- 10.Futagawa T, Akiba H, Kodama T, et al. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int Immunol. 2002;14:275–86. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
- 11.Myers L, Lee SW, Rossi RJ, et al. Combined CD137 (4-1BB) and adjuvant therapy generates a developing pool of peptide-specific CD8 memory T cells. Int Immunol. 2006;18:325–33. doi: 10.1093/intimm/dxh371. [DOI] [PubMed] [Google Scholar]
- 12.Saoulli K, Lee SY, Cannons JL, et al. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J Exp Med. 1998;187:1849–62. doi: 10.1084/jem.187.11.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson SJ, Messer RJ, Carmody AB, Mittler RS, Burlak C, Hasenkrug KJ. CD137 costimulation of CD8+ T cells confers resistance to suppression by virus-induced regulatory T cells. J Immunol. 2008;180:5267–74. doi: 10.4049/jimmunol.180.8.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi BK, Kim YH, Kwon PM, et al. 4-1BB functions as a survival factor in dendritic cells. J Immunol. 2009;182:4107–15. doi: 10.4049/jimmunol.0800459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kober J, Leitner J, Klauser C, et al. The capacity of the TNF family members 4-1BBL, OX40L, CD70, GITRL, CD30L and LIGHT to costimulate human T cells. Eur J Immunol. 2008;38:2678–88. doi: 10.1002/eji.200838250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elpek KG, Yolcu ES, Franke DD, Lacelle C, Schabowsky RH, Shirwan H. Ex vivo expansion of CD4+CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4-1BB signaling. J Immunol. 2007;179:7295–304. doi: 10.4049/jimmunol.179.11.7295. [DOI] [PubMed] [Google Scholar]
- 17.Wilcox RA, Flies DB, Zhu G, et al. Provision of antigen and CD137 signaling breaks immunological ignorance, promoting regression of poorly immunogenic tumors. J Clin Invest. 2002;109:651–9. doi: 10.1172/JCI14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabu C, Quemener A, Jacques Y, Echasserieau K, Vusio P, Lang F. Production of recombinant human trimeric CD137L (4-1BBL). Cross-linking is essential to its T cell co-stimulation activity. J Biol Chem. 2005;280:41472–81. doi: 10.1074/jbc.M506881200. [DOI] [PubMed] [Google Scholar]
- 19.Green NM. Avidin and streptavidin. Methods Enzymol. 1990;184:51–67. doi: 10.1016/0076-6879(90)84259-j. [DOI] [PubMed] [Google Scholar]
- 20.Koksoy S, Elpek KG, Yolcu ES, Shirwan H. Tolerance to rat heart grafts induced by intrathymic immunomodulation is mediated by indirect recognition primed CD4+CD25+ Treg cells. Transplantation. 2005;79:1492–7. doi: 10.1097/01.tp.0000159870.01567.02. [DOI] [PubMed] [Google Scholar]
- 21.Ji H, Chang EY, Lin KY, Kurman RJ, Pardoll DM, Wu TC. Antigen-specific immunotherapy for murine lung metastatic tumors expressing human papillomavirus type 16 E7 oncoprotein. Int J Cancer. 1998;78:41–5. doi: 10.1002/(sici)1097-0215(19980925)78:1<41::aid-ijc8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 22.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–38. doi: 10.1084/jem.20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von BH. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–27. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 24.Bruder D, Westendorf AM, Hansen W, et al. On the edge of autoimmunity: T-cell stimulation by steady-state dendritic cells prevents autoimmune diabetes. Diabetes. 2005;54:3395–401. doi: 10.2337/diabetes.54.12.3395. [DOI] [PubMed] [Google Scholar]
- 25.de Vries IJ, Lesterhuis WJ, Scharenborg NM, et al. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res. 2003;9:5091–100. [PubMed] [Google Scholar]
- 26.Bonifaz LC, Bonnyay DP, Charalambous A, et al. In vivo targeting of antigens to maturing dendritic cells via the DEC-205 receptor improves T cell vaccination. J Exp Med. 2004;199:815–24. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sancho D, Mourao-Sa D, Joffre OP, et al. Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest. 2008;118:2098–110. doi: 10.1172/JCI34584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He LZ, Crocker A, Lee J, et al. Antigenic targeting of the human mannose receptor induces tumor immunity. J Immunol. 2007;178:6259–67. doi: 10.4049/jimmunol.178.10.6259. [DOI] [PubMed] [Google Scholar]
- 29.Carter RW, Thompson C, Reid DM, Wong SY, Tough DF. Preferential induction of CD4+ T cell responses through in vivo targeting of antigen to dendritic cell-associated C-type lectin-1. J Immunol. 2006;177:2276–84. doi: 10.4049/jimmunol.177.4.2276. [DOI] [PubMed] [Google Scholar]
- 30.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 31.den Haan JM, Kraal G, Bevan MJ. Cutting edge: Lipopolysaccharide induces IL-10-producing regulatory CD4+ T cells that suppress the CD8+ T cell response. J Immunol. 2007;178:5429–33. doi: 10.4049/jimmunol.178.9.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Mierlo GJ, den Boer AT, Medema JP, et al. CD40 stimulation leads to effective therapy of CD40(-) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc Natl Acad Sci U S A. 2002;99:5561–6. doi: 10.1073/pnas.082107699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bennett SR, Carbone FR, Toy T, Miller JF, Heath WR. B cells directly tolerize CD8(+) T cells. J Exp Med. 1998;188:1977–83. doi: 10.1084/jem.188.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 2005;307:1630–4. doi: 10.1126/science.1108003. [DOI] [PubMed] [Google Scholar]
- 35.den Haan JM, Lehar SM, Bevan MJ. CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J Exp Med. 2000;192:1685–96. doi: 10.1084/jem.192.12.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pooley JL, Heath WR, Shortman K. Cutting edge: intravenous soluble antigen is presented to CD4 T cells by CD8- dendritic cells, but cross-presented to CD8 T cells by CD8+ dendritic cells. J Immunol. 2001;166:5327–30. doi: 10.4049/jimmunol.166.9.5327. [DOI] [PubMed] [Google Scholar]
- 37.Hochrein H, Shortman K, Vremec D, Scott B, Hertzog P, O'Keeffe M. Differential production of IL-12, IFN-alpha, and IFN-gamma by mouse dendritic cell subsets. J Immunol. 2001;166:5448–55. doi: 10.4049/jimmunol.166.9.5448. [DOI] [PubMed] [Google Scholar]
- 38.Wilson NS, Behrens GM, Lundie RJ, et al. Systemic activation of dendritic cells by Tolllike receptor ligands or malaria infection impairs cross-presentation and antiviral immunity. Nat Immunol. 2006;7:165–72. doi: 10.1038/ni1300. [DOI] [PubMed] [Google Scholar]
- 39.Smith CM, Wilson NS, Waithman J, et al. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5:1143–8. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 40.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–6. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 41.Dudziak D, Kamphorst AO, Heidkamp GF, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–11. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]
- 42.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 43.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179:1427–30. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 45.Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 2008;68:5206–15. doi: 10.1158/0008-5472.CAN-07-6484. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Wang Q, Wang X, et al. Anti-CD137 monoclonal antibody promotes the direct anti-tumor effect mediated by peripheral blood-derived human dendritic cells in vitro. Cell Mol Immunol. 2004;1:71–6. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.