Abstract
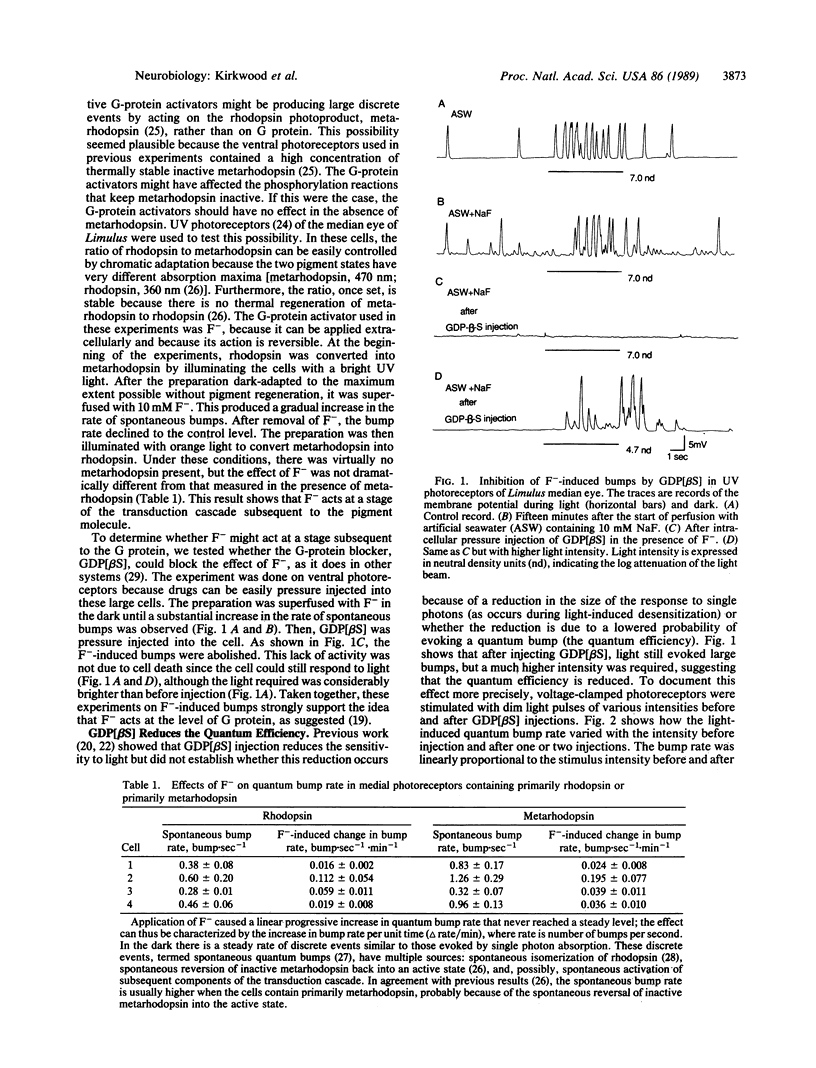
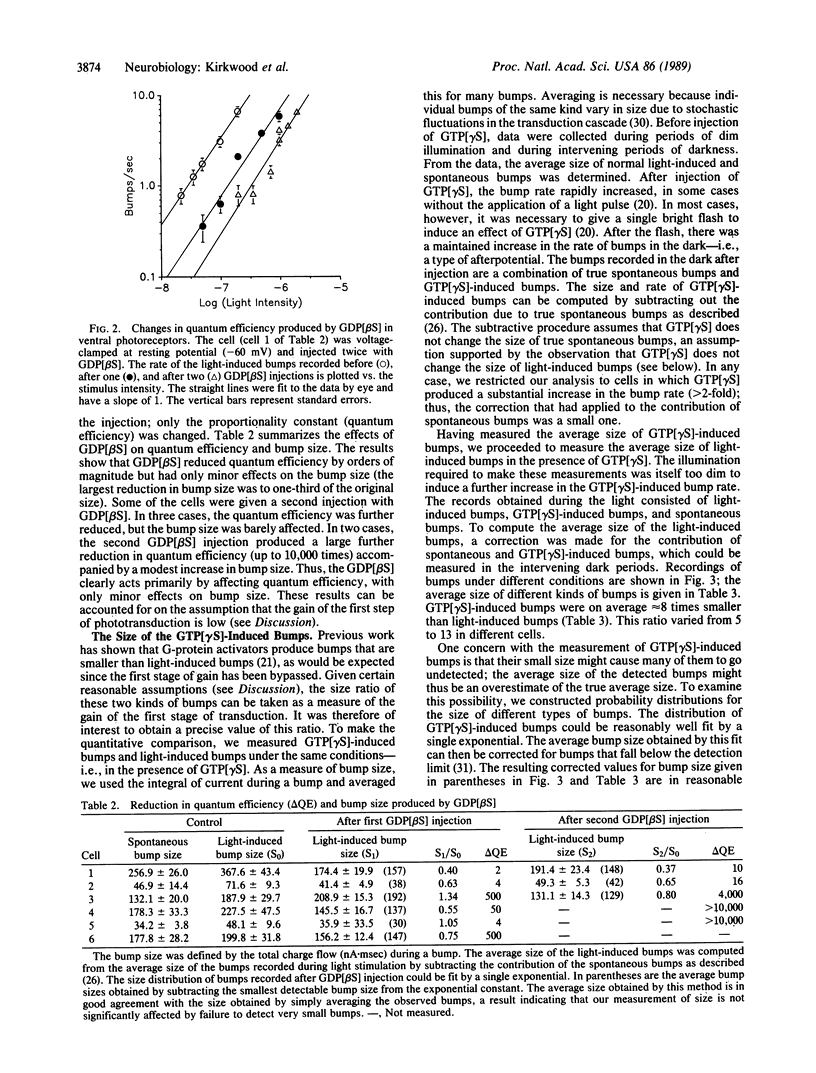
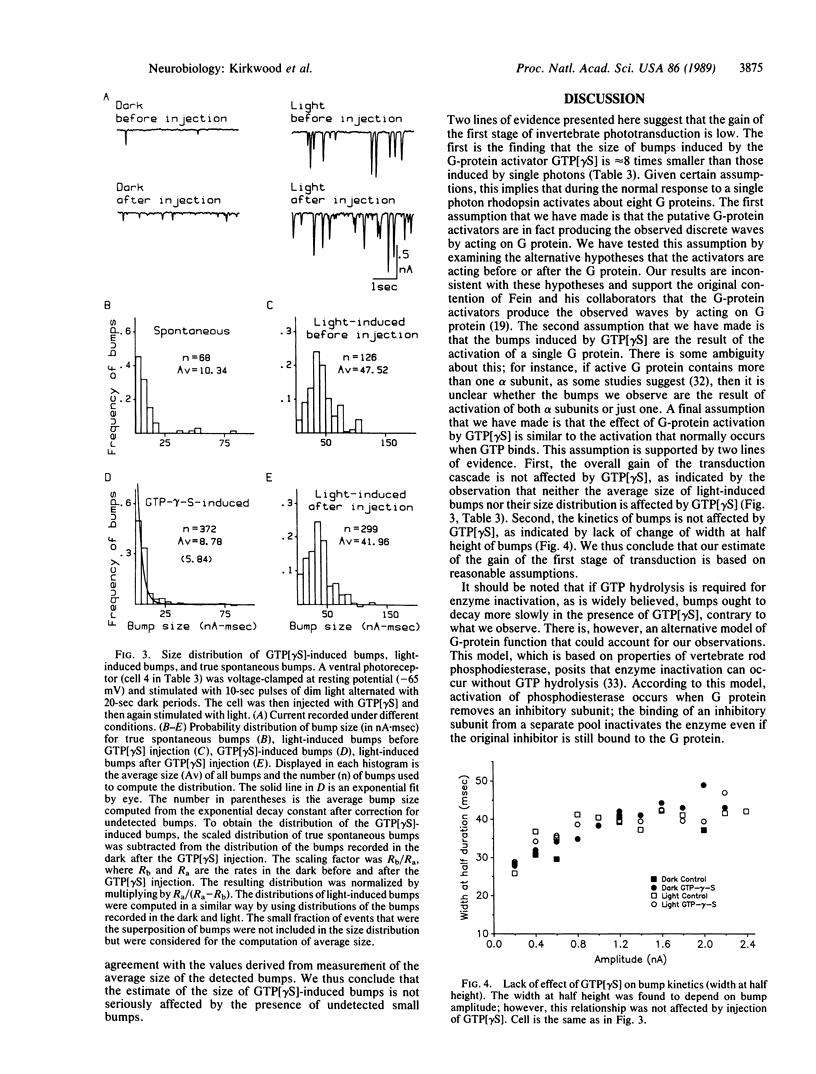
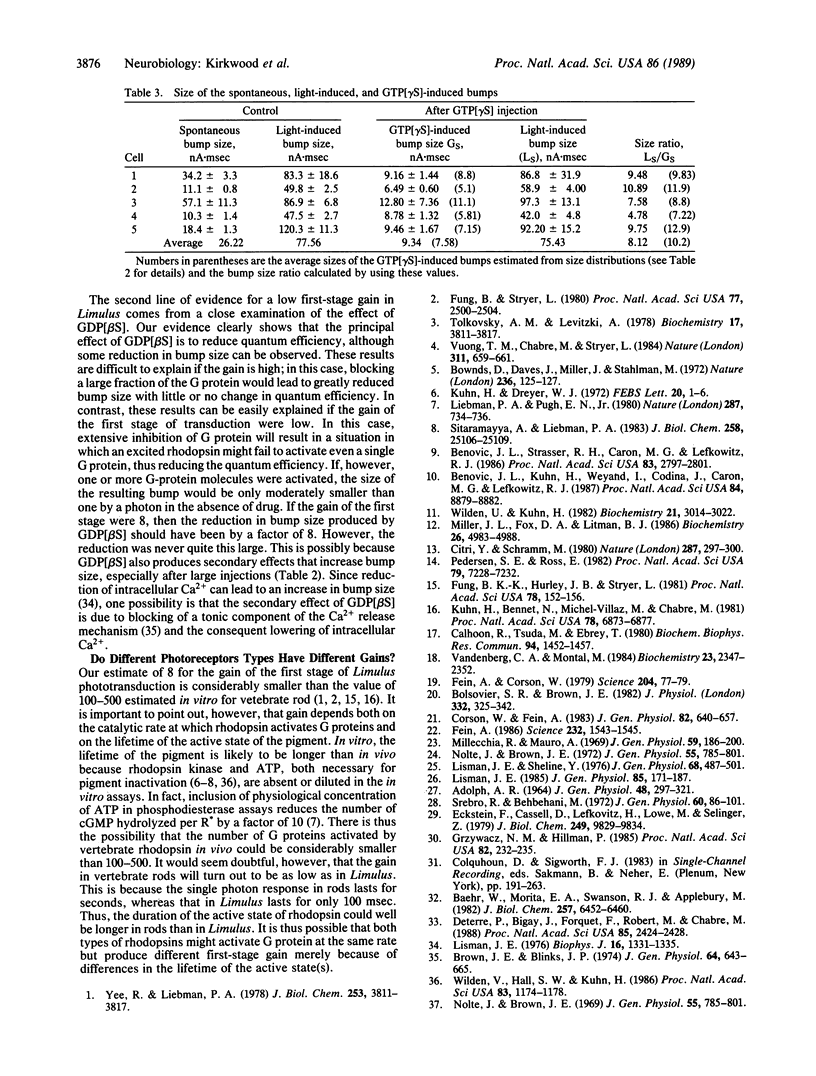
Previous work by others on Limulus photoreceptors has shown that application of a variety of guanine nucleotide-binding regulatory protein (G protein) activators produces discrete waves of depolarization similar to those generated by single photos, but smaller in size. We investigated whether these events might originate at a site other than the G protein. Initiation of the events did not depend on the state of the visual pigment, suggesting that the events do not originate at the pigment level. The events could be blocked by the G-protein blocker guanosine 5'-[beta-thio]diphosphate (GDP[betaS]) and thus support the conclusion that these discrete events are due to the activation of G protein itself. Quantitative measurements indicate that the average size of these events is approximately 8 times smaller than that evoked by single photons under the same conditions. Given certain reasonable assumptions, these results imply that the gain of the first stage of transduction in vivo is approximately 8, a value considerably lower than that measured in vitro in vertebrate rods (gain, 100-500). Furthermore, independent evidence for a low first-stage gain in Limulus is derived from the observation that GDP[betaS] barely affects the size of the response to single photons, but greatly reduces the probability that a photon evokes a response. These results can be explained if rhodopsin normally activates only a few G proteins.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADOLPH A. R. SPONTANEOUS SLOW POTENTIAL FLUCTUATIONS IN THE LIMULUS PHOTORECEPTOR. J Gen Physiol. 1964 Nov;48:297–322. doi: 10.1085/jgp.48.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehr W., Morita E. A., Swanson R. J., Applebury M. L. Characterization of bovine rod outer segment G-protein. J Biol Chem. 1982 Jun 10;257(11):6452–6460. [PubMed] [Google Scholar]
- Benovic J. L., Kühn H., Weyand I., Codina J., Caron M. G., Lefkowitz R. J. Functional desensitization of the isolated beta-adrenergic receptor by the beta-adrenergic receptor kinase: potential role of an analog of the retinal protein arrestin (48-kDa protein). Proc Natl Acad Sci U S A. 1987 Dec;84(24):8879–8882. doi: 10.1073/pnas.84.24.8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benovic J. L., Strasser R. H., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci U S A. 1986 May;83(9):2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolsover S. R., Brown J. E. Injection of guanosine and adenosine nucleotides into Limulus ventral photoreceptor cells. J Physiol. 1982 Nov;332:325–342. doi: 10.1113/jphysiol.1982.sp014416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bownds D., Dawes J., Miller J., Stahlman M. Phosphorylation of frog photoreceptor membranes induced by light. Nat New Biol. 1972 May 24;237(73):125–127. doi: 10.1038/newbio237125a0. [DOI] [PubMed] [Google Scholar]
- Brown J. E., Blinks J. R. Changes in intracellular free calcium concentration during illumination of invertebrate photoreceptors. Detection with aequorin. J Gen Physiol. 1974 Dec;64(6):643–665. doi: 10.1085/jgp.64.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoon R., Tsuda M., Ebrey T. G. A light-activated GTPase from octopus photoreceptors. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1452–1457. doi: 10.1016/0006-291x(80)90582-3. [DOI] [PubMed] [Google Scholar]
- Citri Y., Schramm M. Resolution, reconstitution and kinetics of the primary action of a hormone receptor. Nature. 1980 Sep 25;287(5780):297–300. doi: 10.1038/287297a0. [DOI] [PubMed] [Google Scholar]
- Corson D. W., Fein A. Chemical excitation of Limulus photoreceptors. I. Phosphatase inhibitors induce discrete-wave production in the dark. J Gen Physiol. 1983 Nov;82(5):639–657. doi: 10.1085/jgp.82.5.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deterre P., Bigay J., Forquet F., Robert M., Chabre M. cGMP phosphodiesterase of retinal rods is regulated by two inhibitory subunits. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2424–2428. doi: 10.1073/pnas.85.8.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Fein A. Blockade of visual excitation and adaptation in Limulus photoreceptor by GDP-beta-S. Science. 1986 Jun 20;232(4757):1543–1545. doi: 10.1126/science.3487116. [DOI] [PubMed] [Google Scholar]
- Fein A., Corson D. W. Both photons and fluoride ions excite limulus ventral photoreceptors. Science. 1979 Apr 6;204(4388):77–79. doi: 10.1126/science.34877. [DOI] [PubMed] [Google Scholar]
- Fung B. K., Hurley J. B., Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A. 1981 Jan;78(1):152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzywacz N. M., Hillman P. Statistical test of linearity of photoreceptor transduction process: Limulus passes, others fail. Proc Natl Acad Sci U S A. 1985 Jan;82(1):232–235. doi: 10.1073/pnas.82.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok-Keung Fung B., Stryer L. Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc Natl Acad Sci U S A. 1980 May;77(5):2500–2504. doi: 10.1073/pnas.77.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H., Bennett N., Michel-Villaz M., Chabre M. Interactions between photoexcited rhodopsin and GTP-binding protein: kinetic and stoichiometric analyses from light-scattering changes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6873–6877. doi: 10.1073/pnas.78.11.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H., Dreyer W. J. Light dependent phosphorylation of rhodopsin by ATP. FEBS Lett. 1972 Jan 15;20(1):1–6. doi: 10.1016/0014-5793(72)80002-4. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Pugh E. N., Jr ATP mediates rapid reversal of cyclic GMP phosphodiesterase activation in visual receptor membranes. Nature. 1980 Oct 23;287(5784):734–736. doi: 10.1038/287734a0. [DOI] [PubMed] [Google Scholar]
- Lisman J. E. Effects of removing extracellular Ca2+ on excitation and adaptation in Limulus ventral photoreceptors. Biophys J. 1976 Nov;16(11):1331–1335. doi: 10.1016/S0006-3495(76)85777-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E., Sheline Y. Analysis of the rhodopsin cycle in limulus ventral photoreceptors using the early receptor potential. J Gen Physiol. 1976 Nov;68(5):487–501. doi: 10.1085/jgp.68.5.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. The role of metarhodopsin in the generation of spontaneous quantum bumps in ultraviolet receptors of Limulus median eye. Evidence for reverse reactions into an active state. J Gen Physiol. 1985 Feb;85(2):171–187. doi: 10.1085/jgp.85.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. L., Fox D. A., Litman B. J. Amplification of phosphodiesterase activation is greatly reduced by rhodopsin phosphorylation. Biochemistry. 1986 Sep 9;25(18):4983–4988. doi: 10.1021/bi00366a002. [DOI] [PubMed] [Google Scholar]
- Nolte J., Brown J. E. The spectral sensitivities of single receptor cells in the lateral, median, and ventral eyes of normal and white-eyed Limulus. J Gen Physiol. 1970 Jun;55(6):787–801. doi: 10.1085/jgp.55.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte J., Brown J. E. The spectral sensitivities of single receptor cells in the lateral, median, and ventral eyes of normal and white-eyed Limulus. J Gen Physiol. 1970 Jun;55(6):787–801. doi: 10.1085/jgp.55.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S. E., Ross E. M. Functional reconstitution of beta-adrenergic receptors and the stimulatory GTP-binding protein of adenylate cyclase. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7228–7232. doi: 10.1073/pnas.79.23.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srebro R., Behbehani M. Light adaptation of discrete waves in the Limulus photoreceptor. J Gen Physiol. 1972 Jul;60(1):86–101. doi: 10.1085/jgp.60.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolkovsky A. M., Levitzki A. Coupling of a single adenylate cyclase to two receptors: adenosine and catecholamine. Biochemistry. 1978 Sep 5;17(18):3811–3817. doi: 10.1021/bi00611a021. [DOI] [PubMed] [Google Scholar]
- Vandenberg C. A., Montal M. Light-regulated biochemical events in invertebrate photoreceptors. 2. Light-regulated phosphorylation of rhodopsin and phosphoinositides in squid photoreceptor membranes. Biochemistry. 1984 May 22;23(11):2347–2352. doi: 10.1021/bi00306a004. [DOI] [PubMed] [Google Scholar]
- Vuong T. M., Chabre M., Stryer L. Millisecond activation of transducin in the cyclic nucleotide cascade of vision. Nature. 1984 Oct 18;311(5987):659–661. doi: 10.1038/311659a0. [DOI] [PubMed] [Google Scholar]
- Wilden U., Hall S. W., Kühn H. Phosphodiesterase activation by photoexcited rhodopsin is quenched when rhodopsin is phosphorylated and binds the intrinsic 48-kDa protein of rod outer segments. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1174–1178. doi: 10.1073/pnas.83.5.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilden U., Kühn H. Light-dependent phosphorylation of rhodopsin: number of phosphorylation sites. Biochemistry. 1982 Jun 8;21(12):3014–3022. doi: 10.1021/bi00541a032. [DOI] [PubMed] [Google Scholar]


