Abstract
Pantothenate kinase–associated neurodegeneration (PKAN), a progressive neurodegenerative disorder, is associated with impairment of pantothenate kinase function. Pantothenate kinase is the first enzyme required for de novo synthesis of CoA, an essential metabolic cofactor. The pathophysiology of PKAN is not understood, and there is no cure to halt or reverse the symptoms of this devastating disease. Recently, we and others presented a PKAN Drosophila model, and we demonstrated that impaired function of pantothenate kinase induces a neurodegenerative phenotype and a reduced lifespan. We have explored this Drosophila model further and have demonstrated that impairment of pantothenate kinase is associated with decreased levels of CoA, mitochondrial dysfunction, and increased protein oxidation. Furthermore, we searched for compounds that can rescue pertinent phenotypes of the Drosophila PKAN model and identified pantethine. Pantethine feeding restores CoA levels, improves mitochondrial function, rescues brain degeneration, enhances locomotor abilities, and increases lifespan. We show evidence for the presence of a de novo CoA biosynthesis pathway in which pantethine is used as a precursor compound. Importantly, this pathway is effective in the presence of disrupted pantothenate kinase function. Our data suggest that pantethine may serve as a starting point to develop a possible treatment for PKAN.
Keywords: coenzyme A, mitochondria, PKAN, oxidative stress, lifespan
CoA is a ubiquitous and essential cofactor for various metabolic reactions, including the tricarboxylic acid cycle and fatty acid metabolism (1). The canonical pathway leading to de novo synthesis of CoA starting at vitamin B5 (also known as pantothenate or pantothenic acid, further referred to as VitB5) is well known. All genes encoding the CoA biosynthetic enzymes have been identified and are highly conserved between different species (1–6) (Fig. 1A).
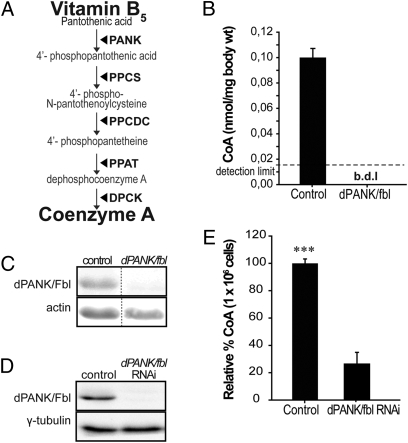
Fig. 1.
dPANK/Fbl impairment leads to reduced levels of CoA. (A) Scheme of canonical de novo CoA biosynthesis pathway. Vitamin B5 (pantothenic acid) is converted into CoA by the consecutive action of five enzymes: PANK, pantothenate kinase (EC2.7.1.33); PPCS, phosphopantotenoylcysteine synthase (EC6.3.2.5); PPCDC, phospho-N-pantothenoylcysteine decarboxylase (EC4.1.1.36); PPAT, phosphopantetheine adenylyltransferase (EC2.7.7.3); and DPCK, dephospho-CoA kinase (EC2.7.1.24)). (B) HPLC was used to measure levels of CoA in wild-type adult flies and in dPANK/fbl homozygous mutants at 6 days of age. (C) Western blot analysis was used to examine levels of dPANK/Fbl protein in wild types and dPANK/fbl mutants at 6 days of age. Actin was used as a loading control. (D) Western blot analysis was used to measure dPANK/Fbl protein levels in S2 cells 4 days after addition of dPANK/fbl dsRNA. As a control, cells were treated with mock ds-RNA. (E) HPLC was used to detect levels of CoA in control Drosophila Schneider's S2 cells and in S2 cells 7 days after dPANK/fbl RNAi treatment. ***P < 0.001 (Student's t test).
The biosynthesis of CoA, especially the CoA biosynthetic enzyme pantothenate kinase (PANK; EC 2.7.1.33), received renewed interest after the discovery that the Hallervorden-Spatz syndrome, a hereditary disease mainly affecting children, is caused by a mutation in the human PANK2 gene, one of the four human pantothenate kinase genes (PANK1-4), rendering the enzyme inactive (7). Accordingly, this syndrome has been referred to pantothenate kinase–associated neurodegeneration (PKAN). This finding uncovered a completely unknown role of CoA biosynthesis in cellular functioning. Patients with the autosomal recessive disorder PKAN show progressive impairment of speech, locomotor, and cognitive function (8). The pathophysiology of PKAN is not understood, and there is no cure to revert or delay the neurodegeneration. It is not known whether there are decreased levels of CoA in the affected tissues and thus whether decreased levels of CoA coincide with impaired neurological and locomotor function. Although a Pank2 mouse knock-out has been generated, this murine model did not show any signs of neurodegeneration (9), leaving the question unanswered as to whether decreased levels of CoA are causative in PKAN.
Recently, we and others have demonstrated that mutations in Drosophila CoA biosynthesis enzymes, including the Drosophila homolog of PANK2 (further referred to as dPANK/fbl mutants), induce a neurodegenerative phenotype; and these flies can be used as a model for PKAN-related research (2, 4, 10). Drosophila is not only a powerful model to understand the mechanisms of various human neurodegenerative diseases (11), but Drosophila disease models are also of value to identify compounds that are able to rescue disease-associated characteristics (12).
In the present study, we used the Drosophila dPANK/fbl mutants and dPANK/Fbl down-regulated Drosophila cultured S2 cells to address the following questions: (i) Does depletion of dPANK/Fbl correlate with decreased levels of CoA? (ii) If dPANK/Fbl depletion does induce decreased levels of CoA, are there ways to restore CoA levels in this background? (iii) If we are able to restore CoA levels, does this lead to a rescue of the phenotypes induced by dPANK/Fbl depletion?
Our results show that dPANK/Fbl depletion results in a significant decrease of CoA. Furthermore, we tested several compounds for their potential to restore CoA levels in the presence of impaired dPANK/Fbl function. One of the compounds tested was pantethine (the disulphide of pantetheine). Previously, it has been demonstrated that purified enzymatic extracts were able to convert both pantethine and pantetheine into 4′-phosphopantetheine (13–15), an intermediate in the canonical de novo CoA biosynthesis pathway (Fig. 1A). However, it has never been tested whether this alternative pathway is functional in a PANK-impaired background, although this knowledge is highly relevant in light of a possible PKAN therapy. Feeding pantethine to dPANK/fbl mutant flies or adding pantethine to dPANK/Fbl–down-regulated S2 cells restored CoA levels and rescued nearly all tested phenotypes, including the neurodegenerative phenotype. Our data further indicate that pantethine rescued mitochondrial abnormalities in hPANK2-depleted mammalian cells. Our results strongly suggest that pantethine can serve as a precursor compound to generate CoA even in the absence of a functional pantothenate kinase. Our findings may serve as a starting point to develop a possible treatment for PKAN.
Results
dPANK/fbl Mutant Flies Show Reduced Levels of CoA.
Pantothenate kinase is the enzyme required for the first step in the canonical biosynthetic route of CoA (Fig. 1A). In hypomorphic dPANK/fbl mutant flies the dPANK/Fbl protein content was severely decreased (Fig. 1C). Although there is a dynamic turnover of CoA in numerous intracellular metabolic reactions, there is only one route known that leads to the de novo synthesis of CoA (1). Therefore, we hypothesized that low levels of CoA caused the phenotype of dPANK/fbl mutants. Indeed, HPLC analysis clearly revealed significantly lower levels of CoA in homozygous dPANK/fbl mutants compared with wild type (Fig. 1B). To further test the effect of impaired function of dPANK/fbl on CoA levels, dPANK/Fbl protein levels were down-regulated in Drosophila S2 cells by RNAi (experimental setup in Fig. S2). Four days after the addition of dPANK/fbl dsRNA, dPANK/Fbl protein levels were strongly decreased (Fig. 1D). Under these circumstances, CoA levels were also significantly decreased to 24% of levels of control cells (Fig. 1E), and cell counts were significantly lower as compared with control cells (Fig. 2A). This suggested that de novo synthesis of CoA is required for maintenance of normal levels of CoA in Drosophila cells and accordingly for normal cell growth in culture.
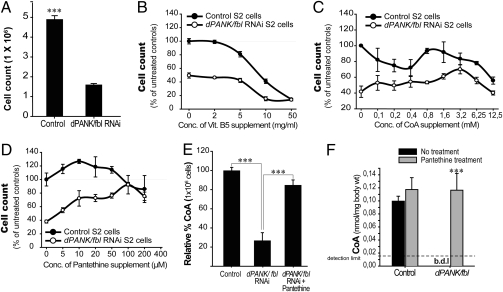
Fig. 2.
Pantethine rescues cell count of dPANK/Fbl-depleted cells. (A) Control and dPANK/fbl RNAi-treated cells were counted and plated in equal numbers (0.35·106 cells·mL) 4 days after dPANK/fbl RNAi treatment, and proliferation was assayed by counting cells 3 days later. ***P < 0.001 (Student's t test). Error bars indicate SEM. Control cells and dPANK/fbl RNAi-treated cells were plated, and it was tested whether VitB5 (B), CoA (C), or pantethine (D) addition to the medium rescued the cell count of dPANK/Fbl down-regulated cells. 100% represents the number of control cells under normal culturing conditions. (E) CoA levels were measured using HPLC in control cells and in dPANK/fbl RNAi-treated S2 cells (7 days after treatment) with and without addition of 100 μM pantethine. (F) HPLC was used to measure CoA levels in wild types and dPANK/fbl mutants after supplementation of 1.6 mg/mL pantethine to the food. ***P < 0.001 (Student's t test). Error bars indicate SEM. b.d.l, below detection limit.
Pantethine Addition Restores Normal Growth of dPANK/Fbl-Depleted Cells.
Our data strongly suggested that reduced levels of CoA might be the primary cause for the decreased cell count of dPANK/Fbl-depleted cells and for the mutant phenotype of dPANK/fbl homozygous flies. Accordingly, restoring CoA levels in dPANK/Fbl-depleted cells and in dPANK/fbl mutants should lead to a rescue of the related phenotypes. We checked several compounds related to CoA biosynthesis (CoA, VitB5, and pantethine) for their ability to rescue growth of dPANK/Fbl-depleted S2 cells and, when successful, for their ability to restore CoA levels. CoA was tested because adding CoA as a supplement may directly restore CoA levels. VitB5 was tested because adding large doses of VitB5 may compensate for decreased activity of dPANK/Fbl enzyme in a dPANK/fbl hypomorphic mutant background. Pantethine was tested because previously it has been reported that pantethine can be converted into the normally occurring CoA intermediate 4′-phosphopantetheine (13–15) (Fig. 1A). Our results showed that although high concentrations of all compounds were toxic, CoA and pantethine were effective in restoring cell counts of dPANK/Fbl-depleted cells in a concentration-dependent manner, whereas VitB5 was ineffective (Fig. 2 B–D). Because rescue with pantethine was most effective for dPANK/Fbl-depleted cells and pantethine was less toxic compared with CoA, our further analyses were focused on the rescuing potential of pantethine. The optimal effective concentration of pantethine for cells was 100 μM (Fig. 2D).
Pantethine Rescues Levels of CoA in dPANK/Fbl-Depleted Cells and in dPANK/fbl Mutant Flies.
First, we tested whether addition of pantethine to the cell culture medium of dPANK/Fbl down-regulated cells could restore CoA levels. Indeed, a restoration of CoA was observed (Fig. 2E). Next, we addressed the question whether pantethine addition to the food also rescues dPANK/fbl mutants. To identify the effective pantethine concentration in the fly food, various doses of pantethine were tested (Fig. S3). The addition of pantethine at a concentration of 1.6 mg/mL induced a significant increase in climbing activity in dPANK/fbl mutants while inducing only a moderate side affect in wild types. This concentration was used in all further experiments unless indicated otherwise. Concomitantly, levels of CoA were restored upon feeding pantethine directly to dPANK/fbl mutants via the food (Fig. 2F). These data suggested that there is a dPANK/Fbl-independent way to generate CoA from pantethine in both Drosophila S2 cells and in Drosophila dPANK/fbl mutant flies. Our data also indicated that pantethine provided via the food can still exert its CoA levels-restoring function.
Mitochondrial Structure and Function Are Severely Affected in dPANK/fbl Mutants, and Pantethine Rescues These Phenotypes.
Mitochondrial dysfunction is associated with a number of neurodegenerative diseases (16, 17). Several findings suggest that most likely PKAN is also a neurodegenerative disorder associated with impaired mitochondrial function; it has been shown that human PANK2 is localized in mitochondria (18), and that chemical inhibition of pantothenate kinase activity in primary hepatocytes induces abnormal mitochondrial morphology (19). In addition, it was shown that a specific splice isoform of Drosophila PANK/fbl was localized in mitochondria of Drosophila S2 cells (10). Together these data strongly suggest that human PANK2 and Drosophila dPANK/Fbl have a mitochondrial function and that impairment of pantothenate kinase might lead to mitochondrial abnormalities. To investigate this, mitochondrial mor-phology was examined. Flight muscles contain numerous densely packed mitochondria, and therefore this tissue was analyzed. Visual inspection of flight muscles with bright field microscopy revealed that the structure had a more “loose” appearance and contained more ruptures in dPANK/fbl mutants as compared with controls (Fig. 3A). Moreover, the muscular degeneration was progressive with age in dPANK/fbl mutants. Electron microscopic analysis was performed for a more detailed analysis, and this revealed that, in contrast to wild types, mitochondria of dPANK/fbl mutants were severely affected. The mutant mitochondria were swollen and showed damaged cristae and ruptured membranes (Fig. 3B). This analysis showed that low levels of CoA coincide with severely damaged mitochondrial structures in dPANK/fbl mutants. Pantethine feeding significantly reversed the morphological mitochondrial defects (Fig. 3 A and B).
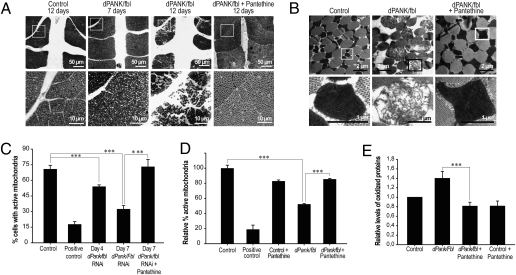
Fig. 3.
Impaired mitochondrial integrity and increased oxidative damage induced by disruption of dPANK/Fbl function is rescued by pantethine. (A) Morphological analysis of wild-type (12 days old) and dPANK/fblmutant (7 days and 12 days old) flight muscles (untreated and treated with pantethine) was performed by light microscopy. (B) Ultrastructural analysis of mitochondria in flight muscle of 12-day-old wild types and dPANK/fbl mutants (untreated and treated with pantethine). (Lower) Higher magnifications of the indicated areas in Upper. (C and D) JC-1 assay was used to quantify mitochondrial function in control cells and in dPANK/fbl RNAi treated cells (C) in the absence and presence of pantethine and in dPANK/fbl mutants (D) in the absence and presence of pantethine. Valinomycin was used as a positive control. (E) Oxyblots were used to measure levels of oxidative damage to proteins in dPANK/fbl mutants and the effect of supplementation of pantethine was investigated. ***P < 0.001 (Student's t test). Error bars indicate SEM.
In addition to this analysis, the more quantitative JC-1 assay (Fig. S1 and SI Text) was used to measure the percentage of functional mitochondria. Under control conditions, 70.8% of S2 cells had functional mitochondria (Fig. 3C). As a positive control for this assay, valinomycin was added to the media, and the percentage of cells with functional mitochondria dropped to 18% (Fig. 3C). Under normal culturing conditions, in dPANK/Fbl down-regulated S2 cells, mitochondrial activity was less than 54% after 4 days of RNAi treatment and was less than 32% after 7 days of RNAi treatment compared with control cells (Fig. 3C). Mitochondrial function was rescued to the levels of control cells after adding pantethine to the medium of dPANK/Fbl-depleted cells (Fig. 3C). A similar assay was performed on isolated mito-chondria from Drosophila flies. The results showed that dPANK/fbl mutants have 42% reduced mitochondrial function at day 6 as compared with control flies (Fig. 3D). Interestingly, feeding pantethine to dPANK/fbl mutants restored their mitochondrial function up to 84% of wild-type controls (Fig. 3D).
Pantethine Reduces Levels of Increased Oxidative Damage of Proteins in dPANK/fbl Mutants.
Previously, we showed that Drosophila CoA mutants displayed an increased sensitivity to oxidative stress (4). Here we investigated whether pantethine was able to reduce increased levels of oxidative stress in dPANK/fbl mutants by using Oxyblot assays (Fig. S4). Clearly, dPANK/fbl mutants showed increased levels of oxidative damage of proteins compared with wild type, and these levels were strongly reduced by addition of pantethine to the food (Fig. 3E).
Pantethine Improves Locomotor Abilities and Rescues Brain Degeneration in dPANK/fbl Mutants.
So far, our results have demonstrated that pantethine restores CoA levels, improves mitochondrial function and reduces levels of oxidative damage in a Drosophila model for PKAN. Next, we investigated whether all these beneficial effects resulted in improvement of locomotor function in dPANK/fbl mu-tants. On average, wild-type larvae were able to crawl 50 cm in 9 min, whereas homozygous dPANK/fbl mutants reached only 20 cm (Fig. 4A). Addition of pantethine to the larval food significantly improved larval crawling abilities, and an average distance of 30 cm was reached (Fig. 4A). Although a strong improvement in larval crawling activity was observed, pantethine feeding was unable to completely rescue the mutant phenotypes. Our data were inconclusive as to whether incomplete rescue was because dPANK/Fbl has additional functions (other than the production of CoA) or whether pantethine has side effects that hampered complete recovery. The latter explanation was supported by our observation that, in wild types, crawling activity was also reduced after pantethine feeding (Fig. 4A).
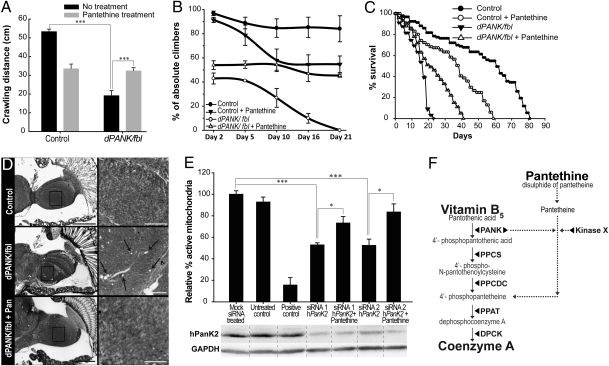
Fig. 4.
Pantethine is a protective compound in mutant flies and human cells. (A) Larval crawling abilities were measured in wild types and in dPANK/fbl mutants untreated and treated with pantethine. ***P < 0.001 (Student's t test). (B) Percentage of climbers was measured in aging wild types and in aging dPANK/fbl mutants untreated and treated with pantethine. (C) Cohorts of more than 120 flies (n = 3) of wild types and dPANK/fbl mutants were followed and survival curves were generated in the absence and presence of pantethine. All four curves were significantly different compared with each other (log-rank test, P < 0.001). (D) Brain morphology was investigated at the light-microscopic level in (12-day-old) wildtypes, dPANK/fbl mutants untreated and treated with pantethine. (Left) Low magnification. (Bars, 100 μm.) (Right) Higher magnifications of boxed areas. (Bars, 20 μm.) Vacuoles are marked by arrows. (E) Human HEK293 cells were treated with two independent siRNAs (siRNA 1 and siRNA 2) directed against human PANK2 mRNA. Western blot analysis with specific hPANK2 antibodies was used to investigate the effect of the RNAi treatment (48 h after RNAi treatment) on hPANK2 protein levels. GAPDH was used as a loading control. Addition of pantethine (100 μM) to the medium simultaneously with the RNAi treatment resulted in restoration of mitochondria function. Valinomycin was used as a positive control. ***P < 0.001; *P < 0.05 (Student's t test). Error bars indicate SEM. (F) Scheme representing possible pathways for CoA biosynthesis from pantethine. Pantethine may be converted to pantetheine; pantetheine may be phosphorylated by a yet-unknown pantetheine kinase, other than pantothenate kinase (indicated by kinase X) Phosphorylated pantetheine (4′-phosphopantetheine) may enter the canonical de novo CoA biosynthesis pathway downstream of PPCDC and upstream of PPAT.
Previously, we demonstrated that dPANK/fbl flies showed reduced ability to climb at a young age (4). Here we assayed whether this reduced ability to climb further deteriorates with age. Climbing tests of wild-type and homozygous dPANK/fbl flies at increasing age (2, 5, 10, 16, and 21 days), showed that mutants not only possessed impaired climbing abilities following eclosion but that they also experienced a steeper decline of the already-reduced climbing activity over time as compared with wild type (Fig. 4B). Pantethine feeding significantly prevented the rapid decline of climbing ability of dPANK/fbl mutant flies (Fig. 4B). Consistent with the data presented in Fig. 4A, pantethine feeding induced a decrease in climbing activity in wild-type flies. In addition to these behavioral assays we tested a possible protective function of pantethine on neurodegeneration more directly by analyzing dPANK/fbl mutant brain tissue. dPANK/fbl mutants show increased numbers of vacuoles in their brains (4), indicating brain degeneration (Fig. 4D). Pantethine also rescues this apparent neurodegenerative phenotype (Fig. 4D and Fig. S8).
Pantethine Increases Lifespan of dPANK/fbl Mutants.
Previously, we have demonstrated that dPANK/fbl mutants showed a severe reduction in lifespan (4). We investigated whether rescue of all of the above-mentioned phenotypes with pantethine also coincides with increased lifespan of dPANK/fbl mutants. The maximal and median lifespan of dPANK/fbl mutants were 23 and 15 days, respectively (Fig. 4C). Under these circumstances, wild-type flies showed maximal and median lifespans of 81 and 45 days, respectively (Fig. 4C). Pantethine feeding increased the maximal lifespan of dPANK/fbl mutants from 23 days to 41 days and the median lifespan from 15 to 22 days (Fig. 4C). Consistent with the data presented in Fig. 4 A and B, pantethine feeding induced a reduction in lifespan in wild-type flies. Regardless of these deleterious side effects of pantethine in wild types, pantethine feeding clearly rescued various relevant abnormalities of dPANK/fbl mutants.
In Mammalian HEK293 Cells with Down-Regulated PANK2 Levels, Pantethine Also Improved Mitochondrial Function.
Finally, we addressed the question of whether pantethine was also capable of rescuing an abnormal phenotype induced by impaired function of endogenous PANK2 in human cells, and for this we used mitochondria integrity as a read-out. First we tested whether down-regulation of PANK2 in HEK293 cells also results in decreased mitochondrial activity [quantified by the mitochondrial JC-1 assay (SI Text)]. Indeed, depletion of human PANK2 using two independent siRNAs resulted in decreased levels of PANK2 and decreased mitochondrial activity (Fig. 4E). Addition of pantethine to the medium of PANK2-depleted cells resulted in a significant rescue of mitochondrial activity (Fig. 4E). These data indicate that, also in human cells, pantethine was capable of protecting (albeit partly) against consequences of impaired PANK2 enzyme function.
Discussion
In the current study, we used a Drosophila model for PKAN to investigate the consequences of impaired pantothenate kinase function and to identify possible protective compounds against the mutant phenotypes. We demonstrate that in Drosophila dPANK/fbl mutants and in dPANK/Fbl down-regulated S2 cells, CoA levels are significantly decreased. Low levels of CoA coincide with impaired mitochondrial integrity, increased levels of oxidized proteins, increased loss of locomotor function, neurodegeneration, and decreased lifespan. Our data are consistent with published data demonstrating that numerous neurodegenerative disorders are tightly linked to mitochondrial dysfunction and increased levels of oxidative stress (16, 17). All of the dPANK/fbl phenotypes, including neurodegeneration, were more or less rescued by addition of the compound pantethine to the food. Our data support that the mechanism underlying pantethine protection in dPANK/fbl flies is specific and not general, because three other neurodegenerative (Parkinson's and two PolyQ) Drosophila models are not rescued by pantethine treatment (Fig. S6). Pantethine has been already proved to be an effective treatment for hyperlipoproteinemia and dyslipidemia in human patients, and a dose of up to 1,200 mg pantethine per day for 24 weeks is tolerated without any side effects (20, 21). Unfortunately it is currently unknown whether pantethine crosses the blood–brain barrier, although this knowledge is highly relevant to develop pantethine further as a possible treatment for PKAN.
For the first time, we show genetic evidence for the existence of a parallel pathway to the canonical de novo CoA biosynthesis starting from pantethine, which at least bypasses the first step of the pathway. We demonstrated that decreased levels of CoA were a clear consequence of impaired dPANK/Fbl function in Drosophila. Although this was an anticipated result, this consequence of impaired pantothenate kinase function has not been investigated in multicellular animals or in human patients. However, there are several reports that indirectly support our observations. Chemical inhibition of PanK1, PanK2, and PanK3 by HoPan in isolated murine hepatocytes resulted in a reduction of de novo CoA synthesis and reduction of total levels of CoA (19). In Arabidopsis thaliana, it was demonstrated that mutations in several genes coding CoA biosynthesis enzymes resulted in impaired CoA biosynthesis and decreased levels of CoA (22–24). Together, these and our data show that impaired function of CoA biosynthesis enzymes (including PANK) lead to a decreased rate of de novo CoA synthesis, and that normal de novo synthesis of CoA is required to maintain the physiological levels of CoA.
After establishing that CoA levels were indeed below detection in the Drosophila PKAN model, we demonstrated that pantethine is a very potent compound that can act as a starting substrate for generating CoA in a dPANK/fbl mutant background. How, exactly, pantethine can be converted into CoA is currently unclear. Classic biochemical studies using cell extracts showed that pantethine can be reduced into pantetheine (25, 26) and that this can be converted into 4′-phosphopantetheine (14). The latter is an intermediate of the canonical de novo biosynthesis pathway and thus here, upstream from PPAT, the CoA de novo synthesis pathways starting from vitB5 and from pantethine may converge (Fig. 4F). This is further supported by experiments showing that the decreased cell counts of dPPCS-depleted cells, but not of dPPAT-depleted cells, is rescued by pantethine (Fig. S7). Possible enzymes that catalyze the phosphorylation of pantetheine have never been genetically identified. However, it has been shown that specific purified enzyme preparations were able to phosphorylate both VitB5 and pantethine with similar kinetics (13). Based on these studies it was assumed, but never tested, that pantothenate kinase is the only enzyme present that can phosphorylate pantetheine. However, these earlier studies did not exclude the presence of additional kinases (other than pantothenate kinase) in the purified enzyme preparations with pantetheine kinase activities. It is also possible that pantethine can be converted into CoA not via 4′-phosphopantetheine but via a completely alternative route. Regardless of the exact route, our data suggest that this pathway can most likely occur independently from pantothenate kinase based on the following. (i) In both dPANK/fbl mutants and in dPANK/fbl down-regulated cells, the levels of dPANK/fbl are severely reduced and it is unlikely that these reduced dPANK/fbl protein levels are responsible for the restoration of CoA levels after pantethine addition. (ii) If some residual pantothenate kinase activity were present in dPANK/fbl mutants and in dPANK/Fbl down-regulated cells, addition of extra VitB5 should have been beneficial also. However, addition of extra vitB5 did not lead to rescue in dPANK/fbl (Fig. S5) mutants and in dPANK/Fbl down-regulated cells (Fig. 2B). Thus all of the above results suggest the presence of an alternative route for de novo synthesis of CoA independent from pantothenate kinase. Apart from whether residual activity of pantothenate kinase is required for pantethine rescue, our findings are still relevant for PKAN-related research because most of the patients with PANK2 gene mutations still have some residual activity of pantothenate kinase (18).
Regardless of the mechanisms behind pantethine toxicity in wild-type cells, the exact pathway of pantethine conversion into CoA, and whether residual activity of pantothenate kinase is required for pantethine rescue, our data at least suggest the existence of an alternative pathway that uses pantethine as a primary compound to generate de novo CoA in the presence of impaired pantothenate kinase function. This knowledge allows the development of a possible future therapy for PKAN.
Material and Methods
Drosophila Strains.
The Drosophila strain y1w1118 was used as a wild-type control (Bloomington Stock Centre). The hypomorphic dPANK/fbl1 mutant flies were used for all assays (2, 4).
Pantethine Supplementation.
D-Pantethine (Sigma) was added at a concentration of 1.6 mg/mL in standard fly food; 100 μM of D-Pantethine was added to S2 cell and HEK293 medium, except where mentioned otherwise.
Physiological Assays.
To study larval crawling, late third instar homozygous dPANK/fbl larvae were placed on 1% nonnutritive agar in a Petri dish. Total distance crawled by the larvae during 9 min was measured. To study the adult lifespan, newly eclosed flies (n > 100, 1 or 2 days old) were collected and raised on standard medium at 25 °C in a dry Petri dish with food (2.29 cm2; with or without pantethine) at the center of the Petri dish. The number of dead flies was counted every 2 days. Each experiment was repeated three times. For a climbing assay, adult flies were used to investigate climbing performance as previously described (27). The experiment was repeated three times (n > 100).
CoA Measurements.
CoA levels were measured from fly extracts (100 flies, 6 days old) or from Drosophila Schneider's S2 cells using HPLC (sample preparation and HPLC analysis are described in SI Text).
Cell Culture and PANK Knockdown.
Drosophila Schneider's S2 Cells were cultured, and RNAi knockdown of dPANK/Fbl was performed as previously described (28) (SI Text). Mammalian cell culture and siRNA knockdown of hPANK2 are described in SI Text.
Electron and Light Microscopy.
Flies were immersed in fixative solution (2.5% glutaraldehyde in 0.1 M cacodylate, pH7.8). Postfixation was performed in 2% OsO4 for 2 h at 4 °C. Dehydration was carried out with graded ethanol series followed by a propylene wash and preembedding in (1:1) propylene:epon solution. Embedding of the flies was performed in EPON. For light microscopy, sections (1–2 μm) were cut using a Reichert Ultracut microtome and stained with Toluidine Blue. For ultrastructural analysis of mitochondria, thin sections (60 nm) were cut from the same samples and analyzed by electron microscopy.
Mitochondrial Assays.
Mitochondria were isolated from 7-day-old flies as previously described (29). For measurement of mitochondrial membrane potential, J-aggregate-forming lipophilic cation (JC-1) was used to evaluate mitochondrial damage (30). The JC-1 assay (Sigma) was performed according to the manufacturer's manual (SI Text and Fig. S1).
Protein Oxidation Detection.
Protein lysates were prepared in RIPA buffer containing 2% β-mercaptoethanol. Total protein solutions were incubated with 2,4-dinitrophenylhydrazine (DNP) according to the OxyBlot protein oxidation detection Kit (Chemicon). The total amount of oxidized proteins was quantified for each sample by measuring chemiluminescence from the whole lane and oxidized protein levels were normalized using β-actin as a loading control (Fig. S4).
Antibodies.
dPANK/Fbl (1:4,000) (4), hPANK2 (1:2,000; a gift from J. Gitschier, University of California–San Francisco), GAPDH (1:10,000; Fitzgerald Industries), β-actin, and γ-tubulin (Sigma) were used. HRP-conjugated antimouse or antirabbit antibodies were used (1:2,000; Amersham) as secondary antibodies.
Supplementary Material
Acknowledgments
We thank Floris Bosveld and Harm Kampinga for stimulating discussions. This work was supported by a VIDI grant (to O.S.), a Neurodegeneration with Brain Iron Accumulation Disorders Association grant (to O.S and S.H.), and a Topmaster grant from the Graduate School GUIDE (to A.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0912105107/DCSupplemental.
References
- 1.Leonardi R, Zhang YM, Rock CO, Jackowski S. Coenzyme A: Back in action. Prog Lipid Res. 2005;44:125–153. doi: 10.1016/j.plipres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Afshar K, Gönczy P, DiNardo S, Wasserman SA. fumble Encodes a pantothenate kinase homolog required for proper mitosis and meiosis in Drosophila melanogaster. Genetics. 2001;157:1267–1276. doi: 10.1093/genetics/157.3.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Begley TP, Kinsland C, Strauss E. The biosynthesis of coenzyme A in bacteria. Vitam Horm. 2001;61:157–171. doi: 10.1016/s0083-6729(01)61005-7. [DOI] [PubMed] [Google Scholar]
- 4.Bosveld F, et al. De novo CoA biosynthesis is required to maintain DNA integrity during development of the Drosophila nervous system. Hum Mol Genet. 2008;17:2058–2069. doi: 10.1093/hmg/ddn105. [DOI] [PubMed] [Google Scholar]
- 5.Daugherty M, et al. Complete reconstitution of the human coenzyme A biosynthetic pathway via comparative genomics. J Biol Chem. 2002;277:21431–21439. doi: 10.1074/jbc.M201708200. [DOI] [PubMed] [Google Scholar]
- 6.Kupke T, Hernández-Acosta P, Culiáñez-Macià FA. 4′-Phosphopantetheine and coenzyme A biosynthesis in plants. J Biol Chem. 2003;278:38229–38237. doi: 10.1074/jbc.M306321200. [DOI] [PubMed] [Google Scholar]
- 7.Zhou B, et al. A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome. Nat Genet. 2001;28:345–349. doi: 10.1038/ng572. [DOI] [PubMed] [Google Scholar]
- 8.Gregory A, Polster BJ, Hayflick SJ. Clinical and genetic delineation of neurodegeneration with brain iron accumulation. J Med Genet. 2009;46:73–80. doi: 10.1136/jmg.2008.061929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuo YM, et al. Deficiency of pantothenate kinase 2 (Pank2) in mice leads to retinal degeneration and azoospermia. Hum Mol Genet. 2005;14:49–57. doi: 10.1093/hmg/ddi005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Z, Li C, Lv S, Zhou B. Pantothenate kinase-associated neurodegeneration: Insights from a Drosophila model. Hum Mol Genet. 2009;18:3659–3672. doi: 10.1093/hmg/ddp314. [DOI] [PubMed] [Google Scholar]
- 11.Lessing D, Bonini NM. Maintaining the brain: Insight into human neurodegeneration from Drosophila melanogaster mutants. Nat Rev Genet. 2009;10:359–370. doi: 10.1038/nrg2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faust K, et al. Neuroprotective effects of compounds with antioxidant and anti-inflammatory properties in a Drosophila model of Parkinson's disease. BMC Neurosci. 2009;10:109. doi: 10.1186/1471-2202-10-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abiko Y. Investigations on pantothenic acid and its related compounds. IX. Biochemical studies. 4. Separation and substrate specificity of pantothenate kinase and phosphopantothenoylcysteine synthetase. J Biochem. 1967;61:290–299. doi: 10.1093/oxfordjournals.jbchem.a128547. [DOI] [PubMed] [Google Scholar]
- 14.Levintow L, Novelli G. The synthesis of coenzyme A from panthetheine: Preparation and properties of panthetheine kinase. J Biol Chem. 1954;207:761–765. [PubMed] [Google Scholar]
- 15.Ward GB, Brown GM, Snell EE. Phosphorylation of pantothenic acid and pantethine by an enzyme from Proteus morganii. J Biol Chem. 1955;213:869–876. [PubMed] [Google Scholar]
- 16.Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 18.Kotzbauer PT, Truax AC, Trojanowski JQ, Lee VM. Altered neuronal mitochondrial coenzyme A synthesis in neurodegeneration with brain iron accumulation caused by abnormal processing, stability, and catalytic activity of mutant pantothenate kinase 2. J Neurosci. 2005;25:689–698. doi: 10.1523/JNEUROSCI.4265-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang YM, et al. Chemical knockout of pantothenate kinase reveals the metabolic and genetic program responsible for hepatic coenzyme A homeostasis. Chem Biol. 2007;14:291–302. doi: 10.1016/j.chembiol.2007.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prisco D, et al. Effect of oral treatment with pantethine on platelet and plasma phospholipids in IIa hyperlipoproteinemia. Angiology. 1987;38:241–247. doi: 10.1177/000331978703800307. [DOI] [PubMed] [Google Scholar]
- 21.Bertolini S, et al. Lipoprotein changes induced by pantethine in hyperlipoproteinemic patients: Adults and children. Int J Clin Pharmacol Ther Toxicol. 1986;24:630–637. [PubMed] [Google Scholar]
- 22.Rubio S, et al. An Arabidopsis mutant impaired in coenzyme A biosynthesis is sugar dependent for seedling establishment. Plant Physiol. 2006;140:830–843. doi: 10.1104/pp.105.072066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubio S, Whitehead L, Larson TR, Graham IA, Rodriguez PL. The coenzyme a biosynthetic enzyme phosphopantetheine adenylyltransferase plays a crucial role in plant growth, salt/osmotic stress resistance, and seed lipid storage. Plant Physiol. 2008;148:546–556. doi: 10.1104/pp.108.124057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tilton GB, Wedemeyer WJ, Browse J, Ohlrogge J. Plant coenzyme A biosynthesis: Characterization of two pantothenate kinases from Arabidopsis. Plant Mol Biol. 2006;61:629–642. doi: 10.1007/s11103-006-0037-4. [DOI] [PubMed] [Google Scholar]
- 25.Durr IF, Cortas N. The reduction of pantethine by an extract of camel intestine. Biochem J. 1964;91:460–463. doi: 10.1042/bj0910460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fisher DH, Szulc ME. Reduction of pantethine in rabbit ocular lens homogenate. J Pharm Biomed Anal. 1997;15:653–662. doi: 10.1016/s0731-7085(96)01878-x. [DOI] [PubMed] [Google Scholar]
- 27.Palladino MJ, Hadley TJ, Ganetzky B. Temperature-sensitive paralytic mutants are enriched for those causing neurodegeneration in Drosophila. Genetics. 2002;161:1197–1208. doi: 10.1093/genetics/161.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Vries HI, et al. Grp/DChk1 is required for G2-M checkpoint activation in Drosophila S2 cells, whereas Dmnk/DChk2 is dispensable. J Cell Sci. 2005;118:1833–1842. doi: 10.1242/jcs.02309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarze SR, Weindruch R, Aiken JM. Oxidative stress and aging reduce COX I RNA and cytochrome oxidase activity in Drosophila. Free Radic Biol Med. 1998;25:740–747. doi: 10.1016/s0891-5849(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 30.Smiley ST, et al. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc Natl Acad Sci USA. 1991;88:3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.