Abstract
We have recently reported that downregulation of miR-199a-5p is necessary and sufficient for inducing upregulation of its targets, including hypoxia-inducible factor-1alpha (Hif-1α) and Sirt1, during hypoxia preconditioning (HPC). Conversely, others and we have reported that miR-199a-5p is upregulated during cardiac hypertrophy. Thus, the objective of this study was to delineate the signaling pathways that regulate the expression of miR-199a-5p and its targets, and their role in myocyte survival during hypoxia. Since HPC is mediated through activation of the AKT pathway, we questioned if AKT is sufficient for inducing downregulation of miR-199a-5p. Our present study shows that overexpression of a constitutively active AKT (caAKT) induced 70% reduction in miR-199a-5p and was associated with a robust increase in HiF-1α (10 ± 2 fold) and Sirt1 (4 ± 0.8 fold) that was reversed by overexpression of miR-199a-5p. Similarly, insulin receptor-stimulated activation of the AKT pathway induced downregulation of miR-199a-5p and upregulation of its targets. In contrast, β-adrenergic receptor (βAR) activation in vitro and in vivo, induced 1.8-3.5-fold increase in miR-199a-5p. Accordingly, we predicted that βAR would antagonize AKT-induced, miR-199a-5p-dependent, upregulation of Hif-1α and Sirt1. Indeed, pre-treating the myocytes with isoproterenol before applying HPC, caAKT, or insulin resulted in 87 ± 3%, 75 ± 15 %, and 100% reductions in Hif-1α expression, respectively, and sensitized the cells to hypoxic injury. Thus, activation of beta-adrenergic signaling counteracts the survival effects of the AKT pathway via upregulating miR-199a-5p.
Keywords: microRNA, miR-199a-5p, Hif-1α, Sirt1, AKT, beta-adrenergic
1. Introduction
We have previously reported that miR-199a-5p is reduced to undetectable levels within minutes of exposing cardiac myocytes to hypoxia or ischemia [1]. We found that miR-199a-5p directly targets and inhibits Hif-1α and Sirt1 and its downregulation is necessary for their enhanced expression during hypoxia/ischemia preconditioning (HPC/IPC) of myocytes. Moreover, knockdown of miR-199a-5p with our miR-199a-eraser fully reproduced the upregulation of both targets and preconditioned the cells against hypoxia. We also demonstrated for the first time that Sirt1 is required for the stabilization of Hif-1α through mediating proteasomal degradation of prolyl hydroxylase-2 (PHD2), a negative regulator of Hif-1α. Thus, functionally inter-dependent molecules may be regulated by a common microRNA (miRNA).
Early HPC/IPC is an immediate cellular reaction to brief cycles of hypoxia/reoxygenation that involves de novo protein, but not mRNA, synthesis [2]. It was first described by Murry et al as a mechanism that protected the heart against subsequent prolonged ischemia- or ischemia/reperfusion (I/R)-induced damage [3]. Central to the preconditioning effect is the protection of mitochondria against hypoxic damage that is a function of the AKT pathway [reviewed in [4]]. Thus, activation of the AKT pathway plays an obligatory role in mediating the effects of early preconditioning [5]. Whereas AKT is well known to phosphorylate and inhibit the proteins BAD and glycogen synthase kinase 3beta (GSK3β), it is not always recognized for its role in inducing upregulation of Hif-1α [6, 7].
Hif-1α is a transcription factor induced by hypoxic conditions in all cell types tested and accounts for the transcription of 89% of genes that are upregulated during hypoxia [8]. Those include erythropoietin [9], vascular endothelial growth factor (VEGF) [10], glucose transporter 1 (Glut-1) [11], glycolytic enzymes [12], hemeoxygenase (HO-1) [13], and inducible nitric oxide (iNOS) [14], among others, all involved in cellular adaptation to hypoxia. However, in the case of severe hypoxia, Hif-1α is also involved in mediating apoptosis, at least partly through activation of Bnip3 [15] and stabilizing p53 [16], although the mechanisms that dissociate its two functions remain unknown.
Hif-1α and its targets are generally considered mediators of late vs. early ischemia preconditioning of the heart. This idea was supported by earlier findings that showed that de novo RNA or protein synthesis was not required for IPC [17]. These results have since been challenged by other studies that demonstrated protein, but not RNA, synthesis is indeed required during IPC [2, 18]. In concordance, Cai et al recently showed that mice heterozygous for Hif-1α fail to exhibit early preconditioning [19], while Eckle et al reported that knockdown of Hif-1α abolished the effect of early ischemia preconditioning [20]. However, since Hif-1α is a transcription factor and transcription is not required for early preconditioning, the mechanism for Hif-1α-mediated early preconditioning remains obscure. Our previous findings show that Hif-1α is not exclusively nuclear during preconditioning; it also associates with the mitochondria. Moreover, its knockdown abrogated HPC-mediated mitochondrial protection during hypoxia, which, for the first, suggests that Hif-1α might play a direct role in mitochondrial protection.
In contrast to the AKT pathway, long-term stimulation of the beta1 adrenergic receptor (β1AR) induces apoptosis of cardiac myocytes [21]. In concordance, sympathetic stimulation of the heart worsens ischemic damage [22–24], while ablation of adenylyl cyclase-5 increases longevity and protects the heart against stress [25]. A plausible mechanism may involve the inhibition of AKT activity by cyclic AMP (cAMP) [26, 27]. In addition, β-adrenergic receptor stimulation inhibits insulin-induced activation of Akt [28–30] and suppresses its protective effect in cardiac myocytes [31]. Here we show that the antagonism between these 2 pathways may be a consequence of their reciprocal effects on miR-199a-5p expression and, therefore, the expression of Hif-1α and Sirt1 target genes.
2. Materials and methods
2.1 Cell cultures and adenovirus Infection
Neonatal cardiac myocytes were prepared from Sprague Dawley rat hearts as previously described, using both pre-plating and percoll gradients for differential separation of myocytes from non-myoctes [32]. Cells were cultured and maintained in Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12, Invitrogen) supplemented with 10% fetal bovine serum all throughout the experimental procedure.
All DNA constructs were delivered to the myocytes via recombinant adenovirus vectors within 24 h of culturing, using 10–20 multiplicity of infection. The dose and duration of stimulation with insulin or isoproterenol (ISO) was as indicated in each figure legend.
2.2. Mice
The development and characterization of mice with cardiac-specific overexpression of β1-AR and β2-AR used in this study are as previously described [33].
2.3. Construction of adenoviruses
Recombinant adenoviruses were constructed, amplified and titered, as previously described by Dr. Frank Graham [34]. The DNA inserts included in this study are, the stem-loop precursor of mmu-miR-199a-1 downstream of a cytomegalovirus promoter, a tandem repeat of the antisense sequence of mature miR-199a-5p downstream of a U6 promoter, and a negative control that includes a stem-loop with a nonsense sequence replacing the mature miR-199a-5p sequence, as previously described [35]. All constructs were custom synthesized by Integrated DNA Technologies (IDT).
2.4. Northern blotting
As previously described [35].
2.5. Western Blotting
Ten microgram of protein samples was analyzed on 4–20% SDS-PAGE (Criterion gels; Bio-Rad). Antibodies used include, anti-phospho-ATK (ser473), anti-phospho-AKT (Thr308), and anti-AKT (Cell Signaling); anti-Hif-1α lpha (Novus Biologicals); anti-Sir-2α (Upstate biotechnology, MA); anti-ILK (Upstate Biotechnology, NY); GSK3-beta (BD Biosciences, CA); anti-myosin-heavy chain (MHC) (Hybridoma Bank, University of Iowa, IO).
2.6. Hypoxia and Hypoxia Preconditioning (HPC)
Cultured myocytes were subjected to hypoxia in a hypoxic chamber (Billups-Rothenberg Inc., CA). The chamber was filled with gas mixture of 95% N and 4.8% ± 0.2% CO2 (Inhalation Therapy, NJ) at 7psi/12,000 kPa filling pressure for 15 minutes. The chamber was then placed in a 37°C incubator for the indicated periods. For hypoxia preconditioning, cultured myocytes were subjected to 4 cycles of 1 h hypoxia followed by 1 h reoxygenation.
2.7. Monitoring mitochondrial membrane potential
Mitochondrial Membrane potential was monitored using JC-1 cationic dye (Molecular Probes, Invitrogen, CA) as recommended by the manufacturer. Briefly, the cells were incubated with JC-1 (0.35ug/ml) for 20 min at 37°C. The cells were then washed with phosphate buffered saline and imaged live.
2.8. Transverse aortic constriction in C57Bl/6 mice
C5BL/6 mice were anesthetized (i.p.) with a mixture of ketamine (0.066 mg/g), xylazine (0.013 mg/g), and acepromazine (0.002 mg/g). The animals were then ventilated via tracheal intubation connected to a rodent ventilator (Harvard Apparatus) with a tidal volume of 0.2 mL and a respiratory rate of 110 breaths per minute. The left chest was opened at the second intercostal space and the thymus glands were superiorly reflected. The transverse thoracic aorta between the innominate artery and left common carotid artery was dissected and a 7-0 nylon suture tied around the aorta against a 27-gauge needle. A control group underwent a sham operation involving thoracotomy and aortic dissection without constricting the aorta.
2.9. Echocardiography
Mice were anaesthetized with 2.5% avertin (0.010 – 0.015ml/g body weight) administered by intraperitoneal injection. Transthoracic echocardiography (Sequoia C256; Acuson, Mountain View, CA) was performed using a 13-MHz linear ultrasound transducer. The chest is shaved. Mice are placed on a warm saline bag in a shallow left lateral position and warm coupling gel is applied to the chest. Electrocardiographic leads are attached to each limb using needle electrodes. Two-dimensional images and M-mode tracing (sweep speed = 100–200 mm/s) are recorded from the parasternal short-axis view at the mid papillary muscle level. Care is taken not to apply too much pressure to the chest wall. The images are recorded on videotape and freeze frames are printed on a Sony color printer, scanned into PC using the Photoshop program (Adobe Photoshop). The images are then analyzed by NIH-Image program. M-mode measurements of left ventricular (LV) internal diameter and wall thickness are made from 3 consecutive beats and averaged using the leading edge-to-leading edge convention adopted by the American Society of Echocardiography. End-diastolic measurements are taken at the time of the apparent maximal LV diastolic dimension. End-systolic measurements are made at the time of the most anterior systolic excursion of the posterior wall. Left ventricular ejection fraction (EF) is calculated by the cubed methods as follows: (d3 – s3)/d3, where d and s represent LV end dimensions of diastole and systole, respectively.
2.10. Hemodynamic measurements
Mice were anesthetized as described above, and a 1.4-French (Millar Instruments) catheter-tip micromanometer catheter is inserted through the right carotid artery into the aorta and then into the LV where pressures, dp/dt, and −dp/dt are recorded.
Statistics
For calculating the probability value between 2 experimental groups, we used an unpaired, 2-tailed, Student t-test. P < 0.05 was considered significant.
3. Results
3.1. MiR-199a-5p is upregulated during cardiac hypertrophy and via β-adrenergic receptor stimulation, but downrgulated by AKT activation in cardiac myocytes
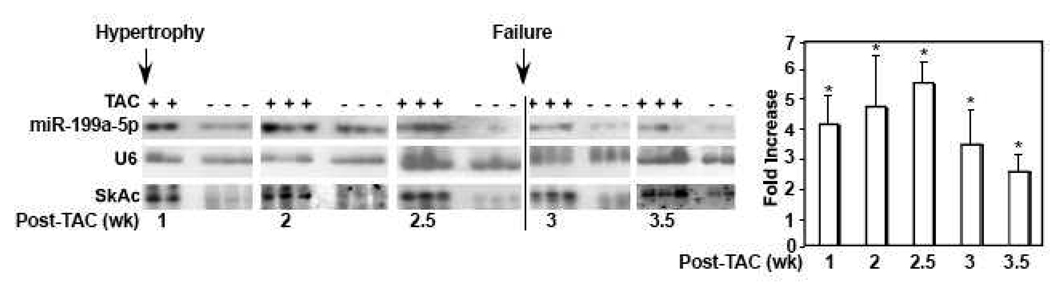
We have previously reported that brief hypoxia or ischemia induces downregulation of miR-199a-5p [1]. In contrast, profiling miRNA in the hypertrophied heart revealed that miR-199a-5p was significantly upregulated [36]. To confirm this finding, we subjected mice to transverse aortic constriction (TAC) or a sham operation for various time intervals. This procedure resulted in cardiac hypertrophic growth within 1 week and cardiac failure 2.5~3 weeks later, as monitored by echocardiography and hemodynamic measurements, and the upregulation of alpha skeletal actin (Fig. 1). In parallel, miR-199a-5p was upregulated 4.8 ± 0.6-fold within 2.5 weeks but declines significantly upon the onset of cardiac failure. The results suggest that miR-199a-5p is positively regulated by a factor(s) that is involved in the development of cardiac hypertrophy, whose effect diminishes during cardiac failure.
Figure 1. MiR-199a-5p is upregulated during cardiac hypertrophy.
Mice were subjected to transverse aortic banding (TAC) or a sham operation. After the indicated time periods RNA was extracted from the left ventricles and analyzed by Northern blotting for miR-199a-5p, alpha skeletal actin (SkAc) as a marker of cardiac hypertrophy, and U6 as an internal control. At each time point cardiac function was assessed by echocardiography and hemodynamic measurements. Any decrease in % ejection fraction, associated with an increase in left ventricular end diastolic pressure, was used as a sign of cardiac failure. MiR-199a-5p signals were quantified by UN-SCAN-IT software and averaged after normalizing each to the corresponding internal control (n=3). The results were graphed as fold increase in miR-199a-5p in TAC vs. Sham hearts. Error bars represent standard deviation. *<0.001 vs. sham.
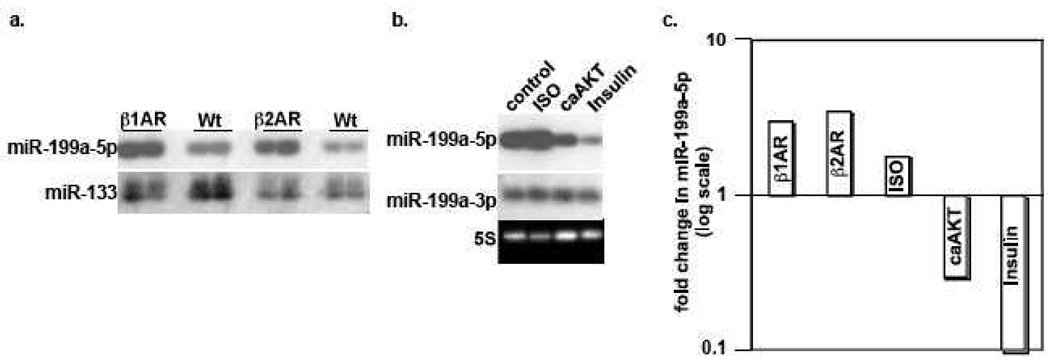
Beta-adrenergic receptor (βAR) stimulation conforms to this pattern of activity, as its desensitization coincides with the onset of cardiac failure. To determine its involvement, we measured miR-199a-5p levels in the β1AR- and β2AR-overexpressing mice hearts. As seen in figure 2a and 2c, these transgenic mice exhibited a 3 and 3.5-fold increase in miR-199a-5p but not in miR-133a. Similarly, stimulation of cultured myocytes with isoproterenol (ISO) resulted in a 1.8-fold increase in miR-199a-5p (Fig. 2b–c).
Figure 2. MiR-199a-5p is reciprocally regulated by βAR stimulation and AKT.
a. RNA was extracted from the hearts of 3 month-old β1AR- and β2AR-overexpressing mice and their wild type (WT) littermates, prior to the onset of any cardiac pathology. These were analyzed by Northern blotting for miR-199a-5p and miR-133 as an internal control (n=2). b. Cultured neonatal cardiac myocytes were treated with 10 μM isoproterenol (ISO), Ad.caAKT, or 200 nM insulin, for 16 h (n=3). RNA was then extracted and analyzed by Northern blotting for miR-199a-5p and miR-199a-3p as an internal control. c. MiR-199a-5p signals were quantified, normalized, and averaged. The results were graphed on a log scale as fold change of miR-199a-5p vs. wild type mice or control samples.
In contrast, preconditioning the myocytes with hypoxia induces downregulation of miR-199a-5p [1], where AKT is a known mediator of ischemia/hypoxia preconditioning [5, 37]. Therefore, we questioned whether activated AKT is sufficient for inducing downregulation of miR-199a-5p. Figure 2b reveals that treatment of cardiac myocytes with a constitutively activated AKT resulted in 70% reduction in miR-199a-5p, suggesting that it is indeed sufficient. We subsequently questioned whether receptor-activation of the AKT pathway is also associated with downregulation of miR-199a-5p. For that purpose we selected insulin, which is a robust activator of the AKT pathway. Moreover, insulin treatment has been reported to confer a preconditioning effect on the heart and protects myocytes against ischemic damage [38, 39]. Figure 2b demonstrates that treatment of myocytes with insulin for 24 h resulted in a robust (95% reduction) in miR-199a-5p. Thus, we concluded that activation of the beta-adrenergic and AKT pathways have distinctly opposite effects on miR-199a-5p expression.
3.2. Activated AKT is sufficient for inducing upregulation of Hif-1α and Sirt1 through a miR-199a-5p-dependent mechanism
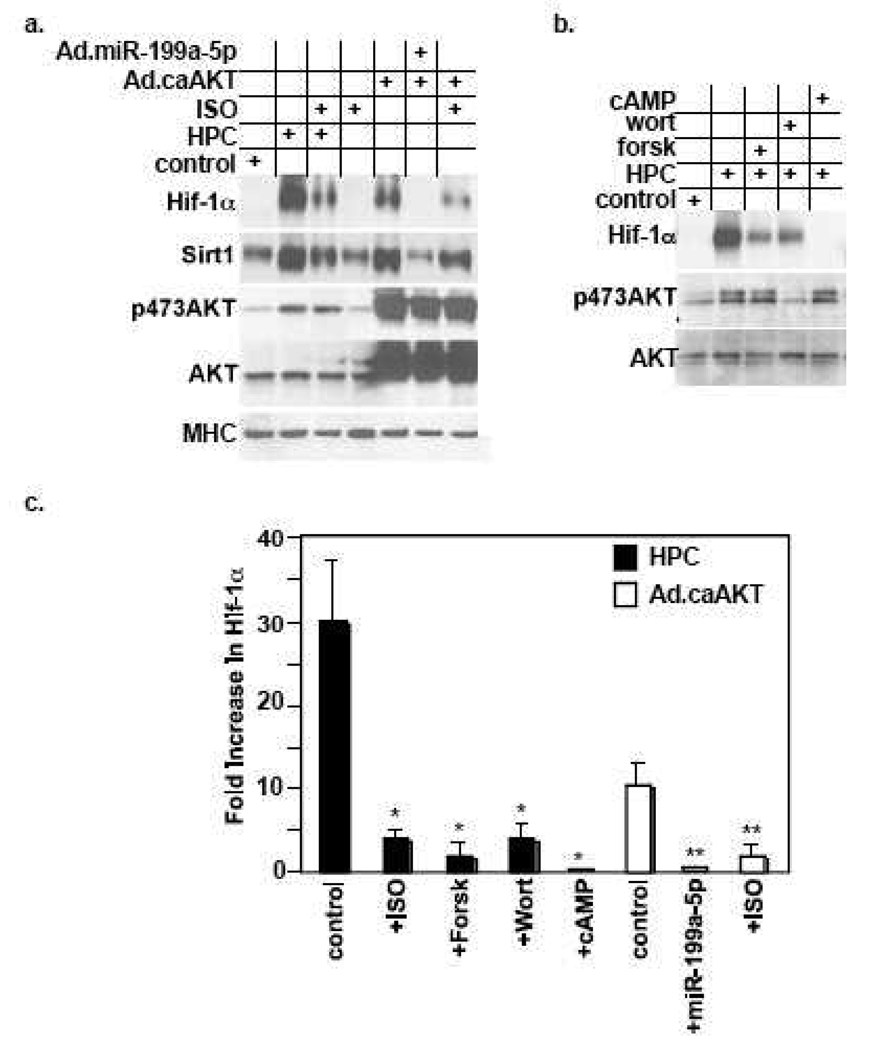
A few studies have implicated the AKT pathway in the upregulation of Hif-1α [6, 40], however, the mechanism is unknown. Additionally, this effect remains unexamined in cardiac myocytes. We showed above that activated AKT was sufficient for inducing downregulation of miR-199a-5p. Thus, we predicted that AKT induces upregulation of the miR-199a-5p targets, Hif-1α and Sirt1, through a miR-199a-5p-dependent mechanism. To test this possibility, we delivered caAKT to the myocytes in the presence of a control or miR-199a-5p-expressing adenovirus. Figure 3a and 3c show that caAKT-induced upregulation of Hif-1α that was completely abrogated by overexpression of miR-199a-5p. Additionally, and for the first time we reveal that AKT induces upregulation of Sirt1 that was similarly abrogated by reconstituting miR-199a-5p. Ultimately, to determine whether AKT mediates HPC-induced Hif-1α , we pretreated the myocytes with wortmannin prior to applying HPC. This resulted in inhibition of AKT phosphorylation and was associated with 87 ± 6 % reduction in Hif-1α (Fig. 3b–c). Thus, AKT is necessary and sufficient for mediating miR-199a-5p-dependent upregulation of Hif-1α and Sirt1 during HPC.
Figure 3. cAMP-dependent signaling antagonizes effects of the AKT pathway.
a. HPC was applied to cardiac myocytes with or without pretreatment with 10 µM ISO for 16 h, as indicated with the + sign. In parallel, cells were treated with Ad.caAKT with or without pretreatment with Ad.miR-199a-5p or 10 µM ISO for 16 h, where indicated with the + sign (n=5). b. HPC was applied to the myocytes with or without pretreatment of the cells with 100 µM forskolin (forsk), 5 mM wortmannin (wort), or a 4 µM cAMP analogue for 16 h, as indicated with the + sign. At the conclusion of all the treatments listed above, protein was extracted and analyzed by Western blotting for the molecules indicated on the left of each panel (n=3). c. The Hif-1α signals were quantified, averaged, and graphed as fold increase over control. Error bars represent standard deviation. *<0.001 vs HPC (no pretreatment). **<0.001 vs Ad.caAKT (no pretreatment).
3.3. Activation of the β-adrenergic receptor pathway blocks HPC- and AKT-induced Hif-1α and Sirt1
We have reported that downregulation of miR-199a-5p was necessary and sufficient for enhancing the expression of both Hif-1α and Sirt1 during HPC [1]. Thus, we hypothesized that any factors, including βAR stimulation, that induce upregulation of miR-199a-5p would counteract its downregulation and subsequently the upregulation of Hif-1α and Sirt1. To test this, we stimulated the myocytes with ISO for 16 h before applying HPC. In figure 3a and 3c, we show that this treatment resulted in 87 ± 3 % reduction in Hif-1α , which was associated with a similar reduction in Sirt1 expression. An equivalent effect was observed following treatment of the myocytes forskolin or cAMP, with the latter resulting in a more complete abrogation of HPC-induced Hif-1α (Fig. 3b–c). To determine whether this antagonism specifically targets the AKT pathway we applied a similar ISO treatment to the myocytes before delivery of caAKT. As observed, this also resulted in 75 ± 15% inhibition of caAKT-induced Hif-1α and Sirt1 (Fig. 3a and 3c). Thus, the results demonstrate that stimulation of the βAR stimulation antagonizes the regulatory effect of the AKT pathway on Hif-1α and Sirt1.
3.4. Isoproterenol antagonizes the protective effect of HPC in cardiac myocytes
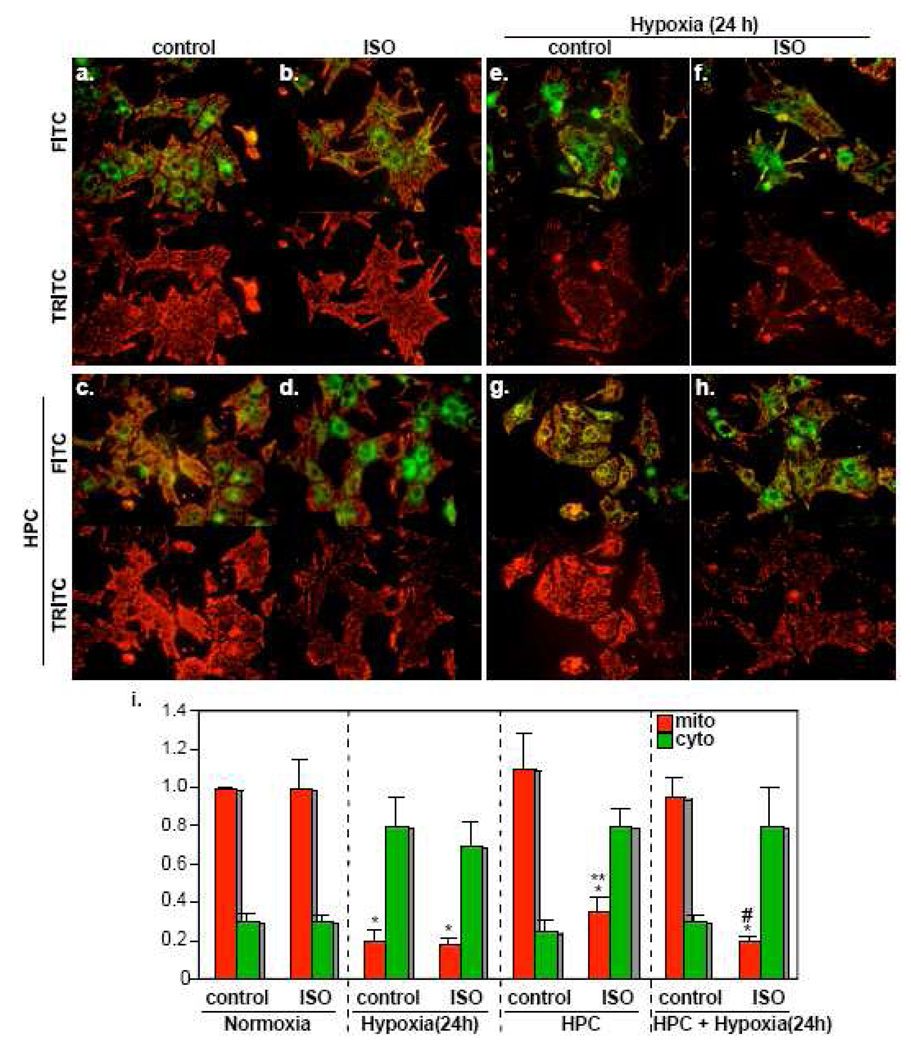
Sympathetic activity has been implicated in cellular damage during myocardial ischemia [41, 42]. Our data suggest that this may be a result of antagonizing the protective effect of the AKT pathway during hypoxia. To test this idea, we applied HPC to the myocytes with or without pretreatment of ISO for 16 h. This procedure was followed by exposure of the cells to 24 h of hypoxia. At its conclusion, the cells were loaded with JC-1 dye to assess the volume of healthy mitochondria (red florescence). On the other hand, cytosolic, monomeric, JC-1 is reflected by the green florescence. Neither the distribution of the green vs red florescence nor the abundance of the latter, which represents the healthy mitochondria, changed after independent ISO, or HPC, treatments (Fig. 4b and 4c, respectively), compared to the control cells (Fig. 4a). Although ISO did not induce detectable mitochondrial damage when applied for 16 h, it did when this was followed by brief hypoxic episodes that were applied for the preconditioning procedure. This is demonstrated by the reduction of the red florescence (Fig. 4d, i). Exposure of non-preconditioned myocytes to 24h hypoxia induced dramatic reduction in intact mitochondria (Fig. 4e and 4f) that was completely reversed by HPC (Fig. 4g). Pretreatment of the cells with ISO blocked the protectiveness of HPC (Fig. 4h). Thus, βAR stimulation did not induce apoptosis within the time frame of this experiment but did abolish the protective effect of HPC and sensitize the myocytes to hypoxic injury.
Figure 4. Isoproterenol inhibits the protective effect of HPC.
Cardiac myocytes were untreated (a, e), stimulated with 10 µM ISO (b, d, f, h), or HPC (c, d, g, h), before exposure to 24 h of hypoxia (e, f, g, h). Following these treatments, the myocytes were loaded with the JC-1 dye and immediately imaged live. The upper panel of each treatment is an image of the cells using the FITC filter, while the lower panel is an image of the same field using the TRITC filter (n=3). i. The red mitochondrial (mito) and the green cytosolic (cyto) signals were quantified using Adobe Photoshop software. The results from 5 fields were averaged and graphed as relative values to the red mitochondrial signal during normoxia adjusted to 1. Error bars represent SEM and *p<0.001 vs control normoxia. ** p<0.01 vs control HPC. # p<0.01 vs control HPC + hypoxia 24 h.
3.5. Insulin induces upregulation of Hif-1α through a miR-199a-5p-dependent mechanism, which is suppressed by isoproterenol
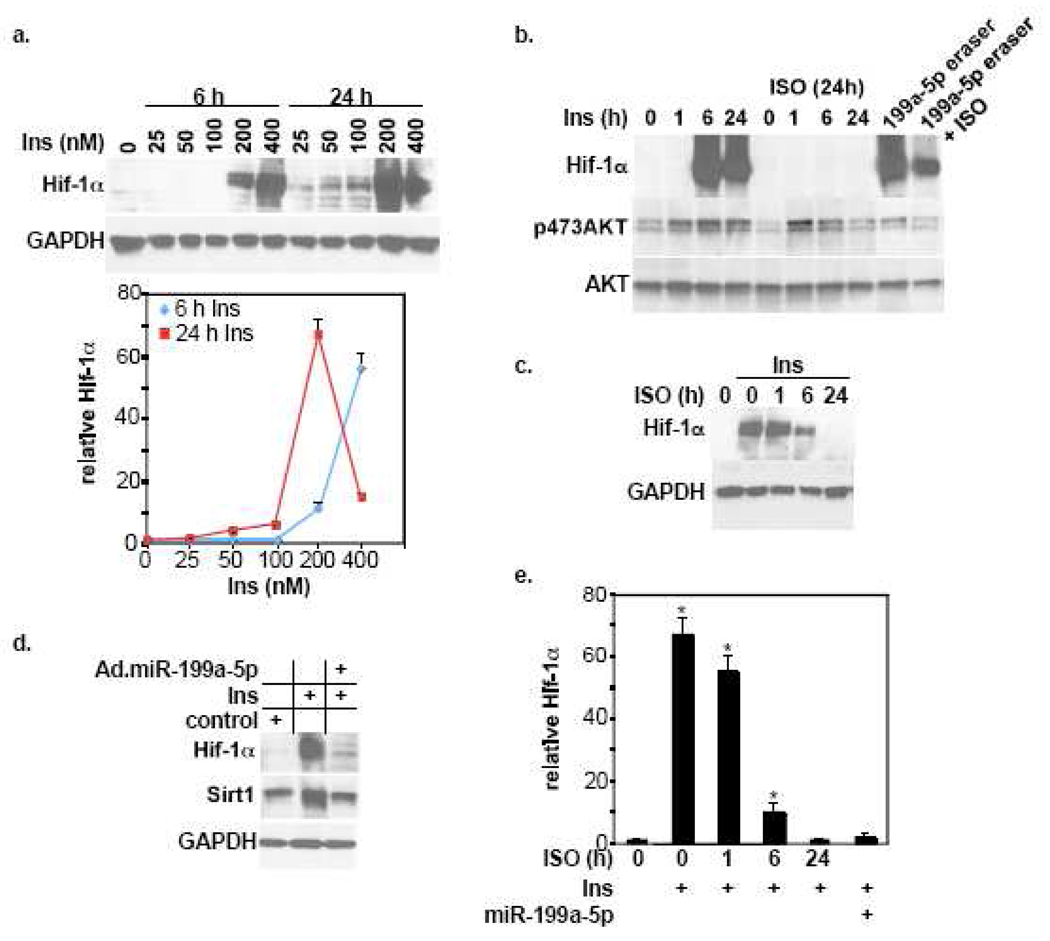
Like HPC, insulin is a cardioprotective hormone that mediates its effects through activation of the AKT pathway and has also been shown to induce upregulation of Hif-1α, although not yet in cardiac myocytes [43–45]. We hypothesized that it induces upregulation of Hif-1α through an AKT-miR-199a-5p-dependent axis that is antagonized by βAR stimulation. Stimulation of cardiac myocytes with insulin shows that it induces upregulation of Hif-1α in a dose- and time-dependent fashion that declined after excessive stimulation with 400 nM for 24 h (Fig. 5a). Pretreatment with ISO completely abolished this effect in a time-dependent manner (Fig. 5b, 5c, 5e). Likewise, overexpression of miR-199a-5p completely inhibited insulin-induced Hif-1α and Sirt1 expression (Fig. 5d–e). We noted that in some experiments pretreatment of the myocytes with ISO appeared to reduce AKT phosphorylation. This suggested that ISO may be inhibiting the induction of Hif-1α through antagonizing AKT activity. However, the results in figure 3a–b, which show that ISO inhibited the effect of caAKT without affecting its level of phosphorylation, argue against this possibility. To further address this, we examined whether ISO stimulation has the capacity to inhibit the effect of direct miR-199a-5p knockdown on Hif-1α expression. For that purpose we used an antisense miR-199a-5p adenovirus expression vector (Ad.miR-199a-5p eraser), which we have previously shown to completely knockdown endogenous miR-199a-5p and, thus, induce a robust upregulation of Hif-1α and Sirt1 [1] (Fig. 5b). This effect was inhibited by ~60% when the myocytes were pretreated with ISO stimulation. These results suggest that the effect of ISO on HPC-, insulin-, and AKT-induced Hif-1α expression is mainly mediated through counteracting the downregulation of miR-199a-5p. In agreement, Northern blot analysis shows that pretreatment of myocytes with ISO not only reversed HPC- or insulin-induced downregulation of miR-199a-5p, but also induced an increase equivalent to that observed following independent ISO stimulation (Fig. 6).
Figure 5. Isoproteronol and miR-199a-5p abrogate insulin-induced upregulation of Hif-1α in cardiac myocytes.
a. Cardiac myocytes were treated with insulin using various doses and durations, as indicated. The Hif-1α signal from 4 different experiments were quantified, normalized to GAPDH, averaged, and plotted relative to its control levels vs. insulin dose and stimulation duration. Error bars represent SEM. b. Cardiac myocytes were treated with insulin for various time intervals or Ad. miR.199a-5p for 24 h, following pretreatment with vehicle or 10 µM ISO for 16 h, as indicated (n=3). c. Myocytes were treated with 10 µM ISO for various time intervals before stimulating them with 200 nM insulin for 24 h (n=4). d. Cells were treated with Ad.miR-199a-5p or a control virus for 20 h followed by 200 nM insulin for 6 h, as indicated by the + sign. At the conclusion of all the treatments listed above, protein was extracted and analyzed by Western blotting for the molecules indicated on the left of each panel (n=3). e. Hif-1α signal from experiments shown in c and d were quantified, normalized to GAPDH, averaged, and plotted relative to its control levels after adjusting it to 1. Error bars represent SEM and *p<0.001 vs. control.
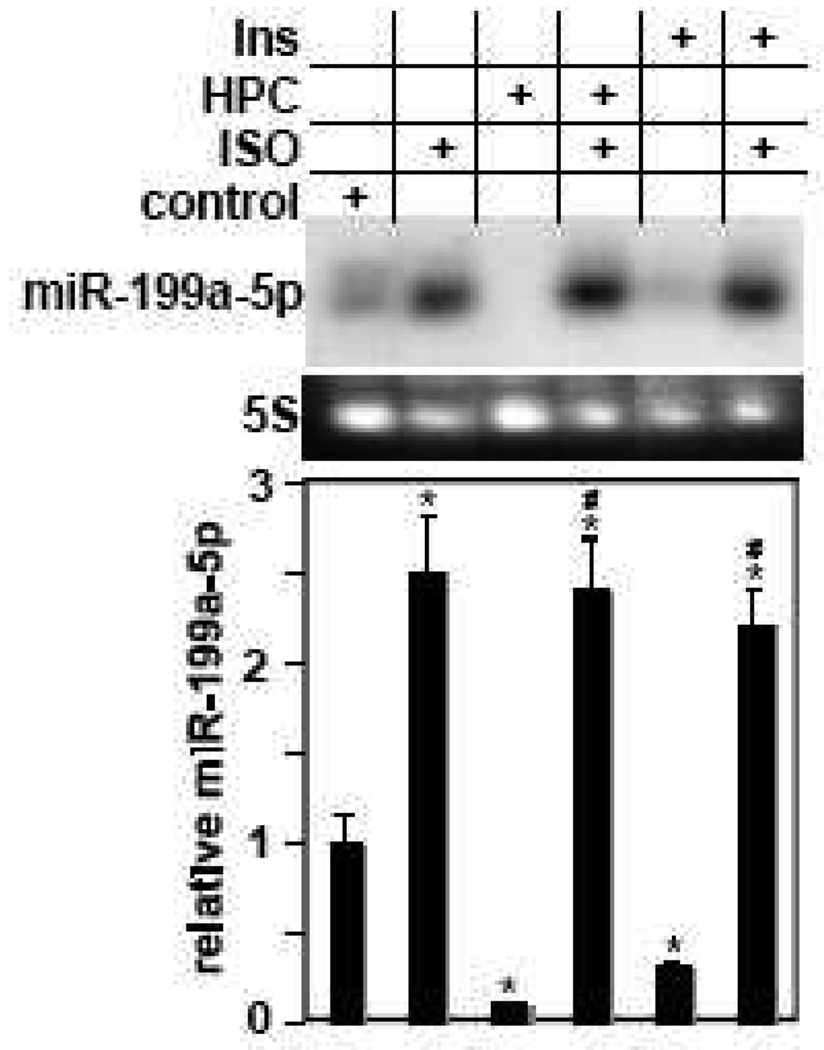
Figure 6. Isoproterenol-induced upregulation of miR-199a-5p counteracts its downregulation by HPC or insulin.
Cardiac myocytes were subjected to HPC or treated with 200 nM insulin for 6 h following treatment with vehicle or 10 µM ISO for 16 h, as indicated by + signs. RNA was extracted and analyzed for miR-199a-5p expression (n=3). MiR-199a-5p signals were quantified, normalized to 5S, averaged, and plotted relative to its control levels after adjusting it to 1. Error bars represent SEM and *p<0.01 vs. control; #p<0.01 vs. HPC.
3.6. Integrin-linked kinase induces upregulation of Hif-1α through a miR-199a-5p-dependent mechanism
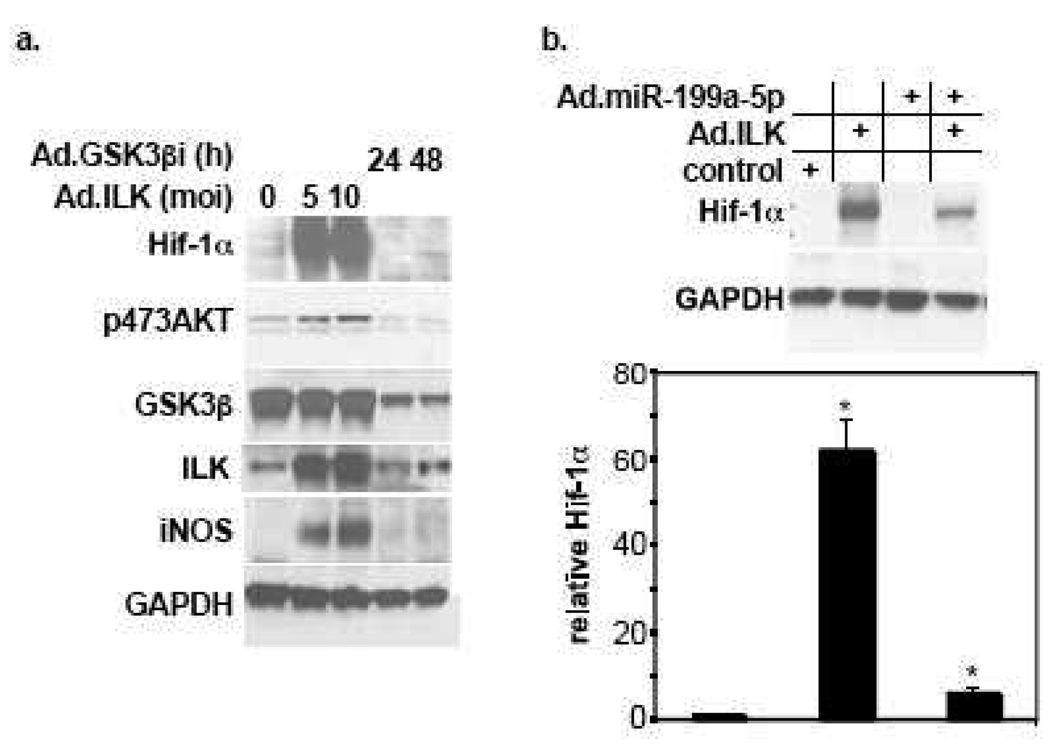
Integrin-linked kinase (ILK) is a known upstream regulator of AKT [46]. A few studies have recently shown that it is also a “hypoxia-responsive” gene that mediates upregulation of Hif-1α and angiogenesis [47, 48]. Thus, to further delineate and validate the pathway that regulates miR-199a-5p and Hif-1α expression, we overexpressed ILK in cardiac myocytes in the presence or absence of exogenous miR-199a-5p. In figure 7 we show that overexpression of ILK is associated with enhanced AKT phosphorylation and was sufficient for inducing a robust increase in Hif-1α (Fig. 7a) that was suppressed by supplementation with exogenous miR-199a-5p (Fig. 7b). Glycogen synthase kinase 3-beta (GSK-3β) is a downstream effector of AKT and has also been identified as a direct downstream substrate of ILK, whose activity is inhibited by phosphorylation. However, unlike activation of AKT, silencing GSK3βby short hairpin knockdown had no influence on Hif-1α ’s expression (Fig. 7a). Thus, similar to caAKT, overexpression of ILK is sufficient for inducing Hif-1α through downregulation of miR-199a-5p. However, inhibition of their downstream target, GSK3β, was not sufficient for mediating that effect.
Figure 7. Overexpression of ILK, but not inhibition of GSK3β, is sufficient for inducing upregulation of Hif-1α.
a. Cardiac myocyte were treated with various doses of Ad.ILK for 20 h or shRNA targeting GSK3β (Ad.GSK3βi) for 24 or 48 h, as indicated (n=2). b. Cardiac myocytes were treated with Ad.miR-199a-5p or a control virus for 16 h before applying Ad.ILK for an additional 20 h. At the conclusion of all the treatments listed above, protein was extracted and analyzed by Western blotting for the molecules indicated on the left of each panel (n=3). Hif-1α signal from experiments shown in a and b were quantified, normalized to GAPDH, averaged, and plotted relative to its control levels after adjusting it to 1. Error bars represent SEM and *p<0.001 vs. control.
4. Discussion
Our previous work shows that downregulation of miR-199a-5p is a prerequisite for the upregulation of Hif-1α during hypoxia or HPC. In this study we show that AKT is both necessary and sufficient for mediating this effect. However, we do not know how the AKT pathway is activated during hypoxia. The integrin-linked kinase, ILK, has been shown to be sensitive to hypoxia, is an activator of AKT, and is also known to induce upregulation of Hif-1α [47, 48]. Thus, it is plausible that integrins and their downstream pathways are activated during hypoxia. In support, overexpression of ILK was sufficient for inducing upregulation of Hif-1α to the same extent seen during HPC and was similarly blocked by replenishing miR-199a-5p.
While reduction in miR-199a-5p levels is required for enhancing the translation of Hif-1α, the protein will remain unstable in the presence of active prolyl hydroxylase-2 (PHD2). PHD2 is constitutively active during normoxia and inhibited by hypoxia. We have previously shown that it is considerably downregulated during hypoxia in cardiac myocytes. Intriguingly, we found that Sirt1 mediates its destruction through a NAD-dependent deactylase function and proteasomal degradation. This explains how Hif-1α is stabilized during normoxia. Thus, a single miRNA may efficiently regulate functionally interdependent genes. The results also show for the first time that insulin and AKT induce upregulation of Sirt1 through a miR-199a-5p-dependent mechanism.
The AKT pathway is activated by an array of receptors, including the tyrosine kinase receptors of insulin and insulin-like growth factor. In accordance, insulin stimulation plays a critical role in cardioprotection. For example, treatment of the heart with insulin preconditions it against ischemia [39], whereas ischemia preconditioning is ineffective in hearts that exhibit insulin resistance [38]. Moreover, treatment of myocytes with insulin protects them against hypoxia-induced apoptosis [49]. Although not well recognized, insulin induces upregulation of Hif-1α in various cell types, including human cancer cells lines and rat skeletal muscle myoblasts [45] through an AKT-dependent pathway [44]. However, this effect has not previously examined in cardiac myocytes. In this study we show that insulin stimulation induces upregulation of Hif-1α in cardiac myocytes in a dose-dependent manner in cultured myocytes via downregulation of miR-199a-5p. Thus, downregulation of miR-199a-5p and upregulation of its targets is a consequence of activation of the AKT pathway by hypoxia or tyrosine kinase receptors. This mechanism might explain how insulin treatment of the heart mimics ischemia preconditioning and protects it against apoptosis.
In contrast to the above results, differential profiling of miRNA revealed a significant increase in miR-199a-5p during cardiac hypertrophy. We also detected upregulation of miR-199a-5p in the hearts of β1 and β2AR transgenic mice. This led us to predict and subsequently confirm that βAR stimulation antagonizes the effect of the AKT pathway through counteracting its downregulation of miR-199a-5p (Fig. 8). Antagonism between these 2 pathways has not been directly documented but there is significant circumstantial evidence. For example, ablation of adenylate cyclase 5 in the heart enhances phosphorylation of AKT [50]. Moreover, beta-adrenergic stimulation antagonizes insulin, and reduces glucose uptake [30] and inhibits the protective effect of insulin in cardiac myocytes [31]. We further show that ISO inhibits the induction of Hif-1α and Sirt1 by caAKT, insulin, or HPC, and the their protective effect during hypoxia. This effect is mediated through neutralizing the downregulation of miR-199a-5p that is required for upregulation of those targets (Fig. 8). This is supported by the fact ISO also inhibited the effect of independent knockdown of miR-199a-5p.
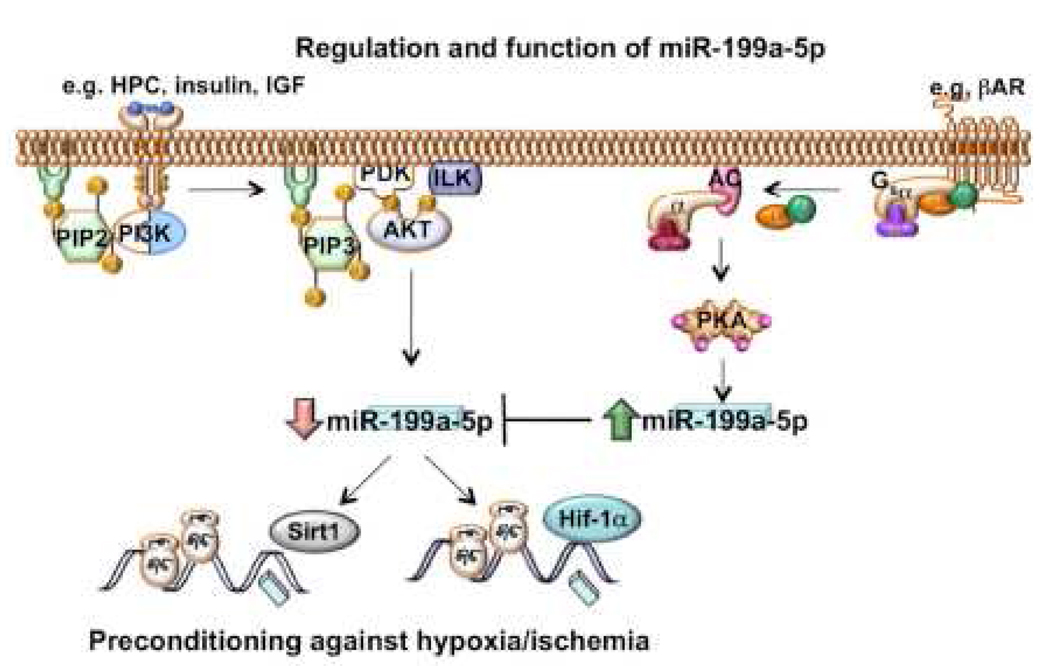
Figure 8. An illustration of the pathways that regulate miR-199a-5p and its targets, as suggested by the data.
Subjecting cells to HPC or stimulation with insulin results in ILK-AKT-dependent downregulation of miR-199a-5p and upregulation of its targets Hif-1α and Sirt1. Conversely, βAR stimulation induces AC-cAMP-dependent upregulation of miR-199a-5p and, thereby, neutralizes the effects of the AKT pathway. Abbreviations: PIP2, phosphatidylinositol 4,5 bisphosphate; PI3K, phosphatidylinositol-3 kinase; PIP3, phosphatidylinositol 3,4,5 trisphosphate; PKD, protein kinase D; AC adenylyl cyclase; PKA, protein kinase A.
Our data provide new insight into the mechanisms underlying the effectiveness of beta-blockers in ameliorating signs of heart failure and reducing cardiac ischemic episodes. This class of drugs is a standard ingredient of the therapeutic regimen in cardiac failure [51] and ischemic heart disease [52], although the exact mechanism of function remains unclear. For a couple of decades there was reluctance in the use of beta-blockers during cardiac failure since they inhibit the positive chronotropic and inotropic effects of the sympathetic system on the heart, which seemed counterproductive in a failing heart [53]. The current hypothesis is that they reverse the desensitization of the βAR and partly restore its functionality. On the other hand, prolonged stimulation of the β1AR has been associated with apoptosis [21], which would be suppressed by beta-blockers. The results presented here demonstrate that βAR stimulation antagonizes the protective effect of the AKT pathway that is mediated by both insulin and HPC, through inhibiting their induction of Hif-1α and Sirt1, which are key elements in a cell’s adaptation to hypoxia (Fig. 8). Although, ISO did not induce apoptosis under the conditions applied in our experiments, it did sensitize the myocytes to brief episodes of hypoxia, which normally precondition the cells against longer exposures. Thus, we propose that antisense knockdown of miR-199a-5p may provide a protective mechanism against cardiac ischemia and the exacerbating effects of sympathetic over activity.
Acknowledgment
We thank Dr. Stephen F. Vatner, Chairman of the Department of Cell Biology and Molecular Medicine for his support. This study is supported in part by the National Institute of Health Grants: 2R01 HL057970 and R01 HL081381 to the corresponding author.
The abbreviations used are
- Hif-1α
hypoxia-inducible factor 1alpha
- Sirt1
sirtuin-1
- HPC
hypoxia preconditioning
- miRNA
microRNA
- IPC
ischemia preconditioning
- TAC
transverse aortic constriction
- caAKT
constitutively active AKT
- ILK
integrin-linked kinase
- βAR
beta-adrenergic receptor
- ISO
isoproterenol
- GSK3β
glycogen synthase-3beta
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rane S, He M, Sayed D, Vashistha H, Malhotra A, Sadoshima J, Vatner DE, Vatner SF, Abdellatif M. Circ Res. 2009;104(7):879–886. doi: 10.1161/CIRCRESAHA.108.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowland RT, Meng X, Cleveland JC, Meldrum DR, Harken AH, Brown JM. J Surg Res. 1997;71(2):155–160. doi: 10.1006/jsre.1997.5142. [DOI] [PubMed] [Google Scholar]
- 3.Murry CE, Jennings RB, Reimer KA. Circulation. 1986;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 4.Miyamoto S, Murphy AN, Brown JH. J Bioenerg Biomembr. 2009;41(2):169–180. doi: 10.1007/s10863-009-9205-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tong H, Chen W, Steenbergen C, Murphy E. Circ Res. 2000;87(4):309–315. doi: 10.1161/01.res.87.4.309. [DOI] [PubMed] [Google Scholar]
- 6.Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK. Cell Growth Differ. 2001;12(7):363–369. [PubMed] [Google Scholar]
- 7.Mottet D, Dumont V, Deccache Y, Demazy C, Ninane N, Raes M, Michiels C. J Biol Chem. 2003;278(33):31277–31285. doi: 10.1074/jbc.M300763200. [DOI] [PubMed] [Google Scholar]
- 8.Wang GL, Semenza GL. Proc Natl Acad Sci U S A. 1993;90(9):4304–4308. doi: 10.1073/pnas.90.9.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semenza GL, Wang GL. Mol Cell Biol. 1992;12(12):5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Mol Cell Biol. 1996;16(9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. J Biol Chem. 2001;276(12):9519–9525. doi: 10.1074/jbc.M010144200. [DOI] [PubMed] [Google Scholar]
- 12.Semenza GL, Roth PH, Fang HM, Wang GL. J Biol Chem. 1994;269(38):23757–23763. [PubMed] [Google Scholar]
- 13.Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AM. J Biol Chem. 1997;272(9):5375–5381. [PubMed] [Google Scholar]
- 14.Jung F, Palmer LA, Zhou N, Johns RA. Circ Res. 2000;86(3):319–325. doi: 10.1161/01.res.86.3.319. [DOI] [PubMed] [Google Scholar]
- 15.Bruick RK. Proc Natl Acad Sci U S A. 2000;97(16):9082–9087. doi: 10.1073/pnas.97.16.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E. Nature. 1998;394(6692):485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 17.Thornton J, Striplin S, Liu GS, Swafford A, Stanley AW, Van Winkle DM, Downey JM. Am J Physiol. 1990;259(6 Pt 2):H1822–H1825. doi: 10.1152/ajpheart.1990.259.6.H1822. [DOI] [PubMed] [Google Scholar]
- 18.Matsuyama N, Leavens JE, McKinnon D, Gaudette GR, Aksehirli TO, Krukenkamp IB. Circulation. 2000;102(19 Suppl 3):III312–III318. doi: 10.1161/01.cir.102.suppl_3.iii-312. [DOI] [PubMed] [Google Scholar]
- 19.Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot K, Wang L, Wei C, Trush MA, Semenza GL. Cardiovasc Res. 2008;77(3):463–470. doi: 10.1093/cvr/cvm035. [DOI] [PubMed] [Google Scholar]
- 20.Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Circulation. 2008;118(2):166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 21.Communal C, Singh K, Pimentel DR, Colucci WS. Circulation. 1998;98(13):1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- 22.Cross HR, Steenbergen C, Lefkowitz RJ, Koch WJ, Murphy E. Circ Res. 1999;85(11):1077–1084. doi: 10.1161/01.res.85.11.1077. [DOI] [PubMed] [Google Scholar]
- 23.Liggett SB, Cresci S, Kelly RJ, Syed FM, Matkovich SJ, Hahn HS, Diwan A, Martini JS, Sparks L, Parekh RR, Spertus JA, Koch WJ, Kardia SL, Dorn GW., 2nd Nat Med. 2008;14(5):510–517. doi: 10.1038/nm1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schomig A, Richardt G. J Cardiovasc Pharmacol. 1990;16(5):S105–S112. [PubMed] [Google Scholar]
- 25.Yan L, Vatner DE, O'Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. Cell. 2007;130(2):247–258. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Jee K, Kim D, Koh H, Chung J. J Biol Chem. 2001;276(16):12864–12870. doi: 10.1074/jbc.M001492200. [DOI] [PubMed] [Google Scholar]
- 27.Wang L, Liu F, Adamo ML. J Biol Chem. 2001;276(40):37242–37249. doi: 10.1074/jbc.M105089200. [DOI] [PubMed] [Google Scholar]
- 28.Doronin S, Wang HyHY, Malbon CC. J Biol Chem. 2002;277(12):10698–10703. doi: 10.1074/jbc.M109432200. [DOI] [PubMed] [Google Scholar]
- 29.Klein J, Fasshauer M, Ito M, Lowell BB, Benito M, Kahn CR. J Biol Chem. 1999;274(49):34795–34802. doi: 10.1074/jbc.274.49.34795. [DOI] [PubMed] [Google Scholar]
- 30.Mulder AH, Tack CJ, Olthaar AJ, Smits P, Sweep FC, Bosch RR. Am J Physiol Endocrinol Metab. 2005;289(4):24. doi: 10.1152/ajpendo.00079.2004. [DOI] [PubMed] [Google Scholar]
- 31.Morisco C, Marrone C, Trimarco V, Crispo S, Monti MG, Sadoshima J, Trimarco B. Cardiovasc Res. 2007;76(3):453–464. doi: 10.1016/j.cardiores.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Abdellatif M, Mcllelan WR, Schneider MD. JBC. 1994;269:15423–15426. [PubMed] [Google Scholar]
- 33.Peter PS, Brady JE, Yan L, Chen W, Engelhardt S, Wang Y, Sadoshima J, Vatner SF, Vatner DE. J Clin Invest. 2007;117(5):1335–1343. doi: 10.1172/JCI29576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Graham FL, Prevec L. Methods in Molecular Biology. Clifton, NJ: The Humana Press Inc.; 1991. [Google Scholar]
- 35.Sayed D, Rane S, Lypowy J, He M, Chen IY, Vashistha H, Yan L, Malhotra A, Vatner D, Abdellatif M. Mol Biol Cell. 2008;18:3272–3282. doi: 10.1091/mbc.E08-02-0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. Circ Res. 2007;100(3):416–424. doi: 10.1161/01.RES.0000257913.42552.23. [DOI] [PubMed] [Google Scholar]
- 37.Mocanu MM, Bell RM, Yellon DM. J Mol Cell Cardiol. 2002;34(6):661–668. doi: 10.1006/jmcc.2002.2006. [DOI] [PubMed] [Google Scholar]
- 38.Katakam PV, Jordan JE, Snipes JA, Tulbert CD, Miller AW, Busija DW. Am J Physiol Regul Integr Comp Physiol. 2007;292(2):28. doi: 10.1152/ajpregu.00520.2006. [DOI] [PubMed] [Google Scholar]
- 39.Fuglesteg BN, Tiron C, Jonassen AK, Mjos OD, Ytrehus K. Acta Physiol. 2008;17:17. doi: 10.1111/j.1748-1716.2008.01901.x. [DOI] [PubMed] [Google Scholar]
- 40.Kim CH, Cho YS, Chun YS, Park JW, Kim MS. Circ Res. 2002;90(2):E25–E33. doi: 10.1161/hh0202.104923. [DOI] [PubMed] [Google Scholar]
- 41.Frishman WH. Circulation. 1983;67(6 Pt 2):I11–I18. [PubMed] [Google Scholar]
- 42.Hu A, Jiao X, Gao E, Koch WJ, Sharifi-Azad S, Grunwald Z, Ma XL, Sun JZ. J Pharmacol Exp Ther. 2006;318(2):469–475. doi: 10.1124/jpet.106.102160. [DOI] [PubMed] [Google Scholar]
- 43.Stiehl DP, Jelkmann W, Wenger RH, Hellwig-Burgel T. FEBS Lett. 2002;512(1–3):157–162. doi: 10.1016/s0014-5793(02)02247-0. [DOI] [PubMed] [Google Scholar]
- 44.Treins C, Giorgetti-Peraldi S, Murdaca J, Semenza GL, Van Obberghen E. J Biol Chem. 2002;277(31):27975–27981. doi: 10.1074/jbc.M204152200. [DOI] [PubMed] [Google Scholar]
- 45.Zelzer E, Levy Y, Kahana C, Shilo BZ, Rubinstein M, Cohen B. Embo J. 1998;17(17):5085–5094. doi: 10.1093/emboj/17.17.5085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Proc Natl Acad Sci U S A. 1998;95(19):11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan C, Cruet-Hennequart S, Troussard A, Fazli L, Costello P, Sutton K, Wheeler J, Gleave M, Sanghera J, Dedhar S. Cancer Cell. 2004;5(1):79–90. doi: 10.1016/s1535-6108(03)00281-2. [DOI] [PubMed] [Google Scholar]
- 48.Lee SP, Youn SW, Cho HJ, Li L, Kim TY, Yook HS, Chung JW, Hur J, Yoon CH, Park KW, Oh BH, Park YB, Kim HS. Circulation. 2006;114(2):150–159. doi: 10.1161/CIRCULATIONAHA.105.595918. [DOI] [PubMed] [Google Scholar]
- 49.Aikawa R, Nawano M, Gu Y, Katagiri H, Asano T, Zhu W, Nagai R, Komuro I. Circulation. 2000;102(23):2873–2879. doi: 10.1161/01.cir.102.23.2873. [DOI] [PubMed] [Google Scholar]
- 50.Okumura S, Vatner DE, Kurotani R, Bai Y, Gao S, Yuan Z, Iwatsubo K, Ulucan C, Kawabe J, Ghosh K, Vatner SF, Ishikawa Y. Circulation. 2007;116(16):1776–1783. doi: 10.1161/CIRCULATIONAHA.107.698662. [DOI] [PubMed] [Google Scholar]
- 51.Bristow MR. Circulation. 2000;101(5):558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 52.Bhatt AB, Stone PH. Curr Opin Cardiol. 2006;21(5):492–502. doi: 10.1097/01.hco.0000240588.22086.43. [DOI] [PubMed] [Google Scholar]
- 53.Lohse MJ, Engelhardt S, Eschenhagen T. Circ Res. 2003;93(10):896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]