Abstract
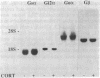
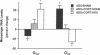
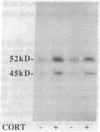
The possibility that glucocorticoids regulate specific guanine nucleotide binding regulatory proteins (G proteins) was investigated in rat cerebral cortex. Corticosterone was administered to normal and bilaterally adrenalectomized rats, and hormone regulation of individual G-protein subunits was investigated in cerebral cortex in three ways: (i) immunoblot analysis of subunit protein, (ii) hybridization blot analysis of subunit mRNA, and (iii) ADP-ribosylation analysis of stimulatory G protein (Gs alpha) subunits. Chronic (7 days) corticosterone administration to normal rats increased levels of Gs alpha immunoreactivity, mRNA, and ADP-ribosylation but decreased levels of inhibitory G protein (Gi alpha) mRNA and tended to decrease levels of Gi alpha immunoreactivity. In contrast, levels of Go alpha and G beta immunoreactivity and mRNA were not influenced by corticosterone treatment. In adrenalectomized rats, corticosterone treatment produced a 25-50% increase in the levels of Gs alpha immunoreactivity, mRNA, and ADP-ribosylation, whereas the hormone produced a 20-35% decrease in the levels of Gi alpha immunoreactivity and mRNA. Adrenalectomy, without corticosterone replacement, produced the opposite effects on Gs alpha and Gi alpha compared to sham-operated controls, indicating that these G proteins are regulated by this class of steroid hormone under physiological conditions in vivo. The results indicate that specific G-protein subunits--namely, Gs alpha and Gi alpha--are under the coordinated control of glucocorticoids in rat brain and demonstrate that G proteins are physiological targets of glucocorticoids in vivo. Possible roles played by these G-protein responses in mediating the effects of glucocorticoids on brain function are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carter A., Bardin C., Collins R., Simons C., Bray P., Spiegel A. Reduced expression of multiple forms of the alpha subunit of the stimulatory GTP-binding protein in pseudohypoparathyroidism type Ia. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7266–7269. doi: 10.1073/pnas.84.20.7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F. H., Bourne H. R. Dexamethasone increases adenylyl cyclase activity and expression of the alpha-subunit of Gs in GH3 cells. Endocrinology. 1987 Nov;121(5):1711–1715. doi: 10.1210/endo-121-5-1711. [DOI] [PubMed] [Google Scholar]
- Duman R. S., Strada S. J., Enna S. J. Effect of imipramine and adrenocorticotropin administration on the rat brain norepinephrine-coupled cyclic nucleotide generating system: alterations in alpha and beta adrenergic components. J Pharmacol Exp Ther. 1985 Aug;234(2):409–414. [PubMed] [Google Scholar]
- Duman R. S., Strada S. J., Enna S. J. Glucocorticoid administration increases receptor-mediated and forskolin-stimulated cyclic AMP accumulation in rat brain cerebral cortical slices. Brain Res. 1989 Jan 16;477(1-2):166–171. doi: 10.1016/0006-8993(89)91404-2. [DOI] [PubMed] [Google Scholar]
- Forray C., Richelson E. Glucocorticoids potentiate the prostaglandin E1-mediated cyclic AMP formation by a cultured murine neuroblastoma clone. J Neurochem. 1985 Jul;45(1):79–85. doi: 10.1111/j.1471-4159.1985.tb05477.x. [DOI] [PubMed] [Google Scholar]
- Foster S. J., Perkins J. P. Glucocorticoids increase the responsiveness of cells in culture to prostaglandin E1. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4816–4820. doi: 10.1073/pnas.74.11.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawler D., Milligan G., Spiegel A. M., Unson C. G., Houslay M. D. Abolition of the expression of inhibitory guanine nucleotide regulatory protein Gi activity in diabetes. Nature. 1987 May 21;327(6119):229–232. doi: 10.1038/327229a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goldsmith P., Rossiter K., Carter A., Simonds W., Unson C. G., Vinitsky R., Spiegel A. M. Identification of the GTP-binding protein encoded by Gi3 complementary DNA. J Biol Chem. 1988 May 15;263(14):6476–6479. [PubMed] [Google Scholar]
- Harley C. B. Hybridization of oligo(dT) to RNA on nitrocellulose. Gene Anal Tech. 1987 Mar-Apr;4(2):17–22. doi: 10.1016/0735-0651(87)90013-6. [DOI] [PubMed] [Google Scholar]
- Harrelson A. L., McEwen B. S. Hypophysectomy increases vasoactive intestinal peptide-stimulated cyclic AMP generation in the hippocampus of the rat. J Neurosci. 1987 Sep;7(9):2807–2810. doi: 10.1523/JNEUROSCI.07-09-02807.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrelson A. L., Rostene W., McEwen B. S. Adrenocortical steroids modify neurotransmitter-stimulated cyclic AMP accumulation in the hippocampus and limbic brain of the rat. J Neurochem. 1987 May;48(5):1648–1655. doi: 10.1111/j.1471-4159.1987.tb05714.x. [DOI] [PubMed] [Google Scholar]
- Itoh H., Toyama R., Kozasa T., Tsukamoto T., Matsuoka M., Kaziro Y. Presence of three distinct molecular species of Gi protein alpha subunit. Structure of rat cDNAs and human genomic DNAs. J Biol Chem. 1988 May 15;263(14):6656–6664. [PubMed] [Google Scholar]
- Johnson G. S., Jaworski C. J. Glucocorticoids increase GTP-dependent adenylate cyclase activity in cultured fibroblasts. Mol Pharmacol. 1983 May;23(3):648–652. [PubMed] [Google Scholar]
- Jones D. T., Reed R. R. Molecular cloning of five GTP-binding protein cDNA species from rat olfactory neuroepithelium. J Biol Chem. 1987 Oct 15;262(29):14241–14249. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., De Kloet E. R., Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986 Oct;66(4):1121–1188. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- Meyer J. S. Biochemical effects of corticosteroids on neural tissues. Physiol Rev. 1985 Oct;65(4):946–1020. doi: 10.1152/physrev.1985.65.4.946. [DOI] [PubMed] [Google Scholar]
- Meyer J. S., Micco D. J., Stephenson B. S., Krey L. C., McEwen B. S. Subcutaneous implantation method for chronic glucocorticoid replacement therapy. Physiol Behav. 1979 May;22(5):867–870. doi: 10.1016/0031-9384(79)90330-5. [DOI] [PubMed] [Google Scholar]
- Mobley P. L., Manier D. H., Sulser F. Norepinephrine-sensitive adenylate cyclase system in rat brain: role of adrenal corticosteroids. J Pharmacol Exp Ther. 1983 Jul;226(1):71–77. [PubMed] [Google Scholar]
- Mobley P. L., Sulser F. Adrenal corticoids regulate sensitivity of noradrenaline receptor-coupled adenylate cyclase in brain. Nature. 1980 Aug 7;286(5773):608–609. doi: 10.1038/286608a0. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D., Chang F. H., Cheever L., Kim M., Diamond I., Gordon A. S. Chronic ethanol causes heterologous desensitization of receptors by reducing alpha s messenger RNA. Nature. 1988 Jun 30;333(6176):848–850. doi: 10.1038/333848a0. [DOI] [PubMed] [Google Scholar]
- Mumby S. M., Kahn R. A., Manning D. R., Gilman A. G. Antisera of designed specificity for subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1986 Jan;83(2):265–269. doi: 10.1073/pnas.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler E. J., Erdos J. J., Terwilliger R., Duman R. S., Tallman J. F. Regulation of G proteins by chronic morphine in the rat locus coeruleus. Brain Res. 1989 Jan 9;476(2):230–239. doi: 10.1016/0006-8993(89)91243-2. [DOI] [PubMed] [Google Scholar]
- Nestler E. J., Terwilliger R. Z., Halm E. Corticosterone increases protein tyrosine kinase activity in the locus coeruleus and other monoaminergic nuclei of rat brain. Mol Pharmacol. 1989 Mar;35(3):265–270. [PubMed] [Google Scholar]
- Rosenthal W., Hescheler J., Trautwein W., Schultz G. Control of voltage-dependent Ca2+ channels by G protein-coupled receptors. FASEB J. 1988 Sep;2(12):2784–2790. doi: 10.1096/fasebj.2.12.2457531. [DOI] [PubMed] [Google Scholar]
- Stryer L., Bourne H. R. G proteins: a family of signal transducers. Annu Rev Cell Biol. 1986;2:391–419. doi: 10.1146/annurev.cb.02.110186.002135. [DOI] [PubMed] [Google Scholar]
- Tamir A., Gill D. M. ADP-ribosylation by cholera toxin of membranes derived from brain modifies the interaction of adenylate cyclase with guanine nucleotides and NaF. J Neurochem. 1988 Jun;50(6):1791–1797. doi: 10.1111/j.1471-4159.1988.tb02480.x. [DOI] [PubMed] [Google Scholar]
- Vallar L., Spada A., Giannattasio G. Altered Gs and adenylate cyclase activity in human GH-secreting pituitary adenomas. Nature. 1987 Dec 10;330(6148):566–568. doi: 10.1038/330566a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Imoto Y., Reeves J. P., Birnbaumer L., Brown A. M. A G protein directly regulates mammalian cardiac calcium channels. Science. 1987 Nov 27;238(4831):1288–1292. doi: 10.1126/science.2446390. [DOI] [PubMed] [Google Scholar]