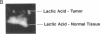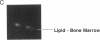Abstract
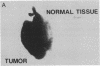
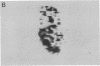
An imaging method is described that makes use of proton double quantum nuclear magnetic resonance (NMR) to construct images based on selected metabolites such as lactic acid. The optimization of the method is illustrated in vitro, followed by in vivo determination of lactic acid distribution in a solid tumor model. Water suppression and editing of lipid signals are such that two-dimensional spectra of lactic acid may be obtained from a radiation-induced fibrosarcoma (RIF-1) tumor in under 1 min and lactic acid images from the same tumor in under 1 hr at 2.0 T. This technique provides a fast and reproducible method at moderate magnetic field strength for mapping biologically relevant metabolites.
Full text
PDF
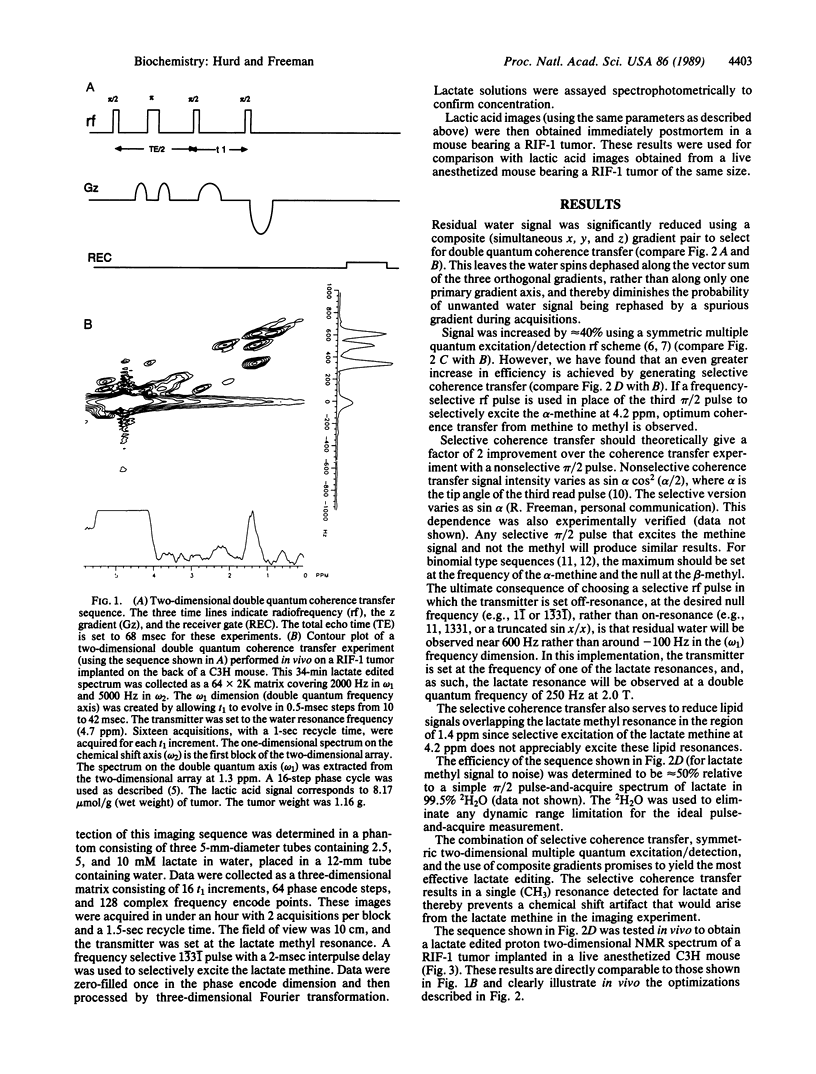
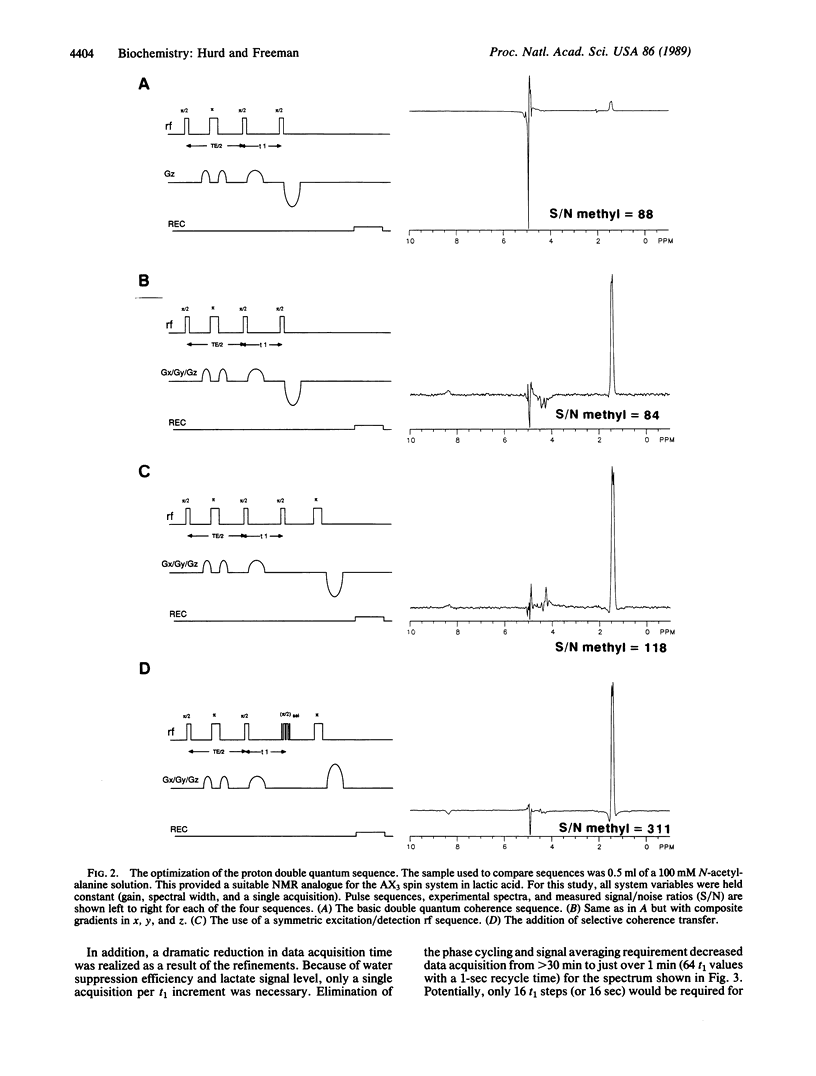
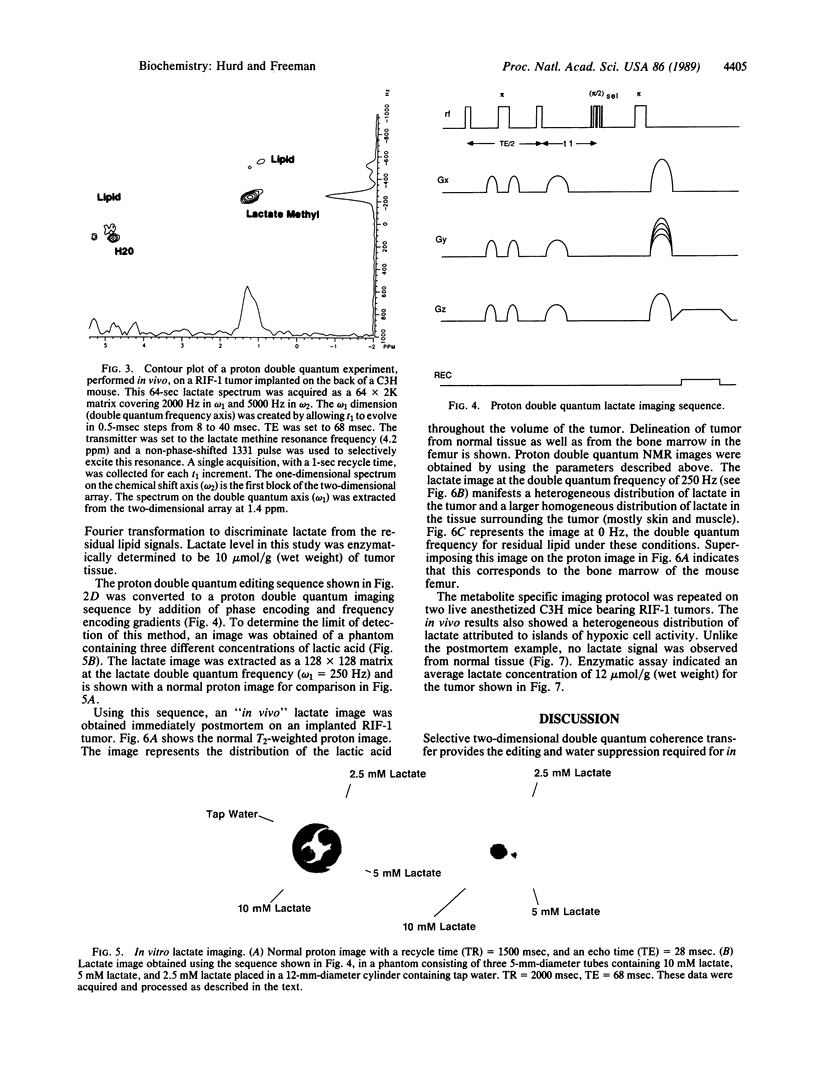

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang L. H., Pereira B. M., Weinstein P. R., Keniry M. A., Murphy-Boesch J., Litt L., James T. L. Comparison of lactate concentration determinations in ischemic and hypoxic rat brains by in vivo and in vitro 1H NMR spectroscopy. Magn Reson Med. 1987 Jun;4(6):575–581. doi: 10.1002/mrm.1910040608. [DOI] [PubMed] [Google Scholar]
- Crockard H. A., Gadian D. G., Frackowiak R. S., Proctor E., Allen K., Williams S. R., Russell R. W. Acute cerebral ischaemia: concurrent changes in cerebral blood flow, energy metabolites, pH, and lactate measured with hydrogen clearance and 31P and 1H nuclear magnetic resonance spectroscopy. II. Changes during ischaemia. J Cereb Blood Flow Metab. 1987 Aug;7(4):394–402. doi: 10.1038/jcbfm.1987.82. [DOI] [PubMed] [Google Scholar]
- Evanochko W. T., Sakai T. T., Ng T. C., Krishna N. R., Kim H. D., Zeidler R. B., Ghanta V. K., Brockman R. W., Schiffer L. M., Braunschweiger P. G. NMR study of in vivo RIF-1 tumors. Analysis of perchloric acid extracts and identification of 1H, 31P and 13C resonances. Biochim Biophys Acta. 1984 Sep 14;805(1):104–116. doi: 10.1016/0167-4889(84)90042-9. [DOI] [PubMed] [Google Scholar]
- Hardy C. J., Dumoulin C. L. Lipid and water suppression by selective 1H homonuclear polarization transfer. Magn Reson Med. 1987 Jul;5(1):58–66. doi: 10.1002/mrm.1910050107. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R., Brown J. M., Gray J. W., Franko A. J., Scoles M. A., Kallman R. F. A new mouse tumor model system (RIF-1) for comparison of end-point studies. J Natl Cancer Inst. 1980 Mar;64(3):595–604. [PubMed] [Google Scholar]
- Vink R., McIntosh T. K., Faden A. I. Nonedited 1H NMR lactate/n-acetyl aspartate ratios and the in vivo determination of lactate concentration in brain. Magn Reson Med. 1988 May;7(1):95–99. doi: 10.1002/mrm.1910070111. [DOI] [PubMed] [Google Scholar]
- WARBURG O. On respiratory impairment in cancer cells. Science. 1956 Aug 10;124(3215):269–270. [PubMed] [Google Scholar]