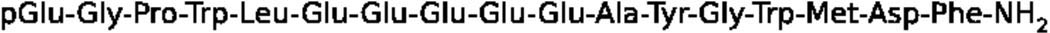Abstract
The gastrointestinal peptide hormone gastrin is responsible for initiating the release of gastric acid in the stomach in response to the presence of food and/or humoral factors such as gastrin releasing peptide. However, it has a role in the growth and maintenance of the gastric epithelium, and has been implicated in the formation and growth of gastric cancers. Hypergastrinemia resulting from atrophic gastritis and pernicious anemia leads to hyperplasia and carcinoid formation in rats, and contributes to tumor formation in humans. Additionally, gastrin has been suspected to play a role in the formation and growth of cancers of the colon, but recent studies have instead implicated gastrin processing intermediates, such as gastrin-17-Gly, acting upon a putative, non-cholecystokinin receptor. This review summarizes the production and chemical structures of gastrin and the processing intermediate gastrin-17-Gly, as well as their activities in the gastrointestinal tract, particularly the promotion of colon cancers.
1. Gastrin processing in the GI tract
John Sydney Edkins postulated in 1905 [1–2] that the acid secretory activity of the stomach could be attributed to the agent gastrin. The gastrin gene is expressed and gastrin secreted in a variety of cells in the body, among them those of the small and large intestine (duodenal, jejunal, ileal, and colonic mucosas), the pancreas, neuroendocrine tissue (the pituitary and hypothalamus, cerebellum, vagal nerve, and adrenal medulla), the genitals and the respiratory tract, where it serves a variety of purposes [3–18]. However, “mature” gastrin is predominantly manufactured by the antral mucosa of the stomach, where G endocrine cells secrete the peptide in response to the presence of amino acids, dietary amines, and calcium in the stomach, for the purpose of stimulating gastric acid secretion [19–25, reviewed in 26]. Neutralization of acid or inhibition of acid secretion also stimulates gastrin release [27–33]. In addition to luminal stimuli, basolateral stimuli of the G cells by GRP or Ach from nerve fibers, or by humoral factors such as EGF, also cause the expression and secretion of gastrin in some species [34–35]. In response to increased acid levels or VIP, D endocrine cells, in turn, secrete somatostatin, which acts to inhibit the secretion of gastrin.
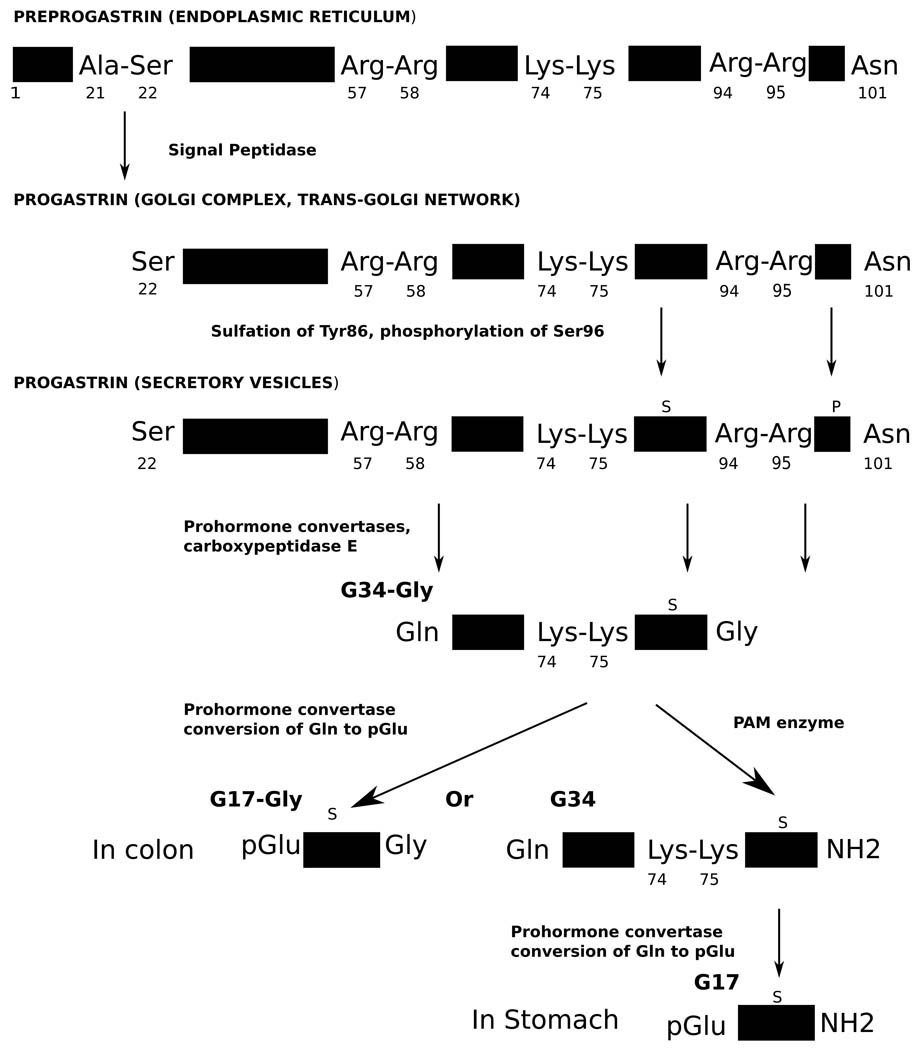
The expression of the gastrin gene and the processing of its polypeptide product in the gastrointestinal tract have been gradually revealed through extraction from gastrin-producing tissues and characterization of its processing intermediates with antibodies, chromatography and peptide analysis tools such as mass spectrometry. Genomic DNA information revealed the gastrin gene structure. The mRNA of the 101-residue gastrin precursor preprogastrin is translated at the endoplasmic reticulum, where an N-terminal signaling sequence is cleaved from the peptide between an alanyl and a seryl residue to yield the 80-residue progastrin [36–38] (Figure 1). Progastrin then passes through the Golgi complex and the trans-Golgi network, where it can be sulfated on Tyr86 and/or phosphorylated on Ser96. It is further packaged into secretory vesicles, in which the dibasic sites Arg57-Arg58 and Arg94-Arg95 are probably cleaved on the C-terminal side by the prohormone convertases PC1/3 and PC2, and the remaining C-terminal basic residues Arg94-Arg95 are removed by carboxypeptidase E to give the peptide G34-Gly [39–41; reviewed 42–43]. Another cleavage by prohormone convertases at Lys74-Lys75 gives G17-Gly [39–41]. Various factors within the secretory vesicles modify these cleavages, including the differential expression of PC1/3 and PC2 and the pH of the vesicle interior (pH > 5.5 may inhibit the maturation of prohormone convertases) [41, 44–46].
Figure 1.
Processing of preprogastrin in G cells.
In addition, the sulfation of Tyr86 and the phosphorylation of Ser96 may affect the cleavage of local dibasic sites, though they have not been shown to affect the biological activity of gastrin, unlike the closely related peptide CCK, which requires sulfation of tyrosine for activity. Sulfation and phosphorylation may both increase processing of progastrin [44, 46], while phosphorylation may also affect the conversion of glycine-extended gastrin intermediates to mature gastrins [47].
The C-terminal glycyl residue of G34-Gly is finally converted to a terminating amide group by the PAM enzyme to give the mature G34, which is additionally sometimes cleaved to give mature G17 [39–40] (Figure 2). G17-Gly is not generally converted directly to G17 by PAM [39]. G17 and G34 are the “classical” gastrins, those that were originally found to stimulate gastric acid secretion, while G17-Gly, G34-Gly, and progastrin are the “non-classical” gastrins, or “processing intermediates,” those peptides were not known to have any biological activity until recently. The ratio of amidated gastrins to processing intermediates varies considerably across the tissues in which the gastrin gene is expressed. Processing intermediates are quite scarce in the gastric antrum, making up only about 5% of gastrin gene products, while in the duodenum the value is 20% [48]. In the colonic mucosa, amidated gastrins are virtually unknown [48]. Additionally, probably due to variable cleavage of the Lys74-Lys75 bond, the amount of G34 compared to G17 varies in different tissues. In the GI tract, region-specific antisera have shown that G17 predominates in the antral mucosa, while G34 is more prevalent in the duodenum [26].
Figure 2.
Primary structure of human G17.
2. Physiological roles of gastrin in the stomach
Ultimately, gastrin has two major roles in the GI tract, the first of which is the well- known stimulation of gastric acid secretion in the stomach. The mature gastrins (as well as a small percentage of non-classical gastrins) are secreted via Ca2+-dependent release in the regulated secretory pathway from G cells in the gastric antrum and, to a lesser extent, the duodenum [49,50]. These mature gastrins act upon ECL cells of the gastric fundus, stimulating the release of histamine, again dependent on intracellular calcium from stores or from the activation of calcium channels [51–59]. Histamine then, via paracrine diffusion, interacts with parietal (oxytinic) cells, stimulating the upregulation of surface H+/K+ proton pumps and thus the secretion of acid [51, 60]. Gastrin itself and acetylcholine also act directly on parietal cells through surface receptors to stimulate acid secretion; in this case, histamine potentiates the activity of gastrin, but this effect is still secondary to the stimulation of acid secretion by histamine [61–65]. Gastrin may also sensitize parietal cells to other acid secretagogues [66].
As acid secretion increases, D endocrine cells release somatostatin, which acts on parietal cells to inhibit acid secretion, and on ECL cells to inhibit histamine secretion, providing a negative feedback control mechanism [30–31, 67–68]. In the duodenum, secretin (through bicarbonate release) and CCK (through the potentiation of secretin) also inhibit acid secretion as stomach contents pass into the small intestine.
Additionally, gastrin promotes the maintenance and proliferation of the gastric epithelium [reviewed in 26, 42–43, 48, 69]. It does this by promoting the proliferation of gastrin receptor-expressing ECL cells, as well as mucous neck cells in the gastric mucosas, that can differentiate into parietal cells, though gastrin does not cause proliferation of parietal cells themselves [70–76]. H2 receptor antagonists and proton pump inhibitors, as well as resection of the acid-secreting mucosa, suppress gastric acid secretion, which promotes elevated gastrin secretion. This promotes increased ECL proliferation [77–80]. Resection of the antral mucosa inhibits this ECL cell hyperplasia, additionally indicating that gastrin is responsible for mucosal proliferation [81]. ECL cells, in turn, may indirectly promote the proliferation of other gastric epithelial cells that do not express the gastrin receptor through the secretion of growth factors such as TGF-α and HB-EGF [82–85, reviewed in 86]. Patients with increased levels of gastrin in circulation show a greater thickness of the gastric and colonic mucosa, as well as a larger mass of parietal and ECL cells [75].
The acceptance of gastrin as a growth factor for the gastric mucosa naturally led to the suggestion that it might play a role in gastrointestinal cancers. Along with the ability to stimulate proliferation, gastrin has been found to have other biological activities, including inhibition of apoptosis [87] and the stimulation of invasion and migration of epithelial cells [88–90]. Conditions such as atrophic gastritis and pernicious anemia, as well as the aforementioned administration of proton pump inhibitors or H2 receptor antagonists, reduce gastric acid secretion significantly (achlorhydria), leading to hypergastrinemia [91–92, reviewed in 48]. The presence of gastrin-secreting neoplasms (gastrinomas), associated with the Zollinger-Ellison syndrome, in the pancreas, duodenum, and the gastric antrum, may also lead to hypergastrinemia [95–100]. The resulting stimulation of ECL cell proliferation from hypergastrinemia frequently leads to ECL cell hyperplasia and carcinoids in rats [91–94]. Similar hypergastrinemia in humans generally leads to hyperplasia only, and progression to tumor formation seems to be condition-specific and require other factors in addition to gastrin [101]. Zollinger-Ellison multiple endocrine neoplasia-1 syndrome patients, for instance, are considerably more likely to have ECL cell carcinoids (up to 30% of those with the disease) than those with atrophic gastritis/pernicious anemia (5%) [101–102]. Colonization of the stomach by the bacteria Helicobacter pylori, a significant factor in the development of atrophic gastritis, causes a decrease in acid secretion, leading to an increase in gastrin secretion. This increase in gastrin stimulates the proliferation of H. pylori that in turn, leads to a further decrease in gastric acid secretion and eventual achlorhydria and hypergastrinemia, leading to gastric atrophy and cancer, as well as the formation of ulcers [103–104]. The small percentage of patients who actually develop cancer, as noted above, indicates that other factors must be involved, including environmental or inherited factors or the inflammatory response [105–107].
It has been suggested that gastrin is itself not a factor in tumor formation, but merely a marker of achlorhydria, which is the actual factor in causing infection and inflammation that leads to carcinogenesis [108–110]. Regardless, gastrin is probably involved in the acceleration of malignant tumor formation [104] and promotion of the growth of gastric tumors [111–115, reviewed in 48].
3. Gastrin and CCK2-R
The actions of the hormones CCK are mediated by two receptors, CCK1-R and CCK2-R (formerly CCK-A and CCK-B). Both belong to the family of G-protein-coupled receptors, (GPCRs) which are characterized by seven trans-plasma membrane helical domains. CCK1-R is distributed widely in the peripheral nervous system and in the upper gastrointestinal tract, and less often in the central nervous system. In the nervous system, CCK1-R is present on vagal afferent neurons and may mediate the physiological response of satiety [116–117]. It also regulates dopamine release that may affect neuropsychiatric disorders such as schizophrenia [118, reviewed in 119]. In the gut, CCK1-R is present in the gastric mucosa (mostly in the basal region of the fundic mucosa) and gallbladder and GI smooth muscle cells, and is probably expressed by pancreatic acini and islets, as well as vagal nerve cells [120–121, reviewed in 122–124]. It mediates the release of pepsinogen from gastric chief cells and somatostatin from D cells to inhibit gastric acid secretion [125–126], gall bladder contraction [127–128], delay of gastric emptying [129–131], the relaxation of the sphincter of Oddi and the lower esophageal sphincter [132], and the secretion of enzymes and hormones from the pancreas [133–138], as well as pancreatic proliferation [139–142].
CCK2-R, on the other hand, is dominant in the central nervous system, where it may be involved in inhibiting dopamine release and provoking the anxiety response [119, 143]. CCK2-R is also found in the upper GI tract in the gastric mucosa (mostly in the midglandular region of the fundic mucosa, where parietal cells are present), where in non-cancerous cells it mediates the activities linked to gastrin above: proliferation of the gastric mucosa, stimulation of histamine from ECL cells, stimulation of acid from parietal cells, as well as inhibition of somatostatin release from D cells [62, 144–145, reviewed in 122–124]. It is also expressed in the pancreas and probably mediates glucagon secretion there in humans [146]. CCK2-R is also expressed in gastric and pancreatic tumor cell lines where it may mediate tumor growth [147–156], while CCK1-R may be present in some pancreatic tumor cell lines but role of the receptor there is unclear [157–159]. Ultimately, the cloning of cDNAs for brain CCK2-R and for the gastrin parietal cell receptor indicated that the two receptors are identical, and thus, that CCK2-R is bound by gastrin with high affinity and mediates its effects in the gastric mucosa [160–162].
Like gastrin, CCK is produced from the processing of a 115-amino acid residue preprohormone into mature C-terminally amidated peptides, occurring as 58- and 8-residue forms [163–164]. The primary structure of gastrin and CCK are related by their identical C-terminal tetrapeptide sequences (Trp-Met-Asp-Phe) and amidation. This sequence is essential for biological activity at both receptor subtypes. Maximum biological activity at the CCK1-R receptor requires at least the seven C-terminal residues of the CCK sequence, with the N-terminal tyrosyl residue sulfated (Tyr52 of CCK-58). Sulfated CCK-8 meets this requirement. G17 (sulfated or not) and non-sulfated CCK-8 bind with 500–1000-fold less affinity, while CCK-4 binds with 10000-fold less affinity [165–166, reviewed in 122]. Binding at CCK2-R is less exclusive. Sulfated and non-sulfated gastrin and CCK-8 bind with nearly equal (sub-nanomolar) affinity to CCK2-R, and CCK-4 binds with micromolar affinity [167, reviewed in 122]. Despite the importance of sulfation to gastrin processing, it has seemingly no role in the biological activity mediated by CCK2-R, in contrast to CCK1-R. C-terminal amidation is, however, essential to high binding affinity, as the processing intermediate G17-Gly has less affinity for CCK2-R than CCK-4 [168–169].
The signal transduction pathways of CCK2-R leading to gastric acid secretion and factors promoting cancer (such as motility, invasion, inflammation, proliferation, and anti-apoptotic action) are varied, with some being typical pathways of other GPCRs, and others novel [reviewed in 69, 122, 124]. Notably, the phosphatidyl inositol pathway has been shown to be triggered in gastric parietal [170–171] and ECL cells [171], AR4-2J pancreatic cells [172–173], normal colonic epithelial cells [174–178] and HT-29 colon cancer cells [179]. Binding of CCK or gastrin activates a pertussis toxin insensitive Gq protein, which dissociates into the Gαq and Gβγ subunits. Gαq is bound to a GDP molecule in the inactive form; this GDP is exchanged for a GTP molecule upon dissociation of the Gq protein [170, 180–181]. Gq then activates the PLC-isoform (among other phospholipases in different cell types), which hydrolyzes plasma membrane-bound PIP2 into the second messengers IP3 and DG [170, 173, 175, 177, 179, 182]. IP3 binds to a ligand-gated Ca2+ channel in the ER and mobilizes Ca2+ from internal stores, while DG activates PKC [176–178, 183–186]. PKC and/or Ca2+ mobilization in turn probably activate a cascade of MAPKs, including ERK1/2 and JNKs in several studied cell types [90, 171–172, 187–189] that have been shown to be involved in cell growth, differentiation, survival, and apoptosis. Using ERK kinase inhibitors, the ERK1/2 pathway activated by CCK receptors has been shown to control cell proliferation, migration, and regulation of gastrin-sensitive gene transcription [88, 187, 190]. CCK2-R has also been shown to initiate MAPK cascades through the activation of tyrosine kinases [reviewed in 124, 191]. In pancreatic AR42J cells and in human gastric cancer cells, CCK2-R activates Src family kinases that in turn, and dependent on PKC, phosphorylate Shc, enabling it to complex with Grb2 and Sos [172, 188, 192–193]. This complex then activates the small GTPase Ras and the protein kinase Raf, which activate the ERK cascade. The ERK cascade might also be initiated by a direct, Ras-independent activation of Raf by PKC [194–195].
Another signaling pathway through the Src kinases is the PI3-K pathway. Activation of the pathway in response to gastrin in cell lines expressing CCK2-R has been reported [190, 196–198]. PI3-K is activated by tyrosine kinases through a complex with the adaptor protein IRS-1, and activates the downstream effector protein Akt [190, 198]. The PI3-K/Akt pathway, as activated by gastrin in pancreatic AR4-2J cells, mediates cell proliferation, anti-apoptotic actions, and cell migration and adhesion [87, 196–197, 199]. Finally, the JAK2/STAT3 pathway has been shown to be directly activated by CCK2-R in rat pancreatic acinar cells, though few GPCRs do so (the pathway is typically initiated by cytokine receptors) [197, 200]. JAK2 is a tyrosine kinase that activates the transcription factor STAT3, which then binds to certain genes. The JAK/STAT pathway is known to mediate anti-apoptosis, cell proliferation, and carcinogenesis.
As discussed previously, cell proliferation may also be stimulated by gastrin in gastric epithelial cells that do not express CCK2-R by transactivation of the EGF receptor. Gastrin stimulates the secretion of HB-EGF, which binds to the EGF receptor on the surface of epithelial cells. This causes tyrosine phosphorylation of the EGF receptor and eventual downstream initiation of the ERK cascade [82, 85].
In addition to the signaling pathways described above that mediate acid secretion, cell proliferation, migration, invasion, and survival, in gastric and colonic cancer cells and intestinal epithelial cells, the PI3-K and MAPK pathways also increase the expression of COX-2 [201–203]. As well as the inflammation mediated by COX-2-formed prostaglandins being a major risk factor for cancer, COX-2 has also been linked to cell proliferation, transformation, invasion, and angiogenesis, all major factors in carcinogenesis. Similarly, the transcription factor NF-κB is activated by CCK2-R leading to the expression of interleukin-8 and other proinflammatory gene products [204].
4. The conformation of gastrin in aqueous and membrane-mimicking solutions
While the C-terminal tetrapeptide fragment of G17 and CCK-8 is essential for biological activity at CCK2-R [205] the secondary and tertiary structure of G17 that affects binding and activation of CCK2-R has been repeatedly investigated using NMR, CD spectroscopy, and computational analysis. ECD as well as NMR spectroscopic analysis of G17 and various C-terminal fragments of G17 up to [Nle15]G17(5–17) (replacement of Met15 with Nle does not significantly affect the conformation or biological activities of the peptide [206]) have shown that the peptide has no secondary and tertiary structure in aqueous solution or in DMSO [207–210] However, subsequent studies in TFE, aqueous solutions containing phospholipids, and aqueous solutions containing SDS micelles have shown G17 and G17 C-terminal analogs adopt a similar, ordered structure in these solvents [207–209, 211–212]. Whether or not this structure represents an active conformation of G17 in the presence of CCK2-R has been the subject of considerable study.
Results of CD and computational studies of the C-terminal tetrapeptide G17(14–17) indicate that the peptide is partially folded in TFE into a C7 structure, with a hydrogen bond between the carbonyl oxygen of Met15 and the amide proton of Phe17 [208–209, 212]. While, in the short gastrin fragments the aromatic side chain of the C-terminal tetrapeptide appear to not be subject to any conformational constraints, extending the chain to [Nle15]G17(10–17) seems to cause a change in the C-terminal environment and rigidity of the Trp14 side chain [208–209]. Extending the N-terminus further to [Nle15]G17(6–17), and then to [Nle15]G17(5–17) strongly enhances the α-helical signature of the CD spectrum, suggesting that a portion of the peptide folds into an α-helix in TFE [208]. While chain extension increases the α-helicity of the peptide, it does not significantly change the local conformation of the C-terminal region [208].
On the basis of the above results and on the propensity of the various amino acid residues to form secondary structure [214], Peggion and associates suggested a model for the conformation of G17 in TFE and membrane-mimicking environments [208]. They concluded that the C-terminal amino acids are unlikely to form α-helical structure, but that since elongation of the peptide through the pentaglutamyl sequence clearly causes conformational changes in the C-terminal region, an interaction must exist between the “head” and the “tail” of the peptide. This implies a β-bend or β-turn as an additional structure in the central portion of the peptide; they suggested Ala-Tyr-Gly-Trp as the most likely sequence to form a turn. The existence of α-helical structure including the residues of the N-terminal portion of the peptide, on the other hand, is strongly supported by the increase in intensity of the CD spectrum when the chain is extended to [Nle15]G17(5–17). Further evidence in favor of this hypothesis is that ionization of the glutamyl side chains by addition of base disrupts the CD-observed helical structure, as with α-helical polyglutamyl sequences [208]. The difference CD spectrum of [Nle15]G17(6–17) and [Nle15]G17(5–17) also indicates rapid onset of an α-helix upon the addition of the N-terminal Leu residue, further supporting the likelihood that the N-terminal region of [Nle15]G17(5–17) forms an α-helix segment [208]. Their model, then, consisted of an α-helix in the pentaglutamyl sequence, β-bend or β-turn in the central region, and, consistent with the earlier conformational calculation of Abillion and associates, a C7 conformation in the biologically essential C-terminal tetrapeptide [208, 213].
Following these CD studies, Mammi and coworkers conducted 1H NMR studies of gastrin peptides in TFE and TFE/H2O mixtures in order to more clearly define and quantify minigastrin structures, as the presence of numerous aromatic residues, which absorb strongly in the UV region, make defining the backbone structure from CD spectra difficult. Despite solubility problems with the longer gastrin fragments ([Nle15]G17(10–17) and ([Nle15]G17(5–17)) in TFE and in 10% TFE/H2O the existence of six hydrogen bonds between backbone amide protons and carbonyl groups in the peptides were deduced [209, 215]. Six amide protons were determined to be solvent shielded based on low temperature coefficients [214]. Two of these are amide protons of two of the Glu residues (though it is not clear which) in the N-terminal portion of the peptide, while the remaining are those of the residues of the essential C-terminal tetrapeptide sequence (Trp14, Nle15 (in their study), Asp16, and Phe17). Based on model proposed by Mammi and associates, they assigned hydrogen bonds between Glu9-NH and Leu5-CO and between Glu10-NH and Glu6-CO, in accordance with the prediction that the N-terminal sequence of minigastrin prior to Gly13 forms a α-helical structure in organic solvent. Because they showed the Ala11 and Tyr12 backbone amide protons are exposed to solvent, they concluded that the α-helix does not extend throughout the peptide, but ends at Glu10. Finally, having considered several possible structures to account for the C-terminal amide protons, they proposed that the C-terminal tetrapeptide sequence forms a 310-helix, with hydrogen bonds between Trp14-NH and Ala11-CO, Nle15-NH and Tyr12-CO, Asp16-NH and Gly13-CO, and Phe17-NH and Trp14-CO. The researchers noted that, while the short two-turn α-helix at the N-terminus would likely be unstable, the existence of a flexible region immediately following allows the possibility of the two helices at opposite ends of the peptide interacting and stabilizing each other [210].
Recently, NMR and MD studies have suggested a more complex structure for G17. Through examination of the backbone dihedral angles and alpha carbon intramolecular distances of [Nle15]G17 solvated in a box of water/decane, Stone and coworkers a 310-helix involving residues Leu5-Glu9 and an α-helix terminating in a type I β-turn from Ala11 to Asp16, with the C-terminal tetrapeptide folding into a type IV β-turn conformation [211,212]. These investigators suggest that this type I β-turn structure seen in Gly13-Asp16 may be indeed be the structure required for binding CCK2-R.
Understanding the relevance of the folded conformation of gastrin to receptor binding and activation is, of course, of great importance, and, thus, the biological activity of the various C-terminal gastrin fragments and fragments have been studied extensively. As shown previously, the conformation of G17 and its C-terminal analogs in phospholipids and SDS micelles is similar to that in TFE, suggesting that the TFE conformation is close to that of these peptides upon entering the membrane environment [208, 213]. Elongation of the chain from the N-terminus of tetragastrin not only changes the conformation, but also the biological potency of the peptide. Extending the chain from [Nle15]G17(11–17) to [Nle15]G17(5–17) increases the biological potency from 20% to 90% of the original activity of G17 [217–218]. This clearly implies that the N-terminal sequence is important in effecting the full biological activity of the peptide, most likely due to the conformational change imparted to the C-terminal tetrapeptide [208]. Additionally, because no ordered structure of the peptide is seen in aqueous solution, if structure is important to the biological activity of G17 and analogs, it should be the structure formed in presence of a lipophilic environment. In continuing ECD and NMR studies, Mammi and associates found that replacing the central Gly residue of [Nle15]G17(5–17) with Ala which is more prone to helix-forming had the effect of extending the α-helix throughout the peptide and changing the environment of the aromatic chromophores of the C-terminus [219]. The analog retains only 10% of the potency of [Nle15]G17(5–17), about the same activity as tetragastrin [220], and has greatly reduced affinity for parietal and nonparietal cells. Replacing the pentaglutamyl sequence with a pentaaspartyl sequence prevents any formation of an α-helix and also significantly reduces potency [221]. These results point to the importance of the suspected “U-shaped” hairpin fold, with the N-terminally-located α-helix stabilizing the active conformation of the C-terminal end, in ensuring the full activity seen in minigastrin and in G17.
5. Structural features required for G17 binding and activation of CCK2-R
The dependence on the C-terminal sequence, Trp-Met-Asp-Phe-NH2, for the biological activity of G17 was discovered shortly after the isolation and structural characterization of gastrin [205]. More detailed studies of G17 revealed the importance of individual residues in addition to that of Gly13 and terminal configurations described above. J. S. Morley synthesized almost 500 tetrapeptide analogs of gastrin and tested their ability to stimulate acid secretion in rats and dogs [222]. Acylation of the N-terminus did not affect activity negatively, but modification of the C-terminal amide group decreased acid secretion. Meanwhile, replacing the Trp14, Met15, or Phe17 residues in some cases resulted in analogs that were still biologically active, but replacing the Asp16 residue always gave a loss of activity. It was concluded that the Trp-Met-Asp residues are essential for receptor binding and that the Phe residue is needed for activation.
Later studies expanded on these findings, using radioligand binding assays in addition to functional assays. Blocking the N-terminus of the tetrapeptide with t-Boc, Aloc, or acylation increased binding affinity and biological activity in one study [223]. Substitution of Trp14 with Ala caused a 1000-fold decrease in binding affinity, as well as a 36% loss of acid secretion [224–225], and removing of the aromatic ring or formylating the indolyl nitrogen also severely decreased binding and biological activity [226–227]. Substitution of Met15 with Ala causes a 100-fold loss in affinity and a 32% loss of acid secretion [224–225], but as aforementioned, it can be replaced with Leu or Nle with no loss of binding or biological activity. Substitution of Asp16 with Ala completely destroyed the peptide’s ability to bind and activate CCK2-R, confirming Morley’s earlier work [224–225]. Finally, substitution of Phe17 with Ala causes a 104-105-fold decrease in affinity [169, 224].
Some groups have also suggested that the binding of metal ions plays an important role in enabling/disabling G17 and G17-Gly activity. Baldwin and colleagues showed that Fe3+ ions bind to both Glu7 and Glu8-Glu9 of the pentaglutamyl sequence. An Ala substitution at position 7 lead to the inability of G17-Gly to stimulate proliferation or migration in IMGE-5 cells in vitro, and the iron chelator desferrioxamine blocks the activity of G17-Gly [228]. Another group showed that C-terminal gastrin fragments bind to calcium ions in TFE, and that these ions can affect the conformation of the peptide [229].
6. Colon cancer and the putative high affinity non-CCK G17-Gly receptor
While it has been established that gastrin is a factor in the development and growth of gastric cancers, there is less consensus about the effect of the peptide on colorectal carcinomas. Exogenous gastrin appears to promote the in vitro growth of some human and rat colorectal cancers, as well as the growth of transplanted mouse and rat tumors in nude mice [115, 230–241, reviewed in 48]. Additionally, exogenous gastrin promotes the growth of some colorectal cancer cell lines, and this growth can be inhibited by gastrin antagonists [242]. Moreover, hypergastrinemia has been considered a risk factor for colorectal carcinoma [243–245]. However, in recent years, investigators have failed to demonstrate that gastrin plays a role in promoting colorectal carcinoma growth. Removal of the gastric fundus, inducing hypergastrinemia, did not result in greater tumor incidence, nor did treatment of transgenic mice, overexpressing gastrin, with the carcinogen azoxymethane produce increased incidence of tumors [246–248]. Other studies failed to show that hypergastrinemia stimulated the growth of the colonic mucosa [249–251]. Finally, the use of the gastrin receptor antagonist CR2945 to block CCK2-R over a period of 52 weeks did not reduce tumor numbers in the colonic mucosa [252–253]. Overall, the studies seem to suggest that, while hypergastrinemic antral mucosa may promote gastric carcinomas, if colorectal carcinomas are stimulated by gastrin or gastrin processing intermediates these would have to be local autocrine or paracrine products [251, 254–255].
Some colorectal cancers were initially thought to synthesize gastrin and to express gastrin receptors [255]. However, receptor autoradiography indicated that CCK2-R was expressed in no more than 4% of colorectal carcinomas, while PCR showed CCK2-R mRNA to be present in only 38% of colorectal carcinomas [256–257]. Equally important was the finding that while gastrin gene product is expressed in the human adult colon [6, 235, 258–262], progastrin is typically not processed to the mature, amidated peptide in colorectal cancers or in the normal colon [6, 260–262]. These revelations, combined with the likelihood that gastrin does not act as an endocrine growth factor through hypersecretion of gastrin elsewhere in the GI tract, strongly suggest that gastrin does not stimulate the colonic mucosa or colorectal cancers either locally or in circulation and that CCK2-R largely does not mediate these effects [reviewed in 124, 255]. Instead, because of the incomplete processing of the gastrin gene product, progastrin and G17-Gly processing intermediates persist in the human adult colon. Resection of colon cancer in one study reduced circulating levels of gastrin precursors, while leaving the levels of circulating mature gastrins unaffected [263], reflecting the production of precursors but not mature gastrin peptides in the colon. Precursors are found in high concentrations in colorectal tumors and in the blood of colorectal cancer patients [260–262, 264, reviewed in 265].
Speculation naturally followed that the incompletely processed peptides might somehow play a role in the stimulation of tumor growth. In fact, gastrin precursors have been shown to stimulate proliferation of colonic mucosa in vitro and in vivo. Gastrin-deficient mice treated with G17-Gly or progastrin had an increase in proliferation of colonic epithelial cells where as mature gastrin had no effect [266–267]. Treatment with G17-Gly also increased aberrant crypt foci and the sensitivity of cells to azoxymethane [268]. Transgenic mice overexpressing progastrin or G17-Gly had increased epithelial cell proliferation and colonic cell hyperplasia, and those mice overexpressing progastrin treated further with a carcinogen had increased predisposition to neoplasia or adenocarcinoma [248, 266, 269–270]. Additionally, some groups have shown that gastrin precursors stimulate proliferation of cells that do not express CCK receptors [271–275]. However, there is still some controversy regarding the effect of precursors. Some groups have not found evidence of precursors as growth factors [276], while another group suggests that the precursors merely potentiate the effect of mature gastrin [277–279].
The ability of gastrin precursors to bind and stimulate proliferation of normal and cancerous colonic cells in the absence of CCK2-R suggests the presence of a putative receptor or receptors that mediate these effects. In fact, high (nanomolar) affinity binding sites have been demonstrated for G17 and G17-Gly on primary and cultured tissues [275, 280–281]. HT-29 cancer cells were shown to possess a nanomolar affinity receptor in studies using radioiodinated G17(2–17)-Gly and G17, respectively [274, 279]. Additionally, saturation binding studies with 67 primary colon cancer specimens showed 57% to have a site to which gastrin binds with high affinity [281]. However, some studies have instead shown a low (micromolar) affinity binding site; Yang and coworkers revealed the presence of a micromolar affinity site for G17 and G17-Gly on DLD-1 human colon cancer cells [282] and Imdahl et al., which showed a micromolar affinity site on primary colorectal cancer cell lines [283].
The effect of G17-Gly binding on the proliferation of colon cancer cells has been a central topic of investigation of our own laboratory in recent years. Following the discovery of micromolar sites on DLD-1 cells, research was conducted to further characterize the G17-Gly binding receptor using optimal conditions in vitro [284]. G17 and G17-Gly were modified by replacing Met15 with a leucyl residue to prevent oxidation. It was found that [3H]G17-Gly bound both intact DLD-1 cells and DLD-1 cell membranes, in a saturable and displaceable manner. Both [Leu15]G17 and [Leu15]G17-Gly were able to displace the tritiated G17-Gly dose-dependently. Non-linear regression analysis showed that, in addition to a low affinity (micromolar) binding site for each peptide, both peptides bound at high affinity, with [Leu15]G17-Gly binding with subnanomolar affinity and [Leu15]G17 with nanomolar affinity on DLD-1 membranes. A subsequent experiment using intact DLD-1 cells and a smaller concentration of the higher specific activity peptide [125I-Tyr12, Leu15]G17-Gly to better resolve the two binding sites also showed that [Leu15]G17-Gly displaced the radiopeptide from two binding sites with nanomolar and micromolar affinity. Finally, [3H]CCK-8 that was shown to bind CHO-K1 cells expressing CCK2-R failed to bind the DLD-1 membranes, and CCK-8 failed to displace [125I-Tyr12, Leu15]G17-Gly from the intact DLD-1 cells. The observations of the failure of CCK-8 to bind DLD-1 membrane or cells as well as the relative similarity of the binding affinities of [Leu15]G17-Gly and [Leu15]G17 clearly indicate the presence of not just non-CCK receptors on the surface of these cells, but possibly a heterogeneous population of such receptors. More curiously, DLD-1 cell proliferation studies with these peptides showed that both stimulated dose-dependent, biphasic growth of cell populations, with cell number reaching a maximum at 10−9 M for [Leu15]G17-Gly and at 10−8 M for [Leu15]G17, while cells treated with micromolar concentrations showed no significant increase in number over controls. This suggests that at low concentrations of these ligands the observed high affinity sites mediate the proliferatory effect, but when ligand concentrations are increased, the low affinity site is bound and mediates an effect countering that of the high affinity site.
Another study examined the importance of the N- and C-terminal residues of [Leu15]G17-Gly and [Leu15]G17 in the promotion of activity in DLD-1 cells, and extended the investigation to include the HT-29 colon cancer cell line [285]. [3H]G17-Gly bound to HT-29 cell membranes, and [Leu15]G17-Gly displaced it in a dose dependent manner. However, only one low (micromolar) affinity site was seen with non-linear regression analysis, in disagreement with previous studies which showed the presence of a nanomolar site for G17-Gly on intact HT-29 cells. In experiments with intact HT-29 cells and [125I-Tyr12, Leu15]G17-Gly as the radioligand, [Leu15]G17-Gly was seen to bind to two sites with nanomolar and micromolar affinity [285]. The discrepancy between the two experiments was attributed to the relatively low population of high affinity sites compared to low affinity sites, and the inability to resolve them using the lower specific-activity tritiated peptide. A further cell proliferation experiment, however, again revealed increased cell numbers at nanomolar concentrations of [Leu15]G17-Gly and a biphasic growth effect at higher concentrations, as with the DLD-1 proliferation study. Once again, CCK-8 failed to displace radioligand binding, as did the CCK2-R antagonist L365-260.
Several other important observations were made. [Leu15]G17(2–17)-Gly, an analog created by omitting the N-terminal pyroglutamatyl residue, was able to displace [3H]G17-Gly in competition experiments with DLD-1 membranes. The analog bound to two sites, but at about an order of magnitude lower affinity than that of [Leu15]G17-Gly to both. Conversely, first omission the entire N-terminal pentapeptide preceding the central pentaglutamyl sequence ([Leu15]G17(6–17)-Gly), then the pentaglutamyl sequence as well ([Leu15]G17(11–17)-Gly) resulted in binding only one site at micromolar and supermicromolar affinity, respectively. Lastly, a cell proliferation experiment using HT-29 cells showed that the analog G17(1–12), created by omitting only the C-terminal sequence 13 to 17 of G17 following the pentaglutamyl sequence, stimulated an increase in cell number with a EC50 at nanomolar concentration. However, unlike the experiments with [Leu15]G17-Gly, no decrease in cell number was seen at higher concentrations. Combined with the previous proliferation data, this result supports the hypothesis that the high affinity receptor for G17 and G17-Gly mediates the proliferatory effect of these peptides. In all, the binding and proliferation results obtained further suggest that N-terminal residues 1–5 are essential for binding the high affinity site, while the C-terminal 13–17 residues may be important for binding the low affinity site.
Further study of the N-terminal region of G17 in our laboratory has led to some surprising observations. G17(1–12) was seen to stimulate the proliferation of DLD-1 cells in a nonbiphasic manner, similar to that of the HT-29 cells treated with G17(1–12) previously [286]. However, instead of binding selectively to one site, G17(1–12) continued to bind to both high and low affinity sites, with affinities similar to those of the full sequence G17-Gly, suggesting that the peptide activates the high affinity, growth promoting site, while acting as an antagonist at the low affinity site [287]. However, the C-terminally truncated-peptide is sufficient to bind both sites, even without the C-terminal tetrapeptide so vital to activity at the CCK2 receptor. Secondly, truncating G17(1–12) to G17(1–6)-NH2 produced a peptide capable of binding a single site with micromolar affinity and the ability to stimulate proliferation of DLD-1 cells [287]. Furthermore, amidated and nonamidated N-terminal peptides as short as G17(1–4) were able to bind a single site on DLD-1 cells albeit with very low affinity (∼10−5 M). However, these shorter peptides were not able to stimulate proliferation of cells [286].
Structural studies using ECD and VCD spectroscopy as well as MD simulations in a variety of aqueous and membrane-mimicking solvents revealed the presence of a β-turn structure from pGlu1-Trp4 in these N-terminal peptides of increasing frequency with peptide length [288]. As peptides shorter than G17(1–4) peptides were not seen to bind to a receptor on DLD-1 cells, the 1–4 sequence appears to be important for binding. Additionally, higher affinity for the binding site on DLD-1 cells as well as stimulation of proliferation of DLD-1 cells by G17(1–6)-NH2 and G17(1–12) may be attributed to the C-terminal amidation and longer sequence, respectively [286–287].
Recent studies have provided some insight into the signaling pathways activated by the putative G17-Gly/G17 receptor leading to cell proliferation. Ogunwobi and Beales showed that MAPK and NF-κB inhibitors significantly reduced the proliferatory effect of G17-Gly administered to HT-29 cells [289]. The activation of ERK in particular supported an earlier report of ERK signaling by G17-Gly in gastric epithelial cells [290]. CCK2-R activation also triggers the MAPK, ERK, and NF-κB pathways leading to cell proliferation, among other functions. Unlike CCK2-R, however, using the COX-2 inhibitor celecoxib it was shown that the G17-Gly/G17 pathway is COX-independent. An anti-apoptotic effect of G17-Gly dependent on these pathways was also shown using celecoxib, and a further study revealed that the PI3-K/Akt, JNK and JAK/STAT pathways to be involved as well [291].
ACKNOWLEDGMENTS
This work was supported by NIH-INBRE grant (1 P20 RR16469) and the Carpenter Endowed Chair in Biochemistry, Creighton University.
Abbreviations
- Ach
acetylcholine
- CCK
cholecystokinin
- COX-2
cyclooxygenase-2
- [3H]CCK8
[3H]cholecystokinin-8
- DG
1,2-diacylglycerol
- DMSO
dimethyl sulfoxide
- ECD
electronic circular dichroism
- ECL
enterochromaffin-like
- EGF
epidermal growth factor
- ERK
extracellular signal-related kinase
- G17
gastrin-17
- G17-Gly
gastrin-17-Gly
- GRP
gastrin releasing peptide
- HB-EGF
heparin binding epidermal growth factor
- IP
inositol phosphate
- IP3
inositol 1,4,5-trisphosphate
- JNK
c-jun NH2-terminal kinase
- MAPK
mitogen activated protein kinase
- MD
molecular dynamics
- NMR
nuclear magnetic resonance
- PAM
peptidyl-glycine α-amidating monooxygenase
- PI3-K
phosphatidylinositol 3-kinase
- PIP2
phosphatidyl inositol 4,5-bisphosphate
- PKC
protein kinase C
- PLC-β
phospholipase C-β
- SDS
sodium dodecyl sulfate
- TFE
2,2,2-trifluoroethanol
- TGF-α
transforming growth factor-α
- VCD
vibrational circular dichroism
- VIP
vasoactive intestinal peptide
References
- 1.Edkins JS. On the chemical mechanism of gastric secretion. Proc R Soc Lond B Biol Sci. 1905;76:376. [Google Scholar]
- 2.Edkins JS. The chemical mechanism of gastric secretion. J Physiol (London) 1906;34:133–144. doi: 10.1113/jphysiol.1906.sp001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchan AMJ, Polak JM, Solcia E, Pearse AGE. Localisation of intestinal gastrin in a distinct endocrine cell type. Nature (London) 1979;277:142–144. doi: 10.1038/277138a0. [DOI] [PubMed] [Google Scholar]
- 4.Larsson LI, Rehfeld JF. A peptide resembling COOH-terminal tetrapeptide amide of gastrin from a new gastrointestinal endocrine cell type. Nature (London) 1979;277:575–578. doi: 10.1038/277575a0. [DOI] [PubMed] [Google Scholar]
- 5.Luttichau HR, van Solinge WW, Nielsen FC, Rehfeld JF. Developmental expression of the gastrin and cholecystokinin genes in rat colon. Gastroenterology. 1993;104:1092–1098. doi: 10.1016/0016-5085(93)90278-k. [DOI] [PubMed] [Google Scholar]
- 6.van Solinge WW, Nielsen FC, Friis-Hansen L, Falkmer UG, Rehfeld RF. Expression but incomplete maturation of progastrin in colorectal carcinomas. Gastroenterology. 1993;104:1099–1107. doi: 10.1016/0016-5085(93)90279-l. [DOI] [PubMed] [Google Scholar]
- 7.Bardram L. Progastrin in serum from Zollinger-Ellison patients. An indicator of malignancy? Gastroenterology. 1990;98:1420–1426. doi: 10.1016/0016-5085(90)91071-d. [DOI] [PubMed] [Google Scholar]
- 8.Brand SJ, Klarlund J, Schwartz TW, Rehfeld JF. Biosynthesis of tyrosine O-sulfated gastrins in rat antral mucosa. J Biol Chem. 1984;259:13246–13252. [PubMed] [Google Scholar]
- 9.Larsson LI, Rehfeld JF, Sundler F, Hakanson R. Pancreatic gastrin in foetal and neonatal rats. Nature (London) 1976;262:609–610. doi: 10.1038/262609a0. [DOI] [PubMed] [Google Scholar]
- 10.Larsson LI, Rehfeld JF. Pituitary gastrins occur in corticotrophs and melanotrophs. Science. 1981;213:768–770. doi: 10.1126/science.6266012. [DOI] [PubMed] [Google Scholar]
- 11.Rehfeld JF. Localisation of gastrins to neuro- and adenohypophysis. Nature (London) 1978;271:771–773. doi: 10.1038/271771a0. 1978. [DOI] [PubMed] [Google Scholar]
- 12.Rehfeld JF. Accumulation of nonamidated preprogastrin and preprocholecystokinin products in porcine pituitary corticotrophs. Evidence of post-translational control of cell differentiation. J Biol Chem. 1986;261:5841–5847. [PubMed] [Google Scholar]
- 13.Rehfeld JF, Hansen HF, Larsson LI, Stengaard-Pedersen K, Thorn NA. Gastrin and cholecystokinin in pituitary neurons. Proc Natl Acad Sci USA. 1984;81:1902–1905. doi: 10.1073/pnas.81.6.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rehfeld JF. Progastrin and its products in the cerebellum. Neuropeptides. 1991;20:239–245. doi: 10.1016/0143-4179(91)90014-a. [DOI] [PubMed] [Google Scholar]
- 15.Uvnas-Wallensten K, Rehfeld JF, Larsson LI, Uvnas B. Heptadecapeptide gastrin in the vagal nerve. Proc Natl Acad Sci USA. 1977;74:5707–5710. doi: 10.1073/pnas.74.12.5707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rehfeld JF, Bardram L, Hilsted L. Gastrin in human bronchogenic carcinomas: constant expression but variable processing of progastrin. Cancer Res. 1989;49:2840–2843. [PubMed] [Google Scholar]
- 17.van Solinge WW, Odum L, Rehfeld JF. Ovarian cancers express and process progastrin. Cancer Res. 1993;53:1823–1830. [PubMed] [Google Scholar]
- 18.Schalling M, Persson H, Pelto-Huikko M, Odum L, Ekman P, Gottlieb C, Hokfelt T, Rehfeld JF. Expression and localization of gastrin messenger RNA and peptide in spermatogenic cells. J Clin Invest. 1990;86:660–669. doi: 10.1172/JCI114758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGuigan JI. Gastrin mucosal intracellular localization of gastrin by immunofluorescence. Gastroenterology. 1968;55:315–327. [PubMed] [Google Scholar]
- 20.McArthur KE, Isenberg JI, Hogan DL, Dreier SJ. Intravenous infusion of L-isomers of phenylalanine and tryptophan stimulates gastric acid secretion at physiologic plasma concentrations in normal subjects and after parietal cell vagotomy. J Clin Invest. 1983;71:1254–1262. doi: 10.1172/JCI110875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor IL, Bryne WJ, Christie DL, Ament ME, Walsh JH. Effect of individual L-amino acids on gastric acid secretion and serum gastrin and pancreatic polypeptide release in humans. Gastroenterology. 1982;83:272–278. [PubMed] [Google Scholar]
- 22.Eysselein VE, Kovacs TOG, Kleibeuker JH, Maxwell V, Reedy T, Walsh JH. Regulation of gastric acid secretion by gastrin in duodenal ulcer patients and healthy subjects. Gastroenterology. 1992;102:1142–1148. [PubMed] [Google Scholar]
- 23.Lichtenberger LM, Nelson AA, Graziani LA. Amine trapping: physical explanation for the inhibitory effect of gastric acidity on the postprandial release of gastrin. Studies on rats and dogs. Gastroenterology. 1986;90:1223–1231. doi: 10.1016/0016-5085(86)90389-6. [DOI] [PubMed] [Google Scholar]
- 24.Lehy T, Accary JP, Labeille D, Dubrasquet M. Chronic administration of bombesin stimulates antral gastrin cell proliferation in the rat. Gastroenterology. 1983;84:914–919. [PubMed] [Google Scholar]
- 25.Dial EJ, Huang J, Delansorne R, Lichtenberger LM. Monoamine oxidase: an important intracellular regulator of gastrin release in the rat. Gastroenterology. 1986;90:1018–1023. doi: 10.1016/0016-5085(86)90881-4. [DOI] [PubMed] [Google Scholar]
- 26.Walsh JH. Gastrin. In: Walsh JH, Dockray GJ, editors. Gut peptides: biochemistry and physiology. New York: Raven Press; 1994. pp. 75–121. pp. 75–121. [Google Scholar]
- 27.Richardson CT, Feldman M. Effect of histamine and cimetidine on amino acid meal-stimulated gastrin release at a controlled intragastric pH in healthy human beings. Regul Pept. 1985;10:339–344. doi: 10.1016/0167-0115(85)90046-1. [DOI] [PubMed] [Google Scholar]
- 28.Lind T, Cederberg C, Forssell H, Olausson M, Olbe L. Relationship between reduction of gastric acid secretion and plasma gastrin concentration during omeprazole treatment. Scand J Gastroenterol. 1988;23:1259–1266. doi: 10.3109/00365528809090202. [DOI] [PubMed] [Google Scholar]
- 29.Simoens C, Woussen-Colle MC, De Graef J. Effect of acute suppression of acid secretion by omeprazole on postprandial gastrin release in conscious dogs. Gastroenterology. 1989;97:837–845. doi: 10.1016/0016-5085(89)91486-8. [DOI] [PubMed] [Google Scholar]
- 30.Brand SJ, Stone DL. Reciprocal regulation of antral gastrin and somatostatin gene expression by omeprazole-induced achlorhydria. J Clin Invest. 1988;82:1059–1066. doi: 10.1172/JCI113662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu SV, Giraud A, Mogard M, Sumii K, Walsh JH. Effects of inhibition of gastric secretion on antral gastrin and somatostatin gene expression in rats. Am J Physiol Gastrointest Liver Physiol. 1990;258:G788–G793. doi: 10.1152/ajpgi.1990.258.5.G788. [DOI] [PubMed] [Google Scholar]
- 32.Tari A, Wu V, Sumii M, Sachs G, Walsh JH. Regulation of rat gastric H+/K+ -ATPase α-subunit mRNA by omeprazole. Biochim Biophys Acta Gene Struct Expression. 1991;1129:49–56. doi: 10.1016/0167-4781(91)90211-4. [DOI] [PubMed] [Google Scholar]
- 33.Allen JM, Bishop AE, Daly MJ, Larsson J, Carlsson E, Polak JM, Bloom SR. Effect of inhibition of acid secretion on the regulatory peptides in the rat stomach. Gastroenterology. 1986;90:970–977. doi: 10.1016/0016-5085(86)90875-9. [DOI] [PubMed] [Google Scholar]
- 34.Varner AA, Modlin IM, Walsh JH. High potency of bombesin for stimulation of human gastrin release and gastric acid secretion. Regul Pept. 1981;1:289–296. doi: 10.1016/0167-0115(81)90052-5. [DOI] [PubMed] [Google Scholar]
- 35.Hirschowitz BI, Molina E. Relation of gastric acid and pepsin secretion to serum gastrin levels in dogs given bombesin and gastrin-17. Am J Physiol Gastrointest Liver Physiol. 1983;244:G546–G551. doi: 10.1152/ajpgi.1983.244.5.G546. [DOI] [PubMed] [Google Scholar]
- 36.Desmond H, Pauwels S, Varro A, Gregory H, Young J, Dockray GJ. Isolation and characterization of the intact gastrin precursor from a gastrinoma. FEBS Lett. 1987;210:185–188. doi: 10.1016/0014-5793(87)81334-0. [DOI] [PubMed] [Google Scholar]
- 37.Huebner VD, Jiang RL, Lee TD, Legesse K, Walsh JH, Shively JE, Chew P, Azumi T, Reeve JR., Jr Purification and structural characterization of progastrin-derived peptides from a human gastrinoma. J Biol Chem. 1991;266:12223–12227. [PubMed] [Google Scholar]
- 38.Reeve JR, Jr, Walsh JH, Tompkins RK, Hawke D, Shively JE. Amino terminal fragments of human progastrin from gastrinoma. Biochem Biophys Res Commun. 1984;123:404–409. doi: 10.1016/0006-291x(84)90428-5. [DOI] [PubMed] [Google Scholar]
- 39.Varro A, Voronina S, Dockray GJ. Pathways of processing of the gastrin precursor in rat antral mucosa. J Clin Invest. 1995;95:1642–1649. doi: 10.1172/JCI117839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varro A, Dockray GJ, Bate GW, Vaillant C, Higham A, Armitage E, Thompson DG. Gastrin biosynthesis in the antrum of patients with pernicious anemia. Gastroenterology. 1997;112:733–741. doi: 10.1053/gast.1997.v112.pm9041234. [DOI] [PubMed] [Google Scholar]
- 41.Macro JA, Bate GW, Varro A, Vaillant C, Seidah NG, Dimaline R, Dockray GJ. Regulation by gastric acid of the processing of progastrin-derived peptides in rat antral mucosa. J Physiol. 1997;502:409–419. doi: 10.1111/j.1469-7793.1997.409bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dockray GJ. Gastrin and gastric epithelial physiology. J Physiol. 1999;518:315–324. doi: 10.1111/j.1469-7793.1999.0315p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dockray GJ, Varro A, Dimaline R, Wang T. The gastrins: their production and biological activities. Annu Rev Physiol. 2001;63:119–139. doi: 10.1146/annurev.physiol.63.1.119. [DOI] [PubMed] [Google Scholar]
- 44.Bishop L, Dimaline R, Blackmore C, Deavall D, Dockray GJ, Varro A. Modulation of the cleavage of the gastrin precursor by phosphorylation. Gastroenterology. 1998;115:1154–1162. doi: 10.1016/s0016-5085(98)70086-1. [DOI] [PubMed] [Google Scholar]
- 45.Voronina S, Henry J, Vaillant C, Dockray GJ, Varro A. Amine precursor uptake and decarboxylation: significance for processing of the rat gastrin precursor. J Physiol. 1997;501:363–374. doi: 10.1111/j.1469-7793.1997.363bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bundgaard JR, Vuust J, Rehfeld JF. New consensus features for tyrosine O-sulfation determined by mutational analysis. J Biol Chem. 1997;272:21700–21705. doi: 10.1074/jbc.272.35.21700. [DOI] [PubMed] [Google Scholar]
- 47.Varro A, Nemeth J, Bridson J, Lonovics J, Dockray GJ. Modulation of posttranslational processing of gastrin precursor in dogs. Am J Physiol Gastrointest Liver Physiol. 1990;258:G904–G909. doi: 10.1152/ajpgi.1990.258.6.G904. [DOI] [PubMed] [Google Scholar]
- 48.Rehfeld JF, van Solinge WW. The tumor biology of gastrin and cholecystokinin. Adv Cancer Res. 1994;63:295–347. doi: 10.1016/s0065-230x(08)60403-0. [DOI] [PubMed] [Google Scholar]
- 49.Campos RV, Buchan AM, Meloche RM, Pederson RA, Kwok YN, Coy DH. Gastrin secretion from human antral G cells in culture. Gastroenterology. 1990;99:36–44. doi: 10.1016/0016-5085(90)91226-v. [DOI] [PubMed] [Google Scholar]
- 50.Watson SA, Grabowska AM, El-Zaatari M, Takhar A. Gastrin – active participant or bystander in gastric carcinogenesis? Nature Rev Cancer. 2006;6:936–946. doi: 10.1038/nrc2014. [DOI] [PubMed] [Google Scholar]
- 51.Waldum HL, Sandvik AK, Brenna E, Petersen H. Gastrin-histamine sequence in the regulation of gastric acid secretion. Gut. 1991;32:698–701. doi: 10.1136/gut.32.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soll AH, Chuang C-N, Chen MCY. The isolation and characterization of fundic endocrine cells. In: Hakanson R, Sundler F, editors. The stomach as an endocrine organ. Amsterdam: Elsevier Science; 1991. pp. 71–78. [Google Scholar]
- 53.Chuang CN, Tanner M, Chen MC, Davidson S, Soll AH. Gastrin induction of histamine release from primary cultures of canine oxyntic mucosal cells. Am J Physiol Gastrointest Liver Physiol. 1992;263:G460–G465. doi: 10.1152/ajpgi.1992.263.4.G460. [DOI] [PubMed] [Google Scholar]
- 54.Prinz C, Kajimura M, Scott DR, Mercier F, Helander HF, Sachs G. Histamine secretion from rat enterochromaffinlike cells. Gastroenterology. 1993;105:449–461. doi: 10.1016/0016-5085(93)90719-s. [DOI] [PubMed] [Google Scholar]
- 55.Prinz C, Scott DR, Hurwitz D, Helander HF, Sachs G. Gastrin effects on isolated rat enterochromaffin-like cells in primary culture. Am J Physiol Gastrointest Liver Physiol. 1994;267:G663–G675. doi: 10.1152/ajpgi.1994.267.4.G663. [DOI] [PubMed] [Google Scholar]
- 56.Prinz C, Zanner R, Gerhard M, Mahr S, Neumayer N, Hohne-Zell B, Gratzl M. The mechanism of histamine secretion from gastric enterochromaffin-like cells. Am J Physiol Cell Physiol. 1999;277:C845–C855. doi: 10.1152/ajpcell.1999.277.5.C845. [DOI] [PubMed] [Google Scholar]
- 57.Kitano M, Norlen P, Hakanson R. Gastric submucosal microdialysis: a method to study gas. Regul Pept. 2000;86:113–123. doi: 10.1016/s0167-0115(99)00096-8. [DOI] [PubMed] [Google Scholar]
- 58.Zeng N, Walsh JH, Kang T, Helander KG, Helander HF, Sachs G. Selective ligand-induced intracellular calcium changes in a population of rat isolated gastric endocrine cells. Gastroenterology. 1996;110:1835–1846. doi: 10.1053/gast.1996.v110.pm8964409. [DOI] [PubMed] [Google Scholar]
- 59.Sachs G, Zeng N, Prinz C. Physiology of isolated gastric endocrine cells. Annu Rev Physiol. 1997;59:243–256. doi: 10.1146/annurev.physiol.59.1.243. [DOI] [PubMed] [Google Scholar]
- 60.Forte JG, Yao X. The membrane-recruitment-and-recycling hypothesis of gastric HCl secretion. Trends Cell Biol. 1996;6:45–48. doi: 10.1016/0962-8924(96)81009-9. [DOI] [PubMed] [Google Scholar]
- 61.Black JW, Shankley NP. How does gastrin act to stimulate oxyntic cell secretion? Trends Pharmacol Sci. 1987;8:486–490. [Google Scholar]
- 62.Soll AH, Amirian DA, Thomas LP, Reedy TJ, Elashoff JD. Gastrin receptors on isolated canine parietal cells. J Clin Invest. 1984;73:1434–1447. doi: 10.1172/JCI111348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Urushidani T, Forte JG. Signal transduction and activation of acid secretion in the parietal cell. J Membr Biol. 1997;159:99–111. doi: 10.1007/s002329900274. [DOI] [PubMed] [Google Scholar]
- 64.Kaise M, Muraoka A, Yamada J, Yamada T. Epidermal growth factor induces H+, K+-ATPase α-subunit gene expression through an element homologous to the 3’ half-site of the c-fos serum response element. J Biol Chem. 1995;270:18637–18642. doi: 10.1074/jbc.270.31.18637. [DOI] [PubMed] [Google Scholar]
- 65.Tari A, Yamamoto G, Yonei Y, Sumii M, Sumii K, Haruma K, Kajiyama G, Wu V, Sachs G, Walsh JH. Effect of histamine on rat gastric H(+)-K(+)-ATPase α-subunit expression. Am J Physiol Gastrointest Liver Physiol. 1994;266:G444–G450. doi: 10.1152/ajpgi.1994.266.3.G444. [DOI] [PubMed] [Google Scholar]
- 66.Friis-Hansen L, Sundler F, Li Y, Gillespie PJ, Saunders TL, Greenson JK, Owyang C, Rehfeld JF, Samuelson LC. Impaired gastric acid secretion in gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 1998;274:G561–G568. doi: 10.1152/ajpgi.1998.274.3.G561. [DOI] [PubMed] [Google Scholar]
- 67.Dimaline R, Evans D, Varro A, Dockray GJ. Reversal by omeprazole of the depression of gastrin of gastrin cell function by fasting in the rat. J Physiol. 1991;433:483–493. doi: 10.1113/jphysiol.1991.sp018439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez V, Curi AP, Torkian B, Schaeffer JM, Wilkinson HA, Walsh JH, Tache Y. High basal gastric acid secretion in somatostatin receptor subtype 2 knockout mice. Gastroenterology. 1998;114:1125–1132. doi: 10.1016/s0016-5085(98)70417-2. [DOI] [PubMed] [Google Scholar]
- 69.Rozengurt E, Walsh JH, Gastrin CCK, signaling, and cancer Annu Rev Physiol. 2001;63:49–76. doi: 10.1146/annurev.physiol.63.1.49. [DOI] [PubMed] [Google Scholar]
- 70.Mahr S, Neumayer N, Kolb HJ, Schepp W, Classen M, Prinz C. Growth factor effects on apoptosis of rat gastric enterochromaffin-like cells. Endocrinology. 1998;139:4380–4390. doi: 10.1210/endo.139.10.6248. [DOI] [PubMed] [Google Scholar]
- 71.Ryberg B, Tielemans Y, Axelson J, Carlsson E, Hakanson R, Mattsson H, Sundler F, Willems G. Gastrin stimulates the self-replication rate of enterochromaffinlike cells in the rat stomach: effects of omeprazole, ranitidine and gastrin-17 in intact and antrectomized rats. Gastroenterology. 1990;99:935–942. doi: 10.1016/0016-5085(90)90610-d. [DOI] [PubMed] [Google Scholar]
- 72.Bordi C, D’Adda T, Azzoni C, Ferraro G. Pathogenesis of ECL cell tumors in humans. Yale J Biol Med. 1998;71:273–284. [PMC free article] [PubMed] [Google Scholar]
- 73.Delle Fave G, Helander H, Holt S, Modlin IM, Powers R, Solcia E, Soll A, Tielemans Y, Wright NA. Acid suppression and gastric mucosal cell biology. Dig Dis Sci. 1994;39:1843–1852. doi: 10.1007/BF02088113. [DOI] [PubMed] [Google Scholar]
- 74.Lamberts R, Creutzfeldt W, Struber HG, Brunner G, Solcia E. Long term omeprazole therapy in peptic ulcer disease: gastrin, endocrine cell growth and gastritis. Gastroenterology. 1993;104:1356–1370. doi: 10.1016/0016-5085(93)90344-c. [DOI] [PubMed] [Google Scholar]
- 75.Jensen RT. Involvement of cholecystokinin/gastrin-related peptides and their receptors in clinical gastrointestinal disorders. Pharmacol Toxicol. 2002;91:333–350. doi: 10.1034/j.1600-0773.2002.910611.x. [DOI] [PubMed] [Google Scholar]
- 76.Willems G, Vansteenkiste Y, Limbosch JM. Stimulating effect of gastrin on cell proliferation kinetics in canine fundic mucosa. Gastroenterology. 1972;62:583–589. [PubMed] [Google Scholar]
- 77.Tielemans Y, Axelson J, Sundler F, Willems G, Hakanson R. Serum gastrin concentration affects the self replication rate of the enterochromaffin like cells in the rat stomach. Gut. 1990;31:274–278. doi: 10.1136/gut.31.3.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Axelson J, Hakanson R, Rosengren E, Sundler F. Hypergastrinemia induced by acid blockade evokes enterochromaffin-like (ECL) cell hyperplasia in chicken, hamster and guinea pig stomach. Cell Tiss Res. 1988;254:511–516. doi: 10.1007/BF00226500. [DOI] [PubMed] [Google Scholar]
- 79.Ryberg B, Bishop AE, Bloom SR, Carlsson E, Hakanson R, Larsson H, Mattsson H, Polak JM, Sundler F. Omeprazole and rantidine, antisecretagogues with different modes of action, are equally effective in causing hyperplasia of enterochromaffin-like cells in rat stomach. Regul Pept. 1989;25:235–246. doi: 10.1016/0167-0115(89)90265-6. [DOI] [PubMed] [Google Scholar]
- 80.Ryberg B, Carlsson E, Hakanson R, Lundell L, Mattsson H, Sundler F. Effects of partial resection of acid-secreting mucosa on plasma gastrin and enterochromaffin-like cells in the rat stomach. Digestion. 1990;45:102–108. doi: 10.1159/000200230. [DOI] [PubMed] [Google Scholar]
- 81.Larsson H, Carlsson E, Mattsson H, Lundell L, Sundler F, Sundell G, Wallmark B, Watanabe T, Hakanson RP. Plasma gastrin and gastric enterochromaffinlike cell activation and proliferation. Studies with omeprazole and rantidine in intact and antrectomized rats. Gastroenterology. 1986;90:391–399. doi: 10.1016/0016-5085(86)90938-8. [DOI] [PubMed] [Google Scholar]
- 82.Miyazaki Y, Shinomura Y, Tsutsui S, Zushi S, Higashimoto Y, Kanayama S, Higashiyama S, Taniguchi N, Matsuzawa Y. Gastrin induces heparin-binding epidermal growth factor-like growth factor in rat gastric epithelial cells transfected with gastrin receptor. Gastroenterology. 1999;116:78–89. doi: 10.1016/s0016-5085(99)70231-3. [DOI] [PubMed] [Google Scholar]
- 83.Miyaoka Y, Kadowaki Y, Ishihara S, Ose T, Fukuhara H, Kazumori H, Takasawa S, Okamoto H, Chiba T, Kinoshita Y. Transgenic overexpression of Reg protein caused gastric cell proliferation and differentiation along parietal cell and chief cell lineages. Oncogene. 2004;23:3572–3579. doi: 10.1038/sj.onc.1207333. [DOI] [PubMed] [Google Scholar]
- 84.Varro A, Noble PJ, Wroblewski LE, Bishop L, Dockray GJ. Gastrin-cholecystokinin(B) receptor expression in AGS cells is associated with direct inhibition and indirect stimulation of cell proliferation via paracrine activation of the epidermal growth factor receptor. Gut. 2002;50:827–833. doi: 10.1136/gut.50.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sinclair NF, Ai W, Raychowdhury R, Bi M, Wang TC, Koh TJ, McLaughlin JT. Gastrin regulates the heparin binding epidermal-like growth factor promoter via a PKC/EGFR dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 2004;286:G992–G999. doi: 10.1152/ajpgi.00206.2002. [DOI] [PubMed] [Google Scholar]
- 86.Dockray GJ, Dimaline R, Varro A. Gastrin: old hormone, new functions. Pflugers Arch - Eur J Physiol. 2005;449:344–355. doi: 10.1007/s00424-004-1347-5. [DOI] [PubMed] [Google Scholar]
- 87.Todisco A, Ramamoorthy S, Witham T, Pausawasdi N, Srinivasan S, Dickinson CJ, Askari FK, Krametter D. Molecular mechanisms for the antiapoptotic action of gastrin. Am J Physiol Gastrointest Liver Physiol. 2001;280:G298–G307. doi: 10.1152/ajpgi.2001.280.2.G298. [DOI] [PubMed] [Google Scholar]
- 88.Noble PJ, Wilde G, White MR, Pennington SR, Dockray GJ, Varro A. Stimulation of gastrin-CCKB receptor promotes migration of gastric AGS cells via multiple paracrine pathways. Am J Physiol Gastrointest Liver Physiol. 2003;284:G75–G84. doi: 10.1152/ajpgi.00300.2002. [DOI] [PubMed] [Google Scholar]
- 89.Wroblewski LE, Pritchard DM, Carter S, Varro A. Gastrin-stimulated gastric epithelial cell invasion: the role and mechanism of increased matrix metalloproteinase 9 expression. Biochem J. 2002;365:873–879. doi: 10.1042/BJ20020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Todisco A, Takeuchi Y, Urumov A, Yamada J, Stepan VM, Yamada T. Molecular mechanisms for the growth factor action of gastrin. Am J Physiol Gastrointest Liver Physiol. 1997;273:G891–G898. doi: 10.1152/ajpgi.1997.273.4.G891. [DOI] [PubMed] [Google Scholar]
- 91.Carlsson E, Larsson H, Matsson H, Ryberg B, Sundell G. Pharmacology and toxicology of omeprazole – with special reference to the effects on the gastric mucosa. Scand J Gastroenterol Supp. 1986;118:31–38. doi: 10.3109/00365528609090884. [DOI] [PubMed] [Google Scholar]
- 92.Mattsson H, Havu N, Brautigam J, Carlsson K, Lundell L, Carlsson E. Partial gastric corpectomy results in hypergastrinemia and development of gastric enterochromaffin-like cell carcinoids in the rat. Gastroenterology. 1991;100:311–319. doi: 10.1016/0016-5085(91)90197-s. [DOI] [PubMed] [Google Scholar]
- 93.Creutzfeldt W. The achlorhydria-carcinoid sequence: role of gastrin. Digestion. 1988;39:61–79. doi: 10.1159/000199609. [DOI] [PubMed] [Google Scholar]
- 94.Hakanson R, Sundler F. Proposed mechanism of induction of gastric carcinoids: the gastrin hypothesis. Eur J Clin Invest. 1990;20:S65–S71. doi: 10.1111/j.1365-2362.1990.tb01780.x. [DOI] [PubMed] [Google Scholar]
- 95.Gregory RA, Tracy HJ, French JM, Sircus W. Extraction of a gastrin-like substance from a pancreatic tumour in a case of Zollinger-Ellison syndrome. Lancet. 1960;1(7133):1045–1048. doi: 10.1016/s0140-6736(60)90932-6. [DOI] [PubMed] [Google Scholar]
- 96.Pipeleers-Marichal MK, Somers G, Willems G, Foulis A, Imrie C, Bishop AE, Polak JM, Hacki WH, Stamm B, Heitz PU, Kloppel G. Gastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. N Engl J Med. 1990;322:723–727. doi: 10.1056/NEJM199003153221103. [DOI] [PubMed] [Google Scholar]
- 97.Larsson LI, Ljungberg O, Sundler F, Svensson SO, Rehfeld JF, Stadil F, Holst JJ. Antor-pyloric gastrinoma associated with pancreatic nesidioblastosis and proliferation of islets. Virchows Arch A: Pathol Anat. 1973;360:305–314. doi: 10.1007/BF00548351. [DOI] [PubMed] [Google Scholar]
- 98.Royston CMS, Brew DSJ, Garnham JR, Stagg BH, Polak J. The Zollinger-Ellison syndrome due to an infiltrating tumour of the stomach. Gut. 1972;13:638–642. doi: 10.1136/gut.13.8.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thompson NW, Vinik AI, Eckhauser FE. Microgastrinomas of the duodenum. A cause of failed operations for the Zollinger-Ellison syndrome. Ann Surg. 1989;209:396–404. doi: 10.1097/00000658-198904000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jacobsen O, Bardram L, Rehfeld JF. The requirement for gastrin measurements. Scand J Clin Lab Invest. 1986;46:272–274. doi: 10.3109/00365518609083693. [DOI] [PubMed] [Google Scholar]
- 101.Rindi G, Bordi C, Rappel S, La Rosa S, Stolte M, Solcia E. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World J Surg. 1996;20:168–172. doi: 10.1007/s002689900026. [DOI] [PubMed] [Google Scholar]
- 102.Peghini PL, Annibale B, Azzoni C, Milione M, Corleto VD, Gibril F, Venson DJ, Delle Fave G, Bordi C, Jensen RT. Effect of chronic hypergastrinemia on human enterochromaffin-like cells: insights from patients with sporadic gastrinomas. Gastroenterology. 2002;123:68–85. doi: 10.1053/gast.2002.34231. [DOI] [PubMed] [Google Scholar]
- 103.Chowers MY, Keller N, Tal R, Barshack I, Lang R, Bar-Meir S, Chowers Y. Human gastrin: a helicobacter pylori-specific growth factor. Gastroenterology. 1999;117:1113–1118. doi: 10.1016/s0016-5085(99)70396-3. [DOI] [PubMed] [Google Scholar]
- 104.Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, Coffey RJ, Ito S, Varro A, Dockray GJ, Fox JG. Synergistic interaction between hypergastrinemia and Helicobacter infection in a mouse model of gastric cancer. Gastroenterology. 2000;118:36–47. doi: 10.1016/s0016-5085(00)70412-4. [DOI] [PubMed] [Google Scholar]
- 105.Israel DA, Salama N, Arnold CN, Moss SF, Ando T, Wirth HP, Tham KT, Camorlinga M, Blaser MJ, Falkow S, Peek RM., Jr Helicobacter pylori strain-specific differences in genetic content, identified by microarray, influence host inflammatory responses. J Clin Invest. 2001;107:611–620. doi: 10.1172/JCI11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Houghton J, Fox JG, Wang TC. Gastric cancer: laboratory bench to clinic. J Gastroenterol Hepatol. 2002;17:495–502. doi: 10.1046/j.1440-1746.2002.02770.x. [DOI] [PubMed] [Google Scholar]
- 107.Kodama K, Sumii K, Kawano M, Kido T, Nojima K, Sumii M, Haruma K, Yoshihara M, Chayama K. Gastric juice nitrite and vitamin C in patients with gastric cancer and atrophic gastritis: is low acidity solely responsible for cancer risk? Eur J Gastroenterol Hepatol. 2003;15:987–993. doi: 10.1097/00042737-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 108.Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhea. J Hosp Infect. 2003;54:243–245. doi: 10.1016/s0195-6701(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 109.Martinsen TC, Bergh K, Waldum HL. Gastric juice: a barrier against infectious diseases. Basic Clin Pharmacol Toxicol. 2005;96:94–102. doi: 10.1111/j.1742-7843.2005.pto960202.x. [DOI] [PubMed] [Google Scholar]
- 110.Sarker SA, Gyr K. Non-immunological defence mechanisms of the gut. Gut. 1992;33:987–993. doi: 10.1136/gut.33.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ishizuka J, Martinez J, Townsend CM, Thompson JC. The effect of gastrin on growth of human stomach cancer cells. Ann Surg. 1992;215:528–534. doi: 10.1097/00000658-199205000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Watson SA, Morris DL, Durrant LG, Robertson JF, Hardcastle JD. Inhibition of gastrin-stimulated growth of gastrointestinal tumour cells by octreotide and the gastrin/cholecystokinin receptor antagonists, proglumide and lorglumide. Eur J Cancer. 1992;28A:1462–1467. doi: 10.1016/0959-8049(92)90544-c. [DOI] [PubMed] [Google Scholar]
- 113.Borch K, Renvall H, Kullman E, Wilander E. Wilander, E. Gastric carcinoid associated with the syndrome of hypergastrinemic atrophic gastritis. A prospective analysis of 11 cases. Am J Surg Pathol. 1987;11:435–444. doi: 10.1097/00000478-198706000-00004. [DOI] [PubMed] [Google Scholar]
- 114.Kobori O, Vuillot MT, Martin F. Growth responses of rat stomach cancer cells to gastro-entero-pancreatic hormones. Int J Cancer. 1982;30:65–67. doi: 10.1002/ijc.2910300112. [DOI] [PubMed] [Google Scholar]
- 115.Watson SA, Durrant LG, Morris DL. Gastrin: growth enhancing effects on human gastric and colonic tumour cells. Br J Cancer. 1989;59:554–558. doi: 10.1038/bjc.1989.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Crawley JN, Corwin RL. Biological actions of cholecystokinin. Peptides. 1994;15:731–755. doi: 10.1016/0196-9781(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 117.Barrachina MD, Martinez V, Wang L, Wei JY, Tache Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci USA. 1997;94:10455–10460. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hokfelt T, Rehfeld JF, Skirboll L, Ivemark B, Goldstein M, Markey K. Evidence for coexistence of dopamine and CCK in meso-limbic neurones. Nature (London) 1980;285:476–478. doi: 10.1038/285476a0. [DOI] [PubMed] [Google Scholar]
- 119.Crawley JN. Cholecystokinin-dopamine interactions. Trends Pharmacol Sci. 1991;12:232–236. doi: 10.1016/0165-6147(91)90558-a. [DOI] [PubMed] [Google Scholar]
- 120.Mantyh CR, Pappas TN, Vigna SR. Localization of cholecystokinin A and cholecystokinin B/gastrin receptors in the canine upper gastrointestinal tract. Gastroenterology. 1994;107:1019–1030. doi: 10.1016/0016-5085(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 121.Reubi JC, Waser B, Laederach U, Stettler C, Friess H, Halter F, Schmassman A. Localization of cholecystokinin A and cholecystokinin B-gastrin receptors in the human stomach. Gastroenterology. 1997;112:1197–1205. doi: 10.1016/s0016-5085(97)70131-8. [DOI] [PubMed] [Google Scholar]
- 122.Wank SA. Cholecystokinin receptors. Am J Physiol Gastrointest Liver Physiol. 1995;269:G628–G646. doi: 10.1152/ajpgi.1995.269.5.G628. [DOI] [PubMed] [Google Scholar]
- 123.Wank SA. G protein-coupled receptors in gastrointestinal physiology I. CCK receptors: an exemplary family. Am J Physiol Gastrointest Liver Physiol. 1998;274:G607–G613. doi: 10.1152/ajpgi.1998.274.4.g607. [DOI] [PubMed] [Google Scholar]
- 124.Dufresne M, Seva C, Fourmy D. Cholecystokinin and gastrin receptors. Physiol Rev. 2006;86:805–847. doi: 10.1152/physrev.00014.2005. [DOI] [PubMed] [Google Scholar]
- 125.Lloyd KC, Raybould HE, Walsh JH. Cholecystokinin inhibits gastric acid secretion through type “A” cholecystokinin receptors and somatostatin in rats. Am J Physiol Gastrointest Liver Physiol. 1992;263:G287–G292. doi: 10.1152/ajpgi.1992.263.3.G287. [DOI] [PubMed] [Google Scholar]
- 126.Qian J-M, Rowley WH, Jensen RT. Gastrin and CCK activate phospholipase C and stimulate pepsinogen release by interacting with two distinct receptors. Am J Physiol Gastrointest Liver Physiol. 1993;264:G718–G728. doi: 10.1152/ajpgi.1993.264.4.G718. [DOI] [PubMed] [Google Scholar]
- 127.Bitar KN, Makhlouf GM. Receptors on smooth muscle cells: characterization by contraction and specific antagonists. Am J Physiol Gastrointest Liver Physiol. 1982;242:G400–G407. doi: 10.1152/ajpgi.1982.242.4.G400. [DOI] [PubMed] [Google Scholar]
- 128.Suzuki S, Takiguchi S, Sato N, Kanai S, Kawanami T, Yoshida Y, Miyasaka K, Takata Y, Funakoshi A, Noda T. Importance of CCK-A receptor for gallbladder contraction and pancreatic secretion: a study in CCK-A knockout mice. Jpn J Physiol. 2001;51:585–590. doi: 10.2170/jjphysiol.51.585. [DOI] [PubMed] [Google Scholar]
- 129.Fried M, Erlacher U, Schwizer W, Lochner C, Koerfer J, Beglinger C, Jansen JB, Lamers CB, Harder F, Bischof-Delaloye A, Stadler GA, Rovati L. Role of cholecystokinin in the regulation of gastric emptying and pancreatic enzyme secretion in humans. Studies with the cholecystokinin-receptor antagonist loxiglumide. Gastroenterology. 1991;101:503–511. doi: 10.1016/0016-5085(91)90031-f. [DOI] [PubMed] [Google Scholar]
- 130.Scarpignato C, Kisfalvi I, D’Amato M, Varga G. Effect of dexloxiglumide and spiroglumide, two new CCK-receptor antagonists, on gastric emptying and secretion in the rat: evaluation of their receptor selectivity in vivo. Aliment Pharmacol Ther. 1996;10:411–419. doi: 10.1111/j.0953-0673.1996.00411.x. [DOI] [PubMed] [Google Scholar]
- 131.Schwizer W, Borovicka J, Junz P, Fraser R, Kreiss C, D’Amato M, Crelier G, Boesiger P, Fried M. Role of cholecystokinin in the regulation of liquid gastric emptying and gastric motility in humans: studies with the CCK antagonist loxiglumide. Gut. 1997;41:500–504. doi: 10.1136/gut.41.4.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Behar J, Bianchini P. Effect of cholecystokinin and the octapeptide of cholecystokinin in the feline sphincter of Oddi and gallbladder. J Clin Invest. 1980;66:1231–1239. doi: 10.1172/JCI109974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cantor P, Olsen O, Gertz BJ, Gjorup I, Worning H. Inhibition of cholecystokinin-stimulated pancreaticobiliary output in man by the cholecystokinin receptor antagonist MK-329. Scand J Gastroenterol. 1991;26:627–637. doi: 10.3109/00365529109043637. [DOI] [PubMed] [Google Scholar]
- 134.Li Y, Hao Y, Owyang C. High-affinity CCK-A receptors on the vagus nerve mediate CCK-stimulated pancreatic secretion in rats. Am J Physiol Gastrointest Liver Physiol. 1997;273:G679–G685. doi: 10.1152/ajpgi.1997.273.3.G679. [DOI] [PubMed] [Google Scholar]
- 135.Konturek SJ, Tasler J, Obtulowicz W. Effect of atropine on pancreatic responses to endogenous and exogenous cholecystokinin. Am J Dig Dis. 1972;17:911–917. doi: 10.1007/BF02239529. [DOI] [PubMed] [Google Scholar]
- 136.Karlsson S, Ahren B. CCKA receptor antagonism inhibits mechanisms underlying CCK-8-stimulated insulin release in isolated rat islets. Eur J Pharmacol. 1991;202:253–257. doi: 10.1016/0014-2999(91)90301-6. [DOI] [PubMed] [Google Scholar]
- 137.Karlsson S, Ahren B. Effects of three different cholecystokinin receptor antagonists on basal and stimulated insulin and glucagon secretion in mice. Acta Physiol Scand. 1989;135:271–278. doi: 10.1111/j.1748-1716.1989.tb08577.x. [DOI] [PubMed] [Google Scholar]
- 138.Konturek JW, Stoll R, Gutwinska-Konturek M, Konturek SJ, Domschke W. Cholecystokinin in the regulation of gastric acid and endocrine pancreatic secretion in humans. Scand J Gastroenterol. 1993;28:401–407. doi: 10.3109/00365529309098239. [DOI] [PubMed] [Google Scholar]
- 139.Miyasaka K, Ohta M, Masuda M, Funakoshi A. Retardation of pancreatic regeneration after partial pancreatectomy in a strain of rats without CCK-A receptor gene expression. Pancreas. 1997;14:391–399. doi: 10.1097/00006676-199705000-00010. [DOI] [PubMed] [Google Scholar]
- 140.Miyasaka K, Ohta M, Tateishi K, Jimi A, Funakoshi A. Role of cholecystokinin-A (CCK-A) receptor in pancreatic regeneration after pancreatic duct occlusion: a study in rats lacking CCK-A receptor gene expression. Pancreas. 1998;16:114–123. doi: 10.1097/00006676-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 141.Moralejo DH, Ogino T, Kose H, Yamada T, Matsumoto K. Genetic verification of the role of CCK-AR in pancreatic proliferation and blood glucose and insulin regulation using a congenic rat carrying CCK-AR null allele. Res Commun Mol Pathol Pharmacol. 2001;109:259–274. [PubMed] [Google Scholar]
- 142.Moralejo DH, Ogino T, Zhu M, Toide K, Wei S, Wei K, Yamada T, Mizuno A, Matsumoto K, Shima K. A major quantitative trait locus co-localizing with cholecystokinin type A receptor gene influences poor pancreatic proliferation in a spontaneously diabetogenic rat. Mamm Genome. 1998;9:794–798. doi: 10.1007/s003359900869. [DOI] [PubMed] [Google Scholar]
- 143.Harro J, Vasar E, Bradwejn J. CCK in animal and human research on anxiety. Trends Pharmacol Sci. 1993;14:244–249. doi: 10.1016/0165-6147(93)90020-k. [DOI] [PubMed] [Google Scholar]
- 144.Nakata H, Matsui T, Ito M, Taniguchi T, Naribayashi Y, Arima N, Nakamura A, Kinoshita Y, Chihara K, Hosoda S, Chiba T. Cloning and characterization of gastrin receptor from ECL carcinoid tumor of Mastomys natalensis. Biochem Biophys Res Commun. 1992;187:1151–1157. doi: 10.1016/0006-291x(92)91317-j. [DOI] [PubMed] [Google Scholar]
- 145.Johnson LR. The trophic action of gastrointestinal hormones. Gastroenterology. 1976;70:278–288. [PubMed] [Google Scholar]
- 146.Saillan-Barreau C, Dufresne M, Clerc P, Sanchez D, Corominola H, Moriscot C, Guy-Crotte O, Escrieut C, Vaysse N, Gomis R, Tarasova N, Fourmy D. Evidence for a functional role of the cholecystokinin-B/gastrin receptor in the human fetal and adult pancreas. Diabetes. 1999;48:2015–2021. doi: 10.2337/diabetes.48.10.2015. [DOI] [PubMed] [Google Scholar]
- 147.Clerc P, Dufresne M, Saillan C, Chastre E, Andre T, Escrieut C, Kennedy K, Vaysse N, Gespach C, Fourmy D. Differential expression of the CCK-A and CCK-B/gastrin receptor genes in human cancers of the esophagus, stomach and colon. Int J Cancer. 1997;72:931–936. doi: 10.1002/(sici)1097-0215(19970917)72:6<931::aid-ijc2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 148.Henwood M, Clarke PA, Smith AM, Watson SA. Expression of gastrin in developing gastric adenocarcinoma. Br J Surg. 2001;88:564–568. doi: 10.1046/j.1365-2168.2001.01716.x. [DOI] [PubMed] [Google Scholar]
- 149.Konturek PC, Konturek SJ, Bielanski W, Karczewska E, Pierzchalski P, Duda A, Starzynska T, Marlicz K, Popiela T, Hartwich A, Hahn EG. Role of gastrin in gastric cancerogenesis in Helicobacter pylori infected humans. J Physiol Pharmacol. 1999;50:857–873. [PubMed] [Google Scholar]
- 150.McWilliams DF, Watson SA, Crosbee DM, Michaeli D, Seth R. Coexpression of gastrin and gastrin receptors (CCK-B and ΔCCK-B) in gastrointestinal tumour cell lines. Gut. 1998;42:795–798. doi: 10.1136/gut.42.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Okada N, Kubota A, Imamura T, Suwa H, Kawaguchi Y, Ohshio G, Seino Y, Imamura M. Evaluation of cholecystokinin, gastrin, CCK-A receptor, and CCK-B/gastrin receptor gene expression in gastric cancer. Cancer Lett. 1996;106:257–262. doi: 10.1016/0304-3835(96)04325-x. [DOI] [PubMed] [Google Scholar]
- 152.Reubi JC, Waser B, Schmassman A, Laissue JA. Receptor autoradiographic evaluation of cholecystokinin, neurotensin, somatostatin and vasoactive intestinal peptide receptors in gastrointestinal adenocarcinoma samples: where are they really located? Int J Cancer. 1999;81:376–386. doi: 10.1002/(sici)1097-0215(19990505)81:3<376::aid-ijc11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 153.Zhou JJ, Chen ML, Zhang QZ, Hu JK, Wang WL. Coexpression of cholecystokinin-B/gastrin receptor and gastrin gene in human gastric tissues and gastric cancer cell line. World J Gastroenterol. 2004;10:791–794. doi: 10.3748/wjg.v10.i6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Clerc P, Leung-Theung-Long S, Wang TC, Dockray GJ, Bouisson M, Delisle MB, Vaysee N, Pradayrol L, Fourmy D, Dufresne M. Expression of CCK2 receptors in the murine pancreas: proliferation, transdifferentiation of acinar cells, and neoplasia. Gastroenterology. 2002;122:428–437. doi: 10.1053/gast.2002.30984. [DOI] [PubMed] [Google Scholar]
- 155.Clerc P, Saillan-Barreau C, Desbois C, Pradayrol L, Fourmy D, Dufresne M. Transgenic mice expressing cholecystokinin 2 receptors in the pancreas. Pharmacol Toxicol. 2002;91:321–326. doi: 10.1034/j.1600-0773.2002.910609.x. [DOI] [PubMed] [Google Scholar]
- 156.Saillan-Barreau C, Clerc P, Adato M, Escrieut C, Vaysse N, Fourmy D, Dufresne M. Transgenic CCK-B/gastrin receptor mediates murine exocrine pancreatic secretion. Gastroenterology. 1998;115:988–996. doi: 10.1016/s0016-5085(98)70271-9. [DOI] [PubMed] [Google Scholar]
- 157.Moonka R, Zhou W, Bell RH., Jr Cholecystokinin-A receptor messenger RNA expression in human pancreatic cancer. J Gastrointest Surg. 1999;3:134–140. doi: 10.1016/s1091-255x(99)80022-5. [DOI] [PubMed] [Google Scholar]
- 158.Weinberg DS, Ruggeri B, Barber MT, Biswas S, Miknyocki S, Waldman SA. Cholecystokinin A and B receptors are differentially expressed in normal pancreas and pancreatic adenocarcinoma. J Clin Invest. 1997;100:597–603. doi: 10.1172/JCI119570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Weinberg DS, So C, Ruggeri B, Biswas S, Barber M, Waldman S. Human cholecystokinin-A receptor is not an oncofetal protein. Dig Dis Sci. 2000;45:538–543. doi: 10.1023/a:1005449308536. [DOI] [PubMed] [Google Scholar]
- 160.Kopin AS, Lee Y-M, McBride EW, Miller LJ, Lu M, Lin HY, Jr, Kolakowski LF, Beinborn M. Expression cloning and characterization of the canine parietal cell gastrin receptor. Proc Natl Acad Sci USA. 1992;89:3605–3609. doi: 10.1073/pnas.89.8.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pisegna JR, de Weerth A, Wank SA. Molecular cloning of the human brain and gastric cholecystokinin receptor: structure, functional expression and chromosomal localization. Biochem Biophys Res Commun. 1992;189:296–303. doi: 10.1016/0006-291x(92)91557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wank SA, Pisegna JR, de Weerth A. Brain and gastrointestinal cholecystokinin receptor family: structure and functional expression. Proc Natl Acad Sci USA. 1992;89:8691–8695. doi: 10.1073/pnas.89.18.8691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Deschenes RJ, Lorenz LJ, Haun RS, Roos BA, Collier KJ, Dixon JE. Cloning and sequence analysis of a cDNA encoding rat preprocholecystokinin. Proc Natl Acad Sci USA. 1984;81:726–730. doi: 10.1073/pnas.81.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Reeve JR, Eysselein V, Ho FJ, Chew P, Vigna SR, Liddle RA, Evans C. Natural and synthetic CCK-58. Ann NY Acad Sci. 1994;713:11–21. doi: 10.1111/j.1749-6632.1994.tb44047.x. [DOI] [PubMed] [Google Scholar]
- 165.Jensen RT, Lemp GF, Gardner JD. Interactions of COOH terminal fragments of cholecystokinin with receptors on dispersed acini from guinea pig pancreas. J Biol Chem. 1982;257:5554–5559. [PubMed] [Google Scholar]
- 166.Gardner JD, Jensen RT. Secretagogue receptors on pancreatic acinar cells. In: Johnson LR, editor. Physiology of the gastrointestinal tract. 2nd edition. New York: Raven Press; 1987. pp. 1109–1127. [Google Scholar]
- 167.Saito A, Sankaran H, Goldfine ID, Williams JA. Characterization of receptors for cholecystokinin and related peptides in mouse cerebral cortex. J Neurochem. 1981;37:483–490. doi: 10.1111/j.1471-4159.1981.tb00481.x. [DOI] [PubMed] [Google Scholar]
- 168.Denyer J, Gray J, Wong M, Stolz M, Tate S. Molecular and pharmacological characterization of the human CCKB receptor. Eur J Pharmacol. 1994;268:29–41. doi: 10.1016/0922-4106(94)90117-1. [DOI] [PubMed] [Google Scholar]
- 169.Ahmed S, Wibowo F, Gembitsky DS, Bozso Zs, Lovas S, Murphy RF. Importance of the C-terminal phenylalanine of gastrin for binding to the human CCK2 receptor. J Pept Res. 2001;58:332–337. doi: 10.1034/j.1399-3011.2001.00942.x. [DOI] [PubMed] [Google Scholar]
- 170.Roche S, Bali JP, Magous R. Involvement of a pertussis toxin-sensitive G protein in the action of gastrin on gastric parietal cells. Biochim Biophys Acta. 1990;1055:287–294. doi: 10.1016/0167-4889(90)90045-f. [DOI] [PubMed] [Google Scholar]
- 171.Kinoshita Y, Nakata H, Kishi K, Kawanami C, Sawada M, Chiba T. Comparison of the signal transduction pathways activated by gastrin in enterochromaffin-like and parietal cells. Gastroenterology. 1998;115:93–100. doi: 10.1016/s0016-5085(98)70369-5. [DOI] [PubMed] [Google Scholar]
- 172.Daulhac L, Kowalski-Chauvel A, Pradayrol L, Vaysee N, Seva C. Ca2+ and protein kinase C-dependent mechanisms involved in gastrin-induced Shc/Grb2 complex formation and P44-mitogen-activated protein kinase activation. Biochem J. 1997;325:383–389. doi: 10.1042/bj3250383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Seva C, Scemama JL, Pradayrol L, Sarfati PD, Vaysee N. Coupling of pancreatic gastrin/cholecystokinin-B (G/CCKB) receptors to phospholipase C and protein kinase C in AR4-2J tumoral cells. Regul Pept. 1994;52:31–38. doi: 10.1016/0167-0115(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 174.Malecka-Panas E, Tureaud J, Majumdar AP. Gastrin activates tyrosine kinase and phospholipase C in isolated rat colonocytes. Acta Biochim Pol. 1996;43:539–546. [PubMed] [Google Scholar]
- 175.Yassin RR, Abrams JT. Gastrin induces IP3 formation through phospholipase Cγ1 and pp60c-src kinase. Peptides. 1998;19:47–55. doi: 10.1016/s0196-9781(97)00276-3. [DOI] [PubMed] [Google Scholar]
- 176.Yassin RR, Clearfield HR, Little KM. Gastrin’s trophic effect in the colon: identification of a signaling pathway mediated by protein kinase C. Peptides. 1993;14:1119–1124. doi: 10.1016/0196-9781(93)90164-c. [DOI] [PubMed] [Google Scholar]
- 177.Yassin RR, Little KM. Early signaling mechanism in colonic epithelial cell response to gastrin. Biochem J. 1995;311:945–950. doi: 10.1042/bj3110945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yassin RR, Murthy SNS. Possible involvement of protein kinase C in mediating gastrin-induced response in rat colonic epithelium. Peptides. 1991;12:925–927. doi: 10.1016/0196-9781(91)90039-r. [DOI] [PubMed] [Google Scholar]
- 179.Ishizuka J, Townsend CM, Jr, Bold RJ, Martinez J, Rodriguez M, Thompson JC. Effects of gastrin on 3’,5’-cyclic adenosine monophosphate, intracellular calcium, and phosphatidylinositol hydrolysis in human colon cancer cells. Cancer Res. 1994;54:2129–2135. [PubMed] [Google Scholar]
- 180.Roche S, Bali JP, Galleyrand JC, Magous R. Characterization of a gastrin-type receptor on rabbit gastric parietal cells using L-365,260 and L-364,718. Am J Physiol Gastrointest Liver Physiol. 1991;260:G182–G188. doi: 10.1152/ajpgi.1991.260.2.G182. [DOI] [PubMed] [Google Scholar]
- 181.Roche S, Gusdinar T, Bali JP, Magous R. Biphasic kinetics of inositol 1,4,5-triphosphate accumulation in gastrin-stimulated parietal cells. Effects of pertussis toxin and extracellular calcium. FEBS Lett. 1991;282:147–151. doi: 10.1016/0014-5793(91)80465-f. [DOI] [PubMed] [Google Scholar]
- 182.del Valle JY, Tsunoda Y, Williams JA, Yamada T. Regulation of [Ca2+]i by secretagogue stimulation of canine gastric parietal cells. Am J Physiol Gastrointest Liver Physiol. 1992;262:G420–G426. doi: 10.1152/ajpgi.1992.262.3.G420. [DOI] [PubMed] [Google Scholar]
- 183.Tsunoda Y, Takeda H, Asaka M, Nakagaki I, Sasaki S. Initial and sustained calcium mobilizations in the parietal cell during stimulations with gastrin, inositol triphosphate, phorbol ester and exogenous diacylglycerol. FEBS Lett. 1988;232:83–89. doi: 10.1016/0014-5793(88)80391-0. [DOI] [PubMed] [Google Scholar]
- 184.Tsunoda Y, Takeda H, Otaki T, Asaka M, Nakagaki I, Sasaki S. Intracellular Ca2+ shift and signal transduction from the tubulovasicular portion of gastric parietal cells during gastrin stimulation or Ca2+ ionophore treatment: comparison between luminescent and fluorescent probes and electron probe X-ray microanalyzer. Biochem Cell Biol. 1988;66:279–287. doi: 10.1139/o88-037. [DOI] [PubMed] [Google Scholar]
- 185.Tsunoda Y, Yodozawa S, Tashiro Y. Heterogeneous distribution of free calcium and propagation of calcium transient in gastric parietal cells revealed by digital imaging microscopy. J Histochem Cytochem. 1989;37:999–1005. doi: 10.1177/37.7.2732461. [DOI] [PubMed] [Google Scholar]
- 186.Stepan VM, Dickinson CJ, del Valle JY, Matsushima M, Todisco A. Cell type-specific requirement of the MAPK pathway for the growth factor action of gastrin. Am J Physiol Gastrointest Liver Physiol. 1999;276:G1363–G1372. doi: 10.1152/ajpgi.1999.276.6.G1363. [DOI] [PubMed] [Google Scholar]
- 187.Stepan VM, Tatewaki M, Matsushima M, Dickinson CJ, del Valle JY, Todisco A. Gastrin induces c-fos gene transcription via multiple signaling pathways. Am J Physiol Gastrointest Liver Physiol. 1999;276:G415–G424. doi: 10.1152/ajpgi.1999.276.2.G415. [DOI] [PubMed] [Google Scholar]
- 188.Hocker M, Henihan RJ, Rosewicz S, Riecken EO, Zhang Z, Koh TJ, Wang TC. Gastrin and phorbol 12-myristate 13-acetate regulate the human histidine decarboxylase promoter through Raf-dependent activation of extracellular signal-regulated kinase-related signaling pathways in gastric cancer cells. J Biol Chem. 1997;272:27015–27024. doi: 10.1074/jbc.272.43.27015. [DOI] [PubMed] [Google Scholar]
- 189.Hocker M. Molecular mechanisms of gastrin-dependent gene regulation. Ann NY Acad Sci. 2004;1014:97–109. doi: 10.1196/annals.1294.010. [DOI] [PubMed] [Google Scholar]
- 190.Daulhac L, Kowalski-Chauvel A, Pradayrol L, Vaysee N, Seva C. Src-family tyrosine kinases in activation of ERK-1 and p85/p110-phosphatidylinositol 3-kinase by G/CCKB receptors. J Biol Chem. 1999;274:20657–20663. doi: 10.1074/jbc.274.29.20657. [DOI] [PubMed] [Google Scholar]
- 191.Yassin RR. Signaling pathways mediating gastrin’s growth-promoting effects. Peptides. 1999;20:885–898. doi: 10.1016/s0196-9781(99)00077-7. [DOI] [PubMed] [Google Scholar]
- 192.Seva C, Kowalski-Chauvel A, Blanchet JS, Vaysee N, Pradayrol L. Gastrin induces tyrosine phosphorylation of Shc proteins and their association with the Grb2/Sos complex. FEBS Lett. 1996;378:74–78. doi: 10.1016/0014-5793(95)01414-4. [DOI] [PubMed] [Google Scholar]
- 193.Nicke B, Tseng MJ, Fenrich M, Logsdon CD. Adenovirus-mediated gene transfer of RasN17 inhibits specific CCK actions on pancreatic acinar cells. Am J Physiol Gastrointest Liver Physiol. 1999;276:G499–G506. doi: 10.1152/ajpgi.1999.276.2.G499. [DOI] [PubMed] [Google Scholar]
- 194.Seufferlein T, Withers DJ, Broad S, Herget T, Walsh JH, Rozengurt E. The human CCKB/gastrin receptor transfected into rat1 fibroblasts mediates activation of MAP kinase, p74raf-1 kinase, and mitogenesis. Cell Growth Differ. 1995;6:383–393. [PubMed] [Google Scholar]
- 195.Bierkamp C, Kowalski-Chauvel A, Dehez S, Fourmy D, Pradayrol L, Seva C. Gastrin mediated cholecystokinin-2 receptor activation induces loss of cell adhesion and scattering in epithelial MDCK cells. Oncogene. 2002;21:7656–7670. doi: 10.1038/sj.onc.1205999. [DOI] [PubMed] [Google Scholar]
- 196.Ferrand A, Kowalski-Chauvel A, Bertrand C, Pradayrol L, Fourmy D, Dufresne M, Seva C. Involvement of JAK2 upstream of the PI 3-kinase in cell-cell adhesion regulation by gastrin. Exp Cell Res. 2004;301:128–138. doi: 10.1016/j.yexcr.2004.07.037. [DOI] [PubMed] [Google Scholar]
- 197.Kowalski-Chauvel A, Pradayrol L, Vaysee N, Seva C. Gastrin stimulates tyrosine phosphorylation of insulin receptor substrate 1 and its association with Grb2 and the phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26356–26361. doi: 10.1074/jbc.271.42.26356. [DOI] [PubMed] [Google Scholar]
- 198.Kowalski-Chauvel A, Pradayrol L, Vaysee N, Seva C. Tyrosine phosphorylation of insulin receptor substrate-1 and activation of the PI 3-kinase pathway by glycine-extended gastrin precursors. Biochem Biophys Res Commun. 1997;236:687–692. doi: 10.1006/bbrc.1997.6975. [DOI] [PubMed] [Google Scholar]
- 199.Harris JC, Clarke PA, Awan A, Jankowski J, Watson SA. An antiapoptotic role for gastrin and the gastrin/CCK-2 receptor in Barrett’s esophagus. Cancer Res. 2004;64:1915–1919. doi: 10.1158/0008-5472.can-03-2713. [DOI] [PubMed] [Google Scholar]
- 200.Ferrand A, Kowalski-Chauvel A, Bertrand C, Escrieut C, Mathieu A, Portolan G, Pradayrol L, Fourmy D, Dufresne M, Seva C. A novel mechanism for JAK2 activation by a G protein-coupled receptor, CCK2-R: implication of this signaling pathway in pancreatic tumor models. J Biol Chem. 2005;280:10710–10715. doi: 10.1074/jbc.M413309200. [DOI] [PubMed] [Google Scholar]
- 201.Colucci R, Blandizzi C, Tanini M, Vassalle C, Breschi MC, Del Tacca M. Gastrin promotes human colon cancer cell growth via CCK-2 receptor mediated cyclooxygenase-2 induction and prostaglandin E2 production. Br J Pharmacol. 2005;144:338–348. doi: 10.1038/sj.bjp.0706053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Guo YS, Cheng JZ, Jin GF, Gutkind JS, Hellmich MR, Townsend CM., Jr Gastrin stimulates cyclooxygenase-2 expression in intestinal epithelial cells through multiple signaling pathways. Evidence for involvement of ERK5 kinase and transactivation of the epidermal growth factor receptor. J Biol Chem. 2002;277:48755–48763. doi: 10.1074/jbc.M209016200. [DOI] [PubMed] [Google Scholar]
- 203.Slice LW, Hodikian R, Zhukova E. Gastrin and EGF synergistically induce cyclooxygenase-2 expression in Swiss 3T3 fibroblasts that express CCK2. J Cell Physiol. 2003;196:454–463. doi: 10.1002/jcp.10304. [DOI] [PubMed] [Google Scholar]
- 204.Hiraoka S, Miyazaki Y, Kitamura S, Toyota M, Kiyohara T, Shinomura Y, Mukaida N, Matsuzawa Y. Gastrin induces CXC chemokine expression in gastric epithelial cells through activation of NF-κB. Am J Physiol Gastrointest Liver Physiol. 2001;281:G735–G742. doi: 10.1152/ajpgi.2001.281.3.G735. [DOI] [PubMed] [Google Scholar]
- 205.Tracy HJ, Gregory RA. Physiological properties of a series of synthetic peptides structurally related to gastrin I. Nature (London) 1964;204:935–938. doi: 10.1038/204935a0. [DOI] [PubMed] [Google Scholar]
- 206.Morley JS, Tracy HJ, Gregory RA. Structure-function relationships in the active C-terminal tetrapeptide sequence of gastrin. Nature (London) 1965;207:1356–1359. doi: 10.1038/2071356a0. [DOI] [PubMed] [Google Scholar]
- 207.Peggion E, Jaeger E, Knof S, Moroder L, Wuensch E. Conformational aspects of gastrin-related peptides: a circular dichroism study. Biopolymers. 1981;20:633–652. doi: 10.1002/bip.1981.360200402. [DOI] [PubMed] [Google Scholar]
- 208.Peggion E, Foffani MT, Wunsch E, Moroder L, Borin G, Goodman M, Mammi S. Conformational properties of gastrin fragments of increasing chain length. Biopolymers. 1985;24:647–666. doi: 10.1002/bip.360250110. [DOI] [PubMed] [Google Scholar]
- 209.Mammi S, Goodman M, Peggion E, Foffani MT, Moroder L, Wuensch E. Conformational studies on gastrin related peptides by high resolution 1H-NMR. Int J Peptide Protein Res. 1986;27:145–152. doi: 10.1111/j.1399-3011.1986.tb01804.x. [DOI] [PubMed] [Google Scholar]
- 210.Torda HJ, Baldwin GS, Norton RS. High-resolution proton nuclear magnetic resonance studies of human gastrin. Biochemistry. 1985;24:1720–1727. doi: 10.1021/bi00328a023. [DOI] [PubMed] [Google Scholar]
- 211.Stone SR, Mierke DF, Jackson GE. Evidence for a C-terminal structural motif in gastrin and its bioactive fragments in membrane mimetic media. Peptides. 2007;28:1561–1571. doi: 10.1016/j.peptides.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 212.Stone SR, Giragossian C, Mierke DF, Jackson GE. Further evidence for a C-terminal structural motif in CCK2 receptor active peptide hormones. Peptides. 2007;28:2211–2222. doi: 10.1016/j.peptides.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 213.Wu CS, Hachimori A, Yang JT. Lipid-induced ordered conformation of some peptide hormones and bioactive oligopeptides: predominance of helix over beta form. Biochemistry. 1982;21:4556–4562. doi: 10.1021/bi00262a007. [DOI] [PubMed] [Google Scholar]
- 214.Mammi S, Mammi NJ, Foffani MT, Peggion E, Moroder L, Wunsch E. Conformation of human little gastrin and minigastrin analogs in surfactant solution. Biopolymers. 1987;26:S1–S10. doi: 10.1002/bip.360260005. [DOI] [PubMed] [Google Scholar]
- 215.Abillon E, Chuong PPV, Fromageot P. Conformational calculations on gastrin C-terminal tetrapeptide. Int J Peptide Protein Res. 1981;17:480–485. doi: 10.1111/j.1399-3011.1981.tb02017.x. [DOI] [PubMed] [Google Scholar]
- 216.Chou PY, Fasman GD. Beta-turns in proteins. J Mol Biol. 1977;115:135–175. doi: 10.1016/0022-2836(77)90094-8. [DOI] [PubMed] [Google Scholar]
- 217.Mammi S, Mammi NJ, Peggion E. Conformational studies of human des-Trp1, Nle12-minigastrin in water-trifluoroethanol mixtures by 1H NMR and circular dichroism. Biochemistry. 1988;27:1374–1379. doi: 10.1021/bi00404a043. [DOI] [PubMed] [Google Scholar]
- 218.Gohring W, Moroder L, Borin GF, Lobbia A, Bali JP, Wunsch E. [Synthesis of peptides with gastrinlike activity. Studies on the structure-activity relationship of the natural hormone human little gastrin I.] Hoppe Seylers Z Physiol Chem. 1984;365:83–94. doi: 10.1515/bchm2.1984.365.1.83. [DOI] [PubMed] [Google Scholar]
- 219.Mammi S, Foffani MT, Peggion E, Galleyrand JC, Bali JP, Simonetti M, Gohring W, Moroder L, Wunsch E. Conformational and biological properties of the Ala10 analogue of human des-Trp1, Nle12-minigastrin. Biochemistry. 1989;28:7182–7188. doi: 10.1021/bi00444a008. [DOI] [PubMed] [Google Scholar]
- 220.Previero A, Mourier G, Bali JP, Lignon MT, Moroder L. N alphaglycosylgastrin-related peptides. Synthesis, characterization and biological activity. Hoppe Seylers Z Physiol Chem. 1982;363:813–818. doi: 10.1515/bchm2.1982.363.2.813. [DOI] [PubMed] [Google Scholar]
- 221.Wunsch E, Scharf R, Peggion E, Foffani MT, Bali JP. Conformational properties of gastrin peptides: synthesis, biological activity, and preliminary CD characterization of the (Asp)5 analog of des-Trp1, Leu12-minigastrin. Biopolymers. 1986;25:229–234. doi: 10.1002/bip.360250204. [DOI] [PubMed] [Google Scholar]
- 222.Morley JS. Structure-function relationships in gastrin-like peptides. Proc R Soc Lond B Biol Sci. 1968;170:97–111. doi: 10.1098/rspb.1968.0028. [DOI] [PubMed] [Google Scholar]
- 223.Weng JH, Blommaert AGS, Moizo L, Bado A, Ducos B, Bohme A, Garbay C, Roques BP. Role of N- and C-terminal substituents on the CCK-B agonist-antagonist pharmacological profile of Boc-Trp-Phg-Asp-Nal-NH2 derivatives. Bioorg Med Chem. 1996;4:563–573. doi: 10.1016/0968-0896(96)00050-8. [DOI] [PubMed] [Google Scholar]
- 224.Black JW, Kalindjian SB. Gastrin agonists and antagonists. Pharmacol Toxicol. 2002;91:275–281. doi: 10.1034/j.1600-0773.2002.910602.x. [DOI] [PubMed] [Google Scholar]
- 225.Low CMR, Black JW, Broughton HB, Buck IM, Davies JMR, Dunstone DJ, Hull RAD, Kalindjian SB, McDonald IM, Pether MJ, Shankley NP, Steel KIM. Development of peptide 3D structure mimetics: rational design of novel peptoid cholecystokinin receptor antagonists. J Med Chem. 2000;43:3505–3517. doi: 10.1021/jm000937a. [DOI] [PubMed] [Google Scholar]
- 226.Magous R, Bali J, Moroder L, Previero A. Effect of Nin-formylation of the tryptophan residue on gastrin (HG-13) binding and on gastric acid secretion. Eur J Pharmacol. 1982;77:11–16. doi: 10.1016/0014-2999(82)90528-3. [DOI] [PubMed] [Google Scholar]
- 227.Yabe Y, Morita A, Miura C, Kobayashi S, Baba Y. Synthesis and biological activity of tetragastrin analogues modifying the tryptophan residue. Chem Pharm Bull. 1977;10:2731–2734. doi: 10.1248/cpb.25.2731. [DOI] [PubMed] [Google Scholar]
- 228.Pannequin J, Barnham KJ, Hollande F, Shulkes A, Norton RS, Baldwin GS. Ferric ions are essential for the biological activity of the hormone glycine-extended gastrin. J Biol Chem. 2002;277:48602–48609. doi: 10.1074/jbc.M208440200. [DOI] [PubMed] [Google Scholar]
- 229.Peggion E, Foffani MT, Wunsch E, Moroder L, Goodman M, Mammi S. Interaction of calcium ions with gastrin fragments of increasing chain length. Biopolymers. 1986;25:135–152. doi: 10.1002/bip.360250110. [DOI] [PubMed] [Google Scholar]
- 230.Beauchamp RD, Townsend SM, Singh P, Glass EJ, Thompson JC. Proglumide, a gastrin receptor antagonist inhibits growth of colon cancer and enhances survival in mice. Ann Surg. 1985;202:303–309. doi: 10.1097/00000658-198509000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.McGregor DB, Jones RD, Karlin DA, Romsdahl MM. Trophic effects of gastrin on colorectal neoplasms in rat. Ann Surg. 1982;195:219–223. doi: 10.1097/00000658-198202000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sirinek KR, Levine BA, Moyer MP. Pentagastrin stimulates in vitro growth of normal and malignant human colon epithelial cells. Am J Surg. 1985;149:35–38. doi: 10.1016/s0002-9610(85)80006-4. [DOI] [PubMed] [Google Scholar]
- 233.Winsett OE, Townsend CM, Glass EJ, Thompson JC. Gastrin stimulates growth of colon cancer. Surgery (St.Louis) 1986;99:302–307. [PubMed] [Google Scholar]
- 234.Elwyn KE, Jones RD, Romsdahl MM. Inhibitory effects of secretin on gastrin-stimulated rat colon neoplasms. Cancer (Philadelphia) 1985;55:1186–1189. doi: 10.1002/1097-0142(19850315)55:6<1186::aid-cncr2820550608>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 235.Hoosein NM, Kiener PA, Curry RL, Brattain MG. Evidence for autocrine growth stimulation of cultured colon tumor cells by a gastrin/cholecystokinin-like peptide. Exp Cell Res. 1990;186:15–21. doi: 10.1016/0014-4827(90)90204-n. [DOI] [PubMed] [Google Scholar]
- 236.Imdahl A, Eggstein S, Crone C, Farthmann EH. Growth of colorectal carcinoma cells: regulation in vitro by gastrin, pentagastrin and the gastrin-receptor antagonist proglumide. J Cancer Res Clin Oncol. 1989;115:388–392. doi: 10.1007/BF00400968. [DOI] [PubMed] [Google Scholar]
- 237.Karlin DA, McBath M, Jones RD, Elwyn KE, Romsdahl MM. Hypergastrinemia and colorectal carcinogenesis in the rat. Cancer Lett. 1985;29:73–78. doi: 10.1016/0304-3835(85)90125-9. [DOI] [PubMed] [Google Scholar]
- 238.Kusyk CJ, McNeil NO, Johnson LR. Stimulation of growth of a colon cancer cell line by gastrin. Am J Physiol Gastrointest Liver Physiol. 1986;251:G597–G601. doi: 10.1152/ajpgi.1986.251.5.G597. [DOI] [PubMed] [Google Scholar]
- 239.Lamote J, Willems G. Stimulating effect of pentagastrin on cancer cell proliferation kinetics in chemically induced colon cancer in rats. Regul Pept. 1988;20:1–9. doi: 10.1016/0167-0115(88)90052-3. [DOI] [PubMed] [Google Scholar]
- 240.Singh P, Walker JP, Townsend CM, Thompson JC. Role of gastrin and gastrin receptors on the growth of a transplantable mouse colon carcinoma (MC-26) in BALB/c mice. Cancer Res. 1986;46:1612–1616. [PubMed] [Google Scholar]
- 241.Sumiyoshi H, Yasui W, Ochiai A, Tahara E. Effects of gastrin on tumor growth and cyclic nucleotide metabolism in xenotransplantable human gastric and colonic carcinomas in nude mice. Cancer Res. 1984;44:4276–4280. [PubMed] [Google Scholar]
- 242.Baldwin GS, Shulkes A. Gastrin, gastrin receptors and colorectal cancer. Gut. 1998;42:581–584. doi: 10.1136/gut.42.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.D’Agostino L, Pignata S, Tritto G, D’Adamo G, Contegiacomo A, Daniele B, Calderopoli R, Pizzi C, Squame G, Mazzacca G. Hypergastrinemia in rats with azoxymethane-induced colon cancers. Int J Cancer. 1995;61:223–226. doi: 10.1002/ijc.2910610214. [DOI] [PubMed] [Google Scholar]
- 244.Sobhani I, Lehy T, Laurent-Puig P, Cadiot G, Ruszniewski P, Mignon M. Chronic endogenous hypergastrinemia in humans: evidence for a mitogenic effect on the colonic mucosa. Gastroenterology. 1993;105:22–30. doi: 10.1016/0016-5085(93)90006-x. [DOI] [PubMed] [Google Scholar]
- 245.Thorburn CM, Friedman GD, Dickinson CJ, Vogelman JH, Orentreich N, Parsonnet J. Gastrin and colorectal cancer: a prospective study. Gastroenterology. 1998;115:275–280. doi: 10.1016/s0016-5085(98)70193-3. [DOI] [PubMed] [Google Scholar]
- 246.Oscarson J, Veen H, Ross J, Malt R. Dimethylhydrazine induced colonic neoplasia: dissociation from endogenous gastrin levels. Surgery. 1982;91:525–530. [PubMed] [Google Scholar]
- 247.Chen D, Destree M, Hakanson R, Willems G. Endogenous hypergastrinemia does not promote growth of colonic mucosa or of transplanted colon adenocarcinoma in rats. Eur J Gastroenterol Hepatol. 1998;10:293–299. doi: 10.1097/00042737-199804000-00004. [DOI] [PubMed] [Google Scholar]
- 248.Singh P, Velasco M, Given R, Wargovich M, Varro A, Wang TC. Mice overexpressing progastrin are predisposed for developing aberrant colonic crypt foci in response to AOM. Am J Physiol Gastroenterol Liver Physiol. 2000;278:G390–G399. doi: 10.1152/ajpgi.2000.278.3.G390. [DOI] [PubMed] [Google Scholar]
- 249.Hakanson R, Blom H, Carlsson E, Larsson H, Ryberg B, Sundler F. Hypergastrinemia produces trophic effects in stomach but not in pancreas and intestine. Regul Pept. 1986;13:225–233. doi: 10.1016/0167-0115(86)90041-8. [DOI] [PubMed] [Google Scholar]
- 250.Hakanson R, Axelson J, Ekman R, Sundler F. Hypergastrinemia evoked by omprazole stimulates growth of gastric mucosa but not of pancreas or intestines in hamster, guinea pig and chicken. Regul Pept. 1988;23:105–115. doi: 10.1016/0167-0115(88)90426-0. [DOI] [PubMed] [Google Scholar]
- 251.Pinson DM, Havu N, Sztern MI, Mattsson H, Looney GA, Kimler BF, Hurwitz A. Drug-induced hypergastrinemia: absence of trophic effects on colonic carcinoma in rats. Gastroenterology. 1995;108:1068–1074. doi: 10.1016/0016-5085(95)90204-x. [DOI] [PubMed] [Google Scholar]
- 252.Fontana MG, Donato F, Villanacci V, Ghirardi M, Moneghini D, Di Betta E, Salerni B. Inhibitory effect of a gastrin receptor antagonist, CR 2495, on 1,2-dimethylhydrazine-induced colorectal cancer in mice. Eur Surg Res. 1999;31:406–411. doi: 10.1159/000008719. [DOI] [PubMed] [Google Scholar]
- 253.Fontana MG, Moneghini D, Villanacci V, Donato F, Rindi G. Effect of cholecystokinin-B gastrin receptor blockade on chemically induced colon carcinogenesis in mice: follow up at 52 weeks. Digestion. 2002;65:35–40. doi: 10.1159/000051929. [DOI] [PubMed] [Google Scholar]
- 254.Rehfeld JF, Hilstead L. Gastrin and cancer. Adv Clin Chem. 1992;29:239–262. doi: 10.1016/s0065-2423(08)60226-7. [DOI] [PubMed] [Google Scholar]
- 255.Aly A, Shulkes A, Baldwin GS. Gastrins, cholecystokinins and gastrointestinal cancer. Biochimica et Biophysica Acta. 2004;1704:1–10. doi: 10.1016/j.bbcan.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 256.Reubi JC, Waser B, Schmassmann A, Laissue JA. Receptor autoradiographic evaluation of cholecystokinin, neurotensin, somatostatin and vasoactive intestinal peptide receptors in gastro-intestinal adenocarcinoma samples: where are they really located? Int J Cancer. 1999;81:376–386. doi: 10.1002/(sici)1097-0215(19990505)81:3<376::aid-ijc11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 257.Schmitz F, Otte JM, Stechele HU, Reimann B, Banasiewicz T, Folsch UR, Schmidt WE, Herzig KH. CCK-2/gastrin receptors in human colorectal cancer. Eur J Clin Investig. 2001;31:812–820. doi: 10.1046/j.1365-2362.2001.00870.x. [DOI] [PubMed] [Google Scholar]
- 258.Baldwin GS, Casey A, Mantamadiotis T, McBride K, Sizeland AM, Thumwood CM. PCR cloning and sequence of gastrin mRNA from carcinoma cell lines. Biochem Biophys Res Commun. 1990;170:691–697. doi: 10.1016/0006-291x(90)92146-q. [DOI] [PubMed] [Google Scholar]
- 259.Baldwin GS, Zhang Q-X. Measurement of gastrin and transforming growth factor alpha messenger RNA levels in colonic carcinoma cell lines by quantitative polymerase chain reaction. Cancer Res. 1992;52:2261–2267. [PubMed] [Google Scholar]
- 260.Kochman ML, Del Valle J, Dickinson CJ, Borland CR. Post-translational processing of gastrin in neoplastic human colonic tissues. Biochem Biophys Res Commun. 1992;189:1165–1169. doi: 10.1016/0006-291x(92)92326-s. [DOI] [PubMed] [Google Scholar]
- 261.Nemeth J, Taylor B, Pauwels S, Varro A, Dockray GJ. Identification of progastrin derived peptides in colorectal carcinoma extracts. Gut. 1993;34:90–95. doi: 10.1136/gut.34.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ciccotosto G, McLeish A, Hardy K, Shulkes A. Expression processing and secretion of gastrin in patients with colorectal carcinoma. Gastroenterology. 1995;109:1142–1153. doi: 10.1016/0016-5085(95)90572-3. [DOI] [PubMed] [Google Scholar]
- 263.Konturek PC, Bielanski W, Konturek SJ, Hartwich A, Pierzchalski P, Gonciarz M, Marlicz K, Starzynska T, Zuchowicz M, Darasz Z, Götze JP, Rehfeld JF, Hahn EG. Progastrin and cyclooxygenase-2 in colorectal cancer. Dig Dis Sci. 2002;47:1984–1991. doi: 10.1023/a:1019652224424. [DOI] [PubMed] [Google Scholar]
- 264.Siddheshwar R, Gray J, Kelly S. Plasma levels of progastrin but not amidated gastrin or glycine extended gastrin are elevated in patients with colorectal carcinoma. Gut. 2001;48:47–52. doi: 10.1136/gut.48.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Smith AM, Watson SA. Review article: gastrin and colorectal cancer. Ailment Pharmacol Ther. 2000;14:1231–1247. doi: 10.1046/j.1365-2036.2000.00842.x. [DOI] [PubMed] [Google Scholar]
- 266.Koh TJ, Dockray GJ, Varro A, Cahill RJ, Dangler CA, Fox JG, Wang TC. Overexpression of glycine-extended gastrin in transgenic mice results in increased colonic proliferation. J Clin Invest. 1999;103:1119–1126. doi: 10.1172/JCI4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ottewell PD, Varro A, Dockray GJ, Kirton CM, Watson AJ, Wang TC, Dimaline R, Pritchard DM. COOH-terminal 26-amino acid residues of progastrin are sufficient for stimulation of mitosis in murine colonic epithelium in vivo. Am J Physiol Gastrointest Liver Physiol. 2005;288:G541–G549. doi: 10.1152/ajpgi.00268.2004. [DOI] [PubMed] [Google Scholar]
- 268.Aly A, Shulkes A, Baldwin GS. Short term infusion of glycine-extended gastrin(17) stimulates both proliferation and formation of aberrant crypt foci in rat colonic mucosa. Int J Cancer. 2001;94:307–313. doi: 10.1002/ijc.1483. [DOI] [PubMed] [Google Scholar]
- 269.Cobb S, Wood T, Ceci J, Varro A, Velasco M, Singh P. Intestinal expression of mutant and wild-type progastrin significantly increases colon carcinogenesis in response to azooxymethane in transgenic mice. Cancer. 2004;100:1311–1323. doi: 10.1002/cncr.20094. [DOI] [PubMed] [Google Scholar]
- 270.Singh P, Velasco M, Given R, Varro A, Wang TC. Progastrin expression predisposes mice to colon carcinomas and adenomas in response to a chemical carcinogen. Gastroenterology. 2000;119:162–171. doi: 10.1053/gast.2000.8527. [DOI] [PubMed] [Google Scholar]
- 271.Baldwin GS. The role of gastrin and cholecystokinin in normal and neoplastic gastrointestinal growth. J Gastroenterol Hepatol. 1995;10:215–232. doi: 10.1111/j.1440-1746.1995.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 272.Hollande F, Imdahl A, Mantamadiotis T, Ciccotosto GD, Shulkes A, Baldwin GS. Glycine-extended gastrin acts as an autocrine growth factor in a nontransformed colon cell line. Gastroenterology. 1997;113:1576–1588. doi: 10.1053/gast.1997.v113.pm9352860. [DOI] [PubMed] [Google Scholar]
- 273.Litvak DA, Hellmich MR, Iwase K, Evers BM, Martinez J, Amblard M, Townsend CM., Jr JMV1155: a novel inhibitor of glycine-extended progastrin-mediated growth of a human colon cancer in vivo. Anticancer Res. 1999;19:45–49. [PubMed] [Google Scholar]
- 274.Singh P, Owlia A, Espeijo R, Dai B. Novel gastrin receptors mediate mitogenic effects of gastrin and processing intermediates of gastrin on Swiss 3T3 fibroblasts. Absence of detectable cholecystokinin CCK-A and CCK-B receptors. J Biol Chem. 1995;270:8429–8438. doi: 10.1074/jbc.270.15.8429. [DOI] [PubMed] [Google Scholar]
- 275.Stepan VM, Sawada M, Todisco A, Dickinson CJ. Glycine-extended gastrin exerts growth-promoting effects on human colon cancer cells. Mol Med. 1999;5:147–159. [PMC free article] [PubMed] [Google Scholar]
- 276.Lindstrom E, Bjorkqvist M, Boketoft A, Chen D, Zhao CM, Kimura K, Hakanson R. Neurohormonal regulation of histamine and pancreastatin secretion from isolated rat stomach ECL cells. Regul Pept. 1997;71:73–86. doi: 10.1016/s0167-0115(97)01018-5. [DOI] [PubMed] [Google Scholar]
- 277.Cui G, Koh TJ, Chen D, Zhao CM, Takaishi S, Dockray GJ, Varro A, Rogers AB, Fox JG, Wang TC. Overexpression of glycine-extended gastrin inhibits parietal cell loss and atrophy in the mouse stomach. Cancer Res. 2004;64:8160–8166. doi: 10.1158/0008-5472.CAN-04-0876. [DOI] [PubMed] [Google Scholar]
- 278.Chen D, Zhao CM, Dockray GJ, Varro A, Van Hoek A, Sinclair NF, Wang TC, Koh TJ. Glycine-extended gastrin synergizes with gastrin 17 to stimulate acid secretion in gastrin-deficient mice. Gastroenterology. 2000;119:756–765. doi: 10.1053/gast.2000.16480. [DOI] [PubMed] [Google Scholar]
- 279.Higashide S, Gomez G, Greeley GH, Jr, Townsend CM, Jr, Thompson JC. Glycine-extended gastrin potentiates gastrin-stimulated gastric acid secretion in rats. Am J Physiol Gastrointest Liver Physiol. 1996;270:G220–G224. doi: 10.1152/ajpgi.1996.270.1.G220. [DOI] [PubMed] [Google Scholar]
- 280.Mauss S, Niederau C, Hengels KJ. Effects of gastrin, proglumide, loxiglumide and L-365,260 on growth of human colon carcinoma cells. Anticancer Res. 1994;14:215–220. [PubMed] [Google Scholar]
- 281.Upp JR, Singh P, Townsend CM, Jr, Thompson JC. Clinical significance of gastrin receptors in human colon cancers. Cancer Res. 1989;49:488–492. [PubMed] [Google Scholar]
- 282.Yang C-H, Ford J, Karelina Y, Shulkes A, Xiao S-D, Baldwin GS. Identification of a 70-kDa gastrin-binding protein on DLD-1 human colorectal carcinoma cells. Int J Biochem Cell Biol. 2001;33:1071–1079. doi: 10.1016/s1357-2725(01)00077-2. [DOI] [PubMed] [Google Scholar]
- 283.Imdahl A, Mantamadiotis T, Eggstein S, Farthmann EH, Baldwin GS. Expression of gastrin, gastrin/CCK-B and gastrin/CCK-C receptors in human colorectal carcinomas. J Cancer Res Clin Oncol. 1995;121:661–666. doi: 10.1007/BF01218524. [DOI] [PubMed] [Google Scholar]
- 284.Ahmed S, Budai B, Heredi-Szabo K, Farkas J, Toth G, Murphy RF, Lovas S. High and low affinity receptors mediate growth effects of gastrin and gastrin-Gly on DLD-1 human colonic carcinoma cells. FEBS Lett. 2004;556:199–203. doi: 10.1016/s0014-5793(03)01408-x. [DOI] [PubMed] [Google Scholar]
- 285.Ahmed S, Murphy RF, Lovas S. Importance of N- and C-terminal regions of gastrin-Gly for preferential binding to high and low affinity gastrin-Gly receptors. Peptides. 2005;26:1207–1212. doi: 10.1016/j.peptides.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 286.Copps J, Murphy RF, Lovas S. Bioactivity of analogs of the N-terminal region of gastrin-17. 2009 doi: 10.1016/j.peptides.2009.09.012. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Copps J, Ahmed S, Murphy RF, Lovas S. Gastrin 1–6 promotes growth of colon cancer cells through non-CCK receptors. Peptides. 2007;28:632–635. doi: 10.1016/j.peptides.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 288.Copps J, Murphy RF, Lovas S. The structure of bioactive gastrin-17 analogs of the N-terminal region. 2009 doi: 10.1016/j.peptides.2009.09.016. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ogunwobi OO, Beales ILP. Glycine-extended gastrin stimulates proliferation and inhibits apoptosis in colon cancer cells via cyclo-oxygenasse-independent pathways. Reg Peptides. 2005;134:1–8. doi: 10.1016/j.regpep.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 290.Hollande F, Choquet A, Blanc EM, Lee DJ, Bali JP, Baldwin GS. Involvement of phosphatidylinositol 3-kinase and mitogen-activated protein kinases in glycine-extended gastrin-induced dissociation and migration of gastric epithelial cells. J Biol Chem. 2001;276:40402–40410. doi: 10.1074/jbc.M105090200. [DOI] [PubMed] [Google Scholar]
- 291.Beales ILP, Ogunwobi O. Glycine-extended gastrin inhibits apoptosis in colon cancer cells via separate activation of Akt and JNK pathways. Mol Cell Endocrinology. 2006;247:140–149. doi: 10.1016/j.mce.2005.12.050. [DOI] [PubMed] [Google Scholar]