Abstract
Cocaine is a highly abused drug without effective pharmacotherapies to treat it. It interacts with sigma (σ) receptors, providing logical targets for the development of medications to counteract its actions. Cocaine causes toxic and stimulant effects that can be categorized as acute effects such as convulsions and locomotor hyperactivity and subchronic effects including sensitization and place conditioning. In the present study, 3-(4-(4-cyclohexylpiperazin-1-yl)butyl)benzo[d]thiazole-2(3H)-thione (CM156), a novel compound, was developed and tested for interactions with σ receptors using radioligand binding studies. It was also evaluated against cocaine-induced effects in behavioral studies. The results showed that CM156 has nanomolar affinities for each of the σ receptor subtypes in the brain and much weaker affinities for non-σ binding sites. Pretreatment of male Swiss-Webster mice with CM156, before administering either a convulsive or locomotor stimulant dose of cocaine, led to a significant attenuation of these acute effects. CM156 also significantly reduced the expression of behavioral sensitization and place conditioning evoked by subchronic exposure to cocaine. The protective effects of CM156 are consistent with σ receptor-mediated actions. Together with previously reported findings, the data from CM156 and related σ compounds indicate that σ receptors can be targeted to alleviate deleterious actions of cocaine.
Cocaine abuse and addiction remain serious problems in the United States, with no effective pharmacotherapies for their treatment (Carrera et al., 2004). Cocaine has powerful psychostimulant effects that involve the modification of activity in neural circuits, including dopaminergic, glutamatergic, and cholinergic systems in the brain reward system (Wise, 2002). The acute effects of cocaine are manifested soon after exposure and include locomotor hyperactivity, stereotyped behaviors, and toxicities such as convulsions and lethality upon overdose (Balster, 1988). Chronic exposure to cocaine may result in changes in reinforcing, discriminative stimulus, sensitizing, and rewarding effects (Balster, 1988; Maurice et al., 2002), which are accompanied by neuroadaptations.
Within the established pharmacological profile of cocaine, σ receptors have gained attention as potential targets for drug discovery of anticocaine agents (Maurice et al., 2002; Matsumoto et al., 2003). These receptors are recognized as unique proteins with two subtypes, σ1 and σ2 (Hayashi and Su, 2005). As distinctive proteins expressed on the endoplasmic reticulum, σ1 receptors possess chaperone-like properties (Hayashi and Su, 2007), act as intracellular amplifiers for signal transduction (Su and Hayashi, 2003), and can exert modulatory effects on many cellular activities and functions (Hayashi and Su, 2005), including modulation of catecholaminergic, glutamatergic, and cholinergic systems in the brain (Werling et al., 2007). These effects appear to involve translocation of σ1 receptors between different cellular compartments (Morin-Surun et al., 1999; Hayashi and Su, 2003) and protein–protein interactions (Hayashi and Su, 2001, 2007). In contrast, less is known about σ2 receptors. They are slightly smaller than σ1 receptors and have not been cloned (Matsumoto, 2007a). Although truly selective σ2 compounds are not yet available, they are under development (Mésangeau et al., 2008).
Earlier evidence of cocaine interacting with σ receptors stems from receptor binding assays (Sharkey et al., 1988; Matsumoto et al., 2002; Maurice et al., 2002) and studies involving antisense oligonucleotides (Romieu et al., 2000; Matsumoto et al., 2001, 2002) and antagonists for σ receptors (McCracken et al., 1999a,b; Matsumoto et al., 2001, 2002, 2007b; Maurice et al., 2002; Mésangeau et al., 2008). Although antagonists of σ receptors have been synthesized and evaluated using both in vitro and in vivo methods, a major drawback is that many of them are not purely σ-selective (Matsumoto, 2007; Newman and Coop, 2007). Besides σ receptors, many compounds also bind to dopamine transporters, opioid receptors, or N-methyl-d-aspartate (NMDA) receptors (Matsumoto, 2007a). Studies using nonselective compounds can confound the interpretation of the role of σ receptors and lead to confusing phenomena in behavioral tests. For example, σ-preferring compounds, including NPC 16377, BD1008, BD1047, BD1063, and AC927 block the locomotor stimulant effects of cocaine (Witkin et al., 1993; McCracken et al., 1999a,b; Matsumoto et al., 2001, 2008; Liu et al., 2005). However, nonselective compounds such as haloperidol and 3-(3-hydroxyphenyl)-N-(1-propyl)piperidine may not significantly affect cocaine-induced locomotor effects, although they have high affinity for σ receptors (Witkin et al., 1993; Maurice et al., 2002). Highly selective σ compounds therefore represent critical experimental tools to determine the role of σ receptors in cocaine-induced effects and may also facilitate the development of anticocaine agents. Currently available compounds with high σ selectivity include BD1063 (Matsumoto et al., 1995), NE-100 (Chaki et al., 1994), and AC927 (Matsumoto et al., 2008), but additional σ-selective ligands are still needed.
In an effort to develop selective σ receptor antagonists, CM156 and related substituted piperazines were designed and synthesized (Mésangeau et al., 2008). In the present study, CM156 was first evaluated in radioligand binding assays to determine its selectivity for σ receptors. CM156 was then tested in cocaine-induced behavioral models including acute effects of convulsions and locomotor activity, and subchronic effects of sensitization and place conditioning.
Materials and Methods
Drugs and Chemicals.
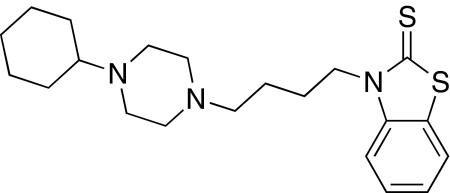
CM156 was synthesized as described previously (Mésangeau et al., 2008). The structure of this ligand is shown in Fig. 1. Cocaine hydrochloride was obtained from Sigma-Aldrich (St. Louis, MO). The radioligands were obtained from PerkinElmer Life and Analytical Sciences (Boston, MA). All other compounds and reagents used for the radioligand binding assays were purchased from Sigma-Aldrich and Aldrich Chemical Co. (Milwaukee, WI).
Fig. 1.
Chemical structure of CM156.
Subjects.
Male Sprague-Dawley rats (175–225 g; Harlan, Indianapolis, IN) were used for the radioligand binding assays. Male Swiss-Webster or C57/BL6 mice (21–30 g; Harlan) were used for the behavioral experiments. The mice were housed in groups of five, with a 12:12-h light/dark cycle and ad libitum food and water. The animals were randomly assigned to their treatment groups. Mice from at least two different shipments were tested on different days to form the final dataset for each experimental group. All procedures involving animals were performed as approved by the Institutional Animal Care and Use Committee at the location where the studies were conducted.
Competition Binding Assays.
The radioligand binding assays were conducted in rat brain homogenates using methods published previously in detail (Matsumoto et al., 1995, 2008). The affinity of CM156 for σ receptors was determined as described previously (Mésangeau et al., 2008). In brief, σ1 receptors were labeled using 5 nM [3H](+)-pentazocine; σ2 receptors were labeled with 3 nM [3H]di-o-tolylguanidine in the presence of 300 nM (+)-pentazocine to mask σ1 receptors. Nonspecific binding was determined in the presence of 10 μM haloperidol. Ten concentrations of CM156 (0.1–1000 nM) were included in the assays and incubated for 120 min at 25°C to measure their ability to displace the radioligands from their binding sites.
Because many historic ligands for σ receptors also bind to non-σ sites (Newman and Coop, 2007), the relative selectivity of CM156 was determined. The affinities of CM156 for opioid, NMDA, dopamine (D2), and 5-HT2 receptors were measured in homogenates from rat brain minus cerebellum using previously published methods (Matsumoto et al., 1995, 2008). In brief, opioid receptors were labeled with 2 nM [3H]bremazocine; nonspecific binding was determined with 10 μM levallorphan. NMDA receptors were labeled with 5 nM [3H]1-[1-(2-thienyl)cyclohexyl]piperidine (TCP); nonspecific binding was determined with 10 μM cyclazocine. Dopamine D2 receptors were labeled with 5 nM [3H](−)-sulpiride; nonspecific binding was determined with 1 μM haloperidol. 5-HT2 receptors were labeled with 2 nM [3H]ketanserin; nonspecific binding was determined with 1 μM mianserin. The incubations were conducted for 60 min at 25°C for the dopamine and opioid receptor assays, for 30 min at 37°C for the 5-HT2 receptor assays and for 60 min at 4°C for the NMDA receptor assays.
Because cocaine interacts with monoamine transporters, the affinities of CM156 at these sites were also determined using previously published methods (Matsumoto et al., 2008). In brief, dopamine transporters were labeled in rat striata using 0.5 nM [3H]WIN35,428; nonspecific binding was determined with 50 μM cocaine. Serotonin transporters were labeled in rat brainstem using 0.2 nM [3H]paroxetine; nonspecific binding was determined with 1.5 μM imipramine. Norepinephrine transporters were labeled in rat cerebral cortex using 0.5 nM [3H]nisoxetine; nonspecific binding was determined with 4 μM desipramine.
All of the assays were terminated with the addition of ice-cold buffer and vacuum filtration through glass fiber filters. Counts were extracted from the filters using Ecoscint cocktail (National Diagnostics, Manville, NJ) for at least 8 h before counting.
To further evaluate the selectivity of CM156 for σ receptors, the compound was characterized by NovaScreen/Caliper Life Sciences (Hanover, MD) against additional binding sites. Details of each assay condition can be accessed through Caliper's web site at www.caliperls.com.
Cocaine-Induced Convulsions.
The dose-response curve for cocaine-induced convulsions was determined by injecting mice (n = 25) with various doses of cocaine (0–70 mg/kg i.p.). To probe for anticonvulsant actions against cocaine, mice (n = 40) were injected with CM156 (0–10 mg/kg i.p.) 15 min before administration of a dose of cocaine that produced convulsions in 100% of the mice (70 mg/kg i.p.). After an injection with cocaine, the animals were placed in individual plastic containers (59 × 43 × 13 cm) and observed for the next 30 min for the occurrence of convulsions. Convulsions were operationally defined as clonic or tonic limb movements, which were accompanied by the loss of righting reflexes for at least 5 s, and/or popcorn jumping. The number and proportion of animals exhibiting convulsions during the 30-min testing period were recorded.
Locomotor Activity.
To measure the locomotor stimulant effects of cocaine, mice were first acclimated for 30 min to the room where the experiment was conducted and then to the Plexiglas enclosures of an automated activity monitoring system for 30 min (San Diego Instruments, San Diego, CA). The mice (n = 30) were then administered cocaine (0–20 mg/kg i.p.). Locomotor activity (ambulatory movements, fine movements, and rearing) was quantified for the subsequent 30 min as disruptions in the 16 × 16 photobeam array that circumscribed each Plexiglas enclosure. The dose of cocaine that produced the peak level of locomotor activity (20 mg/kg i.p.) was selected for use in the subsequent antagonism portion of the study.
To determine whether CM156 itself affects locomotor activity, acclimated mice (n = 42) were injected with a dose of CM156 (0–20 mg/kg i.p.), and locomotor activity was measured for the next 30 min. This part of the study was conducted to confirm that CM156 produced effects no different from saline when administered alone, as would be expected of a σ receptor antagonist (Matsumoto et al., 2003).
For the antagonism experiments, mice (n = 54) were acclimated to the activity monitors for 15 min. The animals were then injected with a dose of CM156 (0–20 mg/kg i.p.) and returned to the activity monitors. After a 15-min pretreatment period, a locomotor stimulant dose of cocaine (20 mg/kg i.p.) was administered, and locomotor activity was quantified for the subsequent 30 min.
Behavioral Sensitization.
For the development of sensitization studies, mice (n = 24) were injected intraperitoneally once a day for 5 consecutive days with one of the following treatments: saline + saline, saline + cocaine, CM156 + cocaine, or CM156 + saline. The two injections making up each treatment were separated by a 15-min pretreatment period; the dose of cocaine used was 10 mg/kg and the dose of CM156 was 20 mg/kg. Treatments on days 1 to 5 were followed by a 10-day drug-free period, and then all of the mice were challenged on day 15 with cocaine (10 mg/kg i.p.).
For the expression of sensitization studies, mice (n = 48) were injected once a day for 5 consecutive days with either saline + saline or saline + cocaine (10 mg/kg i.p.). After a 10-day drug-free period, the effects of CM156 (20 mg/kg i.p.) on the expression of the cocaine (10 mg/kg) response were measured by injecting one of the following treatments on day 15: saline + cocaine or CM156 + cocaine. For both the development and expression of sensitization experiments, locomotor activity was monitored on days 1, 2, 3, 4, 5, and 15 for 30 min immediately after the treatments by using an automated activity monitoring device (San Diego Instruments).
Place Conditioning.
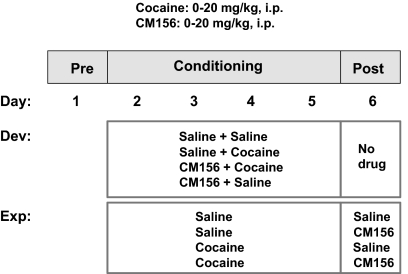
The conditioning chambers were made of Plexiglas (45 × 24 × 20.5 cm) painted gray on one half and black with white vertical stripes on the other half. The floor of the chamber was smooth Plexiglas in half of the chamber, and the floor in the other half was overlaid with a plastic mat containing textured stripes approximately 3 mm apart. The movements of the mice were monitored with an automated video tracking system (SMART; San Diego Instruments). Preconditioning, conditioning, and postconditioning sessions were conducted to measure the rewarding effects of the drug treatments, with time lines depicted in Fig. 2.
Fig. 2.
Time line for place conditioning studies. The animals received no treatments during the preconditioning phase (Pre, day 1); their lack of preference for a particular side of the conditioning chambers was confirmed. For the development (Dev) of place conditioning studies, mice received their designated treatment during the conditioning phase (days 2–5), and their behavioral responses were measured in the absence of drug treatment on the postconditioning day (Post, day 6). For the expression (Exp) of place conditioning studies, mice received either cocaine or saline during the conditioning phase (days 2–5), and on the postconditioning day (day 6) they received saline or antagonist (CM156) before the behavioral measurements.
For the preconditioning session, mice were allowed access to the entire chamber for 30 min. The time spent on each half of the chamber was measured to confirm that they did not have a preference for a particular side. The criterion for an unbiased response and to proceed to the conditioning phase was less than two-thirds of the time spent on a particular side of the chamber.
For the conditioning sessions, the mice received a test drug and were subsequently confined to half of the chamber for 30 min, whereas on alternate sessions the mice received saline and were confined to the other half of the chamber. The mice received a total of eight pairings during the conditioning phase (four drug and four saline sessions). The side of the chamber in which the animals received their drug treatment was assigned randomly and counterbalanced. To determine the dose response for cocaine-induced conditioned place preference, mice (n = 24) were treated with cocaine (0–20 mg/kg i.p.) as the drug during the conditioning phase. To determine whether CM156 alone induced place conditioning, mice (n = 42) were treated with CM156 (0–10 mg/kg i.p.) as the drug during the conditioning phase. To determine whether CM156 could attenuate the development of cocaine-induced conditioned place preference, mice (n = 35) were treated with CM156 (0–10 mg/kg i.p.) + cocaine (20 mg/kg i.p.), as the drug, during the conditioning phase. The mice received saline and were confined to the other side of the chamber during alternate (nondrug) sessions.
For the postconditioning session, mice were allowed access to the entire chamber for 30 min, and the time spent in each half was recorded. For the development of place conditioning studies, no drug was administered for the postconditioning session. For the expression of place conditioning studies, CM156 (0–20 mg/kg; n = 28) was administered 15 min before the postconditioning session. The conditioned score was calculated by subtracting the preconditioning time from the postconditioning time on the side of the chamber in which the mice were trained to drug during the conditioning phase.
Liver Microsomal Assays.
The metabolic stability of CM156 was evaluated in liver microsomes prepared from rat, monkey, and human by XenoTech, LLC (Lenexa, KS). Using a liquid handling system (Tecan, Research Triangle Park, NC), CM156 (5 μM) was incubated at 37°C in 50 mM potassium phosphate buffer (pH 7.4), 3 mM MgCl2, and 1 mM EDTA (pH 7.4), in the presence and absence of cofactor, NADPH-generating system [1 mM NADP (pH 7.4), 5 mM glucose 6-phosphate (pH 7.4), and 1 unit/ml glucose-6-phosphate dehydrogenase]. Reactions were initiated by adding the cofactor mix and terminated at designated time points (0, 15, 30, and 60 min) with the addition of stop reagent (0.2 ml of 200 ng/ml 1-hydroxymidazolam-d4). Precipitated protein was removed by centrifugation at 920g for 10 min at 10°C. The supernatants were collected and analyzed by using liquid chromatography/mass spectrometry.
Data Analysis.
The data from the binding assays were analyzed by using Prism (GraphPad Software Inc., San Diego, CA). Apparent Ki values were calculated by using the Cheng-Prusoff equation and Kd values that were determined in separate saturation binding assays. The data from the convulsion studies were analyzed with Fisher's exact tests (InStat; GraphPad Software Inc.). The data from the locomotor, sensitization, and place conditioning studies were evaluated with analyses of variance. Post hoc comparisons were performed by using Dunnett's tests (comparisons against the vehicle control) or Tukey-Kramer multiple comparisons tests (pairwise comparisons among all groups). For the liver microsomal assays, time 0 incubations served as a control (100% value). The in vitro half-life of CM156 was estimated by using an exponential decay method. In vitro intrinsic clearance was calculated from the in vitro half-life data by using standard equations and the following physiological parameters: microsomal protein/liver (milligrams per gram) for human (45), rat, (45), monkey (49); and liver/body weight (grams per kilogram) for human (20), rat (40), monkey (15). In addition, the following assumptions were made when calculating in vitro intrinsic clearance: the pharmacokinetics of CM156 obeyed the well stirred model, and the nominal concentration of substrate in the incubation was essentially equal to the unbound substrate concentration. For all of the analyses, p < 0.05 was considered statistically significant.
Results
Competition Binding Assays.
Table 1 summarizes the affinities of CM156 for radioligand binding sites. CM156 had high affinity for both σ1 and σ2 receptors in the nanomolar and subnanomolar range. Compared with its high affinity for σ receptors, CM156 had ≥1000-fold weaker affinity for nearly 80 non-σ binding sites tested. Exceptions included nanomolar affinity for the following non-σ sites: α1 adrenergic receptors (695 nM), dopamine D4.2 receptors (53 nM), histamine H1 receptors (853 nM), and type L (benzothiazepine) calcium channels (751 nM). In contrast, no significant binding (>10,000 nM) was observed at the following receptors: adenosine, adrenergic (α2, β), angiotensin (AT1, AT2), bradykinin (BK2), cannabinoid (CB1, CB2), cholecystokinin (CCKA, CCKB), corticotrophin-releasing factor, endothelin (ETA, ETB), estrogen, GABA (GABAA, GABAB), galanin, glucocorticoid, glutamate (AMPA, kainate, NMDA agonist, NMDA/glycine, NMDA/phencyclidine, mGluR1, mGluR5), glycine, leukotriene (LTB4, LTD4), muscarinic (M1), neurokinin (NK1, NK3), nicotinic (muscle, neuronal), opioid, oxytocin, platelet-activating factor, testosterone, thromboxane, thyrotropin-releasing hormone, vasoactive intestinal peptide, and vasopressin. In addition, no significant binding (>10,000 nM) was observed at the following ion channels: calcium (type N) and potassium (ATP-sensitive, voltage-activated VI). No significant binding (>10,000 nM) was observed at the following other targets: norepinephrine transporters, acetylcholine esterase, choline acetyltransferase, glutamic acid decarboxylase, monoamine oxidase (MAOA, MAOB), and nitric-oxide synthase.
TABLE 1.
Binding affinities of CM156
Affinities (Ki values in nanomolar) were determined in tissue or cell homogenates. The values represent the mean ± S.E.M. from replicate assays. Values of >10,000 signify that there was less than 30% displacement of the radioligand at that concentration.
| Radioligand | Nonspecific Binding | Tissue | Ki | |
|---|---|---|---|---|
| σ Receptors | ||||
| σ1 | 5 nM [3H](+)-Pentazocine | 10 μM Haloperidol | Rat brain | 1.3 ± 0.4 |
| σ2 | 3 nM [3H]Di-o-tolylguanidine | 10 μM Haloperidol | Rat brain | 0.6 ± 0.1 |
| Monoamine transporters | ||||
| Dopamine | 0.5 nM [3H]WIN 35,428 | 50 μM Cocaine | Rat striatum | 1175 ± 100 |
| Serotonin | 0.2 nM [3H]Paroxetine | 1.5 μM Imipramine | Rat brainstem | 1402 ± 152 |
| Norepinephrine | 0.5 nM [3H]Nisoxetine | 4 μM Desipramine | Rat cerebral cortex | >10,000 |
| Other neurotransmitter receptors | ||||
| Adenosine | 4.0 nM [3H]5′-N-Ethylcarboxamidoadenosine | 1 μM 5′-N-Ethylcarboxamidoadenosine | Bovine striatum | >10,000a |
| Adrenergic, α1 | 0.3 nM [3H]7-MeOxy-prazosin | 1 μM Phentolamine | Rat forebrain | 695a |
| Adrenergic, α2 | 1 nM [3H]RX 821002 | 1 μM Phentolamine | Rat cerebral cortex | >10,000a |
| Adrenergic, β1 | 0.04 nM [125I](−)-Iodocyanopindolol | 3 μM Alprenolol | Human neuroepithelioma | >10,000a |
| Cannabinoid, CB1 | 0.5 nM [3H]CP 55940 | 1 μM HU-210 | Human recombinant embryonic kidney 293 cells | >10,000a |
| Cannabinoid, CB2 | 0.5 nM [3H]CP 55940 | 1 μM HU-210 | Human recombinant CHO cells | >10,000a |
| Dopamine, nonselective | 0.3 nM [3H]Spiperone | 1 μM Spiperone | Bovine striatum | >10,000a |
| Dopamine D1 | 0.18 nM [3H]SCH 23390 | 1 μM SCH 23390 | LHD1 cells | >10,000a |
| Dopamine D2 | 5 nM [3H](−)-Sulpiride | 1 μM Haloperidol | Rat brain | 1041 ± 9 |
| 0.21 nM [3H]YM-09151-2 | 1 μM Chlorpromazine | CHOp cells | >10,000a | |
| Dopamine D3 | 0.21 nM [3H]YM-09151-2 | 1 μM Chlorpromazine | CHOp cells | >10,000a |
| Dopamine D4.2 | 0.15 nM [3H]Spiperone | 1 μM Haloperidol | Human recombinant CHO-K1 cells | 53a |
| GABAA, agonist site | 5 nM [3H]GABA | 1 μM GABA | Bovine cerebellum | >10,000a |
| GABAA, benzodiazepine α1 | 1 nM [3H]Flunitrazepam | 0.5 μM Flumazenil | Bovine cortex | >10,000a |
| GABAB | 1 nM [3H]CGP 54626A | 100 μM Baclofen | Rat cerebral cortex | >10,000a |
| Glutamate, AMPA | 5 nM [3H]AMPA | 100 μM AMPA | Rat forebrain | >10,000a |
| Glutamate, kainate | 10 nM [3H]Kainic acid | 10 μM Kainic acid | Rat forebrain | >10,000a |
| Glutamate, NMDA agonist | 2 nM [3H]CGP 39653 | 300 μM NMDA | Rat forebrain | >10,000a |
| Glutamate, NMDA glycine | 4 nM [3H]MDL-105,519 | 3 μM MDL-105,519 | Rat cortex/hippoc | >10,000a |
| Glutamate, NMDA/phencyclidine | 10 nM [3H]TCP | 100 μM (+)-MK801 | Rat forebrain | >10,000a |
| 5 nM [3H]TCP | 10 μM Cyclazocine | Rat brain | >10,000 | |
| Glutamate, mGluR1 | 20 nM [3H]Quisqualic acid | 1 mM l-Glutamate | Rat cerebellum | >10,000a |
| Glutamate, mGluR5 | 10 nM [3H]2-Methyl-6-(phenylethynyl)pyridine | 10 μM 2-Methyl-6-(phenylethynyl)pyridine | Rat brain | >10,000a |
| Glycine, strychnine | 16 nM [3H]Strychnine | 100 μM Strychnine nitrate | Rat spinal cord | >10,000a |
| Histamine H1 | 2 nM [3H]Pyrilamine | 10 μM Triprolidine | Bovine cerebellum | 853a |
| Histamine H2 | 0.1 nM [125I]aminopotentidine | 3 μM Tiotidine | Guinea pig striatum | 2570a |
| Histamine H3 | 0.2 nM [3H]N-α-Methylhistamine | 0.1 nM R-(−)-α-Methylhistamine | Rat forebrain | 2385a |
| Melatonin | 70 pM [125I]2-Iodomelatonin | 0.1 μM 2-Iodomelatonin | Chicken brain | >10,000a |
| Muscarinic, central | 0.15 nM [3H]Quinuclidinyl benzilate | 0.1 μM Atropine | Rat cerebral cortex | 3080a |
| Muscarinic, peripheral | 0.3 nM [3H]Quinuclidinyl benzilate | 0.1 μM Atropine | Guinea pig bladder | 2155a |
| Muscarinic, M1 | 0.5 nM [3H]N-Methyl scopolamine | 1 μM (−)-Scopolamine | Human recombinant CHO cells | >10,000a |
| Muscarinic, M2 | 0.5 nM [3H]N-Methyl scopolamine | 1 μM Methylscopolamine | Human recombinant CHO cells | 1210a |
| Nicotinic, muscle | 1 nM 125I-α-Bungarotoxin | 10 μM Nicotine | Human TE671 cells | >10,000a |
| Nicotinic, neuronal | 0.05 nM [3H]Epibatidine | 20 nM Epibatidine | Human SK-N-F1 cells | >10,000a |
| Opioid, nonselective | 0.5 nM [3H]Bremazocine | 10 μM Levallorphan | Rat brain | >10,000 |
| 1 nM [3H]Naloxone | 1 μM Naloxone | Rat forebrain | >10,000a | |
| Serotonin, nonselective | 5 nM [3H]Lysergic acid diethylamide | 10 μM Methysergide | Rat cerebral cortex | 2060a |
| 5-HT1A | 0.50 nM [3H]8-Hydroxy-2-dipropylaminotetralin | 1 μM Dihydroergotamine | HA7 cells | 4985 ± 330a |
| 5-HT2 | 2 nM [3H]Ketanserin | 1 μM Mianserin | Rat brain | 1376 ± 159 |
| 5-HT2A | 0.40 nM [3H]Ketanserin | 1 μM Ketanserin | NIH-3T3-GF6 cells | 1129 ± 49a |
| 5-HT2C | 0.40 nM [3H]Mesulergine | 10 μM Mesulergine | NIH-3T3-Po cells | >10,000a |
| Hormones, peptides, steroids | ||||
| Angiotensin II, AT1 | 0.06 nM [125I](Sar1-Ile8)angiotensin | 1 μM Angiotensin II | Human KAN-TS cells | >10,000a |
| Angiotensin II, AT2 | 0.1 nM [125I]Tyr4-angiotensin II | 0.05 μM Angiotensin II | Bovine cerebellum | >10,000a |
| Bradykinin, BK2 | 0.2 nM [3H]Bradykinin | 100 nM Bradykinin trifluoroacetic acid | Guinea pig ileum | >10,000a |
| Cholecystokinin, CCKA | 0.02 nM [125I]CCK-8 | 1 μM CCK-8 | Mouse pancreas | >10,000a |
| Cholecystokinin, CCKB | 0.02 nM [125I]CCK-8 | 1 μM CCK-8 | Mouse forebrain | >10,000a |
| CRF, nonselective | 0.1 nM [125I]Tyr0-oCRF | 1 μM Tyr0-oCRF | Rat cerebral cortex | >10,000a |
| Endothelin, ETA | 0.033 nM 125I-Endothelin-1 | 0.1 μM Endothelin-1 | Human neuroblastoma | >10,000a |
| Endothelin, ETB | 0.025 nM 125I-Endothelin-1 | 0.1 μM Endothelin-1 | Human astrocytoma | >10,000a |
| Estrogen | 0.1 nM 125I-3,7β-Estradiol | 10 nM 17β-Estadiol | Human breast cancer | >10,000a |
| Galanin, nonselective | 0.07 nM 125I-Galanin | 100 nM Galanin (porcine) | Rat brain | >10,000a |
| Glucocorticoid | 1 nM [3H]Dexamethasone | 10 μM Triamcinolone | Human recombinant | >10,000a |
| Neurokinin, NK1 | 1.4 nM [3H]Substance P | 1 μM Substance P | Rat submaxillary gland | >10,000a |
| Neurokinin, NK2 | 0.1 nM [125I-Neurokinin A | 1 μM Neurokinin A | Human recombinant CHO cells | 1510a |
| Neurokinin, NK3 (NKB) | 0.1 nM 125I-Eledoisin | 1 μM Eledoisin | Rat cerebral cortex | >10,000a |
| Oxytocin | 1 nM [3H]Oxytocin | 1 μM Oxytocin | Rat uterus | >10,000a |
| Testosterone, cytosolic | 0.5 nM [3H]Methyltrienolone | 0.7 μM Methyltrienolone | Human LnCAP cells | >10,000a |
| TRH | 2 nM [3H](3MeHis2)TRH | 10 μM TRH | Rat forebrain | >10,000a |
| VIP, nonselective | 0.05 nM [125I]VIP | 1 μM VIP | Rat forebrain | >10,000a |
| Vasopressin 1 | 0.5 nM [3H]Phenylalanyl-3,4,5-v | 1 μM Arg8-Vasopressin | Rat liver | >10,000a |
| Ion channels: | ||||
| Calcium, type L (benzothiazepine) | 5 nM [3H]cis-(+)-Diltiazem | 10 μM Diltiazem | Rat cerebral cortex | 751a |
| Calcium, type L (dihydropyridine) | 0.2 nM [3H]Nitrendipine | 1 μM Nifedipine | Rat cerebral cortex | 1710a |
| Calcium, type N | 0.01 nM 125I-ω-Conotoxin GVIA | 0.1 μM ω-Conotoxin GVIA | Rat cerebral cortex | >10,000a |
| Chloride, GABA (t-butylbicycloorthobenzoate) | 20 nM [3H]t-Butylbicycloorthobenzoate | 10 μM t-Butylbicyclophosphorothionate | Rat cerebral cortex | >10,000a |
| Potassium, ATP-sensitive | 0.2 nM [3H]Glibenclamide | 0.1 μM Glibenclamide | Rat cerebral cortex | >10,000a |
| Potassium, Ca2+ act VI | 0.05 nM 125I-Apamin | 100 nM Apamin | Rat forebrain | >10,000a |
| Potassium, human ether-a-go-go-related gene | 40 nM [3H]Astemizole | 100 μM Terfenadine | CHO cells | >10,000a |
| Sodium, site 2 | 2 nM [3H]Batrachotoxin | 1 mM Aconitine | Rat forebrain | 1535a |
| Enzymes and other miscellaneous | ||||
| Acetylcholine esterase | 0.3 mM [3H]Acethylthiocholine | 100 μM Physostigmine | Human recombinant | >10,000a |
| Choline acetyltransferase | 0.2 nM [14C]Acetyl coenzyme | 0.1 μM Ro 41-1049 | Rat cerebral cortex | >10,000a |
| Glutamic acid decarboxylase | 4 μM [14C]l-Glutamic acid | 100 μM Aminooxy acetic acid | Rat striatum | >10,000a |
| Leukotriene, LTB4 (BLT) | 0.48 nM [3H]Leukotriene B4 | 500 nM Leukotriene B4 | Guinea pig spleen | >10,000a |
| Leukotriene, LTD4 (CysLT1) | 0.2 nM [3H]Leukotriene D4 | 1 μM Leukotriene D4 | Guinea pig lung | >10,000a |
| MAOA oxidase, peripheral | 50 μM [14C]5-HT | 1 μM Ro 41-1049 | Rat liver mitochondria | >10,000a |
| MAOB oxidase, peripheral | 10 μM [14C]Phenylethylamine | 10 μM Ro 16-6491 | Rat liver mitochondria | >10,000a |
| Nitric-oxide synthase (NOS) | 5 nM [3H]l-NG-Nitro-arginine | 100 μM l-NG-Nitro-arginine | Rat brain | 1220a |
| Platelet activating factor | 1.7 nM [3H]Hexadecyl-acetyl-platelet-activating factor | 1 μM C16-platelet-activating factor | Rabbit platelets | >10,000a |
| Thromboxane, TXA2 | 2 nM [3H]SQ 29,548 | 10 μM Pinane-thromboxane | Human platelets | >10,000a |
CHO, Chinese hamster ovary; CRF, corticotrophin-releasing factor; TRH, thyrotropin releasing hormone; VIP, vasoactive intestinal peptide.
These values were determined by NovaScreen. All other values were determined in-house using the assay conditions described under Materials and Methods.
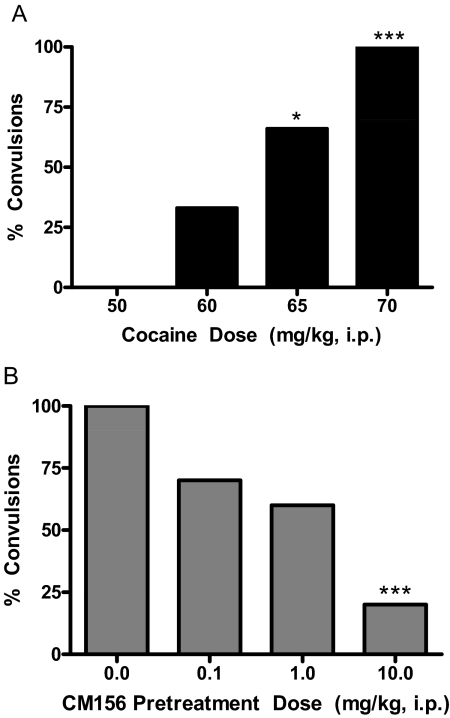
Cocaine-Induced Convulsions.
Cocaine elicited convulsions in a dose-dependent manner, with 100% of the mice convulsing at the 70 mg/kg dose (Fig. 3A). Fisher's exact test revealed that significantly more convulsions were observed at the 65 mg/kg (p < 0.05) and 70 mg/kg (p < 0.001) doses of cocaine compared with saline. Pretreatment with CM156 dose-dependently attenuated the convulsive effects of the 70 mg/kg dose of cocaine (Fig. 3B). Fisher's exact tests confirmed that the reduction in cocaine-induced convulsions produced by the 10 mg/kg dose of CM156 was statistically significant (p < 0.001).
Fig. 3.
Cocaine-induced convulsions. A, administration of cocaine (0–70 mg/kg i.p.) to Swiss-Webster mice produced a dose-dependent increase in the percentage of animals exhibiting convulsions. B, pretreatment of Swiss-Webster mice with CM156 (0–20 mg/kg i.p.), followed 15 min later with a convulsive dose of cocaine (70 mg/kg i.p.) produced a dose-dependent reduction in the convulsive effects of cocaine. *, p < 0.05; ***, p < 0.001; Fisher's exact test.
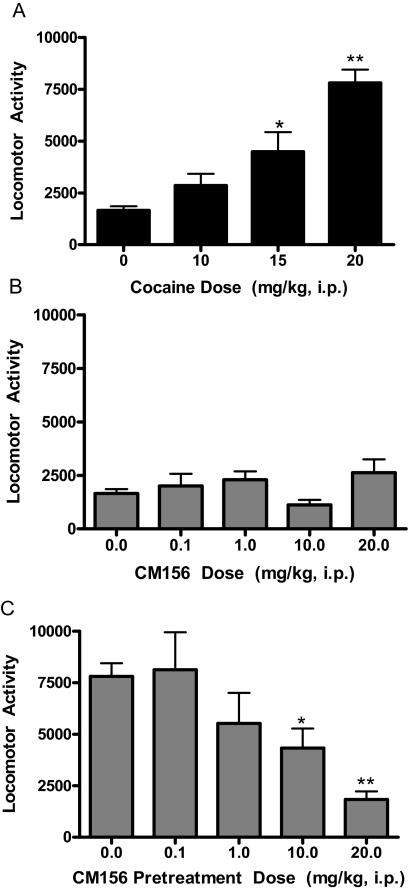
Locomotor Activity.
Cocaine dose-dependently increased locomotor activity (Fig. 4A). In contrast, CM156 had no significant effects on locomotor activity when it was administered alone up to a dose of 20 mg/kg [Fig. 4B; F(4,37) = 2.12; N.S.]. However, when these doses of CM156 were used as a pretreatment to a locomotor stimulatory dose of cocaine (20 mg/kg), there was a dose-dependent reduction in the hyperactivity elicited by cocaine [Fig. 4C; F(4,49) = 6.96, p < 0.0005]. Dunnett's multiple comparisons tests revealed that the following pretreatment doses of CM156 produced effects that significantly reduced the locomotor stimulant effects of cocaine: 10 mg/kg (q = 2.65, p < 0.05) and 20 mg/kg (q = 4.72, p < 0.01).
Fig. 4.
Cocaine-induced locomotor activity. A, administration of cocaine (0–20 mg/kg i.p.) to Swiss-Webster mice produced a dose-dependent increase locomotor activity. B, administration of CM156 (0–20 mg/kg i.p.) to Swiss-Webster mice had no significant effects of locomotor activity. C, pretreatment of Swiss-Webster mice with CM156 (0–20 mg/kg i.p.), followed 15 min later with a locomotor stimulatory dose of cocaine (20 mg/kg i.p.) produced a dose-dependent reduction in the acute stimulant effects of cocaine. *, p < 0.05; **, p < 0.01; post hoc Dunnett's test.
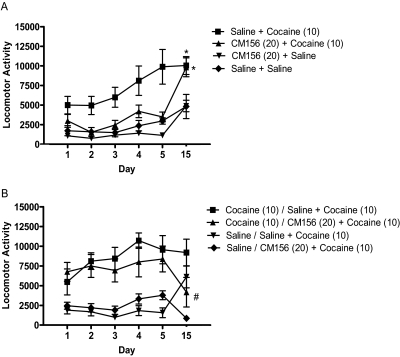
Behavioral Sensitization.
Analysis of variance confirmed a significant difference between the treatment groups in the development of sensitization experiment [F(3,20) = 6.07, p < 0.005]. Repeated administration of cocaine on days 1 to 5 resulted in the development of behavioral sensitization, which was measured as an enhanced response to cocaine on day 15 (Fig. 5A; q = 4.39, p < 0.05). Pretreatment with CM156 before each cocaine exposure on days 1 to 5 attenuated the locomotor stimulatory effects of cocaine on each day (Fig. 5A); the differences were statistically significant on days 2 (q = 5.27, p < 0.01), 3 (q = 4.60, p < 0.05), and 5 (q = 5.47, p < 0.01). However, these pretreatments with CM156 on days 1 to 5 did not prevent the development of cocaine-induced behavioral sensitization, as evidenced by the elevated (sensitized) response to cocaine on day 15 (Fig. 5A). Post hoc tests confirmed that animals treated with CM156 + cocaine on days 1 to 5 exhibited sensitization to cocaine on day 15, as reflected by the significantly greater response to cocaine compared with those treated with saline + saline on days 1 to 5 (q = 4.20, p < 0.05); the magnitude of the sensitization to cocaine between animals treated on days 1 to 5 with saline + cocaine compared with CM156 + cocaine were similar (q = 0.19, N.S.).
Fig. 5.
Cocaine-induced sensitization. A, CM156 had no significant effect on the development of cocaine-induced sensitization. Male Swiss-Webster mice were injected intraperitoneally with saline or CM156 (20 mg/kg) followed 15 min later with saline or cocaine (10 mg/kg), once a day for 5 days. After a 10-day drug-free period, mice were injected with cocaine (10 mg/kg i.p.). Pretreatment with CM156, on days 1 to 5, had no significant effect on the sensitized responses to cocaine observed on day 15. *, p < 0.05, saline + cocaine (10) and CM156 (20) + cocaine (10) versus saline + saline, post hoc tests. B, CM156 significantly attenuated the expression of cocaine-induced sensitization. Male Swiss-Webster mice were injected with saline or cocaine (10 mg/kg i.p.), once a day for 5 days. After a 10-day drug-free period, mice were injected intraperitoneally with saline or CM156 (20 mg/kg) followed 15 min later with cocaine (10 mg/kg). Analysis of variance confirmed that pretreatment with CM156 on day 15 significantly attenuated the expression of the sensitized response to cocaine [#, p < 0.05 versus cocaine (10)/saline + cocaine (10), post hoc tests].
Although CM156 did not prevent the development of cocaine-induced sensitization, it attenuated the expression of the sensitized response (Fig. 5B). Analysis of variance revealed a significant difference between the experimental groups [F(3,20) = 7.85, p < 0.005]. Mice were sensitized to cocaine by injecting them for 5 consecutive days (days 1–5) as in the development of sensitization studies; challenge with cocaine on day 15 revealed the presence of the sensitized response (Fig. 5B). However, in the mice that were pretreated with CM156 before the cocaine challenge on day 15, there was a significant reduction in the expression of cocaine-induced behavioral sensitization (Fig. 5B; q = 4.34, p < 0.05).
Place Conditioning.
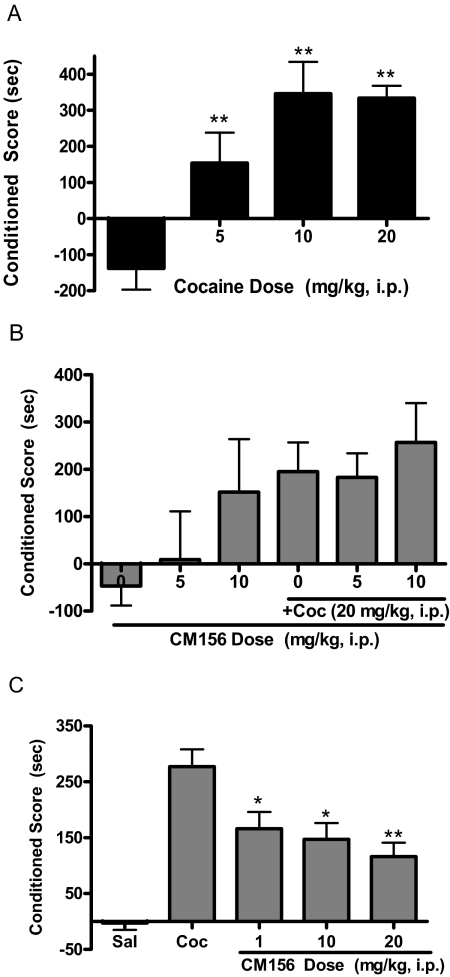
Place conditioning to cocaine was dose-dependent [Fig. 6A; F(3,28) = 15.33, p < 0.0001]. Dunnett's multiple comparison post hoc tests confirmed that all of the tested doses of cocaine differed significantly from saline: 5 mg/kg (q = 3.65, p < 0.01), 10 mg/kg (q = 5.89, p < 0.01), and 20 mg/kg (q = 5.84, p < 0.01). In contrast, CM156 did not elicit significant place conditioning on its own at the doses tested [F(2,39) = 2.37, N.S.].
Fig. 6.
Place conditioning. A, administration of cocaine (0–20 mg/kg i.p.) produced a dose-dependent increase in place conditioning in mice. Mice were injected four times with a dose of cocaine (0–20 mg/kg i.p.) and confined to half of a place conditioning chamber; they received saline on an alternating schedule and were confined to the other side of the chamber. B, CM156 had no significant effect on the development of cocaine-induced place conditioning. Mice were injected with saline or CM156 (5 and 10 mg/kg i.p.) 15 min before saline or cocaine (+Coc; 20 mg/kg i.p.) during conditioning sessions; they remained drug-free on the postconditioning test day. None of the changes were statistically significant. C, CM156 significantly attenuated the expression of cocaine-induced place conditioning. Mice were injected with saline (Sal) or cocaine (Coc; 20 mg/kg i.p.) during the conditioning sessions; they received CM156 (1–20 mg/kg i.p.) 15 min before the postconditioning test session. *, p < 0.05; **, p < 0.01; post hoc tests.
For the development of cocaine-induced place preference studies (Fig. 6B), analysis of variance revealed no significant difference in the ability of CM156 to alter the cocaine-induced response when it was administered as a pretreatment before each cocaine exposure [F(2,32) = 0.18, N.S.]. In contrast, CM156 significantly attenuated the expression of cocaine-induced place conditioning (Fig. 6C). Analysis of variance confirmed an overall significant difference between the experimental groups [F(3,24) = 4.77, p < 0.01]. In addition, post hoc comparisons showed that the reduction in cocaine-induced place preference was statistically significant for all doses of CM156 tested during the postconditioning phase: 1 mg/kg (q = 3.17, p < 0.05), 10 mg/kg (q = 3.22, p < 0.05), and 20 mg/kg (q = 3.54, p < 0.01).
Liver Microsomal Assays.
Table 2 summarizes the percentage of substrate lost, percentage of substrate remaining, half-life, and intrinsic clearance of 5 μM CM156 in vitro in rat, monkey, and human liver microsomes. The half-life values estimated for CM156 in all three species were short, with clearance following the rank order (from high to low) monkey > rat > human.
TABLE 2.
Metabolic stability of CM156 in rat, monkey, and human liver microsomes
Values are the mean of triplicate determinations, except for the zero cofactor values that are the mean of duplicate determinations. CM156 was tested at a concentration of 5 μM. Protein concentration was set at 1.0 mg protein/ml.
| Species | Incubation Time | Cofactor | Area Ratio | % of Loss of Substrate | % Remaining | Half-Life | Clearance |
|---|---|---|---|---|---|---|---|
| min | min | ml/min/kg | |||||
| Rat | 0 | + | 143 | 0.0 | 100 | ||
| 15 | + | 12.7 | 91.2 | 8.8 | |||
| 30 | + | 12.2 | 91.5 | 8.5 | 4.62 | 270 | |
| 60 | + | 11.5 | 92.0 | 8.0 | |||
| 0 | − | 148 | 0.0 | 100 | |||
| 60 | − | 148 | 0.0 | 100 | |||
| Monkey | 0 | + | 140 | 0.0 | 100 | ||
| 15 | + | 3.17 | 97.7 | 2.3 | |||
| 30 | + | 2.11 | 98.5 | 1.5 | 2.8 | 184 | |
| 60 | + | 1.81 | 98.7 | 1.3 | |||
| 0 | − | 146 | 0.0 | 100 | |||
| 60 | − | 144 | 1.4 | 98.6 | |||
| Human | 0 | + | 133 | 0.0 | 100 | ||
| 15 | + | 51.4 | 61.3 | 38.7 | |||
| 30 | + | 15.4 | 88.4 | 11.6 | 10.6 | 58.8 | |
| 60 | + | 7.35 | 94.5 | 5.5 | |||
| 0 | − | 138 | 0.0 | 100 | |||
| 60 | − | 142 | No loss | 103 |
Discussion
The binding data reveal that CM156, a novel substituted piperazine, is one of the most selective, high-affinity σ compounds reported to date. CM156 demonstrates high and preferential affinity for σ receptors, with ≥1000-fold preference for σ subtypes compared with approximately 80 non-σ sites tested. Relative to previously characterized antagonists, such as BD1063 (Matsumoto et al., 1995), NE-100 (Chaki et al., 1994), and AC927 (Matsumoto et al., 2008), CM156 exhibits better affinity and selectivity for σ receptors.
Given the high selectivity and affinity of CM156 for σ receptors and the pattern of data produced by previously studied σ ligands, the significant reductions in cocaine-induced convulsions and locomotor activity by CM156 are consistent with a role for σ receptors in mediating cocaine-induced acute effects. Based on σ actions that have been reported in the literature, several theoretical mechanisms may contribute to the ability of CM156 to block the effects of cocaine. First, CM156 may directly interfere with cocaine's binding to σ receptors. Cocaine binds to σ receptors at physiologically relevant concentrations in the micromolar range (Sharkey et al., 1988; Matsumoto et al., 2002). CM156 has much better nanomolar affinity for σ1 and σ2 receptors and can compete with cocaine for access to these proteins. The notion that interfering with cocaine's access to σ receptors can mitigate its functional effects is supported by earlier studies in which knockdown of σ1 receptors with antisense oligonucleotides prevented cocaine-induced convulsions, locomotor hyperactivity, and place conditioning in mice (Romieu et al., 2000; Matsumoto et al., 2001, 2002). More recent studies further indicate that preferential antagonism of the σ2 subtype also mitigates the effects of cocaine (Matsumoto et al., 2007b; Kaushal, 2008).
Second, after the binding of exogenous or endogenous agonists, σ receptors participate in protein–protein interactions to elicit changes in cellular functions. Thus, CM156 binding to σ receptors may change the conformation or configuration of σ receptors and prevent them from interacting with other proteins. Activation of the σ1 subtype by agonists induces translocation of the protein to different cellular compartments (Morin-Surun et al., 1999; Hayashi and Su, 2003) and facilitates protein–protein interactions that have regulatory roles on cellular events, including potassium channel conductances (Palmer et al., 2007), activities of phospholipase C and protein kinase C (Morin-Surun et al., 1999), and intracellular calcium mobilization (Hayashi et al., 2000). The σ2 subtype, which is enriched in lipid rafts, also seems to participate in protein–protein interactions that are critical for the activation of agonist-stimulated sphingolipid signaling pathways (Gebreselassie and Bowen, 2004). Therefore, CM156 binding may impair σ receptors from forming protein–protein interactions and prevent cocaine from producing its σ-mediated effects on cellular activities.
Third, apart from its interactions with σ receptors, cocaine triggers multiple signal transduction pathways that lead to modifications in the activities of dopaminergic, glutamatergic, and cholinergic systems (Werling et al., 2007). All of these neurotransmitter systems can be modulated through σ receptors. For example, σ receptor agonists increase dopamine release in the striatum and prefrontal cortex, enhance the responsiveness of pyramidal neurons in the hippocampus to NMDA, and enhance the release of acetylcholine from the prefrontal cortex and hippocampus (Werling et al., 2007). Antagonism of σ receptors by CM156 would therefore be expected to modulate and dampen the actions of these neurotransmitter systems that are activated by cocaine. Each of the aforementioned mechanisms may contribute to the actions of CM156 in attenuating the acute effects of cocaine and suggest critical and varied roles for σ receptors in modulating the actions of cocaine.
Upon subchronic exposure to cocaine, CM156 significantly attenuated the expression, but not the development, of cocaine-induced behavioral sensitization and place conditioning. Generally, the development models track the evolution of cocaine-induced neuroadaptations as they occur, whereas the expression models probe the status of established neuroadaptations that have already occurred after repeated cocaine exposures. During the development of behavioral sensitization, CM156 blocked the acute effects of cocaine on each of the 5 consecutive days of psychostimulant drug exposure, but it did not prevent the sensitized behavioral response to cocaine on the challenge day. This implies that CM156 can block the acute stimulant response but that it cannot prevent cocaine-induced neuroadaptations from occurring in brain.
Two possibilities may explain the failure of CM156 to block the development of cocaine-induced neuroadaptations in the behavioral sensitization and place conditioning studies. First, the short half-life of CM156 in vitro is suggestive of rapid metabolism in vivo, which would compromise its ability to prevent behaviors and neuroadaptations that evolve over an extended time course. It should be noted that other σ receptor antagonists have been reported to attenuate the development of sensitization (Matsumoto et al., 2003; Liu and Matsumoto, 2008) and place conditioning (Romieu et al., 2000, 2002) to cocaine. Because the molecular basis of neuroadaptations is mediated by altered gene expression in the brain (McClung and Nestler, 2008), gradually leading to neuroplasticity and persistent structural changes (Kalivas and O'Brien, 2008), a quick clearance of CM156 from the body may permit the progression of cocaine-induced neuroadaptations that evolve after the antagonist has been metabolized.
In addition, pathways independent of σ receptors contribute to cocaine-induced neuroadaptations, including alterations in ΔFosB and D1 receptor-mediated effects (McClung and Nestler, 2008). As indicated in our previous microarray studies, many of the effects of cocaine on gene expression changes are unaltered by σ receptor antagonists such as BD1063 (Liu et al., 2005). Although these non-σ mechanisms could contribute to the inability of CM156 to significantly attenuate the development of cocaine-induced behavioral sensitization, it cannot wholly explain the data because previous studies showed that other σ ligands can mitigate the development of behavioral sensitization to cocaine (Matsumoto et al., 2003; Liu and Matsumoto, 2008). Thus, the inability of CM156 to significantly attenuate the development of cocaine-induced behavioral sensitization is probably linked to its poor metabolic stability.
Although the unfavorable metabolic rate of CM156 may be a critical factor in failing to block the development of cocaine-induced behavioral sensitization and place conditioning, CM156 significantly attenuated the expression of these behaviors, further implicating the involvement of σ receptors in the subchronic effects of cocaine (Romieu et al., 2000, 2002; Liu and Matsumoto, 2008). In the expression of behavioral sensitization and place conditioning studies, neuroadaptations have been induced in the brain after several consecutive days of cocaine exposure. Among the documented changes that may occur is the up-regulation of σ1 receptors (Romieu et al., 2004; Liu and Matsumoto, 2008). On the challenge day, administration of CM156 blocked the sensitized response normally produced by cocaine and the drug-seeking response in the conditioning chambers. CM156 may have successfully mitigated the expression of cocaine-induced behavioral sensitization and place conditioning by interfering with the access of cocaine or an endogenous σ ligand to elevated numbers of σ1 receptors that resulted after repeated cocaine exposures. In addition, preliminary microarray analyses of the effects of CM156 in the expression of cocaine-induced place conditioning studies demonstrate that it can reverse numerous cocaine-induced changes in gene expression that are associated with the behavioral pharmacological responses (unpublished data). CM156 therefore directly or indirectly influences numerous cellular functions that contribute to the expression of place conditioning. Comparable microarray studies have yet to be conducted for the expression of sensitization. The ability of σ receptor antagonism to significantly attenuate the expression of behavioral sensitization and place conditioning, even after an array of cocaine-induced neuroadaptations have been established, thus suggests σ receptors as potential targets for pharmacotherapies in patients already exposed to cocaine.
CM156 interacts with both σ1 and σ2 receptors, and it is likely that its anticocaine effects are mediated through both subtypes. The role of σ1 receptors in reducing the behavioral effects of cocaine is well established in the literature through the use of subtype-selective ligands and antisense oligonucleotides (McCracken et al., 1999a,b; Matsumoto et al., 2001, 2003). Less data are available regarding the σ2 subtype because of the dearth of truly selective ligands, but evidence is accumulating in this regard (Matsumoto et al., 2007; Kaushal, 2008; Mésangeau et al., 2008; Mamolo et al., 2008). Because CM156 shows preferential affinity for σ2 over σ1 receptors, the ability of CM156 to attenuate varied effects of cocaine suggests that additional studies regarding the role of σ2 receptors in anticocaine effects are warranted.
In summary, the results from the binding assays indicate that CM156 is a σ-selective ligand. Furthermore, CM156 produces protective effects against cocaine in behavioral evaluations including both acute and subchronic effects. These results provide further support for the involvement of σ receptors in the actions of cocaine when taken together with previous studies involving antisense oligonucleotides against σ receptors (Matsumoto et al., 2001, 2002; Romieu et al., 2002). Although the rapid metabolic rate of CM156 may limit its further development as a potential medication, additional metabolism studies are needed in vivo to confirm the in vitro trends. Collectively, the results support σ receptors as viable targets for drug discovery of anticocaine agents, and CM156 provides a promising lead compound for alleviating the harmful actions of cocaine.
Acknowledgments
We thank Jie Yu and Jeffrey Diers for technical assistance during some of the behavioral studies. The National Institute on Drug Abuse also arranged for the NovaScreen (radioligand binding) and XenoTech (liver microsomal) assays through contractual agreements with the vendors.
This study was supported by the National Institutes of Health National Institute on Drug Abuse [Grants DA011979, DA013978, DA023205] and the National Institutes of Health National Center for Research Resources [Grant P20-RR021929].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.161398.
- NMDA
- N-methyl-d-aspartate
- NPC 16377
- 6-[6-(4-hydroxypiperidinyl)hexyloxy]-3-methylflavone
- NE-100
- N,N-dipropyl-2-[4-methoxy-3-(2-phenylethoxy)phenyl]ethylamine
- AC927
- 1-(2-phenethyl)piperidine oxalate
- CM156
- 3-(4-(4-cyclohexylpiperazin-1-yl)butyl)benzo[d]thiazole-2(3H)-thione
- D
- dopamine
- 5-HT
- 5-hydroxytryptamine (serotonin)
- TCP
- 1-[1-(2-thienyl)cyclohexyl]piperidine
- WIN35,428
- 2β-carbomethoxy-3β-(4-fluorophenyl)tropane
- AMPA
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AT
- angiotensin
- BK
- bradykinin
- CB
- cannabinoid
- CCK
- cholecystokinin
- ET
- endothelin
- mGluR
- metabotropic glutamate receptor
- LT
- leukotriene
- M
- muscarinic
- NK
- neurokinin
- MAO
- monoamine oxidase
- RX 821002
- 2-(2-methoxy-1,4-benzodioxan-2yl)-2-imidazoline
- CP 55940
- (1R,3R,4R)-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-4-(3-hydroxypropyl)cyclohexan-1-ol
- SCH 23390
- R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4, 5-tetrahydro-1H-3-benzazepine
- YM-09151-2
- nemonapride
- MDL-105,519
- 3-(2-phenyl-2-carboxyethenyl)-4,6-dichloro-1H-indole-2-carboxylic acid
- SQ 29,548
- 7-(3-((2-((phenylamino)carbonyl)hydrazino)methyl)-7-oxabicyclo(2.2.1)hept-2-yl)-5-heptenoic acid
- HU-210
- (−)-7-OH-Δ-6-tetrahydrocannabinol-dimethylheptyl
- MK801
- 5H-dibenzo[a,d]cyclohepten-5,10-imine (dizocilpine maleate)
- Ro 41-1049
- N-(2-aminoethyl)-5-(3-fluorophenyl)-4-thiazolecarboxamide
- Ro 16–6491
- N-(2-aminoethyl)-p-chlorobenzamide
- CGP 39653
- d,l-(E)-2-amino-4-propyl-5-phosphono-3-pentenoic acid
- BD1008
- N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(1-pyrrolidinyl)ethylamine
- BD1047
- N-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine
- BD1063
- 1-[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine.
References
- Balster R. (1988) Pharmacological effects of cocaine relevant to its abuse. NIDA Res Monogr 88:1–13 [PubMed] [Google Scholar]
- Carrera MR, Meijler MM, Janda KD. (2004) Cocaine pharmacology and current pharmacotherapies for its abuse. Bioorg Med Chem 12:5019–5030 [DOI] [PubMed] [Google Scholar]
- Chaki S, Tanaka M, Muramatsu M, Otomo S. (1994) NE-100, a novel potent sigma ligand, preferentially binds to sigma 1 binding sites in guinea pig brain. Eur J Pharmacol 251:R1–R2 [DOI] [PubMed] [Google Scholar]
- Gebreselassie D, Bowen WD. (2004) Sigma-2 receptors are specifically localized to lipid rafts in rat liver membranes. Eur J Pharmacol 493:19–28 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Maurice T, Su TP. (2000) Ca2+ signaling via σ1-receptors: novel regulatory mechanism affecting intracellular Ca2+ concentrations. J Pharmacol Exp Ther 293:788–798 [PubMed] [Google Scholar]
- Hayashi T, Su TP. (2001) Regulating ankyrin dynamics: roles of sigma-1 receptors. Proc Natl Acad Sci USA 98:491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. (2003) Intracellular dynamics of σ-1 receptors (σ1 binding sites) in NG108-15 cells. J Pharmacol Exp Ther 306:726–733 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su T. (2005) The sigma receptor: evolution of the concept in neuropsychopharmacology. Curr Neuropharmacol 3:267–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell 131:596–610 [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O'Brien C. (2008) Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 33:166–180 [DOI] [PubMed] [Google Scholar]
- Kaushal N. (2008) SN79, A Novel Sigma-2 Receptor Antagonist, Attenuates Cocaine-Induced Behaviors in Mice Master's thesis, University of Mississippi, University, MS: [Google Scholar]
- Liu Y, Chen GD, Lerner MR, Brackett DJ, Matsumoto RR. (2005) Cocaine up-regulates Fra-2 and σ-1 receptor gene and protein expression in brain regions involved in addiction and reward. J Pharmcol Exp Ther 314:770–779 [DOI] [PubMed] [Google Scholar]
- Liu Y, Matsumoto RR. (2008) Alterations in fos-related antigen 2 and sigma1 receptor gene and protein expression are associated with the development of cocaine-induced behavioral sensitization: time course and regional distribution studies. J Pharmacol Exp Ther 327:187–195 [DOI] [PubMed] [Google Scholar]
- Mamolo MG, Zampieri D, Zanette C, Florio C, Collina S, Urbano M, Azzolina O, Vio L. (2008) Substituted benzylaminoalkylindoles with preference for the σ2 binding site. Eur J Med Chem 43:2073–2081 [DOI] [PubMed] [Google Scholar]
- Matsumoto RR. (2007a) σ Receptors: historical perspective and background, in Sigma Receptor Chemistry, Cell Biology, and Clinical Implications (Matsumoto RR, Bowen WD, Su T. eds) pp 1–24, Springer, New York: [Google Scholar]
- Matsumoto RR, Bowen WD, Tom MA, Vo VN, Truong DD, De Costa BR. (1995) Characterization of two novel sigma receptor ligands: antidystonic effects in rats suggest sigma receptor antagonism. Eur J Pharmacol 280:301–310 [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Liu Y, Lerner M, Howard EW, Brackett DJ. (2003) Sigma receptors: potential medications development target for anticocaine agents. Eur J Pharmacol 469:1–12 [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, McCracken KA, Friedman MJ, Pouw B, De Costa BR, Bowen WD. (2001) Conformationally restricted analogs of BD1008 and an antisense oligodeoxynucleotide targeting σ1 receptors produce anticocaine effects in mice. Eur J Pharmacol 419:163–174 [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, McCracken KA, Pouw B, Zhang Y, Bowen WD. (2002) Involvement of sigma receptors in the behavioral effects of cocaine: evidence from novel ligands and antisense oligodeoxynucleotides. Neuropharmacology 42:1043–1055 [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Pouw B, Mack AL, Daniels A, Coop A. (2007b) Effects of UMB24 and (+/−)-SM 21, putative sigma2-preferring antagonists, on behavioral toxic and stimulant effects of cocaine in mice. Pharmacol Biochem Behav 86:86–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR, Shaikh J, Wilson LL, Vedam S, Coop A. (2008) Attenuation of methamphetamine-induced effects through the antagonism of sigma (σ) receptors: evidence from in vivo and in vitro studies. Eur Neuropsychopharmacol 18:871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T, Martin-Fardon R, Romieu P, Matsumoto RR. (2002) Sigma1 (σ1) receptor antagonists represent a new strategy against cocaine addiction and toxicity. Neurosci Biobehav Rev 26:499–527 [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. (2008) Neuroplasticity mediated by altered gene expression. Neuropsychopharmacology 33:3–17 [DOI] [PubMed] [Google Scholar]
- McCracken KA, Bowen WD, Matsumoto RR. (1999a) Novel σ receptor ligands attenuate the locomotor stimulatory effects of cocaine. Eur J Pharmacol 365:35–38 [DOI] [PubMed] [Google Scholar]
- McCracken KA, Bowen WD, de Costa BR, Matsumoto RR. (1999b) Two novel σ ligands, BD1047 and LR172, attenuate cocaine-induced toxicity and locomotor activity. Eur J Pharmacol 370:225–232 [DOI] [PubMed] [Google Scholar]
- Mésangeau C, Narayanan S, Green AM, Shaikh J, Kaushal N, Viard E, Xu YT, Fishback JA, Poupaert JH, Matsumoto RR, et al. (2008) Conversion of a highly selective sigma-1 receptor-ligand to sigma-2 receptor preferring ligands with anticocaine activity. J Med Chem 51:1482–1486 [DOI] [PubMed] [Google Scholar]
- Morin-Surun MP, Collin T, Denavit-Saubié M, Baulieu EE, Monnet FP. (1999) Intracellular σ1 receptor modulates phospholipase C and protein kinase C activities in the brainstem. Proc Natl Acad Sci USA 96:8196–8199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AH, Coop A. (2007) Medicinal chemistry: new chemical class and subtype-selective ligands, in Sigma Receptor Chemistry, Cell Biology, and Clinical Implications (Matsumoto RR, Bowen WD, Su T. eds) pp 25–44, Springer, New York: [Google Scholar]
- Palmer CP, Aydar E, Jackson MB. (2007) σ Receptor modulation of ion channels, in Sigma Receptor Chemistry, Cell Biology, and Clinical Implications (Matsumoto RR, Bowen WD, Su T. eds) pp 127–149, Springer, New York: [Google Scholar]
- Romieu P, Martin-Fardon R, Maurice T. (2000) Involvement of σ1 receptor in the cocaine-induced conditioned place preference. NeuroReport 11:2885–2888 [DOI] [PubMed] [Google Scholar]
- Romieu P, Phan VL, Martin-Fardon R, Maurice T. (2002) Involvement of the σ1 receptor in cocaine-induced conditioned place preference: possible dependence on dopamine uptake blockade. Neuropsychopharmacology 26:444–455 [DOI] [PubMed] [Google Scholar]
- Romieu P, Meunier J, Garcia D, Zozime N, Martin-Fardon R, Bowen WD, Maurice T. (2004) The sigma1 (σ1) receptor activation is a key step for the reactivation of cocaine conditioned place preference by drug priming. Psychopharmacology 175:154–162 [DOI] [PubMed] [Google Scholar]
- Sharkey J, Glen KA, Wolfe S, Kuhar MJ. (1988) Cocaine binding at sigma receptors. Eur J Pharmacol 149:171–174 [DOI] [PubMed] [Google Scholar]
- Su TP, Hayashi T. (2003) Understanding the molecular mechanism of sigma-1 receptors: towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr Med Chem 10:2073–2080 [DOI] [PubMed] [Google Scholar]
- Werling LL, Derbez AE, Nuwayhid SJ. (2007) Modulation of classical neurotransmitter systems by σ receptors, in Sigma Receptor Chemistry, Cell Biology, and Clinical Implications (Matsumoto RR, Bowen WD, Su T. eds) pp 195–214, Springer, New York: [Google Scholar]
- Witkin JM, Terry P, Menkel M, Hickey P, Pontecorvo M, Ferkany J, Katz JL. (1993) Effects of the selective sigma receptor ligand, 6-[6-(4-hydroxypiperidinyl)hexyloxy]-3-methylflavone (NPC 16377), on behavioral and toxic effects of cocaine. J Pharmacol Exp Ther 266:473–482 [PubMed] [Google Scholar]
- Wise RA. (2002) Brain reward circuitry: insights from unsensed incentives. Neuron 36:229–240 [DOI] [PubMed] [Google Scholar]