Abstract
Acquired drug resistance to mycotic infections is rapidly emerging as a major medical problem. Opportunistic fungal infections create therapeutic challenges, particularly in high risk immunocompromised patients with AIDS, cancer, and those undergoing transplantation. Higher mortality and/or morbidity rates due to invasive mycosis have been increasing over the last 20 years, and in light of growing resistance to commonly used antibiotics, novel antifungal drugs and approaches are required. Currently there is considerable interest in antifungal peptides that are ubiquitous in plant and animal kingdoms. These small cationic peptides may have specific targets or may be multifunctional in their mechanism of action. On the basis of recent advances in protein engineering and solid phase syntheses, the utility and potential of selected peptides as efficient antifungal drugs with acceptable toxicity profiles are being realized. This review will discuss recent advances in peptide therapy for opportunistic fungal infections.
INTRODUCTION
In an era of increased incidence of fungal infections in immunocompromised patients (1, 2) and greater resistance to “frontline” antifungal therapies (3), there is a growing need to discover new antifungal therapies. Although newer azole derivatives such as voriconazole are more effective and have cidal activity against filamentous fungi such Aspergillus fumigatus (4), these derivatives are fungistatic and not fungicidal against pathogenic yeasts; the inability to kill yeasts leads to resistance to azole in prolonged infections and increases the likelihood that these agents will lack efficacy in severe Candida infections in immunosuppressed patients. Amphotericin B has also been commonly used to treat serious fungal infections, but in contrast to azoles, amphotericin B is fungicidal against yeasts. Nevertheless, resistance to amphotericin B is slowly developing in selected Candida species (5) and there are significant side effects associated with its use, including nephrotoxicity. Although recent antifungal agents, including the peptide-based agents, micafungin and caspofungin, have been developed and are very promising, resistance to these therapies has already been reported (6–8) and will no doubt become more widespread. The development of resistance to current antifungal agents, the limited efficacy, and the side effects associated with several of these agents increase the importance of continued development of new alternative approaches. This review will examine both synthetic and natural peptides as antifungal therapies, and in this context, we will divide this review into peptides that have a primarily antifungal mechanism of action and peptides that broadly inhibit microbes including bacteria, fungi, and enveloped viruses (9). Because there are a large and diverse number of antifungal peptides in nature, we will mainly focus on those that show promise in treating agricultural and human diseases.
Overview of Antimicrobial and Antifungal Peptides
Antimicrobial and antimycotic peptides are small cationic and amphipathic molecules, generally with fewer than 50 amino acids. These peptides are omnipresent and have been isolated from prokaryotes and eukaryotes in the plant, bacterial, fungal, and animal kingdoms (10–13). Nature has strategically placed antimicrobial and antifungal peptides as first line of defenses between the host organism and its surrounding environment, because these peptides are able to inhibit quickly a wide spectrum of infectious microbes without significant toxicity to the host organism. When insects are infected within a short period of time, they secrete an array of cationic peptides to combat the invading organism (14). Although antimicrobial peptides (AMP) are the primary means of combating organisms in lower forms of life, these peptides have an adjunct role to the immune system in phylogenetically more advanced organisms. Indeed, cationic peptides in humans have an important role and they are produced and secreted by several different tissues, including salivary glands, skin, eye, liver, as well as epithelial and platelet cells and neutrophils (15). Several antifungal peptides display selective toxicity for the microbial target by identifying conserved molecular determinants of pathogens (16, 17). A classic example is the echinocandin family which targets 1,3 β glucan synthase, an enzyme essential for cell wall integrity of fungi (18). In most instances, however, AMP are less specific in their targeting and this results in their exhibiting a broad spectrum of inhibitory/cidal activity not only against fungi but also against bacteria and envelope-containing viruses (19). Broad spectrum AMP often target and lyse the membrane of the microbe, yet these peptides frequently have less proclivity to lyse mammalian cell membranes such as those of red blood cells. The interaction between AMP and target microbes is complex, but the positive charge of the peptides is essential to its binding with negatively charged membrane/wall elements such as the mannoproteins in yeasts (19). Moreover, despite targeting and lysing microbial membranes, the potencies and spectra of activities of these broad spectrum AMPs against different classes of microbes vary and depend on the membrane composition of the pathogen and the structure of the peptide. Much remains to be learned about the subtle differences in microbial membranes that may affect efficacy of the AMP.
Although the focus of this review is on the direct action of peptides against disease-causing fungi, accumulating evidence suggests that the antifungal activity of AMPs is multifactorial. For example, AMPs stimulate the immune system in mammals by several mechanisms: 1) activation of T-cells; 2) stimulation of Toll-like receptors; 3) amplifying phagocyte action; 4) activation of dendritic cells; and 5) chemo-attraction of neutrophils (20–25). Moreover, these activated cells and receptors may reduce the growth of fungi in vivo by modifying levels of various cytokines, chemokines, and integrins (26, 27). Thus, similar to other antifungal agents (28, 29), the interplay between antifungal peptides, their modulation of the immune system, and the host immune status will likely determine the efficacy of the peptide. In addition to their role in providing immunosurveillance against pathogens and maintaining a healthy floral milieu, studies have shown the potential of antimicrobial cationic peptides in cancer and gene therapy (30–33). As peptide-engineering methods develop, the potential for producing sufficient amounts of naturally occurring peptides increases significantly. As a result, the antifungal peptides offer promise for future treatment of infectious diseases in a diverse range of organisms including humans. Figure 1 shows representatives of specific and broad-spectrum antifungal peptides as well as their mechanisms of action.
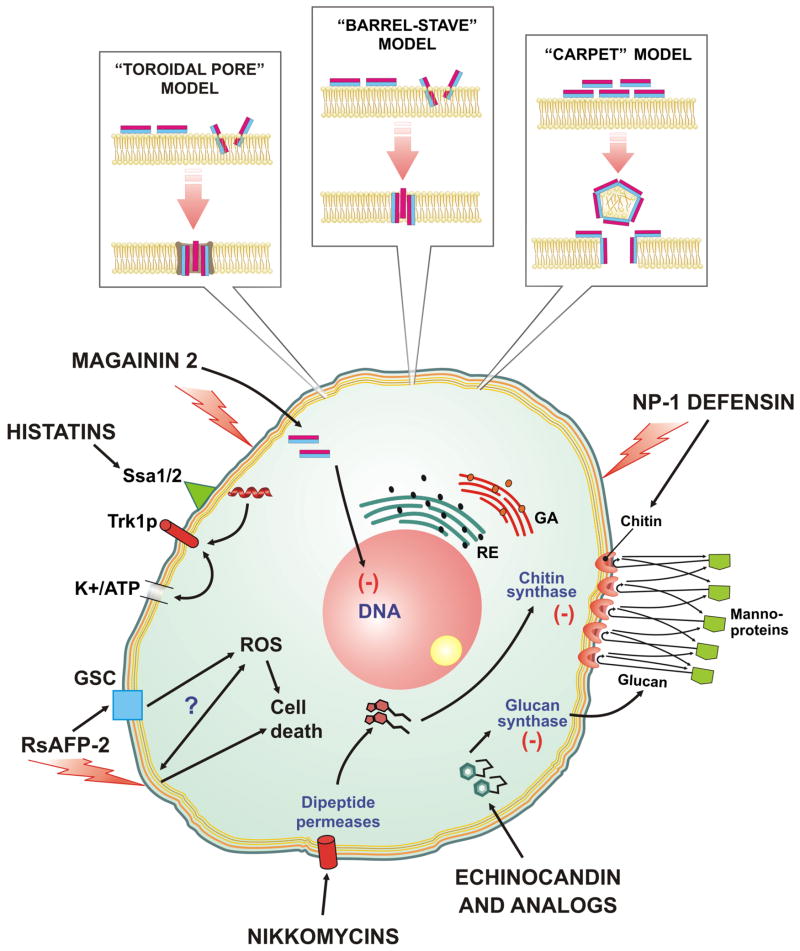
Figure 1. Modes of action proposed for anti-fungi peptides.
The majority of anti-microbial peptides inhibit filamentous fungi and yeasts by membrane lysis: three mechanisms (the toroidal pore, barrel-stave, and carpet) have been proposed to induce lysis of the membranes by AMP. In addition, there are other AMP that are more selective in their antifungal activity. Several anti-fungi peptides disrupt function and/or structure of the cell wall. Defensin NP-1 binds tightly to chitin in the cell wall and this may be important in mediating selective membrane lysis. In addition, chitin biosynthesis is blocked by nikkomycins which enter fungal cells via dipeptide permeases. The lipopeptide echinocandin and its synthetic analogs inhibit synthesis of 1–3 β glucans, a key component of the cell wall. For histatins, one mechanistic concept is based on specific binding with extracellular receptor like Ssa1/2, internalization, and binding to K+ channel transporter Trk1p and as a consequence, efflux of ATP and potassium ions from the cell. MAG-2 magainin kills fungi via cell membrane disruption and DNA damage. The plant defensin, RSAFP-2, causes membrane permeabilization via interaction with glucosylceramide (GSC) and formation of reactive oxygen species (ROS). M – mitochondria; N – nucleus; E – endosomes; L – lysosomes; RE – endoplasmic reticulum; GA – Golgi apparatus.
Because of the disparate structure of antifungal peptides and the incomplete knowledge of their mechanisms of action, classification of the various antifungal peptides is a daunting task. Whereas some lipopeptides (e.g., echinocandins) or histidine-rich (e.g., the linear histatins or branched HK) peptides have primarily antifungal activity, membrane-disrupting peptides (e.g., magainins, protegrins) inhibit a diverse group of microorganisms including fungi, bacteria, and viruses. Structurally, linear cationic antifungal peptides (e.g., LL-37, magainins) form α-helical structures in a hydrophobic milieu while cysteine-containing peptides containing from one to multiple disulfide bonds (e.g., protegrins and defensins) form β-sheet enriched structures. The formation of these α–helical and/or β sheet secondary structures may increase the amphipathicity of the peptides and enable them to act specifically with their targets in the fungal membrane. In addition to histidine-rich peptides such as histatins, other peptides (e.g., apidaecins, indolicidin) have a high percentage of certain amino acids such as proline and tryptophan. Interestingly, potent antifungal linear peptide fragments from larger proteins (e.g., lactoferrin, casein, and lysozyme) are able to inhibit fungi because of multiple direct and indirect effects (34). Notably, for optimal antimicrobial activity, it may be necessary for the peptide structures to undergo post-translational modifications including glycosylation, formation of D-amino acid enantiomers, amidation, halogenation, or phosphorylation (20, 35–39).
Nearly 1200 antimicrobial peptides have now been identified. In this review, we have selected examples of peptides that have antifungal properties with varied mechanisms of action (Fig. 1). For supplementary reviews on antimicrobial peptides, see reviews by Jensen and Hancock (40), Yeaman (41), De Lucca(42), and Bulet (13))
PEPTIDES WITH PRIMARILY ANTIFUNGAL PROPERTIES
Naturally occurring peptides with primarily or exclusively antifungal properties are less ubiquitous than those with broad antimicrobial action. From an evolutionary perspective it was important for nature to form peptides that were functionally omnipotent and could protect all life forms from a great variety of infectious pathogens. Thus, the vast majority of small peptides are actually antimicrobial affecting bacteria, fungi, and enveloped viruses (43). Nevertheless there are some peptides, both natural and synthetic, which exhibit primarily antifungal activity. The potential advantage of these is that the therapeutic window for peptides specific for fungi is greater than that of peptides with broad antimicrobial activity. In contrast to broad spectrum antimicrobial peptides, which induce membrane lysis, most of these antifungal peptides have specific targets that are intracellular, on the cell membrane, or on the cell wall. Because of the diverse targets, the structure of these AMP can vary significantly and includes linear, open ended cysteine-rich cyclic peptides, and cyclic lipopeptides (see Table 1).
Table 1.
Antimicrobial peptides that specifically target fungi
| Name of the group | Representative peptides | Origin | Structure | Mechanism | MW1 | Susceptible strain | References |
|---|---|---|---|---|---|---|---|
| β-glucan synthase inhibitors | Echinocandins Pneumocandins Aculeacins |
Fungi | Cyclic lipopeptides | Inhibition of glucan synthesis | 1000 |
Candida spp. P.carinii Aspergillus spp |
Benz F et al, 1974(47); Fromtling R et al, 1989(50); Iwata K et al, 1982(58) |
| Mulundocandins | Threonine is substituted by serine; and lineoyl with 12- methylmyristoyl | Roy K et al, 1987(60); Hawser S et al, 1999(61) | |||||
| Cell wall chitin inhibitors | Nikkomycins Polyoxins |
Bacteria | Nucleoside peptide antibiotic | Inhibition of chitin synthesis | Nikkomycin Z 495 |
Candida spp. Coccidiodes immitis Balstomyces dermatides Histoplasma capsulatum |
McCarthy P et al, (66); Hector R et al, 1990(67) |
| Aureobasidins | Fungi | cyclic lipophilic depsipeptide with 8 amino acids and an- hydroxyacid | Inhibition of actin and chitin assembly; synthesis of sphingolipids | 1100 |
Candida spp C. neoformans. |
Ikai K et al, 1991(69); Endo M et al, 1997(70); Nagiec M et al, 1997(71) | |
| Membrane-targeting inhibitors | Rs-ARF2 | Plants | α-helical 3-stranded β-sheets, 4 disulphide bridges | Target membrane glycosylcerebroside, induces reactive oxygen species, membrane lysis (?) | 5730 | Candida albicans, C. krusei, A. flavus, Fusarim solani. | Schaaper W et al, 2001(76); Thevissen K et al, 2007(73) |
| Drosomycin | Insects | α-helical 3-stranded β-sheets, 4 disulphide bridges | Target voltage- gated sodium channel (?), Membrane lysis (?) | 5250 |
F. oxysporum N. crassa S.cerevisiae |
Fehlbaum P et al, 1994(74); Bulet P et al, 2005(276); Yuan Y et al, 2007(277); Cohen L et al, 2009(77) | |
| Bacillomycin F Iturin A | Bacteria | Cyclic with lipid-soluble β-amino acid linked to the D/L aa | Lysis by pore formation and leakage of key ions | 1000 |
Aspergillus niger C.albicans F.oxysporum A. flavus F. moniliforme |
Besson F et al, 1984(81) Mhammedi A et al, 1982(82) | |
| Histatins | Histatin 1–12 | Primates | α-helical in hydrophobic environment; increase histidine content | Mechanism uncertain (Target mitochondria vs. Trk1 potassium transporter vs lysis of energized membrane) | Histatin1: 4880; Histatin 3: 4060; Histatin: 5: 3040 | Candida spp. Trichosporon pollulans, Cryptococcus neoformans, A. fumigatus, | Pollock J et al, 1984(88); Helmerhorst E et al, 1999(90); Tsai H et al, 1997(91); Baev D et al, 2004(108); Mochon A et al, 2008(105); Troxler R et al, 1990(278) |
-Approximate molecular weight
1,3-β-Glucan synthesis inhibitors
These specific fungal inhibitors are cyclic lipoproteins that noncompetively inhibit the multiunit membrane-integrated enzyme, β-glucan synthase, critical for cell wall integrity. Inhibition of β-glucan synthase results in destabilizing the cell wall, leading to susceptibility to osmotic stresses and cell lysis. In addition to the cell wall, 1,3- β-glucans have a role in the division septum and assembly of the acropore wall; consequently, these structures are also sensitive to the synthase inhibitors. β-Glucan synthase has a widespread distribution in fungi including Candida, Aspergillus, Cryptococcus, and Pneumocystis species (spp.). Mycelious fungi such as Aspergillus spp., however, are less sensitive to these inhibitors because the synthase is found primarily in tips of the growing hyphae. Inhibition of β-glucan synthase results in negative feedback, causing cell cycle arrest. The family of echinocandins consists of several lipopeptides that differ slightly in their peptide core; this family includes the echinocandins, pneumocandins, molundocandins, aculeacins, and WF11899 (44–46). Notably, members of the echinocandin family are the only peptides thus far approved for severe localized (e.g., pneumonitis) or systemic fungal infections.
Echinocandins and Pneumocandins
Several analogs from these two classes of β-glucan synthase inhibitors have shown promise in preclinical or clinical studies for treatment of invasive and systemic Candida and Aspergillus infections. Of the three subfamilies of echinocandins (B, C and D), analogs of group B have been most useful in the development of antifungal drugs. Echinocandin B, produced by Aspergillus nidulans and A. rugulosus (47), was found to have potent antifungal activity with an MIC between 0.20 and 0.35μg/ml1 for Candida spp. (47). Nevertheless, it was an unsuitable antifungal candidate because it induced lysis of red blood cells. Compared to echinocandins, pneumocandins have a broader spectrum of fungicidal activities (48, 49). This group of cyclic lipopeptides including the most studied, pneumocandin A0 (L-671,329)is produced by Zalerion asboricola and has shown potent activity against Candida spp. and Pneumocystis carinii (50). Similar to echinocandin B, pneumocandin A0 also caused hemolysis. After significant research and screening a number of analogs with varied N-acyl side chain substitutions, the unwanted side effect of hemolysis from these lipopeptides was mitigated with the echinocandin analog, cilofungin (LY121019). While there was no effect on its antifungal activities against A. fumigatus and Candida spp. (51), cilofungin had a 10-fold lower hemolysis than echinocandin B. Nevertheless, clinical trials on cilofungin were stopped because of side effects (52).
Analogs with improved efficacy and safety profile and greater aqueous solubility have now been developed and have received FDA approval for antifungal treatment (53). Currently, there are three approved echinocandins/pneumocandins: caspofungin (second generation pneumocandin, MK-0991) was approved in 2001, micafungin (an echinocandin, FK463) in 2004, while anidulafungin (an echinocandin, LY30366) was most recently approved in 2006. All three drugs have fungicidal activity toward the majority of Candida spp. (C. albicans, C. glabrata, C. tropicalis, C. dubliniensis, and C. krusei) including those that are resistant to fluconazole or amphotericin; moreover, these antifungal lipopeptides are fungistatic toward Aspergillus species. As a representative for these lipopeptides, the MIC of micafungin ranged from 0.0156 to 2 μg/ml for Candida spp. while for Aspergillus spp., the MIC ranged from 0.0078 to 0.0156μg/ml (54). There are a number of fungi in which the microbial activities of these echinocandins/pneumocandins are less effective (e.g., C. parapsilosis, C. guilliermondii, Cryptococcus neoformans, mucormycoses (Rhizopus oryzae), and yeast forms of Histoplasma capsulatum, Blastomyces dermatitidis, and Coccidiodies immitis). Currently, these three lipopeptides have been approved for various Candida infections and caspofungin has been approved to treat invasive aspergillosis when patients are unable to tolerate or are resistant to first-line therapies. Because there are a number of fungi in which the echinocandin family show poor activity, care must be taken to select the appropriate clinical setting to administer these drugs.
Other β-glucan synthase inhibitors
In addition to echinocandins/pneumocandins, there are a number of other related echinocandin family members that will be mentioned briefly. To date, these cyclic lipoprotein analogs have not been clinically approved, and in general, the following classes have reduced antifungal activity and/or greater toxicity compared to the pneumocandins/echinocandins discussed previously.
Aculeacins (A-D, F), isolated from Aspergillus aculeatus, have potent antifungal activity (55–57). In general, aculeacins have a MIC of less than 0.31 μg/ml for most Candida spp., but they are not active against C. tropicalis or most filamentous fungi (58). When aculeacin A was compared with pneumocandin A0, the aculeacin induced significantly more hemolysis at a lower concentration and was slightly less effective in vivo against systemic C. albicans infection in a mouse model (50).
Mulundocandins differ structurally from echinocandins by one amino acid; threonine is substituted by serine, and the lipophilic side chain is 12-methylmyristoyl and not lineoyl (59). Mulundocandins are produced by Aspergillus syndowi var. mulundenis (59, 60) and are effective against C. albicans (MIC, 0.5–4.0 μg/ml), C. glabrata (MIC, 2.0 to 4.0 μg/ml), and C. tropicalis (MIC, 1.0–8.0 μg/ml). Against other species of Candida, mulundocandins are less active and show little to no activity against C. neoformans or filamentous fungi (61).
WF11899A: WF11899A, B and C possess potent anti-Candida activities that are superior to cilofungin, and equivalent to fluconazole; unfortunately, they lyse mouse red blood cells at low concentrations (62).
Inhibitors of Chitin in the Cell Wall
Nikkomycins, produced by Streptomyces tendae and S. ansochromogenes, and polyoxins, produced by Streptomyces cacaoi, are the most widely studied peptidyl nucleoside inhibitors of chitin synthase (63–65). Synthase inhibitors such nikkomycin are structural analogs of uridine diphosphate N-acetylglucosamine, a major constituent of chitin. Absent in vertebrates, chitin is a cell wall component that is essential to maintain the structural integrity of the fungus. Nikkomycins are able to inhibit chitin synthesis in C. albicans both in vitro and in vivo studies (66) and they are not toxic to human cells; they also show significant activity against C. immitis, B. dermatides, and moderate activity against H. capsulatum (67, 68), but these agents are not active against filamentous fungi. Despite their advantages, the use of these inhibitors has been limited because of unfavorable pharmacokinetics.
Aureobasidins: There are 18 members of the Aureobasidins family, produced by Aureobasidium pullulans. They are cyclic depsipeptide lipophilic antibiotics made up of eight amino acids and an α-hydroxyacid (69). Two modes of action have been proposed for aureobasidins: one is based on disruption of cell wall/membranes by altering the assembly of actin and chitin (70) and the other is based on interrupted synthesis of sphingolipids (71). Several members of the Aureobasidin family are active against Candida spp. (e.g., MIC, Aureobasidin A, <0.05 to 0.2 μg/ml), and C. neoformans (e.g., MIC, Aureobasidin A, 0.78 μg/ml) (72).
Membrane-active Selective AMPs
Rs-ARF2, isolated from radish seeds, is a 50 amino-acid residue plant defensin that has an α-helix and three-stranded β-sheets stabilized by four disulfide bridges. Rs-ARF2 shares structural and functional homology with other plant defensins, HsAFP1 and DmAMP1, and the insect defensin, heliomicin (73–75). Notably, these defensins have no significant effect on bacteria, but have marked antifungal effects. Rs-ARF2 causes rapid K+ efflux, Ca2+ uptake, and alkalinization of the medium by targeting the fungus-specific membrane glucosylceramide and by inducing membrane permeability. After targeting the ceramide in the membrane, this defensin also induces reactive oxygen species intracellularly that are toxic to the fungi. Following 7.5 h of incubation, RsAFP2 (10 μM) inhibited C. albicans and C. krusei by 99.8% and 91.1%, respectively (73). C. glabrata, which does not contain this fungus-specific ceramide, was not inhibited. Furthermore, the MICs of RsAFP2 toward A. flavus and Fusarium solani were 0.7 and 0.04 μM, respectively. Analogs of Rs-ARF2, in which arginines replaced neutral amino acids, were more effective against F. solani. Moreover, replacement of cysteines with alpha-aminobutyric acid improved the antifungal potency of Rs-ARF2 (76). Unlike iturins discussed later in this section, Rs-ARF2 and its close analogs have little cytotoxicity to mammalian cells at dosages that are inhibitory to fungal pathogens.
Drosomycin, an insect defensin structurally related to Rs-ARF2, is an inducible 44-residue cysteine-rich peptide produced by Drosophila melanogaster (74, 75). Despite its sequence homology with Rs-ARF2, the underlying antifungal mechanism of drosomycin remains unclear. Based on the interaction of drosomycin with the voltage-gated sodium channel in D. melanogaster, Cohen and colleagues have suggested that this interaction may have a role in the inhibition of microbial pathogens (77). More is understood about the signal transduction pathways that up-regulate drosomycin levels in the hemolymph of D. melanogaster. After insects are challenged by bacteria, drosomycin, under the control of activated Toll receptors, reaches its peak concentration in the hemolymph at 16 h (74, 78); despite its induction by bacteria, this defensin has no significant antibacterial effect (74). Drosomycin has significant fungicidal activity against filamentous fungi (e.g., MIC<5 μM, F. oxysporum, Neurospora crassa) (79) and recent studies have revealed its antiyeast (MIC, 12 μM, S. cerevisiae) activities (80).
Iturins, produced by Bacillus subtilis, are cyclic peptides with a lipid-soluble β-amino acid linked to an array of D and L amino acids. Iturins act on microbial membranes causing pore formation and leakage of key ions of fungi (81), but their antimicrobial activity is limited primarily to fungi, with little effect on bacteria. Unfortunately, iturins are toxic to mammalian cell membranes. In contrast to most other antimicrobial peptides which are cationic, iturins may be anionic (bafilomycin L) or neutral (iturin A). One member of the family, bafilomycin F effectively inhibits Aspergillus niger (MIC, 40 μg/ml), C. Albicans (MIC, 40 μg/ml), C. tropicalis (MIC, 40 μg/ml), and several phytopathogens such as Mycosphaerella pinodes (MIC, 10 μg/ml) (82). In contrast to its potent antifungal activity, bafilomycin F modestly inhibits the bacterium, Micrococus luteus (MIC, 200 μg/ml), but has no inhibitory effect on other bacteria tested (e.g., MIC> 400 μg/ml: Escherichia coli K12, Streptomyces albus G, Staphylococcus aureus). In addition, with a radial diffusion assay, Klich et al. found that Iturin A had marked antifungal activity against phytopathogens and human pathogens such as A. flavus (83); most fungi were inhibited at 7.7 μg/ml with no change in the zone of inhibition for several weeks. Although this group of peptides is effective against dermatomycoses in humans and animals, they induce unacceptable levels of red blood cell lysis (84). Nevertheless, there is a growing interest in substituting toxic pesticides with these peptides for treatment of fungal infections in plants.
Histidine-rich Peptides
Histatins are a group of linear cationic peptides that are isolated from human saliva and have potent and specific biological activity against fungi; their weak amphipathic character, lack of disulfide bonds, and high content of histidines distinguishes them from other cationic peptides (85). Whereas histatins form random polymers in aqueous environments, they adopt α-helical structures in hydrophobic environments and this has been posited to have a role in their antifungal activity (86, 87). A key feature of histatins is their strong candidacidal activity, which includes C. albicans, C. glabrata, C. guillermondii, C. krusei, C. lambica, C. parapsilosis, C. pseudotropicalis, C. stellatoidea, and C. tropicalis (88, 89), C. neoformans and A. fumigatus (90, 91). This family of 12 small (3–4 kD) histidine-rich peptides is created by proteolytic cleavage of histatins-1 and -3 (92) and are exclusively found in saliva of primates (93–95). Of these peptides, histatin 5 has the strongest fungicidal activity with an MIC of 100 μM against C. albicans (96). P-113, a 12-mer amino acid fragment of histatin 5, is the smallest peptide that retains full anticandidal activity compared to its parent peptide (97). In addition to inhibiting yeast cells, histatin 5 and two synthetic variants (Dhar4 and 5) markedly inhibited formation of the drug-resistant biofilm of C. albicans (98–100). Besides mammals, arthropods also produce histidine-rich peptides that are active against certain fungi such as C. albicans but are not active against bacteria (101–103). Compared to histatin 5, synthetic branched histidine-rich peptides are significantly more inhibitory (about 15-fold on molar basis) against C. albicans (104). Moreover, these branched antifungal histidine-rich peptides are highly selective, and have little toxicity toward mammalian cells.
Of interest is the rationale for use of these histidine-rich peptides that provides selective fungicidal activity. Although there are several mechanisms proposed including one study that indicates that histatins interact with an energized membrane (105), most data indicate that histidine-rich peptides must traverse the membrane and interact with an intracellular target (106–108) (see Fig. 1). Interestingly, histidine-rich peptides by our laboratory have been developed as nucleic acid carriers because of their ability to traverse membranes and disrupt acidic endosomes (32, 33, 109). Although we think that the mechanisms of killing fungi by histatins and branched HK polymers are closely linked, this supposition has not yet been proven.
ANTIFUNGAL PEPTIDES WITH WIDE SPECTRUM OF ANTIMICROBIAL ACTIVITY
In contrast to the peptides that specifically target fungi, most antimicrobial peptides affect a number of organisms including bacteria, fungi, and envelope-containing viruses. A common theme with most of these wide-spectrum AMP is that they lyse the membranes of the pathogen. Despite this non-specific mechanism, many of these peptides do not lyse mammalian membranes at concentrations of peptides that can inhibit the pathogen. Because the number of AMP that can affect bacteria, fungi, and viruses is extensive, we will discuss selected examples of linear and cyclic antifungal peptides made by several different species (see Table 2).
Table 2.
Broad-Spectrum Antimicrobial Peptides
| Name of the group | Representative peptides | Origin | Structure | Mechanism | MW1 | Susceptible strain | References |
|---|---|---|---|---|---|---|---|
| Cecropins | 60 members; Cecropin A | Insects | α-helical Tryptophan in the first or second position; C- amidated end | Membrane lysis | 2800–4000 |
Aspergillus spp. Fusarium moniliforme Fusarium oxysporum |
Giacometti A et al, 2001; (121) De Lucca A et al, 1988; (124) |
| Magainins | Magainin 2 | Frogs | α-helical Rich in glycine and serine amino acids | Membrane lysis; DNA damage | 2500–3000 |
C. albicans C. neoformans Saccharomyces cerevisiae |
Zasloff M 1987(111); Morton C et al, 2007(114) |
| Bombinin-like and bombinin H | BLP-1,3 Bombinins H1-7 |
α-helical Glycine-rich; C- terminal amino acid amidated | Membrane lysis | 2300–4000 |
C. albicans C. guillermondii C. tropicalis |
Simmaco M et al, 2009(137) | |
| Dermaseptins | Dermaseptin S1-5 | α-helical Lysine-rich | Membrane lysis; Apoptosis induction | 2500–3500 | A. fugimatus | Mor A et al, 1991(112); Morton C et al, 2007(114) | |
| Cathelicidins (without cysteines) | LL-37; mCRAMP; BMAP-27–28 | Humans, cattle, pigs, goats, mice | α-helical | Membrane lysis | 3000–4000 |
C. albicans C. neoformans |
Boman H et al, 2003(279); Benincasa M et al, 2006(160) |
| Indolicin; Tritrpticin | Extended wedge Rich in tryptophans | Membrane lysis | 1900–2000 |
C. neoformans Candida spp. A. fumigatus |
|||
| PA26 | PA26 | Combinatori al Library | Penatratin-like hexapeptide | Unknown | 950 | Penicillum digitatum, S. cerevisiae | Munoz A et al, 2006(280) |
| Kaxins | dF21-10K | Synthetic design | Non-amphipathic hydrophobic core N-terminal lysine-rich | Membrane- lysis | 1830–2350 | Candida spp. | Burrows H et al, 2006 (175) |
| Thanatin | Thanatin | β-sheet, single disulfide bond forming C- terminal | Membrane lysis | 2400 |
A. fumigatus N. crassa T. mentagrophytes |
Fehlbaum P et al, 1996(176); Bulet P et al, 1999(281) | |
| Cathelicidins (with cysteines) | Protegrins1-5 | Pigs | β-sheet, 2 disulfide bridges | Membrane lysis | 1900–2200 | C. albicans | Kokryakov V et al, 1993 (282) |
| α-Defensins | HNP1-4; HD5-6; NP-1 | Mammals | Cyclic: 3, Disulfide bridges C1-6, C2-4, C3-5 | Membrane lysis | 3000–4000 |
Candida spp. A. fumigatus C. neoformans |
Ganz T et al, 2002 (30); Lehrer RI 2004(283); Levitz S et al, 1986(202) |
| β-Defensins | HBD1-4 Bovine tracheal antimicrobial peptide | Mammals | Cyclic: Disulfide bridges C1-5, C2-4, C3-6 | Membrane lysis | 3500–5000 |
Candida spp A. fumigatus |
Diamond G et al, 1991 (224); Joly S et al, 2004(222) |
| θ-Defensins | rTD1-3 | Rhesus monkeys | Circular octadecapeptides, 2 anti-parallel β-sheets and 3 disulfide bonds | Membrane lysis | 2100 |
C. albicans C. neoformans |
Tang, YQ et al, 1999 (20); Tran, D et al, 2008(225) |
| Gallinacins | Gallinacin-1 and 1 α, gallinacin-2 and -6 | Chicken | β-sheet, rich in lysine and arginine, 3 disulphide bonds | Membrane lysis combined with effect on DNA replication, RNA and protein synthesis | 2000–5000 |
C. albicans S. cereviseae |
Van Dijk A et al, 2007(227) |
| Macrocycles | Kalata Circulin A,B Cyclopsychotride | Plants | Cyclic knot | Membrane lysis | 2800–3400 |
C. kefyr C. tropicalis |
Tam J et al 1999(228), |
| Syringomycins Pseudomycins | Syringomycin E Syringotoxin B Syringostatin A |
Bacteria | Lipodepsinonapeptides | Membrane lysis | 2300–3500 |
Candida spp Aspergillus |
Feigin A et al, 1996(229); Sorensen K et al, 1996(231) |
| Lactoferrin2- dervived peptides | Peptide 2 Lactoferricin | Mammals | Glycosylated protein, two symmetrical lobes (N and C lobes), mixture of α-helix and β-sheet | Membrane lysis | 2600–4660 | C. albicans Crypt. albidus,, Dekkera bruxellensis, Pichia membranifaciens, Saccharomyces cerevisiae, Zygosaccharomyce s bisporus | Gonzalez- Chavez S et al, 2009(284) Ueta E et al; 2001;(242) Viejo-Diaz, M. et al, 2005 #4524}(241) |
| BPI protein2 domain III analogs | XMP.284 XMP. 366 XMP.391 |
Lipid binding protein; the amino-terminal rich in lysine residues | Membrane lysis | 1550 |
Candida spp C. neoformans A. fumigatus Histoplasma capsulatum |
Elsbach, P. et al, 2003(245) |
MW, approximate molecular weight;
The molecular weights of lactoferrin and BPI are 55000 and 80000, respectively
Linear peptides
Small linear primarily α-helical peptides are the most common and well-studied group of antimicrobial peptides and include families such as cecropins (110), magainins (111), and dermaseptins (112). Because α-helical amphipathic peptides differ in amino acid composition as well as in length and positive charge, their antimicrobial activity is likely determined by their global structural components rather than by the specific amino acid sequence (113). The final common pathway for these α-helical amphipathic peptides is primarily disruption of the cell membrane; consequently, these peptides have widespread activity against bacteria, fungi, and membrane-enveloped viruses. Nevertheless, accumulating data suggest that at least some of these peptides also have unique intracellular targets (114–116). In addition, many of them are able to lyse cancer cells at concentrations up to 10-fold lower than those required to lyse normal human cells (117).
Cecropins and cecropin-like peptides have a broad spectrum of antimicrobial activity (bacteria and fungi) and have primarily been isolated from the hemolymph of silkworm moths (118, 119). These peptides range from 29 to 42 amino acids in length and form α-helices in hydrophobic environments such as the plasma membrane. With few exceptions among the insect cecropins, there are two distinguishing characteristics: 1) a tryptophan in the first or second positions; and 2) an amidated C-terminal amino acid (13). Although the primary target of cecropins is the plasma membrane, cecropin A at its microbicidal dose does not affect mammalian cells and numerous studies have shown that this peptide can be administered safely to animals (120–122). At concentrations between 25 and 100 μg/ml cecropin A effectively killed Aspergillus spp., and at concentrations of 12.5 μg/ml the peptide effectively killed Fusarium moniliforme and F. oxysporum (123, 124). Notably, genetically modified rice that expressed cecropin A gene was completely protected from the blast fungus, Magnaporthe grisea (125). In addition, no toxicity was reported in transgenic mice expressing a cecropin-like peptide (e.g., Shiva-1) (122). Thus, cecropins are a promising class of antifungal peptides because of their safe therapeutic profile.
Magainins, isolated from the skin of Xenopus laevis (the African frog), are a family of cationic amphipathic peptides that range between 21 and 26 amino acids in length and are enriched in glycine and serine residues (111, 126, 127). The magainin family of peptides includes magainin I, II, 2, PGLa peptides, xenopsin and the caerulein precursor fragment (128–130). Some of these family members including magainin 2 and PGLa may form heterodimers, which increases their ability to permeabilize the membranes of pathogens (131). In addition to cidal activity of magainins against gram-negative and gram-positive bacteria and protozoa, magainins have antifungal activity against Candida spp., C. neoformans, and Saccharomyces cerevisiae. Magainin 2 (Mag 2) is particularly active against C. neoformans (MIC, 6.25 μg/ml) with greater activity than three other cationic peptides (132). Although Mag 2 has moderate activity toward C. albicans (MIC > 80 μg/ml), it potently inhibits C. glabrata (MIC, 25.0μg/ml), C. tropicalis (MIC, 12.5 μg/ml), and C. krusei (MIC, 12.5–25.0μg/ml) (111, 132). Similar to many other antimicrobial peptides, magainins show selectivity in their cidal activity toward pathogenic fungi (and bacteria). Nevertheless, the therapeutic window for natural magainins is not great enough to allow treatment of systemic infections in humans. As a result, magainin analogs or hybrid peptides with greater activity are being developed (133–135). For example, Avrahami and colleagues showed that conjugating a lipophile with magainins greatly increased their activity against C. neoformans (136). In addition to its membrane-lysis mechanism, Mag 2 has an alternative antifungal action by interfering with the DNA integrity of fungi (114); this study raises the question as to whether many cationic peptides kill fungi by mechanisms other than targeting the membrane.
Bombinin-H and bombinin-like peptides isolated from skin of Bombina genus (137, 138) are glycine-rich, weakly cationic peptides with their C-terminal amino acid amidated (139). In addition to its inhibiting bacteria, bombinin-like peptides (BLP-1, 3) are active against fungi, especially C. albicans (MIC, BLP-1, 3–0.4 μM). Importantly, these peptides have little hemolytic activity (<10%, 15 μM). Bombinin-H peptides have varied antimicrobial and hemolytic activity (139, 140). Bombinins H2 and H4, which damage cell membranes of microbes, were found to be active against C. albicans (MIC, H2, 3.1μM; H4, 1.6 μM) C. guillermondii (MIC, H2, 1.3 μM; H4, 0.7 μM), and C. tropicalis (MIC, H2, 1.1μM; H4, 0.6 μM) (141, 142). While bombinin-H2 induces 11% hemolysis, H4 induces 28 % hemolysis at 15 μM. Other bombinin H peptides, H6 and H7, with greater hydrophobicity, have lower antimicrobial activity and induce greater hemolysis than do H2 and H4. Notably, H2 and H4 peptides are active against the spores of the fungus, Phytophthora nicotianae, with a minimal fungistatic concentration of 10 and 18μM (143). Although some bombinin H peptides differ in their conformations as a result of stereochemistry modifications at the second position, these bombinins have similar antimicrobial activity (144).
Dermaseptins (S and B) have between 28 and 34 amino acids and are lysine-rich peptides with a tryptophan characteristically at position 3. They were identified in the skin of tree frogs of the genus Phyllomedusa (112). By interfering with lipid layers, which leads to osmotic imbalance, dermaseptins lyse a wide spectrum of microorganisms. Remarkably, dermaseptins often exhibit synergy with one another, resulting in 100-fold increase in antimicrobial action compared to dermaseptins separately (145). In addition to their anti-bacterial, antiviral, and anti-protozoa activity, they are cidal to pathogenic fungi (146, 147) including yeasts and some filamentous fungi (A. fumigatus) (148). For example, a synthetic dermaseptin s1 analog, a 16-mer peptide, shows marked activity against C. albicans (MIC, 5.8 μM), and notably this analog has little hemolytic activity (149). Interestingly, the presence of a phenyl group in position 3 selectively inhibits C. albicans more effectively than it inhibits bacteria. In addition to pathogenic fungi, a synthetic derivative of dermaseptin (e.g., MsrA2 (N-Met-dermaseptin B1), elicits strong antimicrobial activities against virulent phytopathogenic fungi and protects transgenic potatoes from a broad spectrum of fungal infections (150).
Metchnikowin is a 26 residue proline rich inducible peptide isolated from Drosophila. This peptide exhibits activity against gram-negative bacteria and filamentous fungi, but does not inhibit gram-positive bacteria. Studies with metchnikowin against fungi have been quite limited, but it has potent activity against N. crassa (0.5 to 1 μM).
LL-37 (CAMP18) is the only member of the cathelicidin family of host defense peptides expressed in humans (151). Members of the cathelicidin family are characterized by a conserved region and a highly variable domain that contains the mature antimicrobial peptide. In some species (cattle, pigs, goats), there are multiple antimicrobial cathelicidin peptides that show dramatic diversity in size, charge, and structure, including α-helical or β-sheet formations. LL-37, however, is an α-helical antimicrobial peptide named for the two N-terminal starting leucines and its length of 37 amino acids; the peptide is widely distributed in humans in skin, gastrointestinal tract, urinary tract and respiratory tract (152, 153). The peptide is expressed by a number of cells including monocytes, neutrophils, NK cells, B and T cells (154–156) and is upregulated by immune stimuli (153) and by Vitamin D (157, 158). Both LL-37 and a close analog, mCRAMP in mice, have a similar MIC range for C. albicans of 15- to 20 μM (159). In one study, mCRAMP was induced by C. albicans at the skin surface in a mouse model, demonstrating that these peptides provide a natural barrier to fungal infections. Other cathelicidin α-helical peptides have shown activity (BMAP-27, 28 from cows) against Candida spp. and C. neoformans but these were less active against filamentous fungi (160). Besides its antifungal activity, LL-37 has potent antimicrobial activity against all isolates of B. pseudomallei independent of their LPS phenotype (161). LL-37 also has an essential role in promoting angiogenesis and as a wound healing agent (162). At higher concentrations, LL-37 induces the production of cytokines and chemokines, and at physiological concentrations LL-37 alters IL-8 production by keratinocytes and bronchial epithelial cells in response to inflammatory conditions (163). Thus, LL-37 plays an important role in epithelial innate immunity (152). In addition to its antimicrobial activity, reduction or excess production of LL-37 has been associated with the skin conditions, atopic dermatitis and rosacea, respectively (164, 165).
Indolicidin and tritrpticin are tryptophan-rich antimicrobial peptides that also belong to the cathelicidin family (166, 167). Indolicidin and tritrpticin are expressed in neutrophils (168) of cow and pigs, respectively. Unlike the α-helical peptides previously discussed, these tryptophan-rich peptides have an extended wedge shape conformation in hydrophobic environments such as the plasma membranes (166, 167, 169). Indolicidin is a 13-amino acid peptide (ILPWKWPWWPWRR) that contains 5 tryptophans (39%) and 3 prolines (25%) and its C-terminal amino acid amidated. In addition to its high activity against S. aureus and E. coli (167), indolicidin has potent antifungal activity against C. neoformans (MIC, 2–4 μg/ml) and good to moderate activity against Candida spp. (MIC,8–32 μg/ml) with the exception of C. guillermondii (MIC>32 μg/ml) (160). In addition, Ahmad and colleagues reported that incorporation of indolicidin within liposomes minimized its toxicity, enabling higher dosages to be administered to cure mice with systemic infections of A. fumigatus; in contrast, maximal tolerated dosages of free indolicidin did not cure mice of such infections (170). Although an important mechanism for killing microbes may be its action as a membrane ionophore, two studies have suggested that the DNA of the microbe is also an important target of indolicidin (115, 116).
Tritrpticin is also a 13-amino acid peptide (VRRFPWWWPFLRR) containing 3 tryptophans (23%), 4 arginines (30%), and 2 prolines (15%). Although no in vivo studies have been done to test the efficacy of tritrpticin, Lawyer et al determined that this peptide had weak activity toward C. albicans (MIC, 1000 μg/ml) and A. fumigatus (250 μg/ml) (166) Unlike the C-terminal amino acid of indolicidin which is amidated, the C-terminal amino acid of tritrpticin is not. When the terminal amino acid of tritrpticin is amidated, antibacterial activity increases 2- to 8-fold while hemolytic activity decreases significantly (171); it is not known whether this increased antimicrobial activity extends to killing fungi. Interestingly, Yang et al found that prolines in tritrpticin were essential for microbial selectivity in that replacing them with alanine markedly increased hemolysis (169).
PAF26, a penetratin-like hexapeptide with the sequence Ac-RKKWFW-NH2, was identified as a lead candidate against phytopathogenic fungi after screening a combinatorial library of hexapeptides. The MIC of PAF26 against Penicillum digitatum, a fungus that causes postharvest decay in fruits, is 4μM. Munoz and colleagues recently found that addition of tryptophan to the N-terminal amino acid increased its activity against yeast (MIC, S. cerevisiae, 16 μM) and gram-negative bacteria (MIC, E. coli, 4 μM) (172). PAF26 and its analogs showed little to no hemolysis at 100 μM. In addition, PAF26 has been fused with magainin to augment their antimicrobial activity (173).
Kaxins are synthetic cationic antimicrobial peptides that have a non-amphipathic hydrophobic core segment (174, 175). By inserting lysines at the N-terminal end and creating D-enatiomers peptides, Burrows and colleagues developed kaxins with potent candidacidal activity with little lysis. One kaxin, dF21-10K (kkkkkkkkkkaafaawaafaa-NH2), showed MIC between 16 and 64 μg/ml against all fluconazole-sensitive and resistant Candida spp. and strains (C. albicans, C. dubliniensis, C. glabrata, C. guillermondii, C. krusei, C. lusitaniae, C. parapsilosis and C. tropicalis) (175). Notably, dF21-10K showed marked activity with complete killing against biofilms formed by C. albicans or C. tropicalis. Although elimination of biofilms by antifungal molecules usually requires concentrations 30–2000 times more than their MIC (100), dF21-10K eradicated biofilms at only 10 times their MIC (175).
Cyclic peptides
The vast majority of broad spectrum cyclic antimicrobial peptides contain between 1 and 4 disulfide bonds and adopt β-sheet enriched structure including beta-hairpin, beta-sheet, or alpha-helix/beta-sheet mixed structures. Most of these AMP contain open-ended cyclic structures formed by internal disulfide bonds but θ-defensins, in addition to internal bonds, form closed-ended cyclic structures. With peptides such as defensins that contain multiple disulfide bonds, formation of the correct bond remains a challenge for developing peptide technologies.
Thanatin is an inducible and nonhemolytic 21-amino acid peptide isolated from the insect, Podisus maculiventris. Compared to other arthropod AMPs, thanatin has broad spectrum antimicrobial activity. Thanatin has a single disulfide bond forming a C-terminal loop that possesses a strong positive charge. Although it has little homology with other arthropod AMP, thanatin does have homology in its amino acid sequence, structure, and biological function with brevenins found in frog secretions. Thanatins are fungicidal against several phytomycotic diseases (e.g., MIC, N. crassa, Botrytis cinerea, Nectria haematococca, Trichoderma viride, Alternaria brassicola, and Fusarium culmorum < 5 μM), and two pathogenic fungi in humans (MIC, A. fumigatus, 10–20 μM; T. mentagrophytes, 20–40 μM) (176). These peptides have no activity against yeasts such as S. cerevisiae or C. albicans. Interestingly, close homologs of thanatin isolated from skin secretions of frogs do have activity against S. cerevisiae and C. albicans (MIC, Brevinin-1E, 4.7 μM) (177)
Protegrins are approximately 2-kD cysteine-rich β-sheet peptides found in neutrophils of pigs. Similar to LL-37 and indolicidin, protegrins belong to the cathelicidin family of peptides, but unlike these two linear peptides, protegrins are cyclic antimicrobial peptides. Protegrins contain 16–18 amino acids and have 2 disulfide bridges which are essential for their antimicrobial activities, especially at physiological salt concentrations (178, 179). In contrast to defensins, protegrins display full antimicrobial activity in the presence of physiologic saline (180). The ability to maintain activity in the presence of salt is critical for AMP to exhibit their antimicrobial effects systemically or perhaps via aerosolized therapy. Although protegrins have limited homology with defensins, they share significant homology with tachyplesins found in hemocytes of horseshoe crabs (181, 182). Protegrins display broad spectrum activity against bacteria, fungi, protozoa, and viruses (20, 179, 183–186) and their primary mechanism of microbial action is due to lysis of the microbial membrane (187). Cho and colleagues examined the anticandidal activity of protegrins 1–5 (188), and found that the protegrins 1–3 and 5 had greater anticandidal activity (MIC range, 2.50–2.85 μM) compared to protegrin 4 (MIC, 4.78 μM). In addition, a D-enantiomer of protegrin-1 had slightly greater activity compared to protegrin-1. Other analogs of protegrin have been made with either greater antimicrobial activity and/or with decreased hemolysis, but these analogues have not been tested against fungi.
Mammalian defensins are a family of cationic peptides containing 6 highly conserved cysteine residues with 3 disulfide bridges (189, 190) that are divided into three subfamilies: α-, β-defensins are found in many mammalian species and θ-defensins are found in Rhesus macaques. The α- and β-defensins differ in amino acid sequence and in the location of disulfide bonds, but their 3-D structures are virtually identical; they both contain 3 antiparallel β-sheets and one α-helix (191). Whereas mammalian defensins usually have antimycotic properties against C. albicans (192, 193), there are distinct variations in their antifungal activities.
α-defensins have between 29 and 36 amino acids in the mature peptide with 3 disulfide bridges between cysteines 1–6, 2–4 and 3–5. Four α-defensins (human neutrophil peptides 1–4; HNP1-4) are constitutively expressed in human neutrophils (30, 194–196), and two other α-defensins, HD 5 and 6, are expressed in specialized epithelial cells (e.g., Paneth cells) of the intestines and female urogenital tract (197–199). In addition to membrane “pores” as a mechanism for microbial inhibition, HNP-1 and HNP-2 are able to inhibit protein, RNA, and DNA synthesis (200, 201). Lehrer et al. compared HNP1-3 defensins for their anti-candidal activity and found that HNP-1 was the most effective. Although HNP-3 at a concentration of 50 μg/ml showed little to no inhibition of C. albicans, HNP-1 greatly reduced the number of colony-forming units and was about 10-fold more effective than was HNP-2 (193). Furthermore, α-defensins from rabbits, NP-1, NP-2, and NP-3, are highly effective against C. albicans (193). Indeed, NP-1 had 10-times greater candidacidal activity compared to HNP-1. In addition to Candida spp, the rabbit defensins killed A. fumigatus (minimal fungicidal concentration, NP-1, 25 μg/ml, NP-2, 50 μg/ml, and NP-3, 100 μg/ml) as determined by a MTT assay (202), and both rabbit NP (MIC, NP-1, 3.75 to 15 μg/ml) and human HNP inhibited the growth of C. neoformans (203, 204). Because NP-1 and 2 defensins bind tightly to chitin and its fragments, this interaction between these peptides and the cell wall of fungi may be important in their cidal activity (202).
β-Defensins are generally larger than their α-counterparts and contain 38–47 amino acids with disulfide bonds between cysteines 1–5, 2–4 and 3–6. The human β-defensin (HBD) family consists of at least 6 members. HBD-1, HBD-2, and HBD-3 are mainly produced by epithelial cells and have an important role in immunity of mucosal and body surfaces (205–210). In contrast to HBD 1–3 which are present in many tissues, HBD-4 is found primarily in the gastric antrum and testes (211) and HBD-5 and 6 are specifically expressed in the epididymis (212). β-Defensins such as HBD-1 may be expressed constitutively (209, 213) or those, including HBD-2 or -3 may be induced by various proinflammatory mediators (e.g., TNF- α, IL-1 β) (214–221). Whereas the antibacterial activity of HBD1-4 has been studied by several groups, antifungal studies of β-defensins has been limited to the inducible HBD-2 and HBD-3 peptides (222). Although there was a strong correlation between HBD-2 and 3 in their inhibition of Candida spp., HBD-3 was generally more effective. The MIC of HBD-2 and -3 for three isolates of several Candida spp. was as follows: C. albicans (MIC range: HBD2-- 4.6–59.2μg/ml; HBD3-- 2.8–7.1μg/ml), C. tropicalis (MIC, HBD2--3.9–13.1 μg/ml; HBD3--3.3–14.4 μg/ml), C. parapsilosis (MIC, HBD2-- 9.3–17.8μg/ml; MIC, HBD3--1.4–12.4 μg/ml), C. krusei (MIC, HBD2--12.2 >250μg/ml; HBD3--2.0–13.7 μg/ml), C. glabrata (MIC, HBD2-- 22.7 >250 μg/ml; HBD3-- 33.8 >250μg/ml) (222). Notably, HBD-3 inhibited the growth of the 3 isolates of C. krusei while 1 of the 3 isolates was resistant to HBD2. Bovine tracheal antimicrobial peptide (TAP), an analog of HBD-2 that is secreted by respiratory epithelial cells, inhibits the C. albicans (MIC, 400 μg/ml (223) vs. MIC, 6–12μg/ml (224)) and the hyphal form of A. fumigatus (400 μg/ml) (223). The discrepancy of MIC may be due to differences in the strains of C. albicans or in different growth media. In addition to their direct antimicrobial properties, β-defensins can modulate the function of immune competent cells (23).
θ-defensins: Three θ-defensins (rTD-1, rTD-2 and rTD-3) were isolated from extracts of granulocytes from Rhesus monkeys (20). These defensins are circular octadecapeptides that contain 2 anti-parallel β sheets and 3 disulfide bonds (20) that are formed by post-translational splicing of 2 nonapeptides which are derived from α-related defensin precursors (20). θ-Defensins possess a wide spectrum of salt-independent antimicrobial activity against bacteria, viruses (both enveloped and nonenveloped), and fungi. Although they share similar sequence and structural homology with protegrins, they induce significantly less hemolysis. Interestingly, θ-defensins differ from other AMP in their interaction with membranes which perhaps explain their reduced proclivity to induce hemolysis. The rTD1-3 defensins have significant activity against C. albicans (MIC, rTD1,2--1μg/ml; rTD3--3μg/ml) (20, 225) and are also active against C. neoformans (20). Their activity against other fungi has not been reported.
Poultry gallinacins, produced by leukocytes, are rich in arginines and lysines, have 3 disulfide bonds, and are functional and structural equivalents to mammalian β-defensins (226). While gallinacin-1 and 1 αinhibited C. albicans, gallinacin-2 did not (226). Strong bactericidal and fungicidal activities (MIC, 8 μg/ml, C. albicans, S. cereviseae) were observed for gallinacin-6, which is expressed in the digestive tract of chickens (227). Because of the potential resistance developing in microbes exposed to low levels of antibiotics, there has been increasing interest in stimulating endogenous AMP such as gallinacin 6 to suppress microbial growth in chickens.
Macrocyclic peptides, isolated from coffee plants, contain 4 end-to-end large cyclic peptides with 6 disulfide bonds (228). Four macrocyclic cystine-knot peptides of 29–31 residues, sharing 45% homology with one another, belong to this family (kalata, circulin A and B (CirA and CirB), and cyclopsychotride (cpt)). In contrast to kalata and CirA, which are relatively ineffective against gram-negative bacteria such as E. coli and Pseudomonas aeruginosa, CirB and cyclopsychotride kills both gram-positive and gram-negative bacteria, in particular E. coli. Although the cyclic peptides were inactive against C. albicans, all peptides in a low salt assay were moderately active against C. kefyr (MIC range-14.0 to 29.0 μM) and two of the four peptides were active against C. tropicalis (MIC, CirA, 21.4μM; Cpt, 56.5μM (228). The antifungal and antibacterial activities of these peptides were significantly diminished when 100 mM NaCL is added to the assay medium. Unless improved analogs are discovered, macrocyclic peptides will likely have a limited role as antifungal agents.
Syringomycins and related peptides are a group of cyclic lipopeptides, called lipodepsinonapeptides, produced by Pseudomonas syringae. This group of peptides induces ion channels that affect membrane function, including membrane potential, protein phosphorylation, and H+-ATPase activity (229, 230). The family members (syringomycin E, syringotoxin B, and syringostatin A) were effective in vitro against a number of isolates of Candida spp. (MIC, 2.5–25 μg/ml), C. neoformans (MIC, 0.8–10 μg/ml) and A. fumigatus (MIC, 5–40 μg/ml) (231). Generally, the 3 cyclic peptides were more effective against yeasts than against filamentous fungi. Moreover, syringotoxin B was the least effective antifungal cyclic peptide. In a subsequent study, syringomycin E was found also to be effective in treating vaginal candidiasis in mice (232).
ANTIMICROBIAL PEPTIDES THAT ARE PROTEOLYTIC FRAGMENTS OF PROTEINS
The majority of peptides derived from proteins such as lactoferrin, BPI, and pepsinogen A have broad-spectrum antimicrobial activity. For example, hydrolysates of lactoferrin have marked activity against gram-negative and gram-positive bacteria (233). Depending on the location of the protein in which the peptides were derived, the peptides may have an α-helical or β-sheet structure. Moreover, these proteolytic peptide fragments frequently have greater antifungal activity than the parent proteins. Although we discuss mammalian peptides from lactoferrin and BPI in this review, amphibian antimicrobial peptides derived from the amino-terminal domains of Pepsinogen A and C have also been identified (234).
Lactoferrin is an iron-binding antimicrobial glycoprotein (78 kDa) that is present in neutrophil granules, in breast milk, and on many mucosal surfaces. (235, 236). Lactoferrin is multifunctional and can influence a wide range of biological processes including immune responses. There are two mechanisms by which lactoferrin is thought to inhibit fungal and bacterial growth: 1) by depriving iron which is essential to growth of fungi and 2) generation of antifungal peptides from proteolytic enzymatic digestion of lactoferrin. Kirkpatrick et al. initially proposed that the iron-binding properties of lactoferrin were responsible for its inhibition of C. albicans (237). Later, Zarember and colleagues showed that iron sequestration had an important role in the inhibition of A. fumigatus by lactoferrin (238). A recombinant lactoferrin (talactoferrin, Agenix) is now in clinical trials for treatment of cancer, diabetic skin ulcers, and sepsis. Enzymatic degradation of lactoferrin results in several antimicrobial/antifungal peptides (lactoferricin H, B; peptide 2; kaliocin-1) (239–242). Except for kaliocin, the antimicrobial peptides are derived from the N-terminal region of lactoferrin. Many of these proteolytic peptides posses stronger antimicrobial activity than did lactoferrin itself (233). The N-terminal lactoferrin-derived peptides that lack the iron-binding domain of the parent protein exert their antifungal activity by affecting permeability of the membrane (243). After the N-terminal amino acid lactoferrin H peptide was first identified, several smaller peptides have been identified with antifungal activity. Viejo-Diaz et al. identified Lfpep (residues 18 to 40 of human lactoferrin) that were candidicidal against fluconazole and amphotericin-resistant Candida spp. (MIC range, 9.3 to 18.7 μM) (241). Another well known antifungal peptide derived from lactoferrin is peptide 2 (242). Ueta and colleagues compared six N-terminal peptides derived from lactoferrin and found that peptide 2 (FKCRRWQWRM) had the most antifungal activity; in two strains of Candida, the MIC of Peptide 2 was approximately 17 μM, which was close to the MIC of miconazole. Interestingly, replacing the single cysteine of peptide 2 with alanine markedly mitigated its anticandida activity. Although Peptide 2 cannot form a cyclic structure, perhaps the single cysteine enables dimers to form, which may be more potent antifungal agents. Moreover, peptide 2 augments the candidacidal activity of polymorphonuclear leukocytes by inducing superoxides (242).
BPI protein domain III analogs
The bactericidal and permeability-increasing (BPI) protein is a 55-kDa cationic protein that is stored in azurophilic granules of neutrophils. BPI inhibits gram-negative bacteria by binding LPS with high affinity and it also shows antifungal activity against H. capsulatum (MIC50: 1 μg/ml; MIC, 80–90 μg/ml) (244). Moreover, BPI together with defensins and cathepsin G are additive in their inhibitory activity against H. capsulatum (244). Compared to the parent protein, proteolytic fragments of BPI have significantly more antifungal activities directed towards Candida spp., C. neoformans, and A. fumigatus (245). On the basis of the BPI protein domain III structure, three synthetic peptides (XMP.284, XMP.366, XMP.391) have been reported to be effective against several Candida species and showed synergistic activity with fluconazole (246). Additionally XMP.391 was found to increase the antifungal activity of amphotericin B against systemic aspergillosis in a murine model (247).
SYNTHETIC STRATEGIES FOR PRODUCING PEPTIDES FOR PRECLINICAL AND CLINICAL APPLICATIONS
Naturally occurring broad-spectrum antimicrobial peptides and their analogs have been examined for their utility as therapeutic antibiotics by many groups, and the ability to produce AMP economically is a key factor that will determine which AMP will receive widespread use for treatment of fungal infections. To date, the only approved antifungal peptides are in the echinocandin family (micafungin, caspofungin, and anidulafungin); precursors of these FDA-approved peptides are isolated from various molds and further modified chemically. Nonetheless, depending on the original source of the peptide, isolation of natural compounds can often be prohibitively expensive and peptide isolates may be sensitive to protease digestion. In many cases, peptide technologies including solid-phase peptide synthesis and recombinant-engineering methods provide mechanistic insight about the peptide and offer the potential of creating more effective antifungal therapies in a cost-saving manner.
Several groups have synthesized peptides by a solid-phase peptide method for their antimicrobial and antifungal studies. The synthesized peptides have included linear (170, 223, 225, 248, 249) and cyclic peptides with one to multiple disulfide bonds (76, 228, 250, 251) and closed-ended cyclic theta defensins peptides (225). Synthesized peptides have ranged in size from 6 (the hexapeptide) to 31 residues. Solid phase peptide syntheses are ideally suited for structure-function studies because the amino acid sequences can rapidly be altered to compare their antimicrobial activities with the parent peptide. For example, Chen and colleagues synthesized 20 analogs of protegrins to gain understanding of the relationship between the primary and secondary structure and the antimicrobial activity (252). From these structural activity relationship (SAR) studies, the IB-367 analog was identified for further clinical investigations. Several such SAR studies have been done to identify analogs with increased efficacy and reduced toxicity (176). In addition to the SAR function studies, several hybrid peptides (e.g., cecropin A-magainin, HP ribosomal peptide-magainin; cecropin A-mellitin) have been made by peptide synthesis and have increased biological activity toward microbes compared to either peptide alone (134, 135, 253). For clinical studies, it is likely that peptide synthesis will prove cost-effective in AMP production for smaller peptides, approximately 25 amino acids or less. Of note, the length of peptide that can be synthesized without significant truncation depends on its amino acid sequence and content. Thus far, analogs of protegrins, histatins, and indolicidin have been made for clinical trials by peptide synthesis (254). Therapeutic peptides with increased length and complexity continue to be made. Improvements in synthesis and development of higher-quality resins for solid-phase synthesis, price reductions for commonly used reagents, and increased competition are enabling efficient and cost-effective production of these peptides. Peptide synthesis is an evolving methodology in which further advances to their production are anticipated.
Recombinant engineering is an alternative technology that has been used to synthesize various antimicrobial peptides (255–260). There is significant overlap in the antimicrobial studies that have been done with recombinant-engineering and solid-phase synthesis technologies. For example, SAR studies and hybrid antimicrobial peptides have also used recombinant engineering methods (133, 173, 261). Recombinant engineering is more appropriate and cost-effective for larger AMPs such as defensins, which have between 30 to 50 amino acids and 3 or more disulfide bonds. Several organisms have been used to synthesize recombinant antimicrobial peptides, including E. coli, baculovirus insect cells, fungi (Pichia Pastoris, S. cerevisiae, Aspergillus spp.) and chloroplast expression systems (262); thus far, expression in E. coli and fungi have yielded promising results. Fusion proteins produced in E.coli are the most widely used in recombinant engineering because of their low cost and the large amount of information on the strains and available plasmids (255). Many antimicrobial peptides are toxic to bacteria so that these peptides are often fused with proteins such as thioredoxin and glutathione-S-transferase (255, 263); in addition to preventing cell lysis, thioredoxin and glutathione-S-transferase aid in disulfide bond formation of the antimicrobial peptide (264). Although most expression plasmids produced low levels of antimicrobial peptides in E. coli, the IPTG-inducible pET-32a(+) expression vector produced levels of HBD-2 reaching 1.3 g/l (265). To express antifungal peptides in yeast and filamentous fungi, resistant strains of these organisms that lack the target to these peptides have been developed. In P. Pastoris, the plant defensin, Psd1, fused with MFa1 signal peptide and a pro-region produced fusion protein levels of 60 mg/L. Expression systems in filamentous fungi have yielded even higher levels of proteins (10 g or more/L). Talactoferrin, which has entered phase III clinical trials for treatment of foot ulcers, sepsis, and cancer, is expressed by Aspergillus spp.
Another synthetic strategy to develop cost-effective antibiotics is to utilize antimicrobial peptide mimetic agents. For example, Beckloff and colleagues developed meta-phenylene ethynylene (mPE) compounds that have an amphipathic structure similar to magainins. mPE exhibited antimicrobial activity at nanomolar concentrations against a variety of bacteria and Candida spp. found in oral infections (266). Other magainin-mimetic compounds such as MSI-751 have also been developed (267). Electron microcopy showed formation of ion channels in pathogenic organisms with these mimetics that was similar to magainins. Moreover, experimental studies to identify the determinants underlying the configuration of these peptides will likely provide adequate information for computer simulation methods to explore their fungicidal mechanism at the atomic level. Interactions between peptides and lipid bilayers are being investigated by molecular dynamics (MD) methods (268). By understanding structural and dynamic properties of peptides and their target, MD-based methodology increases the likelihood for rational design of antimicrobial peptidomimetics (269, 270).
CONCLUDING REMARKS
Both plants and animals are constantly exposed to harmful pathogens present in all environmental niches. Moreover, some organisms lack components related to specialized immunity such as lymphocytes or antibodies. Nonetheless, living organisms have found a way to live and thrive. During the evolutionary process, nature was able not only to create but also strategically localize small antimicrobial peptides with relatively simple modes of action, resulting in survival of life forms on this planet. The pharmaceutical use of these antimicrobial peptides and analogs may also have an important role in treating human infectious diseases because of increased resistance to the widespread use of currently-used antibiotics. Whether resistance to these antimicrobial peptides will occur has been debated, but it is likely that resistance will occur at a reduced rate and will depend on how the antimicrobial peptide is administered. Combination therapy of the antimicrobial peptide with antibiotics or with other peptides will likely reduce the development of resistance markedly. One area that merits further attention and research is the use of cationic peptides to prevent the formation of polymicrobial biofilms. Although there are promising peptide candidates that have demonstrated marked reduction of biofilms caused by bacteria or Candida species (98, 175, 266, 271–273), we are not aware of studies demonstrating the efficacy of these peptides against biofilms caused by multiple organisms (274). Regardless of the effectiveness of naturally occurring peptides as antifungal agents, new peptide strategies offer the potential of developing more potent, specific and non-toxic therapies. Several examples given in this review have shown that modified peptides are more effective than their parent peptides and most AMP in clinical trials are analogs of the parent peptide.
Current antimycotic therapies face many difficulties including fast growing drug-resistance, limited choice of antifungal drugs, and increased fungal infections in immunosuppressed patients with AIDS, organ transplant or cancer. Candidemia in these patients leads to an overall mortality of 40% in the United States (275). Although AMP and their analogs bring the hope of treating these serious antifungal infections more effectively, interactions of new therapies with biological pathways to address safety concerns will need to be addressed. To date, the only antifungal peptides to receive FDA approval are the echinocandin family of β-glucan inhibitors; these inhibitors have been approved for treatment of candidemia, empiric treatment of neutropenic febrile patients, and invasive aspergillosis. In addition, there are a number of other antifungal/antimicrobial peptides in various stages of preclinical and clinical trials, primarily involving topical and aerosolized approaches to infections (see reviews by Gordon (254) and Jenssen and Hancock (40)). With the many promising antifungal peptides that show selective toxicity toward pathogenic fungi, we anticipate that peptide analogs will continue to be developed with increased therapeutic efficacy and have an important role in treating patients with fungal infections.
Acknowledgments
We thank Dr. Pamela Talalay for her careful reading and helpful suggestions of this manuscript. This work was supported by the National Institutes of Health (CA096984) and Maryland Industrial Partnership (4225).
Footnotes
References
- 1.Ostrosky-Zeichner L, et al. Deeply invasive candidiasis. Infect Dis Clin North Am. 2002;16(4):821–35. doi: 10.1016/s0891-5520(02)00034-x. [DOI] [PubMed] [Google Scholar]
- 2.Venkatesan P, Perfect JR, Myers SA. Evaluation and management of fungal infections in immunocompromised patients. Dermatol Ther. 2005;18(1):44–57. doi: 10.1111/j.1529-8019.2005.05001.x. [DOI] [PubMed] [Google Scholar]
- 3.Prasad R, Kapoor K. Multidrug resistance in yeast Candida. Int Rev Cytol. 2005;242:215–48. doi: 10.1016/S0074-7696(04)42005-1. [DOI] [PubMed] [Google Scholar]
- 4.Chandrasekar PH, Cutright J, Manavathu E. Efficacy of voriconazole against invasive pulmonary aspergillosis in a guinea-pig model. J Antimicrob Chemother. 2000;45(5):673–6. doi: 10.1093/jac/45.5.673. [DOI] [PubMed] [Google Scholar]
- 5.Perea S, Patterson TF. Antifungal resistance in pathogenic fungi. Clin Infect Dis. 2002;35(9):1073–80. doi: 10.1086/344058. [DOI] [PubMed] [Google Scholar]
- 6.Hernandez S, et al. Caspofungin resistance in Candida albicans: correlating clinical outcome with laboratory susceptibility testing of three isogenic isolates serially obtained from a patient with progressive Candida esophagitis. Antimicrob Agents Chemother. 2004;48(4):1382–3. doi: 10.1128/AAC.48.4.1382-1383.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hakki M, Staab JF, Marr KA. Emergence of a Candida krusei isolate with reduced susceptibility to caspofungin during therapy. Antimicrob Agents Chemother. 2006;50(7):2522–4. doi: 10.1128/AAC.00148-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson GR, 3rd, et al. Development of caspofungin resistance following prolonged therapy for invasive candidiasis secondary to Candida glabrata infection. Antimicrob Agents Chemother. 2008;52(10):3783–5. doi: 10.1128/AAC.00473-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy KV, Yedery RD, Aranha C. Antimicrobial peptides: premises and promises. Int J Antimicrob Agents. 2004;24(6):536–47. doi: 10.1016/j.ijantimicag.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann JA, et al. Phylogenetic perspectives in innate immunity. Science. 1999;284(5418):1313–8. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 11.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–95. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 12.Broekaert WF, et al. Plant defensins: novel antimicrobial peptides as components of the host defense system. Plant Physiol. 1995;108(4):1353–8. doi: 10.1104/pp.108.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulet P, Stocklin R, Menin L. Anti-microbial peptides: from invertebrates to vertebrates. Immunol Rev. 2004;198:169–84. doi: 10.1111/j.0105-2896.2004.0124.x. [DOI] [PubMed] [Google Scholar]
- 14.Otvos L, Jr, et al. Insect peptides with improved protease-resistance protect mice against bacterial infection. Protein Sci. 2000;9(4):742–9. doi: 10.1110/ps.9.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganz T, Lehrer RI. Antimicrobial peptides of leukocytes. Curr Opin Hematol. 1997;4(1):53–8. doi: 10.1097/00062752-199704010-00009. [DOI] [PubMed] [Google Scholar]
- 16.Thevissen K, Terras FR, Broekaert WF. Permeabilization of fungal membranes by plant defensins inhibits fungal growth. Appl Environ Microbiol. 1999;65(12):5451–8. doi: 10.1128/aem.65.12.5451-5458.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thevissen K, et al. Specific binding sites for an antifungal plant defensin from Dahlia (Dahlia merckii) on fungal cells are required for antifungal activity. Mol Plant Microbe Interact. 2000;13(1):54–61. doi: 10.1094/MPMI.2000.13.1.54. [DOI] [PubMed] [Google Scholar]
- 18.Schmatz DM, et al. Pneumocandins from Zalerion arboricola. IV. Biological evaluation of natural and semisynthetic pneumocandins for activity against Pneumocystis carinii and Candida species. J Antibiot (Tokyo) 1992;45(12):1886–91. doi: 10.7164/antibiotics.45.1886. [DOI] [PubMed] [Google Scholar]
- 19.Jigami Y, Odani T. Mannosylphosphate transfer to yeast mannan. Biochim Biophys Acta. 1999;1426(2):335–45. doi: 10.1016/s0304-4165(98)00134-2. [DOI] [PubMed] [Google Scholar]
- 20.Tang YQ, et al. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science. 1999;286(5439):498–502. doi: 10.1126/science.286.5439.498. [DOI] [PubMed] [Google Scholar]
- 21.Biragyn A, et al. Toll-like receptor 4-dependent activation of dendritic cells by beta-defensin 2. Science. 2002;298(5595):1025–9. doi: 10.1126/science.1075565. [DOI] [PubMed] [Google Scholar]
- 22.Chong-Cerrillo C, et al. Susceptibility of human and murine Chlamydia trachomatis serovars to granulocyte- and epithelium-derived antimicrobial peptides. J Pept Res. 2003;61(5):237–42. doi: 10.1034/j.1399-3011.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 23.Yang D, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286(5439):525–8. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 24.Yang D, et al. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukoc Biol. 2000;68(1):9–14. [PubMed] [Google Scholar]
- 25.Yang D, et al. Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 2002;23(6):291–6. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]
- 26.Durr M, Peschel A. Chemokines meet defensins: the merging concepts of chemoattractants and antimicrobial peptides in host defense. Infect Immun. 2002;70(12):6515–7. doi: 10.1128/IAI.70.12.6515-6517.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hancock RE, Rozek A. Role of membranes in the activities of antimicrobial cationic peptides. FEMS Microbiol Lett. 2002;206(2):143–9. doi: 10.1111/j.1574-6968.2002.tb11000.x. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Ami R, Lewis RE, Kontoyiannis DP. Immunocompromised hosts: immunopharmacology of modern antifungals. Clin Infect Dis. 2008;47(2):226–35. doi: 10.1086/589290. [DOI] [PubMed] [Google Scholar]
- 29.Balloy V, et al. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect Immun. 2005;73(1):494–503. doi: 10.1128/IAI.73.1.494-503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ganz T. Immunology. Versatile defensins. Science. 2002;298(5595):977–9. doi: 10.1126/science.1078708. [DOI] [PubMed] [Google Scholar]
- 31.Okumura K, et al. C-terminal domain of human CAP18 antimicrobial peptide induces apoptosis in oral squamous cell carcinoma SAS-H1 cells. Cancer Lett. 2004;212(2):185–94. doi: 10.1016/j.canlet.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 32.Chen QR, et al. Branched co-polymers of histidine and lysine are efficient carriers of plasmids. Nucleic Acids Res. 2001;29(6):1334–1340. doi: 10.1093/nar/29.6.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leng Q, Mixson AJ. Modified branched peptides with a histidine-rich tail enhance in vitro gene transfection. Nucleic Acids Res. 2005;33(4):e40. doi: 10.1093/nar/gni040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez-Exposito I, Recio I. Protective effect of milk peptides: antibacterial and antitumor properties. Adv Exp Med Biol. 2008;606:271–93. doi: 10.1007/978-0-387-74087-4_11. [DOI] [PubMed] [Google Scholar]
- 35.Kuipers OP, et al. Protein engineering of lantibiotics. Antonie Van Leeuwenhoek. 1996;69(2):161–69. doi: 10.1007/BF00399421. [DOI] [PubMed] [Google Scholar]
- 36.Andreu D, Rivas L. Animal antimicrobial peptides: an overview. Biopolymers. 1998;47(6):415–33. doi: 10.1002/(SICI)1097-0282(1998)47:6<415::AID-BIP2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 37.Cheigh CI, Pyun YR. Nisin biosynthesis and its properties. Biotechnol Lett. 2005;27(21):1641–8. doi: 10.1007/s10529-005-2721-x. [DOI] [PubMed] [Google Scholar]
- 38.Jack RW, Jung G. Lantibiotics and microcins: polypeptides with unusual chemical diversity. Curr Opin Chem Biol. 2000;4(3):310–7. doi: 10.1016/s1367-5931(00)00094-6. [DOI] [PubMed] [Google Scholar]
- 39.Xie L, van der Donk WA. Post-translational modifications during lantibiotic biosynthesis. Curr Opin Chem Biol. 2004;8(5):498–507. doi: 10.1016/j.cbpa.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Jenssen H, Hamill P, Hancock RE. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19(3):491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55(1):27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 42.De Lucca AJ. Antifungal peptides: potential candidates for the treatment of fungal infections. Expert Opin Investig Drugs. 2000;9(2):273–99. doi: 10.1517/13543784.9.2.273. [DOI] [PubMed] [Google Scholar]
- 43.Cole AM, Ganz T. Human antimicrobial peptides: analysis and application. Biotechniques. 2000;29(4):822–6. 828, 830–1. doi: 10.2144/00294rv01. [DOI] [PubMed] [Google Scholar]
- 44.Georgopapadakou NH, Walsh TJ. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob Agents Chemother. 1996;40(2):279–91. doi: 10.1128/aac.40.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurtz MB, Douglas CM. Lipopeptide inhibitors of fungal glucan synthase. J Med Vet Mycol. 1997;35(2):79–86. doi: 10.1080/02681219780000961. [DOI] [PubMed] [Google Scholar]
- 46.Turner WW, Current WL. Echinocandin antifungal agents. In: Strohl WR, editor. Biotechnology of antibiotic. 2. Mercel Dekker, Inc; New York, N.Y: 1997. pp. 315–334. [Google Scholar]
- 47.Benz F, et al. Echinocandin B, ein neuartiges Polypetid Antibioticum aus Aspergillus nidulans var echinlatus: isolierung and Baudsteine. Helv Chim Acta. 1974;57:2459–2477. doi: 10.1002/hlca.19740570818. [DOI] [PubMed] [Google Scholar]
- 48.Schmatz DM, et al. Treatment of Pneumocystis carinii pneumonia with 1,3-beta-glucan synthesis inhibitors. Proc Natl Acad Sci U S A. 1990;87(15):5950–4. doi: 10.1073/pnas.87.15.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmatz DM, et al. Antipneumocystis activity of water-soluble lipopeptide L-693,989 in rats. Antimicrob Agents Chemother. 1992;36(9):1964–70. doi: 10.1128/aac.36.9.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fromtling RA, Abruzzo GK. L-671,329, a new antifungal agent. III. In vitro activity, toxicity and efficacy in comparison to aculeacin. J Antibiot (Tokyo) 1989;42(2):174–8. doi: 10.7164/antibiotics.42.174. [DOI] [PubMed] [Google Scholar]
- 51.Zambias RA, et al. Preparation and structure-activity relationships of simplified analogues of the antifungal agent cilofungin: a total synthesis approach. J Med Chem. 1992;35(15):2843–55. doi: 10.1021/jm00093a018. [DOI] [PubMed] [Google Scholar]
- 52.Gordee RS, et al. Anti-Candida activity and toxicology of LY121019, a novel semisynthetic polypeptide antifungal antibiotic. Ann N Y Acad Sci. 1988;544:294–309. doi: 10.1111/j.1749-6632.1988.tb40415.x. [DOI] [PubMed] [Google Scholar]
- 53.Sucher AJ, Chahine EB, Balcer HE. Echinocandins: the newest class of antifungals. Ann Pharmacother. 2009;43(10):1647–57. doi: 10.1345/aph.1M237. [DOI] [PubMed] [Google Scholar]
- 54.Tawara S, et al. In vitro activities of a new lipopeptide antifungal agent, FK463, against a variety of clinically important fungi. Antimicrob Agents Chemother. 2000;44(1):57–62. doi: 10.1128/aac.44.1.57-62.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mizuno K, et al. Studies on aculeacin. I. Isolation and characterization of aculeacin A. J Antibiot (Tokyo) 1977;30(4):297–302. doi: 10.7164/antibiotics.30.297. [DOI] [PubMed] [Google Scholar]
- 56.Satoi S, et al. Studies on aculeacin. II. Isolation and characterization of aculeacins B, C, D, E, F and G. J Antibiot (Tokyo) 1977;30(4):303–7. doi: 10.7164/antibiotics.30.303. [DOI] [PubMed] [Google Scholar]
- 57.Mizoguchi J, et al. On the mode of action of a new antifungal antibiotic, aculeacin A: inhibition of cell wall synthesis in Saccharomyces cerevisiae. J Antibiot (Tokyo) 1977;30(4):308–13. doi: 10.7164/antibiotics.30.308. [DOI] [PubMed] [Google Scholar]
- 58.Iwata K, et al. In vitro studies of aculeacin A, a new antifungal antibiotic. J Antibiot (Tokyo) 1982;35(2):203–9. doi: 10.7164/antibiotics.35.203. [DOI] [PubMed] [Google Scholar]
- 59.Mukhopadhyay T, et al. Mulundocandin, a new lipopeptide antibiotic. II. Structure elucidation. J Antibiot (Tokyo) 1987;40(3):281–9. doi: 10.7164/antibiotics.40.281. [DOI] [PubMed] [Google Scholar]
- 60.Roy K, et al. Mulundocandin, a new lipopeptide antibiotic. I. Taxonomy, fermentation, isolation and characterization. J Antibiot (Tokyo) 1987;40(3):275–80. doi: 10.7164/antibiotics.40.275. [DOI] [PubMed] [Google Scholar]
- 61.Hawser S, et al. Mulundocandin, an echinocandin-like lipopeptide antifungal agent: biological activities in vitro. J Antibiot (Tokyo) 1999;52(3):305–10. doi: 10.7164/antibiotics.52.305. [DOI] [PubMed] [Google Scholar]
- 62.Iwamoto T, et al. WF11899A, B and C, novel antifungal lipopeptides. II. Biological properties. J Antibiot (Tokyo) 1994;47(10):1092–7. doi: 10.7164/antibiotics.47.1092. [DOI] [PubMed] [Google Scholar]
- 63.Hori M, et al. Studies on the mode of action of polyoxins. VI. Effect of polyoxin B on chitin synthesis in polyoxin-sensitive and resistant strains of Alternaria kikuchiana. J Antibiot (Tokyo) 1974;27(4):260–6. doi: 10.7164/antibiotics.27.260. [DOI] [PubMed] [Google Scholar]
- 64.Brillinger GU. Metabolic products of microorganisms. 181. Chitin synthase from fungi, a test model for substances with insecticidal properties. Arch Microbiol. 1979;121(1):71–4. doi: 10.1007/BF00409207. [DOI] [PubMed] [Google Scholar]
- 65.Chen W, Zeng H, Tan H. Cloning, sequencing, and function of sanF: A gene involved in nikkomycin biosynthesis of Streptomyces ansochromogenes. Curr Microbiol. 2000;41(5):312–6. doi: 10.1007/s002840010141. [DOI] [PubMed] [Google Scholar]
- 66.McCarthy PJ, Troke PF, Gull K. Mechanism of action of nikkomycin and the peptide transport system of Candida albicans. J Gen Microbiol. 1985;131(4):775–80. doi: 10.1099/00221287-131-4-775. [DOI] [PubMed] [Google Scholar]
- 67.Hector RF, Zimmer BL, Pappagianis D. Evaluation of nikkomycins X and Z in murine models of coccidioidomycosis, histoplasmosis, and blastomycosis. Antimicrob Agents Chemother. 1990;34(4):587–93. doi: 10.1128/aac.34.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clemons KV, Stevens DA. Efficacy of nikkomycin Z against experimental pulmonary blastomycosis. Antimicrob Agents Chemother. 1997;41(9):2026–8. doi: 10.1128/aac.41.9.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ikai K, et al. Structure of aureobasidin A. J Antibiot (Tokyo) 1991;44(9):925–33. doi: 10.7164/antibiotics.44.925. [DOI] [PubMed] [Google Scholar]
- 70.Endo M, et al. Fungicidal action of aureobasidin A, a cyclic depsipeptide antifungal antibiotic, against Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1997;41(3):672–6. doi: 10.1128/aac.41.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nagiec MM, et al. Sphingolipid synthesis as a target for antifungal drugs. Complementation of the inositol phosphorylceramide synthase defect in a mutant strain of Saccharomyces cerevisiae by the AUR1 gene. J Biol Chem. 1997;272(15):9809–17. doi: 10.1074/jbc.272.15.9809. [DOI] [PubMed] [Google Scholar]
- 72.Takesako K, et al. Biological properties of aureobasidin A, a cyclic depsipeptide antifungal antibiotic. J Antibiot (Tokyo) 1993;46(9):1414–20. doi: 10.7164/antibiotics.46.1414. [DOI] [PubMed] [Google Scholar]
- 73.Thevissen K, et al. Therapeutic potential of antifungal plant and insect defensins. Drug Discov Today. 2007;12(21–22):966–71. doi: 10.1016/j.drudis.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 74.Fehlbaum P, et al. Insect immunity. Septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides. J Biol Chem. 1994;269(52):33159–63. [PubMed] [Google Scholar]
- 75.Gao B, Zhu SY. Differential potency of drosomycin to Neurospora crassa and its mutant: implications for evolutionary relationship between defensins from insects and plants. Insect Mol Biol. 2008;17(4):405–11. doi: 10.1111/j.1365-2583.2008.00810.x. [DOI] [PubMed] [Google Scholar]
- 76.Schaaper WM, et al. Synthetic peptides derived from the beta2-beta3 loop of Raphanus sativus antifungal protein 2 that mimic the active site. J Pept Res. 2001;57(5):409–18. doi: 10.1034/j.1399-3011.2001.00842.x. [DOI] [PubMed] [Google Scholar]
- 77.Cohen L, et al. Drosomycin, an innate immunity peptide of Drosophila melanogaster, interacts with the fly voltage-gated sodium channel. J Biol Chem. 2009;284(35):23558–63. doi: 10.1074/jbc.M109.023358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lemaitre B, et al. The dorsoventral regulatory gene cassette spatzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86(6):973–83. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 79.Michaut L, et al. Determination of the disulfide array of the first inducible antifungal peptide from insects: drosomycin from Drosophila melanogaster. FEBS Lett. 1996;395(1):6–10. doi: 10.1016/0014-5793(96)00992-1. [DOI] [PubMed] [Google Scholar]
- 80.Zhang ZT, Zhu SY. Drosomycin, an essential component of antifungal defence in Drosophila. Insect Mol Biol. 2009;18(5):549–56. doi: 10.1111/j.1365-2583.2009.00907.x. [DOI] [PubMed] [Google Scholar]
- 81.Besson F, Michel G. Action of the antibiotics of the iturin group on artificial membranes. J Antibiot (Tokyo) 1984;37(6):646–51. doi: 10.7164/antibiotics.37.646. [DOI] [PubMed] [Google Scholar]
- 82.Mhammedi A, et al. Bacillomycin F, a new antibiotic of iturin group: isolation and characterization. J Antibiot (Tokyo) 1982;35(3):306–11. doi: 10.7164/antibiotics.35.306. [DOI] [PubMed] [Google Scholar]
- 83.Klich MA, Lax AR, Bland JM. Inhibition of some mycotoxigenic fungi by iturin A, a peptidolipid produced by Bacillus subtilis. Mycopathologia. 1991;116(2):77–80. doi: 10.1007/BF00436368. [DOI] [PubMed] [Google Scholar]
- 84.Latoud C, et al. Interactions of antibiotics of the iturin group with human erythrocytes. Biochim Biophys Acta. 1986;856(3):526–35. doi: 10.1016/0005-2736(86)90144-6. [DOI] [PubMed] [Google Scholar]
- 85.Brewer D, Hunter H, Lajoie G. NMR studies of the antimicrobial salivary peptides histatin 3 and histatin 5 in aqueous and nonaqueous solutions. Biochem Cell Biol. 1998;76(2–3):247–56. doi: 10.1139/bcb-76-2-3-247. [DOI] [PubMed] [Google Scholar]
- 86.Raj PA, Soni SD, Levine MJ. Membrane-induced helical conformation of an active candidacidal fragment of salivary histatins. J Biol Chem. 1994;269(13):9610–9. [PubMed] [Google Scholar]
- 87.Raj PA, Marcus E, Sukumaran DK. Structure of human salivary histatin 5 in aqueous and nonaqueous solutions. Biopolymers. 1998;45(1):51–67. doi: 10.1002/(SICI)1097-0282(199801)45:1<51::AID-BIP5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 88.Pollock JJ, et al. Fungistatic and fungicidal activity of human parotid salivary histidine-rich polypeptides on Candida albicans. Infect Immun. 1984;44(3):702–7. doi: 10.1128/iai.44.3.702-707.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rayhan R, et al. Antifungal activities of salivary histidine-rich polypeptides against Candida albicans and other oral yeast isolates. Oral Microbiol Immunol. 1992;7(1):51–2. doi: 10.1111/j.1399-302x.1992.tb00020.x. [DOI] [PubMed] [Google Scholar]
- 90.Helmerhorst EJ, et al. Amphotericin B- and fluconazole-resistant Candida spp., Aspergillus fumigatus, and other newly emerging pathogenic fungi are susceptible to basic antifungal peptides. Antimicrob Agents Chemother. 1999;43(3):702–4. doi: 10.1128/aac.43.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tsai H, Bobek LA. Human salivary histatin-5 exerts potent fungicidal activity against Cryptococcus neoformans. Biochim Biophys Acta. 1997;1336(3):367–9. doi: 10.1016/s0304-4165(97)00076-7. [DOI] [PubMed] [Google Scholar]
- 92.Castagnola M, et al. A cascade of 24 histatins (histatin 3 fragments) in human saliva. Suggestions for a pre-secretory sequential cleavage pathway. J Biol Chem. 2004;279(40):41436–43. doi: 10.1074/jbc.M404322200. [DOI] [PubMed] [Google Scholar]
- 93.MacKay BJ, et al. Isolation of milligram quantities of a group of histidine-rich polypeptides from human parotid saliva. Infect Immun. 1984;44(3):688–94. doi: 10.1128/iai.44.3.688-694.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.vanderSpek JC, et al. Localization of the genes for histatins to human chromosome 4q13 and tissue distribution of the mRNAs. Am J Hum Genet. 1989;45(3):381–7. [PMC free article] [PubMed] [Google Scholar]
- 95.Ahmad M, et al. Immunocytochemical localization of histatins in human salivary glands. J Histochem Cytochem. 2004;52(3):361–70. doi: 10.1177/002215540405200307. [DOI] [PubMed] [Google Scholar]
- 96.Raj PA, Edgerton M, Levine MJ. Salivary histatin 5: dependence of sequence, chain length, and helical conformation for candidacidal activity. J Biol Chem. 1990;265(7):3898–905. [PubMed] [Google Scholar]
- 97.Rothstein DM, et al. Anticandida activity is retained in P-113, a 12-amino-acid fragment of histatin 5. Antimicrob Agents Chemother. 2001;45(5):1367–73. doi: 10.1128/AAC.45.5.1367-1373.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.De Prijck K, et al. Candida albicans biofilm formation on peptide functionalized polydimethylsiloxane. Biofouling. 26(3):269–75. doi: 10.1080/08927010903501908. [DOI] [PubMed] [Google Scholar]
- 99.Pusateri CR, Monaco EA, Edgerton M. Sensitivity of Candida albicans biofilm cells grown on denture acrylic to antifungal proteins and chlorhexidine. Arch Oral Biol. 2009;54(6):588–94. doi: 10.1016/j.archoralbio.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Douglas LJ. Candida biofilms and their role in infection. Trends Microbiol. 2003;11(1):30–6. doi: 10.1016/s0966-842x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- 101.Lee SY, et al. A sapecin homologue of Holotrichia diomphalia: purification, sequencing and determination of disulfide pairs. Biol Pharm Bull. 1995;18(3):457–9. doi: 10.1248/bpb.18.457. [DOI] [PubMed] [Google Scholar]
- 102.Lee SY, et al. Purification and cDNA cloning of an antifungal protein from the hemolymph of Holotrichia diomphalia larvae. Biol Pharm Bull. 1995;18(8):1049–52. doi: 10.1248/bpb.18.1049. [DOI] [PubMed] [Google Scholar]
- 103.Iijima R, Kurata S, Natori S. Purification, characterization, and cDNA cloning of an antifungal protein from the hemolymph of Sarcophaga peregrina (flesh fly) larvae. J Biol Chem. 1993;268(16):12055–61. [PubMed] [Google Scholar]
- 104.Zhu J, et al. Synthetic histidine-rich peptides inhibit Candida species and other fungi in vitro: role of endocytosis and treatment implications. Antimicrob Agents Chemother. 2006;50(8):2797–805. doi: 10.1128/AAC.00411-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mochon AB, Liu H. The antimicrobial peptide histatin-5 causes a spatially restricted disruption on the Candida albicans surface, allowing rapid entry of the peptide into the cytoplasm. PLoS Pathog. 2008;4(10):e1000190. doi: 10.1371/journal.ppat.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Helmerhorst EJ, et al. Characterization of histatin 5 with respect to amphipathicity, hydrophobicity, and effects on cell and mitochondrial membrane integrity excludes a candidacidal mechanism of pore formation. J Biol Chem. 2001;276(8):5643–9. doi: 10.1074/jbc.M008229200. [DOI] [PubMed] [Google Scholar]
- 107.Helmerhorst EJ, et al. The cellular target of histatin 5 on Candida albicans is the energized mitochondrion. J Biol Chem. 1999;274(11):7286–91. doi: 10.1074/jbc.274.11.7286. [DOI] [PubMed] [Google Scholar]
- 108.Baev D, et al. The TRK1 potassium transporter is the critical effector for killing of Candida albicans by the cationic protein, Histatin 5. J Biol Chem. 2004;279(53):55060–72. doi: 10.1074/jbc.M411031200. [DOI] [PubMed] [Google Scholar]
- 109.Leng Q, et al. Highly branched HK peptides are effective carriers of siRNA. J Gene Med. 2005 doi: 10.1002/jgm.748. [DOI] [PubMed] [Google Scholar]
- 110.Steiner H, et al. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature. 1981;292(5820):246–8. doi: 10.1038/292246a0. [DOI] [PubMed] [Google Scholar]
- 111.Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987;84(15):5449–53. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mor A, et al. Isolation, amino acid sequence, and synthesis of dermaseptin, a novel antimicrobial peptide of amphibian skin. Biochemistry. 1991;30(36):8824–30. doi: 10.1021/bi00100a014. [DOI] [PubMed] [Google Scholar]
- 113.Dathe M, Wieprecht T. Structural features of helical antimicrobial peptides: their potential to modulate activity on model membranes and biological cells. Biochim Biophys Acta. 1999;1462(1–2):71–87. doi: 10.1016/s0005-2736(99)00201-1. [DOI] [PubMed] [Google Scholar]
- 114.Morton CO, et al. Global phenotype screening and transcript analysis outlines the inhibitory mode(s) of action of two amphibian-derived, alpha-helical, cationic peptides on Saccharomyces cerevisiae. Antimicrob Agents Chemother. 2007;51(11):3948–59. doi: 10.1128/AAC.01007-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hsu CH, et al. Structural and DNA-binding studies on the bovine antimicrobial peptide, indolicidin: evidence for multiple conformations involved in binding to membranes and DNA. Nucleic Acids Res. 2005;33(13):4053–64. doi: 10.1093/nar/gki725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Marchand C, et al. Covalent binding of the natural antimicrobial peptide indolicidin to DNA abasic sites. Nucleic Acids Res. 2006;34(18):5157–65. doi: 10.1093/nar/gkl667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cruciani RA, et al. Antibiotic magainins exert cytolytic activity against transformed cell lines through channel formation. Proc Natl Acad Sci U S A. 1991;88(9):3792–6. doi: 10.1073/pnas.88.9.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Merrifield RB, et al. Design and synthesis of antimicrobial peptides. Ciba Found Symp. 1994;186:5–20. discussion 20–6. [PubMed] [Google Scholar]
- 119.Hultmark D. Immune reactions in Drosophila and other insects: a model for innate immunity. Trends Genet. 1993;9(5):178–83. doi: 10.1016/0168-9525(93)90165-e. [DOI] [PubMed] [Google Scholar]
- 120.Moore AJ, Devine DA, Bibby MC. Preliminary experimental anticancer activity of cecropins. Pept Res. 1994;7(5):265–9. [PubMed] [Google Scholar]
- 121.Giacometti A, et al. Effect of mono-dose intraperitoneal cecropins in experimental septic shock. Crit Care Med. 2001;29(9):1666–9. doi: 10.1097/00003246-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 122.Reed WA, et al. Interleukin 2 promoter/enhancer controlled expression of a synthetic cecropin-class lytic peptide in transgenic mice and subsequent resistance to Brucella abortus. Transgenic Res. 1997;6(5):337–47. doi: 10.1023/a:1018423015014. [DOI] [PubMed] [Google Scholar]
- 123.De Lucca AJ, et al. Fungicidal and binding properties of the natural peptides cecropin B and dermaseptin. Med Mycol. 1998;36(5):291–8. [PubMed] [Google Scholar]
- 124.De Lucca AJ, et al. Fungicidal properties, sterol binding, and proteolytic resistance of the synthetic peptide D4E1. Can J Microbiol. 1998;44(6):514–20. doi: 10.1139/w98-032. [DOI] [PubMed] [Google Scholar]
- 125.Coca M, et al. Enhanced resistance to the rice blast fungus Magnaporthe grisea conferred by expression of a cecropin A gene in transgenic rice. Planta. 2006;223(3):392–406. doi: 10.1007/s00425-005-0069-z. [DOI] [PubMed] [Google Scholar]
- 126.Giovannini MG, et al. Biosynthesis and degradation of peptides derived from Xenopus laevis prohormones. Biochem J. 1987;243(1):113–20. doi: 10.1042/bj2430113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Terry AS, et al. The cDNA sequence coding for prepro-PGS (prepro-magainins) and aspects of the processing of this prepro-polypeptide. J Biol Chem. 1988;263(12):5745–51. [PubMed] [Google Scholar]
- 128.Bevins CL, Zasloff M. Peptides from frog skin. Annu Rev Biochem. 1990;59:395–414. doi: 10.1146/annurev.bi.59.070190.002143. [DOI] [PubMed] [Google Scholar]
- 129.Sures I, Crippa M. Xenopsin: the neurotensin-like octapeptide from Xenopus skin at the carboxyl terminus of its precursor. Proc Natl Acad Sci U S A. 1984;81(2):380–4. doi: 10.1073/pnas.81.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gibson BW, et al. Novel peptide fragments originating from PGLa and the caerulein and xenopsin precursors from Xenopus laevis. J Biol Chem. 1986;261(12):5341–9. [PubMed] [Google Scholar]
- 131.Hara T, et al. Heterodimer formation between the antimicrobial peptides magainin 2 and PGLa in lipid bilayers: a cross-linking study. Biochemistry. 2001;40(41):12395–9. doi: 10.1021/bi011413v. [DOI] [PubMed] [Google Scholar]
- 132.Giacometti A, et al. Antimicrobial activity of polycationic peptides. Peptides. 1999;20(11):1265–73. doi: 10.1016/s0196-9781(99)00131-x. [DOI] [PubMed] [Google Scholar]
- 133.Wang XQ, et al. Synthesis of hybrid antimicrobial peptide CecA-mag gene and it’s secretion expression in Pichia pastoris. Wei Sheng Wu Xue Bao. 2007;47(1):75–8. [PubMed] [Google Scholar]
- 134.Park Y, et al. A Leu-Lys-rich antimicrobial peptide: activity and mechanism. Biochim Biophys Acta. 2003;1645(2):172–82. doi: 10.1016/s1570-9639(02)00541-1. [DOI] [PubMed] [Google Scholar]
- 135.Park Y, Lee DG, Hahm KS. HP(2–9)-magainin 2(1–12), a synthetic hybrid peptide, exerts its antifungal effect on Candida albicans by damaging the plasma membrane. J Pept Sci. 2004;10(4):204–9. doi: 10.1002/psc.489. [DOI] [PubMed] [Google Scholar]
- 136.Avrahami D, Shai Y. Bestowing antifungal and antibacterial activities by lipophilic acid conjugation to D, L-amino acid-containing antimicrobial peptides: a plausible mode of action. Biochemistry. 2003;42(50):14946–56. doi: 10.1021/bi035142v. [DOI] [PubMed] [Google Scholar]
- 137.Simmaco M, et al. A family of bombinin-related peptides from the skin of Bombina variegata. Eur J Biochem. 1991;199(1):217–22. doi: 10.1111/j.1432-1033.1991.tb16112.x. [DOI] [PubMed] [Google Scholar]
- 138.Rozek T, et al. The maculatin peptides from the skin glands of the tree frog Litoria genimaculata: a comparison of the structures and antibacterial activities of maculatin 1.1 and caerin 1.1. J Pept Sci. 1998;4(2):111–5. doi: 10.1002/(sici)1099-1387(199804)4:2<111::aid-psc134>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 139.Simmaco M, Kreil G, Barra D. Bombinins, antimicrobial peptides from Bombina species. Biochim Biophys Acta. 2009;1788(8):1551–5. doi: 10.1016/j.bbamem.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 140.Mangoni ML, et al. Comparative analysis of the bactericidal activities of amphibian peptide analogues against multidrug-resistant nosocomial bacterial strains. Antimicrob Agents Chemother. 2008;52(1):85–91. doi: 10.1128/AAC.00796-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mangoni ML, et al. Structure-function relationships in bombinins H, antimicrobial peptides from Bombina skin secretions. Peptides. 2000;21(11):1673–9. doi: 10.1016/s0196-9781(00)00316-8. [DOI] [PubMed] [Google Scholar]
- 142.Simmaco M, et al. Defense peptides in the amphibian immune system. In: Ascenzi P, Polticelli F, Visca P, editors. Bacterial, Plant, and Animal Toxins. Kerala, India: Research Signpost; 2003. [Google Scholar]
- 143.Mangoni ML, Marcellini HG, Simmaco M. Biological characterization and modes of action of temporins and bombinins H, multiple forms of short and mildly cationic anti-microbial peptides from amphibian skin. J Pept Sci. 2007;13(9):603–13. doi: 10.1002/psc.853. [DOI] [PubMed] [Google Scholar]
- 144.Mignogna G, et al. Antibacterial and haemolytic peptides containing D-alloisoleucine from the skin of Bombina variegata. EMBO J. 1993;12(12):4829–32. doi: 10.1002/j.1460-2075.1993.tb06172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mor A, Hani K, Nicolas P. The vertebrate peptide antibiotics dermaseptins have overlapping structural features but target specific microorganisms. J Biol Chem. 1994;269(50):31635–41. [PubMed] [Google Scholar]
- 146.Lorin C, et al. The antimicrobial peptide dermaseptin S4 inhibits HIV-1 infectivity in vitro. Virology. 2005;334(2):264–75. doi: 10.1016/j.virol.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 147.Hernandez C, et al. Functional and structural damage in Leishmania mexicana exposed to the cationic peptide dermaseptin. Eur J Cell Biol. 1992;59(2):414–24. [PubMed] [Google Scholar]
- 148.Mor A, Nicolas P. Isolation and structure of novel defensive peptides from frog skin. Eur J Biochem. 1994;219(1–2):145–54. doi: 10.1111/j.1432-1033.1994.tb19924.x. [DOI] [PubMed] [Google Scholar]
- 149.Savoia D, et al. Synthesis and antimicrobial activity of dermaseptin S1 analogues. Bioorg Med Chem. 2008;16(17):8205–9. doi: 10.1016/j.bmc.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 150.Osusky M, et al. Genetic modification of potato against microbial diseases: in vitro and in planta activity of a dermaseptin B1 derivative, MsrA2. Theor Appl Genet. 2005;111(4):711–22. doi: 10.1007/s00122-005-2056-y. [DOI] [PubMed] [Google Scholar]
- 151.Nijnik A, Hancock RE. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr Opin Hematol. 2009;16(1):41–7. doi: 10.1097/moh.0b013e32831ac517. [DOI] [PubMed] [Google Scholar]
- 152.Bals R. Epithelial antimicrobial peptides in host defense against infection. Respir Res. 2000;1(3):141–50. doi: 10.1186/rr25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Frohm M, et al. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272(24):15258–63. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 154.Cowland JB, Johnsen AH, Borregaard N. hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett. 1995;368(1):173–6. doi: 10.1016/0014-5793(95)00634-l. [DOI] [PubMed] [Google Scholar]
- 155.Gudmundsson GH, et al. The human gene FALL39 and processing of the cathelin precursor to the antibacterial peptide LL-37 in granulocytes. Eur J Biochem. 1996;238(2):325–32. doi: 10.1111/j.1432-1033.1996.0325z.x. [DOI] [PubMed] [Google Scholar]
- 156.Agerberth B, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96(9):3086–93. [PubMed] [Google Scholar]
- 157.Schauber J, Gallo RL. The vitamin D pathway: a new target for control of the skin’s immune response? Exp Dermatol. 2008;17(8):633–9. doi: 10.1111/j.1600-0625.2008.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Segaert S. Vitamin D regulation of cathelicidin in the skin: toward a renaissance of vitamin D in dermatology? J Invest Dermatol. 2008;128(4):773–5. doi: 10.1038/jid.2008.35. [DOI] [PubMed] [Google Scholar]
- 159.Lopez-Garcia B, et al. Anti-fungal activity of cathelicidins and their potential role in Candida albicans skin infection. J Invest Dermatol. 2005;125(1):108–15. doi: 10.1111/j.0022-202X.2005.23713.x. [DOI] [PubMed] [Google Scholar]
- 160.Benincasa M, et al. Fungicidal activity of five cathelicidin peptides against clinically isolated yeasts. J Antimicrob Chemother. 2006;58(5):950–9. doi: 10.1093/jac/dkl382. [DOI] [PubMed] [Google Scholar]
- 161.Kanthawong S, et al. In vitro susceptibility of Burkholderia pseudomallei to antimicrobial peptides. Int J Antimicrob Agents. 2009;34(4):309–14. doi: 10.1016/j.ijantimicag.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 162.Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75(1):39–48. doi: 10.1189/jlb.0403147. [DOI] [PubMed] [Google Scholar]
- 163.Filewod NC, Pistolic J, Hancock RE. Low concentrations of LL-37 alter IL-8 production by keratinocytes and bronchial epithelial cells in response to proinflammatory stimuli. FEMS Immunol Med Microbiol. 2009;56(3):233–40. doi: 10.1111/j.1574-695X.2009.00571.x. [DOI] [PubMed] [Google Scholar]
- 164.Schittek B, et al. The role of antimicrobial peptides in human skin and in skin infectious diseases. Infect Disord Drug Targets. 2008;8(3):135–43. doi: 10.2174/1871526510808030135. [DOI] [PubMed] [Google Scholar]
- 165.Wollenberg A, Klein E. Current aspects of innate and adaptive immunity in atopic dermatitis. Clin Rev Allergy Immunol. 2007;33(1–2):35–44. doi: 10.1007/s12016-007-0032-9. [DOI] [PubMed] [Google Scholar]
- 166.Lawyer C, et al. Antimicrobial activity of a 13 amino acid tryptophan-rich peptide derived from a putative porcine precursor protein of a novel family of antibacterial peptides. FEBS Lett. 1996;390(1):95–8. doi: 10.1016/0014-5793(96)00637-0. [DOI] [PubMed] [Google Scholar]
- 167.Selsted ME, et al. Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J Biol Chem. 1992;267(7):4292–5. [PubMed] [Google Scholar]
- 168.Falla TJ, Karunaratne DN, Hancock RE. Mode of action of the antimicrobial peptide indolicidin. J Biol Chem. 1996;271(32):19298–303. doi: 10.1074/jbc.271.32.19298. [DOI] [PubMed] [Google Scholar]
- 169.Yang ST, et al. Conformation-dependent antibiotic activity of tritrpticin, a cathelicidin-derived antimicrobial peptide. Biochem Biophys Res Commun. 2002;296(5):1044–50. doi: 10.1016/s0006-291x(02)02048-x. [DOI] [PubMed] [Google Scholar]
- 170.Ahmad I, et al. Liposomal entrapment of the neutrophil-derived peptide indolicidin endows it with in vivo antifungal activity. Biochim Biophys Acta. 1995;1237(2):109–14. doi: 10.1016/0005-2736(95)00087-j. [DOI] [PubMed] [Google Scholar]
- 171.Yang ST, et al. Design of perfectly symmetric Trp-rich peptides with potent and broad-spectrum antimicrobial activities. Int J Antimicrob Agents. 2006;27(4):325–30. doi: 10.1016/j.ijantimicag.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 172.Munoz A, et al. Antimicrobial properties of derivatives of the cationic tryptophan-rich hexapeptide PAF26. Biochem Biophys Res Commun. 2007;354(1):172–7. doi: 10.1016/j.bbrc.2006.12.173. [DOI] [PubMed] [Google Scholar]
- 173.Li GP, Chen YB. Cloning, expression and characterization of a new hybrid AMP gene of Hex-Mag. Wei Sheng Wu Xue Bao. 2007;47(1):115–20. [PubMed] [Google Scholar]
- 174.Stark M, Liu LP, Deber CM. Cationic hydrophobic peptides with antimicrobial activity. Antimicrob Agents Chemother. 2002;46(11):3585–90. doi: 10.1128/AAC.46.11.3585-3590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Burrows LL, et al. Activity of novel non-amphipathic cationic antimicrobial peptides against Candida species. J Antimicrob Chemother. 2006;57(5):899–907. doi: 10.1093/jac/dkl056. [DOI] [PubMed] [Google Scholar]
- 176.Fehlbaum P, et al. Structure-activity analysis of thanatin, a 21-residue inducible insect defense peptide with sequence homology to frog skin antimicrobial peptides. Proc Natl Acad Sci U S A. 1996;93(3):1221–5. doi: 10.1073/pnas.93.3.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Simmaco M, et al. Antimicrobial peptides from skin secretions of Rana esculenta. Molecular cloning of cDNAs encoding esculentin and brevinins and isolation of new active peptides. J Biol Chem. 1994;269(16):11956–61. [PubMed] [Google Scholar]
- 178.Daly NL, et al. Solution structure by NMR of circulin A: a macrocyclic knotted peptide having anti-HIV activity. J Mol Biol. 1999;285(1):333–45. doi: 10.1006/jmbi.1998.2276. [DOI] [PubMed] [Google Scholar]
- 179.Shafer WM, et al. Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci U S A. 1998;95(4):1829–33. doi: 10.1073/pnas.95.4.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lehrer RI, Lichtenstein AK, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–28. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 181.Miyata T, et al. Antimicrobial peptides, isolated from horseshoe crab hemocytes, tachyplesin II, and polyphemusins I and II: chemical structures and biological activity. J Biochem. 1989;106(4):663–8. doi: 10.1093/oxfordjournals.jbchem.a122913. [DOI] [PubMed] [Google Scholar]
- 182.Nakamura T, et al. Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus). Isolation and chemical structure. J Biol Chem. 1988;263(32):16709–13. [PubMed] [Google Scholar]
- 183.Yasin B, et al. Protegrins: structural requirements for inactivating elementary bodies of Chlamydia trachomatis. Infect Immun. 1996;64(11):4863–6. doi: 10.1128/iai.64.11.4863-4866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yasin B, et al. Susceptibility of Chlamydia trachomatis to protegrins and defensins. Infect Immun. 1996;64(3):709–13. doi: 10.1128/iai.64.3.709-713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tamamura H, et al. Synthesis of protegrin-related peptides and their antibacterial and anti-human immunodeficiency virus activity. Chem Pharm Bull (Tokyo) 1995;43(5):853–8. doi: 10.1248/cpb.43.853. [DOI] [PubMed] [Google Scholar]
- 186.Yasin B, et al. Evaluation of the inactivation of infectious Herpes simplex virus by host-defense peptides. Eur J Clin Microbiol Infect Dis. 2000;19(3):187–94. doi: 10.1007/s100960050457. [DOI] [PubMed] [Google Scholar]
- 187.Tam JP, Wu C, Yang JL. Membranolytic selectivity of cystine-stabilized cyclic protegrins. Eur J Biochem. 2000;267(11):3289–300. doi: 10.1046/j.1432-1327.2000.01359.x. [DOI] [PubMed] [Google Scholar]
- 188.Cho Y, et al. Activity of protegrins against yeast-phase Candida albicans. Infect Immun. 1998;66(6):2486–93. doi: 10.1128/iai.66.6.2486-2493.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Owen SM, et al. RC-101, a retrocyclin-1 analogue with enhanced activity against primary HIV type 1 isolates. AIDS Res Hum Retroviruses. 2004;20(11):1157–65. doi: 10.1089/aid.2004.20.1157. [DOI] [PubMed] [Google Scholar]
- 190.Ganz T. Biosynthesis of defensins and other antimicrobial peptides. Ciba Found Symp. 1994;186:62–71. doi: 10.1002/9780470514658.ch4. [DOI] [PubMed] [Google Scholar]
- 191.Zimmermann GR, et al. Solution structure of bovine neutrophil beta-defensin-12: the peptide fold of the beta-defensins is identical to that of the classical defensins. Biochemistry. 1995;34(41):13663–71. doi: 10.1021/bi00041a048. [DOI] [PubMed] [Google Scholar]
- 192.Scott MG, Hancock RE. Cationic antimicrobial peptides and their multifunctional role in the immune system. Crit Rev Immunol. 2000;20(5):407–31. [PubMed] [Google Scholar]
- 193.Lehrer RI, et al. Modulation of the in vitro candidacidal activity of human neutrophil defensins by target cell metabolism and divalent cations. J Clin Invest. 1988;81(6):1829–35. doi: 10.1172/JCI113527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ganz T, Selsted ME, Lehrer RI. Defensins. Eur J Haematol. 1990;44(1):1–8. doi: 10.1111/j.1600-0609.1990.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 195.Ganz T, et al. Posttranslational processing and targeting of transgenic human defensin in murine granulocyte, macrophage, fibroblast, and pituitary adenoma cell lines. Blood. 1993;82(2):641–50. [PubMed] [Google Scholar]
- 196.Wilson CL, et al. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286(5437):113–7. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 197.Selsted ME, et al. Enteric defensins: antibiotic peptide components of intestinal host defense. J Cell Biol. 1992;118(4):929–36. doi: 10.1083/jcb.118.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Jones DE, Bevins CL. Defensin-6 mRNA in human Paneth cells: implications for antimicrobial peptides in host defense of the human bowel. FEBS Lett. 1993;315(2):187–92. doi: 10.1016/0014-5793(93)81160-2. [DOI] [PubMed] [Google Scholar]
- 199.Quayle AJ, et al. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol. 1998;152(5):1247–58. [PMC free article] [PubMed] [Google Scholar]
- 200.Lehrer RI, et al. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Invest. 1989;84(2):553–61. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3(3):238–50. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 202.Levitz SM, et al. In vitro killing of spores and hyphae of Aspergillus fumigatus and Rhizopus oryzae by rabbit neutrophil cationic peptides and bronchoalveolar macrophages. J Infect Dis. 1986;154(3):483–9. doi: 10.1093/infdis/154.3.483. [DOI] [PubMed] [Google Scholar]
- 203.Alcouloumre MS, et al. Fungicidal properties of defensin NP-1 and activity against Cryptococcus neoformans in vitro. Antimicrob Agents Chemother. 1993;37(12):2628–32. doi: 10.1128/aac.37.12.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ganz T, et al. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985;76(4):1427–35. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Bensch KW, et al. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 1995;368(2):331–5. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 206.Weinberg A, Krisanaprakornkit S, Dale BA. Epithelial antimicrobial peptides: review and significance for oral applications. Crit Rev Oral Biol Med. 1998;9(4):399–414. doi: 10.1177/10454411980090040201. [DOI] [PubMed] [Google Scholar]
- 207.Tollin M, et al. Antimicrobial peptides in the first line defence of human colon mucosa. Peptides. 2003;24(4):523–30. doi: 10.1016/s0196-9781(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 208.Cole AM, Dewan P, Ganz T. Innate antimicrobial activity of nasal secretions. Infect Immun. 1999;67(7):3267–75. doi: 10.1128/iai.67.7.3267-3275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Valore EV, et al. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Invest. 1998;101(8):1633–42. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sahasrabudhe KS, et al. Expression of the antimicrobial peptide, human beta-defensin 1, in duct cells of minor salivary glands and detection in saliva. J Dent Res. 2000;79(9):1669–74. doi: 10.1177/00220345000790090601. [DOI] [PubMed] [Google Scholar]
- 211.Garcia JR, et al. Human beta-defensin 4: a novel inducible peptide with a specific salt-sensitive spectrum of antimicrobial activity. FASEB J. 2001;15(10):1819–21. [PubMed] [Google Scholar]
- 212.Yamaguchi Y, et al. Identification of multiple novel epididymis-specific beta-defensin isoforms in humans and mice. J Immunol. 2002;169(5):2516–23. doi: 10.4049/jimmunol.169.5.2516. [DOI] [PubMed] [Google Scholar]
- 213.Shi J, et al. Porcine epithelial beta-defensin 1 is expressed in the dorsal tongue at antimicrobial concentrations. Infect Immun. 1999;67(6):3121–7. doi: 10.1128/iai.67.6.3121-3127.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Schroder JM, Harder J. Human beta-defensin-2. Int J Biochem Cell Biol. 1999;31(6):645–51. doi: 10.1016/s1357-2725(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 215.Harder J, et al. A peptide antibiotic from human skin. Nature. 1997;387(6636):861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 216.Jia HP, et al. Discovery of new human beta-defensins using a genomics-based approach. Gene. 2001;263(1–2):211–8. doi: 10.1016/s0378-1119(00)00569-2. [DOI] [PubMed] [Google Scholar]
- 217.Diamond G, Russell JP, Bevins CL. Inducible expression of an antibiotic peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc Natl Acad Sci U S A. 1996;93(10):5156–60. doi: 10.1073/pnas.93.10.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Schonwetter BS, Stolzenberg ED, Zasloff MA. Epithelial antibiotics induced at sites of inflammation. Science. 1995;267(5204):1645–8. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- 219.Russell JP, et al. Coordinate induction of two antibiotic genes in tracheal epithelial cells exposed to the inflammatory mediators lipopolysaccharide and tumor necrosis factor alpha. Infect Immun. 1996;64(5):1565–8. doi: 10.1128/iai.64.5.1565-1568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Singh PK, et al. Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci U S A. 1998;95(25):14961–6. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zasloff M. Inducing endogenous antimicrobial peptides to battle infections. Proc Natl Acad Sci U S A. 2006;103(24):8913–4. doi: 10.1073/pnas.0603508103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Joly S, et al. Human beta-defensins 2 and 3 demonstrate strain-selective activity against oral microorganisms. J Clin Microbiol. 2004;42(3):1024–9. doi: 10.1128/JCM.42.3.1024-1029.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Lawyer C, et al. Effects of synthetic form of tracheal antimicrobial peptide on respiratory pathogens. J Antimicrob Chemother. 1996;37(3):599–604. doi: 10.1093/jac/37.3.599. [DOI] [PubMed] [Google Scholar]
- 224.Diamond G, et al. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci U S A. 1991;88(9):3952–6. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Tran D, et al. Microbicidal properties and cytocidal selectivity of rhesus macaque theta defensins. Antimicrob Agents Chemother. 2008;52(3):944–53. doi: 10.1128/AAC.01090-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Harwig SS, et al. Gallinacins: cysteine-rich antimicrobial peptides of chicken leukocytes. FEBS Lett. 1994;342(3):281–5. doi: 10.1016/0014-5793(94)80517-2. [DOI] [PubMed] [Google Scholar]
- 227.van Dijk A, et al. The beta-defensin gallinacin-6 is expressed in the chicken digestive tract and has antimicrobial activity against food-borne pathogens. Antimicrob Agents Chemother. 2007;51(3):912–22. doi: 10.1128/AAC.00568-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tam JP, et al. An unusual structural motif of antimicrobial peptides containing end-to-end macrocycle and cystine-knot disulfides. Proc Natl Acad Sci U S A. 1999;96(16):8913–8. doi: 10.1073/pnas.96.16.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Feigin AM, et al. Properties of voltage-gated ion channels formed by syringomycin E in planar lipid bilayers. J Membr Biol. 1996;149(1):41–7. doi: 10.1007/s002329900005. [DOI] [PubMed] [Google Scholar]
- 230.Zhang L, Takemoto JY. Mechanism of action of Pseudomonas syringae phytotoxin, syringomycin. Interaction with the plasma membrane of wild-type and respiratory-deficient strains of Saccharomyces cerevisiae. Biochim Biophys Acta. 1986;861(1):201–4. doi: 10.1016/0005-2736(86)90581-x. [DOI] [PubMed] [Google Scholar]
- 231.Sorensen KN, Kim KH, Takemoto JY. In vitro antifungal and fungicidal activities and erythrocyte toxicities of cyclic lipodepsinonapeptides produced by Pseudomonas syringae pv. syringae. Antimicrob Agents Chemother. 1996;40(12):2710–3. doi: 10.1128/aac.40.12.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Sorensen KN, et al. Efficacy of syringomycin E in a murine model of vaginal candidiasis. J Antibiot (Tokyo) 1998;51(8):743–9. doi: 10.7164/antibiotics.51.743. [DOI] [PubMed] [Google Scholar]
- 233.Tomita M, et al. Potent antibacterial peptides generated by pepsin digestion of bovine lactoferrin. J Dairy Sci. 1991;74(12):4137–42. doi: 10.3168/jds.S0022-0302(91)78608-6. [DOI] [PubMed] [Google Scholar]
- 234.Minn I, Kim HS, Kim SC. Antimicrobial peptides derived from pepsinogens in the stomach of the bullfrog, Rana catesbeiana. Biochim Biophys Acta. 1998;1407(1):31–9. doi: 10.1016/s0925-4439(98)00023-4. [DOI] [PubMed] [Google Scholar]
- 235.Nibbering PH, et al. Human lactoferrin and peptides derived from its N terminus are highly effective against infections with antibiotic-resistant bacteria. Infect Immun. 2001;69(3):1469–76. doi: 10.1128/IAI.69.3.1469-1476.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Lupetti A, et al. Candidacidal activities of human lactoferrin peptides derived from the N terminus. Antimicrob Agents Chemother. 2000;44(12):3257–63. doi: 10.1128/aac.44.12.3257-3263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kirkpatrick CH, et al. Inhibition of growth of Candida albicans by iron-unsaturated lactoferrin: relation to host-defense mechanisms in chronic mucocutaneous candidiasis. J Infect Dis. 1971;124(6):539–44. doi: 10.1093/infdis/124.6.539. [DOI] [PubMed] [Google Scholar]
- 238.Zarember KA, et al. Human polymorphonuclear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion. J Immunol. 2007;178(10):6367–73. doi: 10.4049/jimmunol.178.10.6367. [DOI] [PubMed] [Google Scholar]
- 239.Bellamy W, et al. Identification of the bactericidal domain of lactoferrin. Biochim Biophys Acta. 1992;1121(1–2):130–6. doi: 10.1016/0167-4838(92)90346-f. [DOI] [PubMed] [Google Scholar]
- 240.Bellamy WR, et al. Role of cell-binding in the antibacterial mechanism of lactoferricin B. J Appl Bacteriol. 1993;75(5):478–84. [PubMed] [Google Scholar]
- 241.Viejo-Diaz M, Andres MT, Fierro JF. Different anti-Candida activities of two human lactoferrin-derived peptides, Lfpep and kaliocin-1. Antimicrob Agents Chemother. 2005;49(7):2583–8. doi: 10.1128/AAC.49.7.2583-2588.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ueta E, Tanida T, Osaki T. A novel bovine lactoferrin peptide, FKCRRWQWRM, suppresses Candida cell growth and activates neutrophils. J Pept Res. 2001;57(3):240–9. doi: 10.1111/j.1399-3011.2001.00821.x. [DOI] [PubMed] [Google Scholar]
- 243.Bellamy W, et al. Killing of Candida albicans by lactoferricin B, a potent antimicrobial peptide derived from the N-terminal region of bovine lactoferrin. Med Microbiol Immunol. 1993;182(2):97–105. doi: 10.1007/BF00189377. [DOI] [PubMed] [Google Scholar]
- 244.Newman SL, et al. Identification of constituents of human neutrophil azurophil granules that mediate fungistasis against Histoplasma capsulatum. Infect Immun. 2000;68(10):5668–72. doi: 10.1128/iai.68.10.5668-5672.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Elsbach P, Weiss J. The bactericidal/permeability-increasing protein (BPI), a potent element in host-defense against gram-negative bacteria and lipopolysaccharide. Immunobiology. 1993;187(3–5):417–29. doi: 10.1016/S0171-2985(11)80354-2. [DOI] [PubMed] [Google Scholar]
- 246.Horwitz A, Motchink P, Nadell R. Fungicidal properties from bactericidal/permiability-increasing protein (BPI) act synergistically with fluconazole on a variety of Candida strains. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. American society of Microbilogy; Washington, D.C. 1997. p. 163. [Google Scholar]
- 247.Ammons S, et al. Efficacy of domain III peptide from bactericidal/permeability-increasing protein (BPI) in murine disseminated aspergillosis. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbilogy; Washington, D.C. 1997. p. 29. [Google Scholar]
- 248.Giacometti A, et al. Potential therapeutic role of cationic peptides in three experimental models of septic shock. Antimicrob Agents Chemother. 2002;46(7):2132–6. doi: 10.1128/AAC.46.7.2132-2136.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Nikawa H, et al. Fungicidal effect of three new synthetic cationic peptides against Candida albicans. Oral Dis. 2004;10(4):221–8. doi: 10.1111/j.1601-0825.2004.01010.x. [DOI] [PubMed] [Google Scholar]
- 250.Wade D, et al. Antibacterial peptides designed as analogs or hybrids of cecropins and melittin. Int J Pept Protein Res. 1992;40(5):429–36. doi: 10.1111/j.1399-3011.1992.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 251.Mosca DA, et al. IB-367, a protegrin peptide with in vitro and in vivo activities against the microflora associated with oral mucositis. Antimicrob Agents Chemother. 2000;44(7):1803–8. doi: 10.1128/aac.44.7.1803-1808.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Chen J, et al. Development of protegrins for the treatment and prevention of oral mucositis: structure-activity relationships of synthetic protegrin analogues. Biopolymers. 2000;55(1):88–98. doi: 10.1002/1097-0282(2000)55:1<88::AID-BIP80>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 253.Andreu D, et al. Shortened cecropin A-melittin hybrids. Significant size reduction retains potent antibiotic activity. FEBS Lett. 1992;296(2):190–4. doi: 10.1016/0014-5793(92)80377-s. [DOI] [PubMed] [Google Scholar]
- 254.Gordon YJ, Romanowski EG, McDermott AM. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr Eye Res. 2005;30(7):505–15. doi: 10.1080/02713680590968637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Chen H, et al. Recent advances in the research and development of human defensins. Peptides. 2006;27(4):931–40. doi: 10.1016/j.peptides.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 256.Hong IP, et al. Recombinant expression of human cathelicidin (hCAP18/LL-37) in Pichia pastoris. Biotechnol Lett. 2007;29(1):73–8. doi: 10.1007/s10529-006-9202-8. [DOI] [PubMed] [Google Scholar]
- 257.Huang L, Leong SS, Jiang R. Soluble fusion expression and characterization of bioactive human beta-defensin 26 and 27. Appl Microbiol Biotechnol. 2009;84(2):301–8. doi: 10.1007/s00253-009-1982-z. [DOI] [PubMed] [Google Scholar]
- 258.Jin F, et al. Expression of recombinant hybrid peptide cecropinA(1–8)-magainin2(1–12) in Pichia pastoris: purification and characterization. Protein Expr Purif. 2006;50(2):147–56. doi: 10.1016/j.pep.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 259.Chen H, Xu Z, Cen P. High-level expression of human beta-defensin-2 gene with rare codons in E. coli cell-free system. Protein Pept Lett. 2006;13(2):155–62. doi: 10.2174/092986606775101724. [DOI] [PubMed] [Google Scholar]
- 260.Kim HK, et al. Expression of the cationic antimicrobial peptide lactoferricin fused with the anionic peptide in Escherichia coli. Appl Microbiol Biotechnol. 2006;72(2):330–8. doi: 10.1007/s00253-005-0266-5. [DOI] [PubMed] [Google Scholar]
- 261.De Samblanx GW, et al. Mutational analysis of a plant defensin from radish (Raphanus sativus L.) reveals two adjacent sites important for antifungal activity. J Biol Chem. 1997;272(2):1171–9. doi: 10.1074/jbc.272.2.1171. [DOI] [PubMed] [Google Scholar]
- 262.Chebolu S, Daniell H. Chloroplast-derived vaccine antigens and biopharmaceuticals: expression, folding, assembly and functionality. Curr Top Microbiol Immunol. 2009;332:33–54. doi: 10.1007/978-3-540-70868-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Li Y. Carrier proteins for fusion expression of antimicrobial peptides in Escherichia coli. Biotechnol Appl Biochem. 2009;54(1):1–9. doi: 10.1042/BA20090087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bessette PH, et al. Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proc Natl Acad Sci U S A. 1999;96(24):13703–8. doi: 10.1073/pnas.96.24.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Peng L, et al. Preferential codons enhancing the expression level of human beta-defensin-2 in recombinant Escherichia coli. Protein Pept Lett. 2004;11(4):339–44. doi: 10.2174/0929866043406760. [DOI] [PubMed] [Google Scholar]
- 266.Beckloff N, et al. Activity of an antimicrobial peptide mimetic against planktonic and biofilm cultures of oral pathogens. Antimicrob Agents Chemother. 2007;51(11):4125–32. doi: 10.1128/AAC.00208-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Genco CA, et al. Antimicrobial activity of magainin analogues against anaerobic oral pathogens. Int J Antimicrob Agents. 2003;21(1):75–8. doi: 10.1016/s0924-8579(02)00275-3. [DOI] [PubMed] [Google Scholar]
- 268.La Rocca P, et al. Simulation studies of the interaction of antimicrobial peptides and lipid bilayers. Biochim Biophys Acta. 1999;1462(1–2):185–200. doi: 10.1016/s0005-2736(99)00206-0. [DOI] [PubMed] [Google Scholar]
- 269.La Rocca P, Shai Y, Sansom MS. Peptide-bilayer interactions: simulations of dermaseptin B, an antimicrobial peptide. Biophys Chem. 1999;76(2):145–59. doi: 10.1016/s0301-4622(98)00232-4. [DOI] [PubMed] [Google Scholar]
- 270.Massimiliano M, Colombo G. Molecular Simulation of Peptides: A Useful Tool for the Development of New Drugs and for the Study of Molecular Recognition. In: Cretich M, Chiari M, editors. Peptide Microarrays. Methods and Protocols. Springer; New York, NY: 2009. pp. 77–153. [DOI] [PubMed] [Google Scholar]
- 271.Altman H, et al. In vitro assessment of antimicrobial peptides as potential agents against several oral bacteria. J Antimicrob Chemother. 2006;58(1):198–201. doi: 10.1093/jac/dkl181. [DOI] [PubMed] [Google Scholar]
- 272.Balaban N, et al. A chimeric peptide composed of a dermaseptin derivative and an RNA III-inhibiting peptide prevents graft-associated infections by antibiotic-resistant staphylococci. Antimicrob Agents Chemother. 2004;48(7):2544–50. doi: 10.1128/AAC.48.7.2544-2550.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Helmerhorst EJ, et al. The effects of histatin-derived basic antimicrobial peptides on oral biofilms. J Dent Res. 1999;78(6):1245–50. doi: 10.1177/00220345990780060801. [DOI] [PubMed] [Google Scholar]
- 274.Ryan RP, et al. Interspecies signalling via the Stenotrophomonas maltophilia diffusible signal factor influences biofilm formation and polymyxin tolerance in Pseudomonas aeruginosa. Mol Microbiol. 2008;68(1):75–86. doi: 10.1111/j.1365-2958.2008.06132.x. [DOI] [PubMed] [Google Scholar]
- 275.Sims CR, Ostrosky-Zeichner L, Rex JH. Invasive candidiasis in immunocompromised hospitalized patients. Arch Med Res. 2005;36(6):660–71. doi: 10.1016/j.arcmed.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 276.Bulet P, Stocklin R. Insect antimicrobial peptides: structures, properties and gene regulation. Protein Pept Lett. 2005;12(1):3–11. doi: 10.2174/0929866053406011. [DOI] [PubMed] [Google Scholar]
- 277.Yuan Y, Gao B, Zhu S. Functional expression of a Drosophila antifungal peptide in Escherichia coli. Protein Expr Purif. 2007;52(2):457–62. doi: 10.1016/j.pep.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 278.Troxler RF, et al. Structural relationship between human salivary histatins. J Dent Res. 1990;69(1):2–6. doi: 10.1177/00220345900690010101. [DOI] [PubMed] [Google Scholar]
- 279.Boman HG. Antibacterial peptides: basic facts and emerging concepts. J Intern Med. 2003;254(3):197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 280.Munoz A, Lopez-Garcia B, Marcos JF. Studies on the mode of action of the antifungal hexapeptide PAF26. Antimicrob Agents Chemother. 2006;50(11):3847–55. doi: 10.1128/AAC.00650-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Bulet P, et al. Antimicrobial peptides in insects; structure and function. Dev Comp Immunol. 1999;23(4–5):329–44. doi: 10.1016/s0145-305x(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 282.Kokryakov VN, et al. Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 1993;327(2):231–6. doi: 10.1016/0014-5793(93)80175-t. [DOI] [PubMed] [Google Scholar]
- 283.Lehrer RI. Primate defensins. Nat Rev Microbiol. 2004;2(9):727–38. doi: 10.1038/nrmicro976. [DOI] [PubMed] [Google Scholar]
- 284.Gonzalez-Chavez SA, Arevalo-Gallegos S, Rascon-Cruz Q. Lactoferrin: structure, function and applications. Int J Antimicrob Agents. 2009;33(4):301, e1–8. doi: 10.1016/j.ijantimicag.2008.07.020. [DOI] [PubMed] [Google Scholar]