Linear peptides derived from the HIV gp41 C-terminus (C-peptides), such as the 36-residue Fuzeon, are potent HIV fusion inhibitors.1 These molecules bind to the N-peptide region of gp41 and act as dominant negative inhibitors of an intramolecular protein–protein interaction that powers fusion of the viral and host cell membranes.2–4 The gp41 N-peptide region contains a surface pocket3–5 that is less prone to mutation than other gp41 regions or HIV enzymes.6 This pocket is occupied in the post-fusion state by three α-helical residues found near the gp41 C-terminus: Trp628, Trp631, and Ile635; together, these residues comprise the WWI epitope.3–5 Simple7,8 and constrained9,10 α-peptides, aromatic foldamers,11 peptide–small molecule conjugates,12 and small molecules13 that bind this pocket inhibit gp41-mediated fusion. Here, we describe a set of β3-decapeptides, βWWI-1–4, in which the WWI epitope is presented on one face of a short 14-helix (Figure 1).14 βWWI-1–4 bind to a validated gp41 model in vitro and inhibit viral fusion in cell culture. Our work suggests that β-peptide 14-helices, which are likely to be metabolically stable and protease resistant,15–17 can function as in vivo inhibitors of intramolecular protein–protein interactions.18
Figure 1.
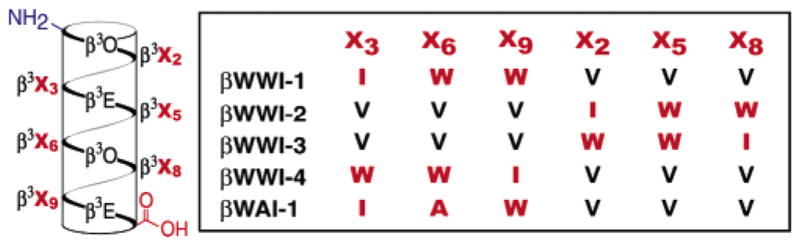
Sequences of βWWI-1–4 and βWAI-1. β3-homoamino acids are identified by the single letter code used for the corresponding α-amino acid. O signifies ornithine.
We synthesized19 four β3-peptides (βWWI-1–4) containing the WWI epitope in both possible orientations on each available face of a β3-decapeptide14 possessing significant 14-helix stability in aqueous solution due to electrostatic macrodipole stabilization20 and side chain–side chain salt bridges.21,22 We also prepared βWAI-1 as a control, as previous work has documented the significant contribution of the central Trp631 to gp41 affinity and viral infectivity.7 The circular dichroism spectra of βWWI-1–4 and βWAI-1 all display the expected minima at 214 nm (Figure 2A).14,20,23 The spectra of βWWI-1–4, but not βWAI-1, also show a transition at 227 nm, which may result from distortions in the 14-helix or the presence of two tryptophan residues in close proximity.24 Two-dimensional NMR spectroscopy in CD3OH confirmed the presence of 14-helix structure in βWWI-1; NOESY spectra showed five of seven possible Cα(i) → Cβ(i+3) NOEs and three of six possible (CN(i) → Cβ(i+3) NOEs, and no NOEs inconsistent with 14-helical structure were observed.19
Figure 2.
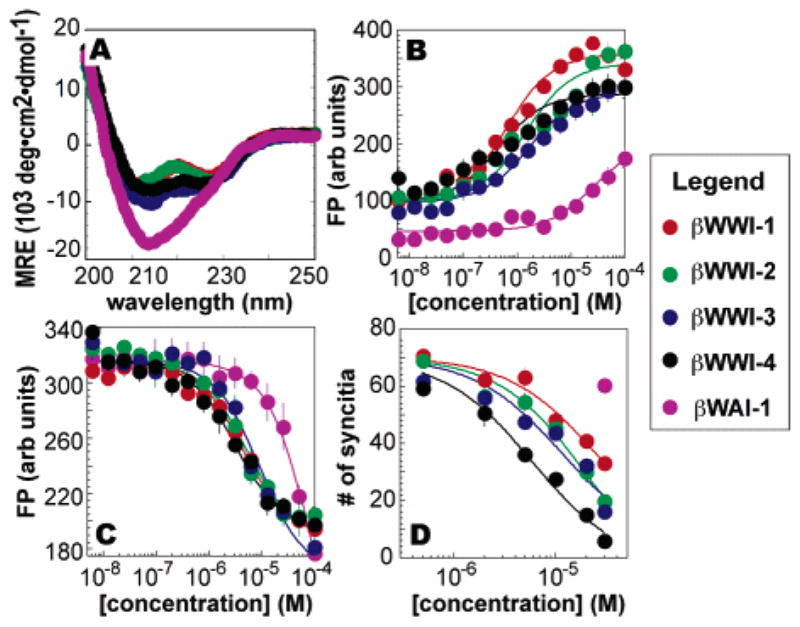
(A) CD spectra of βWWI-1–4 and βWAI-1 at 5 μM in PBC buffer. (B) Fluorescence polarization analysis of the binding of IZN17 and (C) the inhibition of C14wtFlu• IZN17 complexation by βWWI-1–4 and βWAI-1. (D) Inhibition of syncytia formation by βWWI-1–4 and βWAI-1.19
Each β-peptide was fluorescently labeled19 at the N-terminus and used in direct fluorescence polarization (FP) experiments to determine its affinity for the gp41 model IZN17.25 IZN17, which exists as a stable trimer in solution,25 contains 24 residues of an isoleucine zipper26 fused in register to 17 residues from gp41 containing the pocket for the WWI functional epitope.25 All four β-peptides, βWWI-1–4Flu, bound IZN17 well, with equilibrium affinities of 0.75 ± 0.1, 1.0 ± 0.3, 2.4 ± 0.7, and 1.5 ± 0.4 μM, respectively (Figure 2A). Interestingly, in this case, IZN17 affinity is relatively insensitive to the orientation of the WWI epitope relative to either the 14-helix macrodipole or the salt-bridging face.14 The affinity of βWWI-1–4 for IZN17 is nearly identical to that of the highest affinity α-peptide of comparable size (Kd = 1.2 μM).10 Also, βWWI-1 binds IZN17 with significantly higher affinity than it binds carbonic anhydrase II (Kd ≥ 115 μM) or calmodulin (Kd > 100 μM), two globular proteins that recognize hydrophobic and/or helical molecules.19
Two experiments were performed to investigate the binding mode of βWWI-1–4. First we performed competition fluorescence polarization experiments to assess whether βWWI-1–4 competed with C14wtFlu (suc-MTWMEWDR EINNYTCFlu), a fluorescent analogue of a gp41 ligand10 that binds IZN17 with an affinity of 4.1 μM. βWWI-1–4 competed well, with IC50 values of 4.0 ± 0.7, 4.6 ± 0.4, 13 ± 4.1, and 3.3 ± 1.4 μM, respectively (Figure 2C). We also synthesized the βWWI-1 analogue βWAI-1 with alanine in place of the central tryptophan of the WWI epitope. βWAI-1Flu bound IZN17 with lower affinity (Kd ≥ 20 μM) than βWWI-1 and βWAI-1 and competed poorly with C14wtFlu for IZN17 (IC50 = 72.9 ± 5.0 μM).27 These data suggest that the affinity of βWWI-1–4 for IZN17 results from interactions between the WWI epitope and the targeted IZN17 pocket.
βWWI-1–4 were then evaluated for their ability to inhibit gp41-mediated cell–cell fusion in an assay that accurately predicts potency in HIV infectivity assays.9 HeLa cells that express CD4 and a tat inducible β-gal gene28 were co-cultured in the presence of varying concentrations of β-peptides with HXB2 Env-expressing CHO cells29 that express HIV-1 env, tat, and rev. Without inhibitors, these cells fuse and form syncytia that express β-galactosidase and can be detected with 5-bromo-4-chloro-3-indoyl-β-D-galactoside.28 β-Peptides βWWI-1–4 inhibited cell–cell fusion with EC50 values of 27 ± 2.5, 15 ± 1.6, 13 ± 1.9, and 5.3 ± 0.5 μM, respectively, whereas βWAI-1 did not (Figure 2).19 The EC50 values measured for βWWI-1–4 are equal if not better than those measured for L-peptides,10 cyclic D-peptides,9 aromatic foldamers,11 or small molecules.13 Although less potent than Fuzeon (IC50 = 0.11 nM),1 βWWI-1–4 are one-third the size, likely metabolically stable,15 and can be optimized combinatorially. These results suggest that short β-peptide 14-helices can inhibit intramolecular protein–protein interactions in vivo. Molecules, such as βWWI-1–4, could represent leads toward inhibitors or antigens effective against HIV or other viruses, such as SARS,30 Ebola, HRSV, and influenza,31 that employ common fusion mechanisms.
Supplementary Material
Acknowledgments
This work was supported by the NIH (GM65453 and GM74756 to A.S., P01 GM066521 to M.K.), the National Foundation for Cancer Research, the Robert Leet and Clara Guthrie Patterson Trust, and in part by a grant to Yale University, in support of A.S., from the Howard Hughes Medical Institute. B.W. was supported by an NIH training grant in Biological Chemistry. CD4+ HeLa cells were obtained from the AIDS Research and Reference Reagent Program (M. Emerman). HXB2 Env-expressing CHO cells were a gift of M. Krieger.
Footnotes
Supporting Information Available: β-peptide synthesis and binding and cell fusion assays. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Wild CT, Shugars DC, Greenwell TA, McDanal CB, Matthews TJ. Proc Natl Acad Sci USA. 1994;91:9770. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu M, Blacklow SC, Kim PS. Nat Struct Biol. 1995;2:1075. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- 3.Chan DC, Fass D, Berger JM, Kim PS. Cell. 1997;89:263. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 4.Weissenhorn W, Desson A, Harrison SC, Skehel JJ, Wiley DC. Nature. 1997;387:426. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 5.Tan K, Liu JH, Wang JH, Shen S, Lu M. Proc Natl Acad Sci USA. 1997;94:12303. doi: 10.1073/pnas.94.23.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Malim MH, Hauber J, Shu-Yun L, Maizel JV, Cullen BR. Nature. 1989;338:254. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]; (b) Zapp ML, Green MR. Nature. 1989;342:714. doi: 10.1038/342714a0. [DOI] [PubMed] [Google Scholar]
- 7.Chan DC, Chutkowski CT, Kim PS. Proc Natl Acad Sci USA. 1998;95:15613. doi: 10.1073/pnas.95.26.15613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Jin BS, Ryu JR, Ahn K, Yu YG. AIDS Res Hum Retroviruses. 2000;16:1797. doi: 10.1089/08892220050195757. [DOI] [PubMed] [Google Scholar]; (b) Sia SK, Kim PS. Proc Natl Acad Sci USA. 2003;100:9756. doi: 10.1073/pnas.1733910100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckert DM, Malashkevich VN, Hong LH, Carr PA, Kim PS. Cell. 1999;99:103. doi: 10.1016/s0092-8674(00)80066-5. [DOI] [PubMed] [Google Scholar]
- 10.Sia SK, Carr PA, Cochran AG, Malashkevich VN, Kim PS. Proc Natl Acad Sci USA. 2002;99:14664. doi: 10.1073/pnas.232566599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst JT, Kutzki O, Debnath AK, Jiang S, Lu H, Hamilton AD. Angew Chem, Int Ed. 2002;41:278. doi: 10.1002/1521-3773(20020118)41:2<278::aid-anie278>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 12.Ferrer M, Kapoor TM, Strassmaier T, Weissenhorn W, Skehel JJ, Oprian D, Schreiber SL, Wiley DC, Harrison SC. Nature Struct Biol. 1999;6:953. doi: 10.1038/13324. [DOI] [PubMed] [Google Scholar]
- 13.(a) Zhao Q, Ernst JT, Hamilton AD, Debnath AK, Jiang S. AIDS Res Hum Retroviruses. 2002;18:989. doi: 10.1089/08892220260235353. [DOI] [PubMed] [Google Scholar]; (b) Jiang S, Lu H, Liu S, Zhao Q, He Y, Debnath AK. Antimicrob Agents Chemother. 2004;48:4349. doi: 10.1128/AAC.48.11.4349-4359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Debnath AK, Radiga L, Jiang S. J Med Chem. 1999;42:3203. doi: 10.1021/jm990154t. [DOI] [PubMed] [Google Scholar]
- 14.Kritzer JA, Lear JD, Hodsdon ME, Schepartz A. J Am Chem Soc. 2004;126:9468. doi: 10.1021/ja031625a. [DOI] [PubMed] [Google Scholar]
- 15.(a) Seebach D, Overhand M, Kuhnle FNM, Martinoni B, Oberer L, Hommel U, Widmer H. Helv Chim Acta. 1996;79:913. [Google Scholar]; (b) Seebach D, Abele S, Schreiber JV, Martinoni B, Nussbaum AK, Schild H, Schulz H, Hennecke H, Woessner R, Bitsch F. Chimia. 1998;52:734. [Google Scholar]; (c) Frackenpohl J, Arvidsson PI, Schreiber JV, Seebach D. ChemBioChem. 2001;2:445. doi: 10.1002/1439-7633(20010601)2:6<445::aid-cbic445>3.3.co;2-i. [DOI] [PubMed] [Google Scholar]
- 16.Wiegand H, Wirz B, Schweitzer A, Camenisch GP, Perez MIR, Gross G, Woessner R, Voges R, Arvidsson PI, Frackenpohl J, Seebach D. Biopharm Drug Disp. 2002;23:251. doi: 10.1002/bdd.334. [DOI] [PubMed] [Google Scholar]
- 17.Wiegand H, Wirz B, Schweitzer A, Gross G, Perez MIR, Andres H, Kimmerlin T, Reuping M, Seebach D. Chem Biodiv. 2004;1:1812. doi: 10.1002/cbdv.200490136. [DOI] [PubMed] [Google Scholar]
- 18.(a) Hamuro Y, Schneider JP, DeGrado WF. J Am Chem Soc. 1999;121:12200. [Google Scholar]; (b) Porter EA, Wang X, Lee HS, Weisblum B, Gellman SH. Nature. 2000;404:565. doi: 10.1038/35007145. [DOI] [PubMed] [Google Scholar]; (c) Arvidsson PI, Frackenpohl J, Ryder NS, Liechty B, Petersen F, Zimmermann H, Camenisch GP, Woessner R, Seebach D. ChemBioChem. 2001;2:771. doi: 10.1002/1439-7633(20011001)2:10<771::aid-cbic771>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]; (d) Liu D, DeGrado WF. J Am Chem Soc. 2001;123:7553. doi: 10.1021/ja0107475. [DOI] [PubMed] [Google Scholar]; (e) Porter EA, Weisblum B, Gellman SH. J Am Chem Soc. 2002;124:7324. doi: 10.1021/ja0260871. [DOI] [PubMed] [Google Scholar]; (f) Raguse TL, Porter EA, Weisblum B, Gellman SH. J Am Chem Soc. 2002;124:12774. doi: 10.1021/ja0270423. [DOI] [PubMed] [Google Scholar]; (g) Epand RF, Umezawa N, Porter EA, Gellman S, Epand RM. Eur J Biochem. 2003;270:1240. doi: 10.1046/j.1432-1033.2003.03484.x. [DOI] [PubMed] [Google Scholar]; (h) Gademann K, Seebach D. Helv Chim Acta. 2001;84:2924. [Google Scholar]; (i) Gademann K, Ernst M, Hoyer D, Seebach D. Angew Chem. 1999;111:1302. doi: 10.1002/(SICI)1521-3773(19990503)38:9<1223::AID-ANIE1223>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]; Angew Chem, Int Ed. 1999;38:1223. [Google Scholar]; (j) Gademann K, Kimmerlin T, Hoyer D, Seebach D. J Med Chem. 2001;44:2460. doi: 10.1021/jm010816q. [DOI] [PubMed] [Google Scholar]; (k) Seebach D, Rueping R, Arvidsson PI, Kimmerlin T, Micuch P, Noti C, Langenegger D, Hoyer D. Helv Chim Acta. 2001;84:3503. [Google Scholar]; (l) Nunn C, Rueping M, Langenegger D, Schuepbach E, Kimmerlin T, Micuch P, Hurt K, Seebach D, Hoyer D. Naunyn–Schmiedebergs Pharmacol. 2003;367:95. doi: 10.1007/s00210-002-0673-4. [DOI] [PubMed] [Google Scholar]; (m) Arvidsson PI, Ryder NR, Weiss HM, Gross G, Kretz O, Woessner R, Seebach D. ChemBioChem. 2003;4:1345. doi: 10.1002/cbic.200300698. [DOI] [PubMed] [Google Scholar]; (n) Werder M, Hauser H, Abele S, Seebach D. Helv Chim Acta. 1999;82:1774. [Google Scholar]
- 19.See Supporting Information for details.
- 20.Hart SA, Bahadoor ABF, Matthews EE, Qiu XJ, Schepartz A. J Am Chem Soc. 2003;125:4022. doi: 10.1021/ja029868a. [DOI] [PubMed] [Google Scholar]
- 21.(a) Arvidsson PI, Rueping M, Seebach D. Chem Commun. 2001:649. [Google Scholar]; (b) Cheng RP, DeGrado WF. J Am Chem Soc. 2001;123:5162. doi: 10.1021/ja010438e. [DOI] [PubMed] [Google Scholar]; (c) Rueping M, Mahajan YR, Jaun B, Seebach D. Chem–Eur J. 2004;10:1607. doi: 10.1002/chem.200305571. [DOI] [PubMed] [Google Scholar]
- 22.Kritzer JA, Hodsdon ME, Schepartz A. J Am Chem Soc. 2005;127:4118. doi: 10.1021/ja042933r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.(a) Arvidsson PI, Frackenpohl J, Seebach D. Helv Chim Acta. 2003;86:1522. [Google Scholar]; (b) Park JS, Lee HS, Lai JR, Kim BM, Gellman SH. J Am Chem Soc. 2003;125:8539. doi: 10.1021/ja034180z. [DOI] [PubMed] [Google Scholar]; (c) Raguse TL, Lai JR, Gellman SH. J Am Chem Soc. 2003;125:5592. doi: 10.1021/ja0341485. [DOI] [PubMed] [Google Scholar]; (d) Cheng RP, DeGrado WF. J Am Chem Soc. 2002;124:11564. doi: 10.1021/ja020728a. [DOI] [PubMed] [Google Scholar]; (e) Seebach D, Beck AK, Bierbaum DJ. Chem Biodiv. 2004;1:1111. doi: 10.1002/cbdv.200490087. [DOI] [PubMed] [Google Scholar]; (f) Cheng RP, Gellman SH, DeGrado WF. Chem Rev. 2001;101:3219. doi: 10.1021/cr000045i. [DOI] [PubMed] [Google Scholar]; (g) DeGrado WF, Schneider JP, Hamuro Y. J Peptide Res. 1999;54:206. doi: 10.1034/j.1399-3011.1999.00131.x. [DOI] [PubMed] [Google Scholar]
- 24.(a) Persson D, Thoren PE, Lincoln P, Norden B. Biochemistry. 2004;43:11045. doi: 10.1021/bi036054d. [DOI] [PubMed] [Google Scholar]; (b) Freskgard PO, Martensson LG, Jonasson P, Jonsson BH, Carlsson U. Biochemistry. 1994;33:14281. doi: 10.1021/bi00251a041. [DOI] [PubMed] [Google Scholar]; (c) Vuilleumier S, Sancho J, Loewenthal R, Fersht AR. Biochemistry. 1993;32:10303. doi: 10.1021/bi00090a005. [DOI] [PubMed] [Google Scholar]
- 25.Eckert DM, Kim PS. Proc Natl Acad Sci USA. 2001;98:11187. doi: 10.1073/pnas.201392898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki K, Hiroaki H, Kohda D, Tanaka T. Protein Eng. 1998;11:1051. doi: 10.1093/protein/11.11.1051. [DOI] [PubMed] [Google Scholar]
- 27.βWWI-1 analogues βAWI-1 and βWWA-1 bound IZN17 with affinities 3-fold lower than βWWI-1. The relative affinities of these analogues are consistent with previous work documenting the modest contribution of Trp628 and I635 to gp41 affinity and viral infectivity.7
- 28.Kimpton J, Emerman M. J Virol. 1992;66:2232. doi: 10.1128/jvi.66.4.2232-2239.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kozarsky K, Penman M, Basiripour L, Haseltine W, Sodroski J, Krieger M. J Acquired Immune Defic Syndr. 1989;2:163. [PubMed] [Google Scholar]
- 30.(a) Ingallinella P, Bianchi E, Finotto M, Cantoni G, Eckert DM, Supekar VM, Bruckmann C, Carfi A, Pessi A. Proc Natl Acad Sci USA. 2004;101:8709. doi: 10.1073/pnas.0402753101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tripet B, Howard MW, Jobling M, Holmes RK, Holmes KV, Hodges RS. J Biol Chem. 2004;279:20836. doi: 10.1074/jbc.M400759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eckert DM, Kim PS. Annu Rev Biochem. 2001;70:777. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


