Abstract
Herein, we tested a recently-proposed working model of apolipoprotein E (apoE)-mediated sulfatide metabolism/trafficking/homeostasis with two well-characterized amyloid precursor protein (APP) transgenic (Tg) animal models of Alzheimer’s disease (AD) (i.e., APPV717F and APPsw) on a wild-type murine apoE background or after being bred onto an Apoe−/− background. As anticipated, lipidomics analysis demonstrated that the sulfatide levels in brain tissues were reduced beginning at approximately 6 months of age in APPV717F Tg, Apoe+/+ mice and at 9 months of age in APPsw Tg, Apoe+/+ mice relative to their respective non APP Tg littermates. This reduction increased in both APP Tg mice as they aged. In contrast, sulfatide depletion did not occur in APP Tg, Apoe−/− animals relative to the Apoe−/− littermates. The lack of sulfatide depletion in APP Tg, Apoe−/− mice strongly supports the role of apoE in the deficient sulfatide content in APP Tg, Apoe+/+ mice. Collectively, through different animal models of AD, this study provides evidence for an identified biochemical mechanism that may be responsible for the sulfatide depletion at the earliest stages of AD.
Keywords: Alzheimer’s disease, apolipoprotein E, APP transgenic mice, PDAPP, shotgun lipidomics, sphingolipidomics, sulfatide, Tg2576
1. Introduction
Although substantial progress has recently been made toward understanding the pathogenesis of Alzheimer’s disease (AD) (Nagy, 2005; Zetterberg and Andreasson, 2007), the biochemical mechanism(s) responsible for this devastating disease has still not been defined. Prior work has demonstrated that the ε4 allelic variant of apoE is a major genetic risk factor for “sporadic” AD, which accounts for > 99% of AD cases. The mechanism(s) underlying the genetic risk of the apoE4 allele for AD pathogenesis remains to be completely clarified. Both in vitro and more recently in vivo data strongly suggest that the ability of apoE to modify amyloid-β peptide (Aβ) deposition may underlie the role of apoE4 as an AD risk factor (Bales et al., 2002). Furthermore, the involvement of apoE in sulfatide metabolism, trafficking, and homeostasis in the central nervous system (CNS) (Han et al., 2003a; Han, 2007) that has recently been identified may play an important role in multiple early events occurring in the pathogenesis of AD.
Sulfatides are a class of sulfated galactocerebrosides, which are almost exclusively synthesized by oligodendrocytes in the CNS and are present predominantly in the myelin sheath surrounding axons (Vos et al., 1994). The content of sulfatides in the CNS is specifically modulated by apoE in an isoform-dependent fashion through the same metabolic pathways that regulate levels of apoE-containing CNS lipoproteins (Han et al., 2003a). Therefore, a small amount of sulfatides is distributed to the plasma membranes of neurons and other glial cells (Pernber et al., 2002). The majority of the cellular sulfatides taken up through apoE-mediated endocytic pathways are degraded to its basic building blocks (e.g., sulfate, galactose, trans-hexadecenal, sphingosine, etc.) in both endosomes and lysosomes (Zeng et al., 2008).
Substantial amounts of sulfatides accumulate in apoE knockout mouse brain tissue samples due to the termination of the trafficking process of sulfatides (Han et al., 2003a). Accumulation of sulfatides, due to the deficiency in sulfatidase or its co-enzymes (e.g., saponin B) in lysosomes, is responsible for metachromatic leukodystrophy (Molander-Melin et al., 2004; von Figura et al., 2001). Mice deficient in sulfatides and galactosylceramides (GalCer) (generated by knocking out a ceramide galactosyltransferase), generally die by 3 months of age and demonstrate multiple neurological abnormalities including abnormal axonal function, dysmyelinosis, and loss of axonal conduction velocity (Bosio et al., 1996; Bosio et al., 1998; Coetzee et al., 1996; Coetzee et al., 1998). Severe myelin developmental abnormalities, myelin sheath degeneration, and the significant increases of deteriorating nodal/paranodal structures are manifest in sulfatide-null mice (Marcus et al., 2006).
We have demonstrated that sulfatide content precipitously and specifically drops at the earliest clinically recognizable stages of AD in both postmortem brain tissues and freshly-collected cerebrospinal fluid samples in comparison to age-matched cognitively-normal controls (Han et al., 2002; Han et al., 2003b). Furthermore, we have also demonstrated that sulfatide deficiency is specific to AD among other examined neurodegenerative diseases including Parkinson’s disease and dementia with Lewy bodies (Cheng et al., 2003) as well as multiple sclerosis (Han, unpublished data). These findings suggest that sulfatides, and molecules that mediate sulfatide metabolism such as apoE, may play an important role in AD pathogenesis.
To identify the biochemical mechanisms underlying sulfatide depletion at the earliest clinically-recognizable stages of AD and the relationship of sulfatide depletion in AD with the apoE risk factor, we have recently proposed a working model of apoE-mediated sulfatide metabolism, trafficking, and homeostasis based on all of the currently available findings (Scheme 1) (Han, 2007). According to this working model, apoE-associated lipoprotein particles are released from astrocytes and acquire sulfatides from the myelin sheath likely through a “kiss-and-run” mechanism. Then the sulfatide-containing apoE-associated lipoprotein particles can be metabolized and degraded through an endocytic pathway by neuronal cells possessing low-density lipoprotein (LDL) receptors or its family members such as the LDL receptor-related protein (Arelin et al., 2002; Van Uden et al., 2000). Alternatively, these sulfatide-enriched apoE-associated lipoproteins can be transported to destinations in the peripheral system through the cerebrospinal fluid (Han et al., 2003b). It can be anticipated based upon our working model that an increased uptake of these apolipoproteins and/or an increased expression of the receptors can lead to the accelerated degradation of sulfatide-containing, apoE-associated lipoprotein particles, thereby resulting in an accelerated sulfatide depletion. It should be emphasized that apoE4 can exaggerate this process since apoE4-associated lipoprotein particles carry more sulfatides than the lipoprotein particles associated with other apoE isoforms (Han et al., 2003a).
Scheme 1.

A schematic diagram of a proposed working model for the metabolism of apolipoprotein E-associated lipoproteins which mediate sulfatide transport and homeostasis. In the model, apoE-associated lipoprotein particles are released from astrocytes and acquire sulfatides from myelin sheath (likely through a kiss-and-run process). The sulfatide-containing, apoE-associated lipoproteins can then be either metabolized through endocytotic pathway through low-density lipoprotein (LDL) receptor superfamily or transported to cerebrospinal fluid. Therefore, any factors that modulate apoE-associated lipoprotein metabolism can alter sulfatide levels in the central nervous system. The diagram has been modified from the figure in (Han, 2007) with permission.
In the current study, we tested this working model with two well-characterized animal AD-models transgenically expressing the mutants of human amyloid precursor protein (APP), i.e., APPV717F (also called PDAPP) and APPsw (also called Tg2576) transgenic (Tg) mice (Games et al., 1995; Holtzman et al., 2000b; Hsiao et al., 1996; Irizarry et al., 1997a; Irizarry et al., 1997b). It is well documented that substantial increases in the production of Aβ peptides are manifest in these animal models (Games et al., 1995; Holtzman et al., 2000b; Hsiao et al., 1996; Irizarry et al., 1997a; Irizarry et al., 1997b). It has also found that a higher apoE level is present in APP Tg mice relative to the controls in an age-dependent manner (Kuo et al., 2000). According to our working model, this elevated apoE level will facilitate the metabolism of apoE-associated lipoproteins through endocytic pathways, leading to an increased sulfatide trafficking and metabolism in an age-dependent manner. As anticipated, the sulfatide levels were reduced in these animal models in an Aβ pathology-dependent and age-dependent manner. In contrast, shotgun sphingolipidomics analysis demonstrated that sulfatide depletion did not occur in APP mutant, apoE null (Apoe−/−) animals relative to the Apoe−/− controls even though the basal content of sulfatides in both APP Tg, apoE null animals and apoE null controls was higher in comparison to those in their respective apoE-expressing mice. Collectively, through different animal models of AD, we verified our recently proposed working model of apoE-mediated sulfatide metabolism, trafficking, and homeostasis (Scheme 1), thereby providing evidence for the mechanisms that may be responsible for the sulfatide depletion at the earliest stages of AD.
2. Materials and methods
2.1. Materials
Synthetic phospholipids, N-lauroryl sphingomyelin (N12:0 CerPCho), and N-heptadecanoyl ceramide (N17:0 Cer) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL, USA). Semisynthetic N-palmitoyl sulfatide (N16:0 sulfatide) and perdeuterated N-stearoyl GalCer (d35-N18:0 GalCer) were obtained from Matreya, Inc. (Pleasant Gap, PA, USA). It should be pointed out that, herein, the prefix “N” denotes the amide linkage in sphingolipid molecular species. All the solvents were obtained from Burdick and Jackson (Honeywell International Inc., Burdick and Jackson, Muskegon, MI, USA). All other chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA) or as indicated.
2.2. Mouse brain tissues
Wild type (WT) and Apoe−/− mice on either a C57BL/6 background or a B6/SJL background were utilized in this study. APPV717F Tg (heterozygous for the APPV717F transgene), Apoe+/+ and APPV717F Tg, Apoe−/− mice on an outbred background [(Swiss Webster × C57BL/6 × DBA/2) × C57BL/6] and APPsw Tg, Apoe+/+ and APPsw Tg, Apoe−/− mice on a B6/SJL background were produced as described previously (Holtzman et al., 1999, 2000a). At the indicated age, mice were anesthetized with intraperitoneal pentobarbital (150 mg/kg) and were perfused transcardially with 0.1 M phosphate-buffered saline (pH 7.4) at 4 °C. Brain regions were immediately dissected and frozen on dry ice before analysis. The tissue wafers were pulverized into a fine powder with a stainless-steel mortar and pestle. All animal procedures were performed in accordance with the “Guide for the Care and Use of Laboratory Animals” (National Academy of Science, 1996) and were approved by the “Animals Studies Committee” at Washington University.
2.3. Preparation of lipid extracts from brain samples
A powder sample (approximately 10 mg) from each dissected brain tissue was weighed and further homogenized in 0.3 ml of ice-cold diluted (0.1x) phosphate-buffered saline with a Potter-Elvehjem tissue grinder. Protein assays on each individual homogenate were performed. Lipids were extracted by the modified method of Bligh and Dyer (Bligh and Dyer, 1959) as described previously (Cheng et al., 2006; Cheng et al., 2007) in the presence of internal standards which were in a pre-mixed solution for global lipid analysis and included N12:0 CerPCho (2.5 nmol/mg protein), N17:0 Cer (0.8 nmol/mg protein), N16:0 sulfatide (5.0 nmol/mg protein), and d35-N18:0 GalCer (10 nmol/mg protein). Other necessary internal standards for quantitation of individual molecular species of other lipid classes were also added to each brain tissue sample based on protein concentration prior to extraction of lipids. Thus, the lipid content could be normalized to the protein content and quantified directly. These internal standards were selected because they represent ≪ 1% of endogenous cellular lipid molecular species present as demonstrated by electrospray ionization mass spectrometry (ESI/MS) lipid analysis without addition of these internal standards. A part of each lipid extract was treated with lithium methoxide for sphingolipid analysis as previously described (Jiang et al., 2007). Each treated solution was finally reconstituted with a volume of 500 μl/mg of tissue protein in 1:1 chloroform/methanol. All lipid solutions were finally flushed with nitrogen, capped, and stored at −20 °C for ESI/MS (typically analyzed within one week).
2.4. Mass spectrometric analysis of lipids
A triple-quadrupole mass spectrometer (Thermo Scientific TSQ Quantum Ultra Plus, San Jose, CA, USA) equipped with a Nanomate device and Xcalibur system software was utilized in the study as previously described (Han et al., 2004; Yang et al., 2007). The diluted lipid extract was directly infused into the ESI source through the Nanomate device. Typically, a 1-min period of signal averaging in the profile mode was employed for each MS spectrum. For tandem mass spectrometry, a collision gas pressure was set at 1.0 mT, but the collision energy varied with the classes of lipids as described previously (Han et al., 2004; Han and Gross, 2005). Typically, a 2 to 5-min period of signal averaging in the profile mode was employed for each tandem MS mass spectrum. All mass spectra and tandem MS mass spectra were automatically acquired by a customized sequence subroutine operated under Xcalibur software. Data processing including ion peak selection, baseline correction, data transfer, peak intensity comparison, 13C de-isotoping, and quantitation were conducted using a custom programmed Microsoft Excel macros as outlined previously (Han et al., 2004).
2.5. Miscellaneous
Protein concentration was determined with a bicinchroninic acid protein assay kit (Pierce, Rockford, IL, USA) using bovine serum albumin as a standard. Quantitative data were normalized to protein content to minimize any effects of neuron and synapse loss upon quantitative analyses of lipids and are presented as the means ± SD. Differences between mean values were determined by an unpaired Student’s t tests. P < 0.01 was considered significant.
3. Results
3.1. Sulfatide content was depleted in APPsw Tg, Apoe+/+ mouse brain in an age- and region-dependent manner
We have hypothesized that the disturbance of the metabolism of apoE-containing lipoprotein particles due to a requirement of an accelerated clearance of Aβ peptides in APP Tg mouse brain will lead to an increased sulfatide degradation in lysosome, resulting in sulfatide depletion in brain tissues containing myelin sheath (e.g., cortex and cerebellum) (Scheme 1) (Han, 2007). To verify this hypothesis, we determined the levels of sulfatides in both cortex and cerebellum of APPsw Tg, Apoe+/+ mice at 18 months of age in comparison to their WT (i.e., Apoe+/+) littermates by using our newly developed shotgun sphingolipidomics (Jiang et al., 2007).
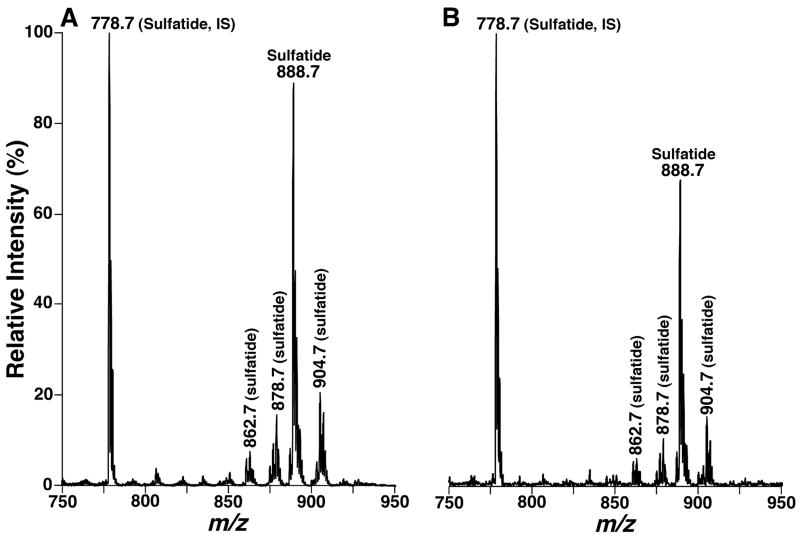
As anticipated, mass spectrometric analysis in a shotgun sphingolipidomics approach demonstrated that the levels of all sulfatide molecular species which contain various fatty acyl chains with or without hydroxy moiety in the cortex of APPsw Tg, Apoe+/+ mice at 18 months of age were decreased in comparison to the counterpart of their WT littermates (Figure 1). This result indicates a non-selective reduction of sulfatide content in APPsw Tg, Apoe+/+ mice relative to the controls. The content of total sulfatides in cortex was significantly reduced from 13.1 ± 1.2 nmol/mg protein in WT littermates to 11.2 ± 0.9 nmol/mg protein in APPsw Tg, Apoe+/+ mice at this age (Figure 2A, P < 0.001, n = 4). In contrast to the sulfatide depletion, shotgun sphingolipidomics analyses did not show significant changes in the content of other sphingolipid classes including CerPCho, GalCer, and Cer examined in cortex of APPsw Tg, Apoe+/+ mice relative to those of WT littermates. These results indicate that the reduction of sulfatide content is not due to the loss of myelin.
Figure 1.
Negative-ion electrospray ionization mass spectra of alkaline-treated lipid extracts of brain cortices from APPsw transgenic mice and their wild type littermates. Cortical lipid extracts of the wild type littermate controls (Panel A) and APPsw Tg, Apoe+/+ mice (Panel B) at 18 months of age were prepared by a modified Bligh and Dyer procedure as described under “Materials and Methods”. The lipid extracts were treated with lithium methoxide as previously described (Jiang et al., 2007). The treated lipid extracts were reconstituted in 1:1 chloroform/methanol and finally diluted to less than 50 pmol of total lipids/μl with chloroform/methanol/isopropanol (1:2:4, v/v/v) prior to infusion with a nanomate device. Mass spectra of alkaline-treated lipid extracts of mouse brain were acquired in the negative-ion model as described under “Materials and Methods”. The molecular species indicated were identified by using multi-dimensional mass spectrometric analysis as previously described (Jiang et al., 2007). “IS” denotes internal standard.
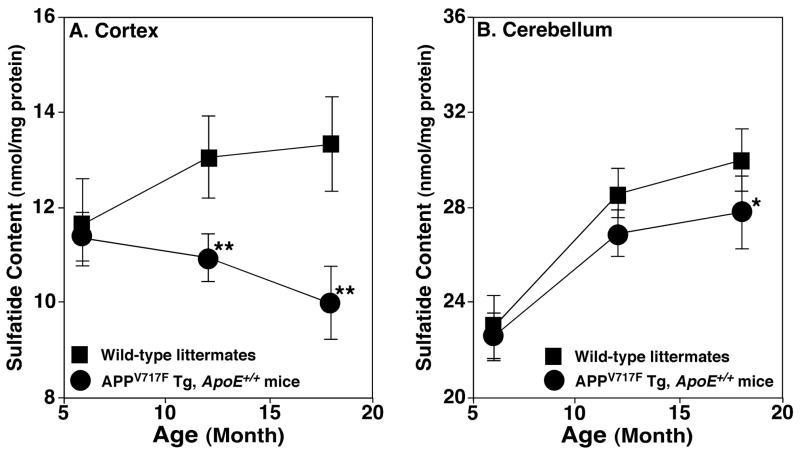
Figure 2.
Temporal changes in the content of total sulfatides in lipid extracts of brain cortices from APPsw transgenic mice and their wild type littermates. Lipid extracts of cortices (Panel A) and cerebella (Panel B) from the wild type littermate controls (solid squares) and APPsw Tg, Apoe+/+ mice (solid circles) at different ages were prepared using a modified Bligh and Dyer method. The content of each individual sulfatide molecular species after identification was determined in comparison to the selected internal standard after 13C de-isotoping as described under “Materials and Methods”. The data points represent mean ± SD from separate preparations of at least four different animals. *P < 0.01 and ** P < 0.001 compared to the wild-type littermates at the same age.
Next, to identify the temporal course of sulfatide depletion in cortex of APPsw Tg, Apoe+/+ mice relative to that of their age-matched WT littermates, we determined the content and composition of sulfatide molecular species in cortex of APPsw Tg, Apoe+/+ mice and their littermates at the selected ages (Figure 2A). We found that the decreased levels of sulfatides in APPsw Tg, Apoe+/+ mouse brain occurred at 9 months of age and the degree of sulfatide depletion increased with aging (Figure 2A). A similar finding was also demonstrated in other cerebral brain regions including hippocampus.
To verify that the demonstrated sulfatide depletion in APPsw Tg, Apoe+/+ mouse cortex is related to Aβ pathology (i.e., Aβ production and/or Aβ plaques), but not to the APP mutant expression, we determined the sulfatide content in cerebellum of APPsw Tg, Apoe+/+ mice. It is well known that the Aβ pathology is relatively less developed in this brain region in AD patients in general (Gearing et al., 1995) and in this animal model in particular (Hsiao et al., 1996; Irizarry et al., 1997a). Shotgun sphingolipidomics analysis showed that the content of sulfatides in this brain region of APPsw Tg, Apoe+/+ mice was not significantly different from that present in their WT littermates although a relatively lower sulfatide content was manifest in this region of the aged APPsw Tg, Apoe+/+ mice in comparison to their WT littermates (Figure 2B).
3.2. Sulfatide depletion occurred earlier and was more significant in APPV717F Tg, Apoe+/+ mouse brain relative to that in APPsw Tg, Apoe+/+ mouse brain
To further test our hypothesis, we next determined the sulfatide levels in another APP Tg mouse model (i.e., APPV717F Tg, Apoe+/+ mice) which develops AD-type plaques and Aβ pathology about 2 months prior to that seen in the APPsw Tg, Apoe+/+ mouse model (Games et al., 1995; Hsiao et al., 1996; Irizarry et al., 1997a; Irizarry et al., 1997b). Shotgun sphingolipidomics analysis showed that sulfatide depletion was also present in the cortex of APPV717F Tg, Apoe+/+ mice and the degree of sulfatide depletion was age-dependent (Figure 3A).
Figure 3.
Temporal changes in the content of total sulfatides in lipid extracts of brain cortices from APPV717F transgenic mice and their wild type littermates. Lipid extracts of cortices (Panel A) and cerebella (Panel B) from the wild type littermate controls (solid squares) and APPV717F Tg, Apoe+/+ mice (solid circles) at different ages were prepared using a modified Bligh and Dyer method. The content of each individual sulfatide molecular species after identification was determined in comparison to the selected internal standard after 13C de-isotoping as described under “Materials and Methods”. The data points represent mean ± SD from separate preparations of at least four different animals. *P < 0.01 and ** P < 0.001 compared to the wild-type littermates at the same age.
Moreover, shotgun sphingolipidomics analysis demonstrated three intriguing points that were related to the depleted sulfatide levels in APPV717F Tg, Apoe+/+ mouse brain samples in comparison to that present in APPsw Tg, Apoe+/+ mice (compare Figure 3 to Figure 2). First, sulfatide deficiency was detected at 6 months of age in APPV717F Tg, Apoe+/+ mouse brain in comparison to that at 9 months in APPsw Tg, Apoe+/+ mice. Second, the deficiency in sulfatide content in both cortex and cerebellum of this animal model relative to its age-matched WT littermates was much more severe in APPV717F Tg, Apoe+/+ mice relative to that in APPsw Tg, Apoe+/+ mice. Third, the changes of the deficient sulfatide content increased substantially as they aged. These observations indicate that sulfatide depletion in APPV717F Tg, Apoe+/+ mouse brain is relatively more severe and occurs at a relatively earlier age in comparison to that in APPsw Tg, Apoe+/+ model.
In contrast to small changes of sulfatide content in cerebellum of APPsw Tg, Apoe+/+ mice, the reduced sulfatide content became apparent at 12 months of age and was significant at 18 months of age in this brain region in APPV717F Tg, Apoe+/+ mice relative to their WT littermates (n = 4, P < 0.01) (Figure 3B). These results further support that sulfatide depletion in APPV717F Tg, Apoe+/+ mouse brain is relatively more severe and occurs at a relatively earlier age in comparison to that in APPsw Tg, Apoe+/+ model. However, similar to APPsw Tg, Apoe+/+ mice, other sphingolipid classes including CerPCho, GalCer, and Cer examined did not show significant changes in both cortex and cerebellum of APPV717F Tg, Apoe+/+ mice at the ages examined relative to those in age-matched WT littermates.
3.3. Cortex sulfatide levels were unchanged in the APP transgenic, apoE-deficient animal models
To identify the underlying cause(s) of the demonstrated sulfatide depletion in brain tissues of two AD animal models and further verify our aforementioned hypothesis, we next performed shotgun sphingolipidomics analysis of lipid extracts from brain tissues of these APP mutant Tg animal models in an apoE-deficient background (i.e., APPsw Tg, Apoe−/− and APPV717F Tg, Apoe−/− mice) in comparison to those from Apoe−/− or Apoe+/+ (i.e., WT) mice. As anticipated, shotgun sphingolipidomics analysis extended our previous observation (Han et al., 2003a) that sulfatide content in brain tissues of apoE null mice accumulated in comparison to the WT mice due to a deficiency in sulfatide transport and degradation (Figure 4). Intriguingly, the sulfatide levels in cortices of both APPsw Tg, Apoe−/− and APPV717F Tg, Apoe−/− mice were essentially identical to that of Apoe−/− mice (Figure 4). These results indicate that apoE plays a key role in sulfatide depletion that occurred in both APPsw Tg, Apoe+/+ and APPV717F Tg, Apoe+/+ mice.
Figure 4.
Temporal changes in the contents of total sulfatides in lipid extracts of brain cortices from APP mutant transgenic mice in an apolipoprotein E deficient background, their apolipoprotein E deficient littermates, as well as wild type mice. Lipid extracts of cortices from APPsw Tg, Apoe−/− mice (open circles, Panel A), APPV717F Tg, Apoe−/− mice (open circles, Panel B), their respective Apoe−/− littermates (open squares), and wild type (i.e., Apoe+/+) mice (solid squares) at different ages were prepared using a modified Bligh and Dyer method. The content of each individual sulfatide molecular species with or without the hydroxy group after identification was determined in comparison to the selected internal standard after 13C de-isotoping as described under “Materials and Methods”. The data points represent mean ± SD from separate preparations of at least four different animals.
4. Discussion
In the current study, we have employed our newly developed shotgun sphingolipidomics (Jiang et al., 2007) to determine the sphingolipidomes of the AD animal models in a systems biology approach. We have demonstrated a selective loss of sulfatide content in multiple brain regions of these animals in an age-dependent manner. We have demonstrated that sulfatide depletion in the AD animal models was in an Aβ level-related fashion in two aspects. First, within the APP Tg mice the degree of sulfatide depletion in the cerebellum was much less than that seen in the cortex. This supports the idea that Aβ in some way is associated with the sulfatide deficiency since Aβ accumulation does not occur to a significant extent in the cerebellum (Gearing et al., 1995). Secondly, between the animal models, the more severe loss of sulfatide content and the earlier onset of this loss in APPV717F Tg, Apoe+/+ mice relative to those in APPsw Tg, Apoe+/+ mice coincided with the difference in onset time of Aβ pathology between the two animal models (Holtzman et al., 2000a; Holtzman et al., 2000b). Accordingly, this study has shown a possible association between Aβ pathology and sulfatide loss in the examined AD animal models.
One potential underlying cause of sulfatide depletion in these AD animal models is that the Aβ peptides that accumulate in the brain directly modulate sulfatide metabolism and result in sulfatide depletion. A Previous study has shown that total Aβ levels in the cortex of APPsw Tg, Apoe+/+ mice are 11.1, 162.1, 385.9 pg of Aβ per μg total protein at 3, 12, and 16 months, respectively. The plaque loads in the mouse brain cortex at the same time points are 0, 2.56, and 19.37 %, respectively (Fryer et al., 2003). We have found no Aβ staining in the cerebellum of these mice until about 18 months where it is still < 0.1% plaque load (Holtzman, unpublished data). In the APPsw Tg, Apoe−/− mice, no fibrillar plaque load develops through 16 months of age (Fryer et al., 2003). Very similar findings are seen in PDAPP mice. Thus, some type of association between the Aβ buildup and sulfatide depletion in the brain likely exists although we do not prove the rise in Aβ is causative. Indeed, further study on this topic is warranted.
Previous studies have also shown that apoE plays a role in sulfatide metabolism, trafficking, and homeostasis (Cheng et al., 2007; Han et al., 2003a). To identify the role of apoE in potentially causing sulfatide depletion in APP mutant Tg mouse brain samples, we performed shotgun sphingolipidomics analysis of lipid extracts from APP mutant Tg mice on an apoE null background. We demonstrated unaltered sulfatide levels in APP Tg, Apoe−/− mice in comparison to non-APP Tg, Apoe−/− mice. Therefore, the unchanged sulfatide levels in APP Tg, Apoe−/− mice compared to Apoe−/− mice suggest that apoE is essential for the depletion in sulfatide levels that occurs in APP Tg mice with age. However, an indirect effect mediated by Aβ buildup in the APP Tg mice is not able to be excluded at this stage since there is a much greater level of Aβ accumulation, in particular fibrillar Aβ, in APP Tg mice in the presence vs. the absence of apoE (Holtzman et al., 2000a; Holtzman et al., 2000b).
It is possible that Aβ accumulation that is present in APP Tg mice is associated with an age-dependent alteration of apoE clearance of the over-produced Aβ peptides. It is well known that apoE-associated lipoproteins play an important role in the process of Aβ clearance either through transport of Aβ-containing apoE-associated lipoproteins to the peripheral circulatory system or by a local endocytic process through LDL super family receptors in the brain (Bales et al., 2002; Kang et al., 2000). Following this line of reasoning, the demand for clearance of the overproduced Aβ peptides through either transport to the circulatory system or local brain degradation in the endocytic vesicular organelles could somehow lead to upregulation of apoE expression. A higher apoE level in APP Tg mice relative to the controls has been reported (Kuo et al., 2000) and has been confirmed in our laboratories (Holtzman, unpublished data).
The determined increase in apoE levels in APP Tg mice supports a possible role for the increased apoE metabolism leading to the loss of sulfatide. Since sulfatide levels in the CNS are mediated by apoE in an apoE isoform-dependent manner, any changes in the metabolism of the apoE-associated lipoproteins, the apoE expression levels, and/or apoE isoforms could theoretically lead to alterations in sulfatide content in the CNS (Scheme 1) (Han, 2007). Therefore, the increased endocytic uptake and endosomal/lysosomal degradation of sulfatides and Aβ-containing, apoE-associated lipoprotein particles (particularly present in a chronic fashion) could result in a deficient content of sulfatides in the APP Tg mice as demonstrated. However, it should be specifically pointed out that the increased apoE levels could be due to a reduced uptake of apoE via endocytosis. If this would be the case, the sulfatide content should be higher as similarly demonstrated in Apoe−/− mouse brain. This is in contrast to our experimental observation that sulfatide is reduced accompanying an increased level of apoE.
Our recent experiments have shown that sulfatides can facilitate the Aβ binding to apoE-associated lipoproteins (likely through a specific interaction between the sulfatide-induced microenvironment at the membrane interface and Aβ peptides), thereby increasing the efficiency of Aβ clearance through the endocytotic pathway (Zeng and Han, 2008). Moreover, we have recently also demonstrated that abnormal sulfatide metabolism can induce cell apoptosis due to endosome-mediated ceramide generation and the accumulation of cytotoxic levels of sulfatides in lysosomes (Zeng et al., 2008). Collectively, although the cellular role of apoE-mediated sulfatide transport in the CNS remains unclear, our recent studies clearly indicate the importance of interactions between Aβ peptides, apoE isoform, and sulfatides in Aβ clearance, sulfatide metabolism, and cell apoptosis.
It is clear that the APPsw and APPV717F Tg mice develop sulfatide deficiency with age in the cortex which is similar to what we observed in AD patients (Han et al., 2002). However, there is minimal to no deficiency of sulfatides in the cerebellum of these animal models. In human AD, we found a more dramatic 91% loss of sulfatides in the cortical gray matter though there also was also a decrease by 63% in cerebellar gray matter (Han et al., 2002). The mechanisms underlying the discrepancy between human AD and the animal models are unclear. Some differences that may contribute to this discrepancy are that in cognitively normal humans, sulfatide levels are significantly higher in the cortex vs. the cerebellum (42.0 vs. 7.2 nmol/mg protein, respectively) whereas in the mouse, the sulfatide content is reversed with there being about 11 nmol/mg protein in the cortex and 23 nmol/mg protein in the cerebellum. This starting difference may alter the effects of the AD pathology. Moreover, while humans with AD develop neuronal loss (Gomez-Isla et al., 1996), the APP Tg mice that we studied do not (Irizarry et al., 1997b). This may also in some way account for the differences observed. In addition, sulfatide content is precipitously depleted even at the earliest clinically-recognizable stages of AD while sulfatide depletion in animal models largely depends on age. This difference suggests that other factors besides Aβ pathology may contribute to the sulfatide loss in human AD. The difference of the altered ceramide content between human AD and the AD animal models also supports the presence of other potential differences between AD and the changes occurring in the brain of APP Tg mice.
Acknowledgments
This work was supported by National Institute on Aging Grants R01 AG23168 (XH), R01 AG31675 (XH), AG13956 (DMH), and Eli Lilly (DMH).
Abbreviations
- AD
Alzheimer’s disease
- apoE
apolipoprotein E
- Apoe
the apoE gene
- APP
amyloid precursor protein
- Cer
ceramide
- CerPCho
sphingomyelin
- CNS
central nervous system
- ESI
electrospray ionization
- GalCer
galactosylceramides
- LDL
low density lipoprotein
- m:n
acyl chain containing m carbons and n double bonds
- MS
mass spectrometry
- SD
statistical deviation
- WT
wild type
Footnotes
Disclosure Statement: There are no actual or potential conflicts of interest, including any financial, personal or other relationships with other people or organizations within three years of beginning the work submitted that could inappropriately influence their work, that need to be disclosed by the authors except DMH who receives research funding from Eli Lilly and is a co-founder of C2N diagnostics.
All animal procedures were performed in accordance with the “Guide for the Care and Use of Laboratory Animals” (National Academy of Science, 1996) and were approved by the “Animals Studies Committee” at Washington University.
References
- Arelin K, Kinoshita A, Whelan CM, Irizarry MC, Rebeck GW, Strickland DK, Hyman BT. LRP and senile plaques in Alzheimer’s disease: colocalization with apolipoprotein E and with activated astrocytes. Brain Res Mol Brain Res. 2002;104:38–46. doi: 10.1016/s0169-328x(02)00203-6. [DOI] [PubMed] [Google Scholar]
- Bales KR, Dodart JC, DeMattos RB, Holtzman DM, Paul SM. Apolipoprotein E, amyloid, and Alzheimer disease. Mol Interv. 2002;2:363–375. doi: 10.1124/mi.2.6.363. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bosio A, Binczek E, Stoffel W. Functional breakdown of the lipid bilayer of the myelin membrane in central and peripheral nervous system by disrupted galactocerebroside synthesis. Proc Natl Acad Sci USA. 1996;93:13280–13285. doi: 10.1073/pnas.93.23.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosio A, Bussow H, Adam J, Stoffel W. Galactosphingolipids and axono-glial interaction in myelin of the central nervous system. Cell Tissue Res. 1998;292:199–210. doi: 10.1007/s004410051051. [DOI] [PubMed] [Google Scholar]
- Cheng H, Xu J, McKeel DW, Jr, Han X. Specificity and potential mechanism of sulfatide deficiency in Alzheimer’s disease: An electrospray ionization mass spectrometric study. Cell Mol Biol. 2003;49:809–818. [PubMed] [Google Scholar]
- Cheng H, Guan S, Han X. Abundance of triacylglycerols in ganglia and their depletion in diabetic mice: Implications for the role of altered triacylglycerols in diabetic neuropathy. J Neurochem. 2006;97:1288–1300. doi: 10.1111/j.1471-4159.2006.03794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Jiang X, Han X. Alterations in lipid homeostasis of mouse dorsal root ganglia induced by apolipoprotein E deficiency: A shotgun lipidomics study. J Neurochem. 2007;101:57–76. doi: 10.1111/j.1471-4159.2006.04342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee T, Fujita N, Dupree J, Shi R, Blight A, Suzuki K, Popko B. Myelination in the absence of galactocerebroside and sulfatide: normal structure with abnormal function and regional instability. Cell. 1996;86:209–219. doi: 10.1016/s0092-8674(00)80093-8. [DOI] [PubMed] [Google Scholar]
- Coetzee T, Dupree JL, Popko B. Demyelination and altered expression of myelin-associated glycoprotein isoforms in the central nervous system of galactolipid-deficient mice. J Neurosci Res. 1998;54:613–622. doi: 10.1002/(SICI)1097-4547(19981201)54:5<613::AID-JNR6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Taylor JW, DeMattos RB, Bales KR, Paul SM, Parsadanian M, Holtzman DM. Apolipoprotein E markedly facilitates age-dependent cerebral amyloid angiopathy and spontaneous hemorrhage in amyloid precursor protein transgenic mice. J Neurosci. 2003;23:7889–7896. doi: 10.1523/JNEUROSCI.23-21-07889.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah E, McConlogue L, Montoya-Zavala M, Mucke L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano P, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Gearing M, Schneider JA, Robbins RS, Hollister RD, Mori H, Games D, Hyman BT, Mirra SS. Regional variation in the distribution of apolipoprotein E and A beta in Alzheimer’s disease. J Neuropathol Exp Neurol. 1995;54:833–841. doi: 10.1097/00005072-199511000-00010. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Holtzman DM, McKeel DW, Jr, Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: potential role in disease pathogenesis. J Neurochem. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- Han X, Cheng H, Fryer JD, Fagan AM, Holtzman DM. Novel role for apolipoprotein E in the central nervous system: Modulation of sulfatide content. J Biol Chem. 2003a;278:8043–8051. doi: 10.1074/jbc.M212340200. [DOI] [PubMed] [Google Scholar]
- Han X, Fagan AM, Cheng H, Morris JC, Xiong C, Holtzman DM. Cerebrospinal fluid sulfatide is decreased in subjects with incipient dementia. Ann Neurol. 2003b;54:115–119. doi: 10.1002/ana.10618. [DOI] [PubMed] [Google Scholar]
- Han X, Yang J, Cheng H, Ye H, Gross RW. Towards fingerprinting cellular lipidomes directly from biological samples by two-dimensional electrospray ionization mass spectrometry. Anal Biochem. 2004;330:317–331. doi: 10.1016/j.ab.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Han X, Gross RW. Shotgun lipidomics: Electrospray ionization mass spectrometric analysis and quantitation of the cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev. 2005;24:367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- Han X. Potential mechanisms contributing to sulfatide depletion at the earliest clinically recognizable stages of Alzheimer’s disease: a tale of shotgun lipidomics. J Neurochem. 2007;103(s1):171–179. doi: 10.1111/j.1471-4159.2007.04708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci USA. 2000a;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, Fagan AM, Mackey B, Tenkova T, Sartorius L, Paul SM, Bales K, Ashe KH, Irizarry MC, Hyman BT. Apolipoprotein E facilitates neuritic and cerebrovascular plaque formation in an Alzheimer’s disease model. Ann Neurol. 2000b;47:739–747. [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT. APPSw transgenic mice develop age-related A beta deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol. 1997a;56:965–973. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- Irizarry MC, Soriano F, McNamara M, Page KJ, Schenk D, Games D, Hyman BT. Abeta deposition is associated with neuropil changes, but not with overt neuronal loss in the human amyloid precursor protein V717F (PDAPP) transgenic mouse. J Neurosci. 1997b;17:7053–7059. doi: 10.1523/JNEUROSCI.17-18-07053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Cheng H, Yang K, Gross RW, Han X. Alkaline methanolysis of lipid extracts extends shotgun lipidomics analyses to the low abundance regime of cellular sphingolipids. Anal Biochem. 2007;371:135–145. doi: 10.1016/j.ab.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang DE, Pietrzik CU, Baum L, Chevallier N, Merriam DE, Kounnas MZ, Wagner SL, Troncoso JC, Kawas CH, Katzman R, Koo EH. Modulation of amyloid beta-protein clearance and Alzheimer’s disease susceptibility by the LDL receptor-related protein pathway. J Clin Invest. 2000;106:1159–1166. doi: 10.1172/JCI11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo YM, Crawford F, Mullan M, Kokjohn TA, Emmerling MR, Weller RO, Roher AE. Elevated A beta and apolipoprotein E in A betaPP transgenic mice and its relationship to amyloid accumulation in Alzheimer’s disease. Mol Med. 2000;6:430–439. [PMC free article] [PubMed] [Google Scholar]
- Marcus J, Honigbaum S, Shroff S, Honke K, Rosenbluth J, Dupree JL. Sulfatide is essential for the maintenance of CNS myelin and axon structure. Glia. 2006;53:372–381. doi: 10.1002/glia.20292. [DOI] [PubMed] [Google Scholar]
- Molander-Melin M, Pernber Z, Franken S, Gieselmann V, Mansson JE, Fredman P. Accumulation of sulfatide in neuronal and glial cells of arylsulfatase A deficient mice. J Neurocytol. 2004;33:417–427. doi: 10.1023/B:NEUR.0000046572.53905.2c. [DOI] [PubMed] [Google Scholar]
- Nagy Z. The last neuronal division: a unifying hypothesis for the pathogenesis of Alzheimer’s disease. J Cell Mol Med. 2005;9:531–541. doi: 10.1111/j.1582-4934.2005.tb00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernber Z, Molander-Melin M, Berthold CH, Hansson E, Fredman P. Expression of the myelin and oligodendrocyte progenitor marker sulfatide in neurons and astrocytes of adult rat brain. J Neurosci Res. 2002;69:86–93. doi: 10.1002/jnr.10264. [DOI] [PubMed] [Google Scholar]
- Van Uden E, Kang DE, Koo EH, Masliah E. LDL receptor-related protein (LRP) in Alzheimer’s disease: towards a unified theory of pathogenesis. Microsc Res Tech. 2000;50:268–272. doi: 10.1002/1097-0029(20000815)50:4<268::AID-JEMT3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- von Figura K, Gieselmann V, Jaeken J. Metachromatic leukodystrophy: Lysosomal disorders. In: Sachdev HS, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited diseases. 8. New York: McGraw-Hill; 2001. pp. 3695–3724. [Google Scholar]
- Vos JP, Lopes-Cardozo M, Gadella BM. Metabolic and functional aspects of sulfogalactolipids. Biochim Biophys Acta. 1994;1211:125–149. doi: 10.1016/0005-2760(94)90262-3. [DOI] [PubMed] [Google Scholar]
- Yang K, Zhao Z, Gross RW, Han X. Shotgun lipidomics identifies a paired rule for the presence of isomeric ether phospholipid molecular species. PLoS ONE. 2007;2:e1368. doi: 10.1371/journal.pone.0001368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Cheng H, Jiang X, Han X. Endosomes and lysosomes play distinct roles in sulfatide-induced neuroblastoma apoptosis: Potential mechanisms contributing to abnormal sulfatide metabolism in related neuronal diseases. Biochem J. 2008;410:81–92. doi: 10.1042/BJ20070976. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Han X. Sulfatides facilitate apolipoprotein E-mediated amyloid-b peptide clearance through an endocytotic pathway. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05481.x. PMID: 18485101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterberg H, Andreasson U. Update on Alzheimer’s and Parkinson’s diseases. Aging Health. 2007;3:305–307. [Google Scholar]