Abstract
Background. Human metapneumovirus (HMPV) is a leading cause of acute respiratory illness (ARI) in children. Population-based incidence rates and comprehensive clinical characterizations of disease have not been established.
Methods. We conducted population-based prospective surveillance for 2 years in 2 US counties of HMPV infection among children <5 years old who were hospitalized with ARI or fever. Nasal and throat specimens obtained with swabs were tested for HMPV by real-time reverse-transcription polymerase chain reaction and genotyped.
Results. Forty-two (3.8%) of 1104 children tested positive for HMPV. The overall annual rate of HMPVassociated hospitalizations per 1000 children <5 years old was 1.2 (95% confidence interval [CI], 0.9–1.6). This rate was highest among infants 0–5 months old (4.9 per 1000 [95% CI, 2.9–7.2]), followed by children 6–11 months old (2.9 per 1000 [95% CI, 1.4–4.7]). The annual rate of hospitalization for HMPV infection was less than that for respiratory syncytial virus infection but similar to that for influenza and parainfluenza virus 3 infection in all age groups. The mean age of children hospitalized with HMPV infection was 6 months. Bronchiolitis, pneumonia, and asthma were the most common diagnoses among children with HMPV infection. All 4 HMPV subgroups were detected during both years at both sites. HPMV infection was most prominent from March through May.
Conclusion. HMPV was detected in 3.8% of children hospitalized with ARI or fever, with a population incidence similar to that of influenza virus and parainfluenza virus 3.
Human metapneumovirus (HMPV) is a recently identified paramyxovirus that is a major cause of upper and lower respiratory tract infections in infants and children worldwide [1–7] and among immunocompromised persons or those with other underlying chronic medical conditions [8–15]. HMPV infection appears to be ubiquitous, as virtually all children are seropositive by the age of 5 years [3, 16–18]. Previous estimates of the percentage of acute pediatric lower respiratory tract infections associated with HMPV range from 5% to 25% [2, 4, 5, 7, 19–21], with differences likely resulting from various geographic areas being studied, seasonal variability in HMPV circulation, methodological differences, and age groups evaluated. Reinfection occurs throughout life, but subsequent infections are more likely to present with upper respiratory symptoms than with lower respiratory symptoms and to be milder in character than primary infection [1, 2].
Despite studies demonstrating the prevalence of HMPV infection, its actual population-wide disease burden is poorly understood. This study had 3 objectives. First, we sought to establish population-based rates of HMPV-associated infection in hospitalized children <5 years old with acute respiratory symptoms or fever. Second, we sought to compare the burden of HMPV infection with that of other respiratory virus infections. Finally, although earlier studies outlined the clinical characteristics of HMPV infection on convenience samples, we wished to comprehensively characterize the clinical presentations from a population-wide perspective.
Methods
Study design. The New Vaccine Surveillance Network was established by the Centers for Disease Control and Prevention to determine the burden of acute respiratory illness (ARI) from vaccine-preventable or potentially preventable agents and to monitor the impact of vaccine use on disease rates [22]. Prospective population-based surveillance was conducted among children <5 years of age who were hospitalized with acute respiratory symptoms or fever anytime from 1 October 2001 through 30 September 2003 in area hospitals that provided care for >95% of hospitalized children in Davidson County, Tennessee (Nashville), and Monroe County, New York (Rochester). The institutional review boards at both study sites, the participating hospitals, and the Centers for Disease Control and Prevention approved the study.
The current study used previously published methods to establish the burden of HMPV infection in hospitalized children [23–25]. Eligible hospitalized children were <5 years of age and had received an admission diagnosis of fever alone or of acute respiratory infection, which was defined as an illness presenting with fever and/or 1 or more of the following symptoms: cough, earache, nasal congestion, rhinorrhea, sore throat, vomiting after coughing, wheezing, and labored, rapid, or shallow breathing. Children were excluded if they had respiratory symptoms that had lasted >14 days at the time of admission, had neutropenia from chemotherapy, were hospitalized elsewhere within the preceding 4 days, or were newborns who had been hospitalized since birth. Study nurses enrolled children admitted to surveillance hospitals from Sunday through Thursday, after obtaining written informed consent. Children were eligible for enrollment if they were admitted within 48 h of an enrollment day. Nasal and throat specimens were obtained with swabs from all children on the day of enrollment (thus within 48 h of admission), combined into a single tube of viral transport medium, and promptly delivered to the research laboratories at the study sites. Demographic and clinical information was collected using a standardized questionnaire, as described elsewhere [22, 26]. We performed statistical comparisons between children infected with HMPV alone and children infected with other specific viruses alone; children with >1 virus detected in their specimens were excluded from analysis. Children were considered to be virus-negative if their specimens tested negative for HMPV, influenza virus, respiratory syncytial virus (RSV), parainfluenza virus (PIV), human rhinovirus (HRV), and human coronavirus by testing methods that are described below. Highrisk conditions were assessed, which included history of asthma, heart disease, sickle cell anemia, cystic fibrosis, diabetes mellitus, and neuromuscular conditions such as seizures, cerebral palsy, or muscular dystrophy. History of asthma was determined by asking the parents if their child had ever received a diagnosis of asthma from any health care provider or by documentation of a previous diagnosis of asthma in the medical record [24].
Molecular testing. Specimens were divided into aliquots and stored at -80°C until processing. RNA was extracted from pooled nasal and throat swab medium. Specimens were tested for HMPV by real-time reverse-transcription polymerase chain reaction (RT-PCR), as described elsewhere [1, 27]. Specimens were also tested for influenza virus, RSV, PIV, HRV, and human coronavirus by real-time RT-PCR [23–25, 28, 29]. Specimens that tested positive for HMPV were subjected to conventional nested RT-PCR to amplify and sequence complementary DNA of the F and G genes, as described elsewhere [1]. HMPV subgroup assignment was made on the basis of either the F or the G gene; either gene reliably identifies the viral subgroup [30]. Sequences were edited and aligned with published HMPV sequences obtained from GenBank by means of the ClustalW algorithm in MacVector software (version 10.0; Accelrys). Phylogenetic analyses were performed using Mega software (version 4) [31]. The data sets were resampled with 500 bootstraps, distances were computed using the maximum composite likelihood method, and phylogeny was inferred using the neighbor-joining method [31]. Analyses that made use of the maximum parsimony and minimum evolution methods yielded similar results (data not shown), and so only the results of the neighbor-joining analysis are shown.
Data analysis. The number of hospitalized children with laboratory-confirmed HMPV infection per 1000 children was calculated as the weighted number of HMPV-positive hospitalized children with ARI or fever divided by the number of children in the county population as determined by the 2000 US Census, multiplied by 1000 [22–26, 29]. Rates were calculated by weighting the observed number of enrolled hospitalized children to account for sampling 5 days per week and eligible patients who were not enrolled. Rates were determined overall and by demographic subsets with 95% confidence intervals (CIs) calculated using 1000 bootstrap samples.
Kruskal-Wallis or Pearson tests were used as appropriate for contingency table analyses of signs and symptoms associated with HMPV infection alone compared with infection with RSV, influenza virus, PIV, or HRV alone, or compared with virusnegative children. Influenza virus included influenza A and B viruses; PIV included PIV types 1, 2, and 3. For comparative analyses, children with coinfections were excluded. Prospectively collected demographic and clinical data were compared among children with different viral infections and virus-negative children. Age was categorized as 1 of 3 groups (<6, 6–23, and >23 months), on the basis of prior studies of respiratory viruses among this population [22, 23, 25, 26, 29]. All analyses were performed using R software (version 2.6.2; see http:// www.R-project.org).
Results
Study population. Of 1298 children admitted to the surveillance hospitals with respiratory symptoms or fever during enrollment days over the 2-year period, 1123 (86.5%) were enrolled. Of 175 children who were not enrolled, 87 (49.7%) had parents and/or guardians who refused participation, 62 (35.4%) had no parents and/or guardians available, 16 (9.1%) were discharged before enrollment, and 10 (5.7%) were not enrolled because of missed admission, unavailability of an interpreter, or refusal of the physician to allow participation. Sufficient specimens for HMPV testing were available for 1104 (98.3%) of 1123 study children. Thus, a total of 1104 children were included in the study.
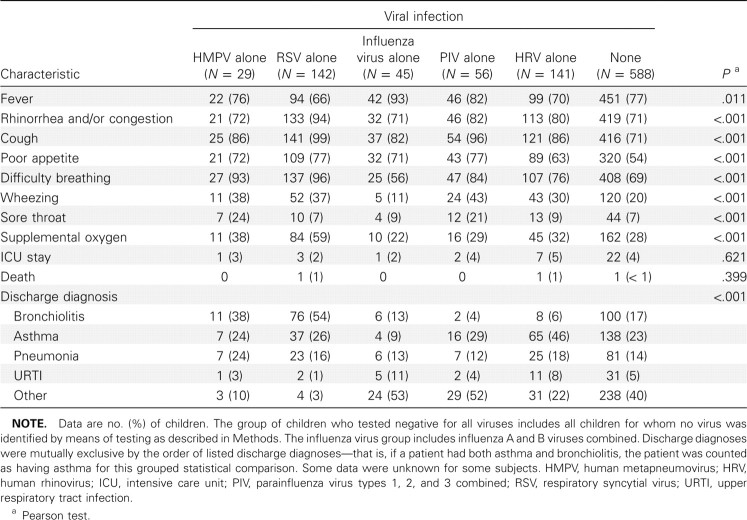
Demographic characteristics of children infected with HMPV alone compared with those of children infected with other sin gle viruses. Of the 1104 specimens tested, 42 (3.8% [95% CI, 2.8%–5.1%]) were positive for HMPV, with similar rates in both study years (4.2% in 2001–2002 and 3.3% in 2002–2003) and at both study sites. Demographic characteristics of children with HMPV, RSV, influenza virus, PIV, or HRV infection alone or with no virus detected are presented in Table 1. The median age of children who tested positive for HMPV alone was 6 months (range, 2–13 months), similar to the median age of those with RSV infection or influenza, whereas children with PIV or HRV infection were older. Sex, race, history of premature birth, and insurance status did not differ between groups. Attendance in out-of-home daycare was less common among children with HMPV infection and those with influenza, compared with those with RSV, PIV, or HRV infection. Children with HMPV infection and children with influenza had slightly higher rates of exposure to household tobacco smoke, compared with children in other groups. Proportions of general high-risk status (including asthma) were higher among children with HMPV or HRV infection, whereas proportions of underlying asthma alone were similar between children with HMPV infection, RSV infection, or no virus identified. Underlying asthma alone was more common among children with HRV infection and less common among children with influenza.
Table 1.
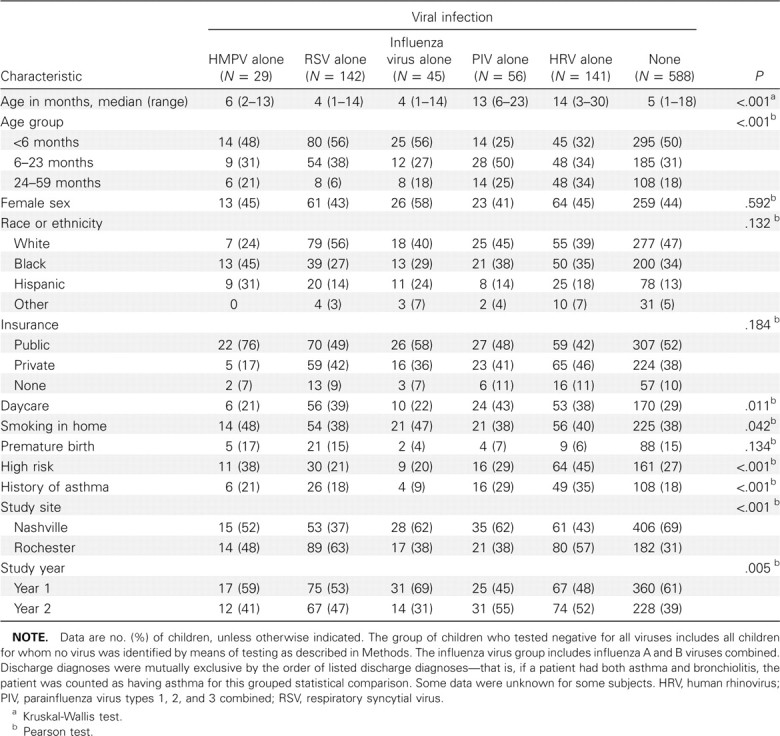
Characteristics of Hospitalized Children with Human Metapneumovirus (HMPV) Infection Compared with Those of Other Study Children
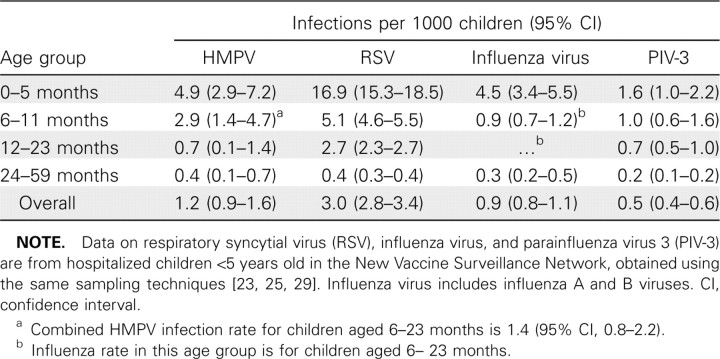
Rates of HMPV-associated hospitalization and seasonal trends. Population-based rates of HMPV infection in hospitalized children are presented in Table 2, along with previously published rates of infection with other common viruses in the New Vaccine Surveillance Network cohort during time periods that overlapped this study period [23, 25, 29]. The overall annual incidence was 1.2 HMPV-associated hospitalizations (95% CI, 0.9–1.6) per 1000 children <5 years of age; the age-specific incidence was highest among younger children: 4.9 (95% CI, 2.9- 7.2) for children 0–5 months of age, 2.9 (95% CI, 1.4–4.7) for children 6–11 months of age, 0.7 (95% CI, 0.1–1.4) for children 12–23 months of age, and 0.4 (95% CI, 0.1–0.7) for children 24–59 months of age. The incidence of HMPV was highest in the 0–5-month-old and 6–11-month-old age groups, although RSV infection remained more common than HMPV infection in these age groups. The incidence of HMPV infection in other age groups was similar to that of influenza and PIV-3 infection.
Table 2.
Incidence of Hospitalization Attributable to Infection with Human Metapneumovirus (HMPV) and Other Common Respiratory Viruses among Children <5 Years Old
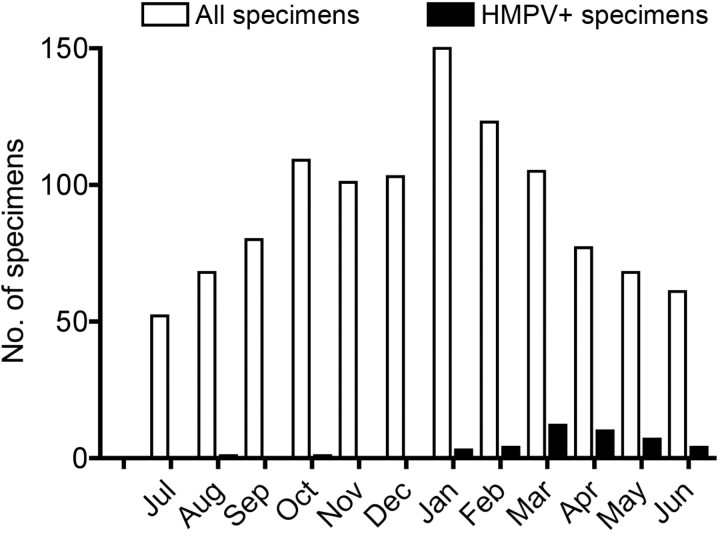
The number of all specimens collected (including HMPVpositive specimens and HMPV-negative specimens) peaked during January, with 55% of all specimens collected between 1 October and 28 February during both study years (Figure 1). However, detection of HMPV in specimens peaked slightly later in both years, with 80% of all HMPV infections occurring between 1 February and 31 May during both study years. The percentage of patients who tested HMPV-positive by month varied from 0 during the summer months to a peak of 15% during April.
Figure 1.
Number of specimens submitted and number of specimens that tested positive for human metapneumovirus (HMPV), by month. Data are combined from 2 years.
Clinical characterization of HMPV infections. Clinical features of children infected with HMPV or other viruses are shown in Table 3. The majority of HMPV-infected children had fever, rhinorrhea, cough, poor feeding, and/or difficulty breathing. Although most of these symptoms were also present in children infected with other respiratory viruses, fever was less common among children with HMPV infection than among children with influenza. Conversely, difficulty breathing and wheezing were more common among HMPV-infected children than among children with influenza or children with no virus detected. Sore throat was most common among children with HMPV or PIV infection. Thirty-eight percent of the HMPVinfected children required supplemental oxygen, and 2% were treated in the intensive care unit; these proportions were similar to those for other viruses. No child with HMPV infection in this study died, and the mean duration of hospitalization was 2 days, similar for all viruses. Thirteen children with HMPV detection had codetection of other viruses, including 6 with HRV detection, 4 with influenza virus detection, 2 with RSV detection, and 1 with PIV-3 detection. The most common discharge diagnoses among children with HMPV infection only were bronchiolitis (38%), pneumonia (24%), asthma (24%), and upper respiratory tract infection (3%). Bronchiolitis was most common among children with HMPV or RSV infection, whereas pneumonia was more common among HMPV-infected children. A discharge diagnosis of asthma was common in all groups but was less frequent among children with influenza (9%) and most frequent among HRV-infected children (46%). Other diagnoses included croup in a substantial number of children with influenza and PIV infection [25, 29] and febrile illness alone in children with no respiratory virus identified.
Table 3.
Clinical Features of Human Metapneumovirus-Positive and Human Metapneumovirus-Negative Hospitalized Children
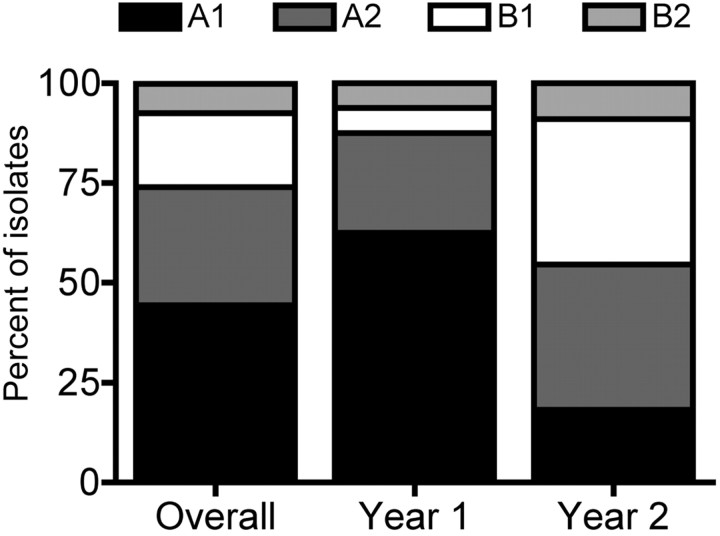
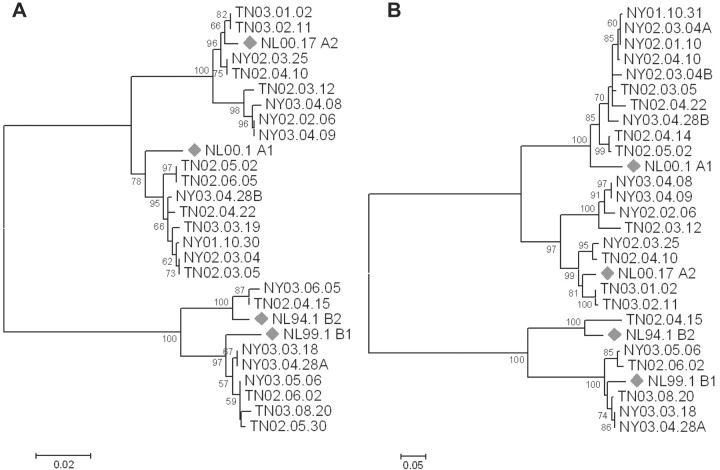
HMPV diversity. HMPV-positive specimens were tested by means of RT-PCR for the F and G genes, and 27 (61%) were genotyped; not all specimens were successfully amplified for F or G by means of RT-PCR because of RNA degradation. All 4 subgroups of HMPV were detected in both years; overall, subgroup A1 was the most prevalent (44.4%), followed by A2 (29.6%), B1 (18.5%), and B2 (7.4%). However, the distribution of subgroups varied between years, with A1 dominant in the first year and A2 and B1 detected with equal frequency in the second year (Figure 2). There were no differences between the prevalence of the subgroups by study site. No differences in disease severity were noted between the subgroups (data not shown). Phylogenetic analysis showed genetic diversity even within each of these subgroups, with analysis of the F gene sequences showing 2 sublineages of the A2 subgroup (Figure 3A) and analysis of the G gene sequences showing 2 sublineages of the B1 subgroup (Figure 3A). Both of these sublineages circulated during the same season and at the same study site and thus were not unique to either site or year.
Figure 2.
Subgroups of the isolates of human metapneumovirus (HMPV) detected during study period.
Figure 3.
Phylogenetic tree depicting the relationships among human metapneumovirus (HMPV) F sequences (A) and G sequences (B) identified in this study. Phylogeny was inferred using the neighbor-joining method with 500 bootstrap replicates, as described in Methods. Branches reproduced in <50% of the bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test is shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. Diamonds , prototypical GenBank strains. The scale bar indicates nucleotide substitutions per site. Sequences identified in this study have been submitted to GenBank.
Discussion
We used prospective, population-based, laboratory-confirmed active surveillance to define the burden of HMPV infection among hospitalized young children. The contribution of HMPV infection to pediatric hospitalization for ARI or fever was substantial, with 1.2 hospitalizations per 1000 children <5 years old per year (95% CI, 0.9–1.6), compared with 3.0 per 1000 (95% CI, 2.8–3.4) for RSV, 0.9 per 1000 (95% CI, 0.8–1.1) for influenza virus, and 0.5 per 1000 (95% CI, 0.4–0.6) for PIV-3 [23, 25, 29]. The rate of HMPV-associated hospitalization was highest among infants 0–5 months old, at 4.9 per 1000 (95% CI, 2.9–7.2), compared with 16.9 per 1000 (95% CI, 15.3–18.5) for RSV, 4.5 per 1000 (95% CI, 3.4–5.5) for influenza virus, and 1.6 per 1000 (95% CI, 1.0–2.2) for PIV-3 [23, 25, 29]. These data suggest that HMPV causes hospitalizations for respiratory symptoms and fever in children <5 years old at rates similar to those of influenza virus and PIV. If we extrapolate from US Census data, then our data would project ∼27,000 HMPV-related hospitalizations of children <5 years of age annually, which underscores the impact of HMPV infection on children. HMPV is rarely detected in asymptomatic young children [2, 3, 32, 33].
Although there have been reports of HMPV disease in children with underlying medical conditions, such as premature birth or asthma, few of these studies have been populationbased and have enrolled as many children as were enrolled in the present study [8, 11–15, 19, 34, 35]. One-third of the hospitalized children with HMPV infection in our study had highrisk conditions, which suggests that these children are at higher risk for hospitalization with HMPV infection. However, twothirds of HMPV-infected hospitalized children were otherwise healthy children. We did not observe an association between HMPV hospitalization and a history of asthma, in contrast to the association between asthma and HRV infection that was previously found in this cohort [24, 36]. Consistent with previous reports, HMPV infection was associated most frequently with a diagnosis of bronchiolitis [2, 5, 7, 19, 21]. The presenting symptoms of HMPV infection in this cohort generally were not distinct from those of children infected with other viruses, consistent with other reports [1, 2, 5, 7, 19, 21, 37, 38]. However, very few HMPV-infected children (5%) had fever alone without respiratory symptoms.
HMPV infection was most prominent at both study sites during the late winter and spring months, with peak illness rates in March, April, and May. Overall, HMPV infection accounted for up to 15% of all hospitalizations for children <5 years old with ARI or fever with a late spring distribution and was more common than influenza or RSV infection during this time period [23, 25]. However, the lack of distinctive clinical symptoms and the overlap with other community respiratory virus infections highlights the potential utility of rapid, sensitive diagnostic tests such as RT-PCR to detect HMPV and other respiratory viruses.
We identified all 4 subgroups of HMPV during each year and at each study site, although the distribution of subgroups varied by year. The extent of antigenic variability between HMPV subgroups is not clear in animals or in humans [30, 39–41], but the presence of multiple HMPV strains in a single season could affect the design of vaccines or prophylactic antibodies if antigenic variability leads to partial immune escape. We did not find that specific subgroups were associated with more severe disease (data not shown); some studies have suggested this phenomenon [42], whereas others have not [43]. Correlation between infecting subtype and disease severity has been shown for RSV infection [44, 45]. A postulated mechanism for alternating circulation of different HMPV subgroups is population immune pressure [46, 47]. Long-term, prospective, population-based surveillance is needed to establish whether different subgroups of HMPV vary in virulence and whether circulation patterns are the result of immune pressure.
Study limitations. Despite the strength of our prospective population-based surveillance system, our study had limitations. First, surveillance was performed for only 2 years at only 2 geographically distant sites (representing 2 US regions, Northeast and South). Greater regional and temporal differences in viral circulation may have been evident if the study included more years or sites, because other studies have reported substantial year-to-year variability in HMPV infection prevalence [1]. Second, institutional differences in medical practice might have contributed to variation in HMPV-related hospitalization rates, and although enrolled and nonenrolled children did not differ in demographic characteristics, unknown biases may have affected our HMPV infection burden estimates. Coinfections were present in a minority of children, but the clinical importance of these coinfections is not clear. HRV is identified frequently in asymptomatic individuals and thus is especially difficult to interpret as a codetected virus [48–50]. A large number of children in the study did not have a virus identified by means of the testing used; this could be attributable to false negative test results or to the presence of other pathogens for which we did not test. Nearly all of the HMPV-infected children had ARI symptoms and not fever alone, but we enrolled children with fever alone in addition to children with ARI. Thus, because the population-based incidence was calculated with all hospitalized children, including those with fever alone, we may have underestimated the true incidence of infection with HMPV (and other viruses) among children with ARI. Finally, this study only assessed hospitalized children, and the impact of HMPV infection on emergency department and outpatient visits is unknown.
Conclusion. HMPV infection is a leading cause of hospitalization for ARI among children <5 years of age, with a population-based incidence similar to that of influenza and PIV-3 infection. The majority of HMPV-associated hospitalizations occurred among otherwise healthy children. These data suggest a need for preventive and therapeutic strategies for HMPV infection and highlight the need to consider HMPV infection as a cause of ARI among hospitalized children, especially in the spring.
Acknowledgments
We thank the children who participated in this study, their parents, and all of the members of the New Vaccine Surveillance Network (NVSN). The NVSN acute respiratory illness inpatient study collaborators were as follows: Linda Anderson, Charlene Freundlich, Gladys Lathan, Gerry Lofthus, Andrea Marino, Rebecca Martinez, Kenneth Schnabel, Lynne Shelly, Jennifer Carnahan, and Christina Albertin (University of Rochester); Diane Kent, Ann Clay, Ayesha Khan, Nayleen Whitehead, Amy Podsiad, and Jody Peters (Vanderbilt University); and Larry Anderson, John Copeland, Marika Iwane, Ben Schwartz, and Fran Walker (Centers for Disease Control and Prevention).
Footnotes
Financial support: National Institutes of Health (grant R03 AI-054790 to J.V.W.); MedImmune (research grant to J.E.C.); Centers for Disease Control and Prevention (cooperative agreements U38/CCU417958 and U01/IP000022 with Vanderbilt University and U38/CCU217969 and U01/IP000017 with University of Rochester).
Disclaimer: This publication and its contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
Potential conflicts of interest: J.V.W. has served as a consultant for MedImmune and Novartis. K.M.E. receives research funding from Sanofi-Pasteur, Wyeth, Novartis, and CSL. G.A.W. has served as a consultant for MedImmune. M.R.G. has received research funding from MedImmune and Merck. C.B.H. has served as a consultant for and received research support from MedImmune. J.E.C. has served as a consultant for Anaptys, Immunobiosciences, Mapp, MedImmune, and Novartis and has received research support from MedImmune, Mapp, Alnylam, and Sanofi-Pasteur. C.K.W., C.-F.Y., D.S., D.Y., and R.R.S. were employees of MedImmune at the time of this study. All other authors report no potential conflicts.
Role of the industry collaborator: MedImmune provided research support to J.E.C. De-identified specimens were shipped to MedImmune, which performed extraction and real-time reverse-transcription polymerase chain reaction partial sequencing. Results of real-time human metapneumovirus testing and sequencing and extracted nucleic acids were returned to Vanderbilt University. The study was designed, the data were analyzed, and the paper was written by the authors affiliated with Vanderbilt University and the New Vaccine Surveillance Network.
References
- 1.Williams JV, Wang CK, Yang CF, et al. The role of human metapneumovirus in upper respiratory tract infections in children: a 20-year experience. J Infect Dis. 2006;193:387–395. doi: 10.1086/499274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams JV, Harris PA, Tollefson SJ, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med. 2004;350:443–450. doi: 10.1056/NEJMoa025472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Hoogen BG, de Jong JC, Groen J, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mullins JA, Erdman DD, Weinberg GA, et al. Human metapneumovirus infection among children hospitalized with acute respiratory illness. Emerg Infect Dis. 2004;10:700–705. doi: 10.3201/eid1004.030555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McAdam AJ, Hasenbein ME, Feldman HA, et al. Human metapneumovirus in children tested at a tertiary-care hospital. J Infect Dis. 2004;190:20–26. doi: 10.1086/421120. [DOI] [PubMed] [Google Scholar]
- 6.Mackay IM, Bialasiewicz S, Jacob KC, et al. Genetic diversity of human metapneumovirus over 4 consecutive years in Australia. J Infect Dis. 2006;193:1630–1633. doi: 10.1086/504260. [DOI] [PubMed] [Google Scholar]
- 7.Boivin G, De Serres G, Cote S, et al. Human metapneumovirus infections in hospitalized children. Emerg Infect Dis. 2003;9:634–640. doi: 10.3201/eid0906.030017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Englund JA, Boeckh M, Kuypers J, et al. Brief communication: fatal human metapneumovirus infection in stem-cell transplant recipients. Ann Intern Med. 2006;144:344–349. doi: 10.7326/0003-4819-144-5-200603070-00010. [DOI] [PubMed] [Google Scholar]
- 9.Falsey AR, Erdman D, Anderson LJ, Walsh EE. Human metapneumovirus infections in young and elderly adults. J Infect Dis. 2003;187:785–790. doi: 10.1086/367901. [DOI] [PubMed] [Google Scholar]
- 10.erna G, Vitulo P, Rovida F, et al. Impact of human metapneumovirus and human cytomegalovirus versus other respiratory viruses on the lower respiratory tract infections of lung transplant recipients. J Med Virol. 2006;78:408–416. doi: 10.1002/jmv.20555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamelin ME, Cote S, Laforge J, et al. Human metapneumovirus infection in adults with community-acquired pneumonia and exacerbation of chronic obstructive pulmonary disease. Clin Infect Dis. 2005;41:498–502. doi: 10.1086/431981. [DOI] [PubMed] [Google Scholar]
- 12.Martinello RA, Esper F, Weibel C, Ferguson D, Landry ML, Kahn JS. Human metapneumovirus and exacerbations of chronic obstructive pulmonary disease. J Infect. 2006;53:248–254. doi: 10.1016/j.jinf.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vicente D, ontes M, Cilla G, Perez-Trallero E. Human metapneumovirus and chronic obstructive pulmonary disease. Emerg Infect Dis. 2004;10:1338–1339. doi: 10.3201/eid1007.030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams JV, Crowe JE, Jr, Enriquez R, et al. Human metapneumovirus infection plays an etiologic role in acute asthma exacerbations requiring hospitalization in adults. J Infect Dis. 2005;192:1149–1153. doi: 10.1086/444392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams JV, Martino R, Rabella N, et al. A prospective study comparing human metapneumovirus with other respiratory viruses in adults with hematologic malignancies and respiratory tract infections. J Infect Dis. 2005;192:1061–1065. doi: 10.1086/432732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebihara T, Endo R, Kikuta H, Ishiguro N, Ishiko H, obayashi K. Comparison of the seroprevalence of human metapneumovirus and human respiratory syncytial virus. J Med Virol. 2004;72:304–306. doi: 10.1002/jmv.10572. [DOI] [PubMed] [Google Scholar]
- 17.Pavlin JA, Hickey AC, Ulbrandt N, et al. Human metapneumovirus reinfection among children in Thailand determined by ELISA using purified soluble fusion protein. J Infect Dis. 2008;198:836–842. doi: 10.1086/591186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf DG, akay-Rones Z, Fadeela A, Greenberg D, Dagan R. High seroprevalence of human metapneumovirus among young children in Israel. J Infect Dis. 2003;188:1865–1867. doi: 10.1086/380100. [DOI] [PubMed] [Google Scholar]
- 19.Esper F, Martinello RA, Boucher D, et al. A 1-year experience with human metapneumovirus in children aged <5 years. J Infect Dis. 2004;189:1388–1396. doi: 10.1086/382482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sloots TP, Mackay IM, Bialasiewicz S, et al. Human metapneumovirus, Australia, 2001–2004. Emerg Infect Dis. 2006;12:1263–1266. doi: 10.3201/eid1208.051239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van den Hoogen BG, van Doornum GJ, Fockens JC, et al. Prevalence and clinical symptoms of human metapneumovirus infection in hospitalized patients. J Infect Dis. 2003;188:1571–1577. doi: 10.1086/379200. [DOI] [PubMed] [Google Scholar]
- 22.Iwane MK, Edwards KM, Szilagyi PG, et al. Population-based surveillance for hospitalizations associated with respiratory syncytial virus, influenza virus, and parainfluenza viruses among young children. Pediatrics. 2004;113:1758–1764. doi: 10.1542/peds.113.6.1758. [DOI] [PubMed] [Google Scholar]
- 23.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller EK, Edwards KM, Weinberg GA, et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol. 2009;123:98–104. doi: 10.1016/j.jaci.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poehling KA, Edwards KM, Weinberg GA, et al. The underrecognized burden of influenza in young children. N Engl J Med. 2006;355:31–40. doi: 10.1056/NEJMoa054869. [DOI] [PubMed] [Google Scholar]
- 26.Griffin MR, Walker FJ, Iwane MK, Weinberg GA, Staat MA, Erdman DD. Epidemiology of respiratory infections in young children: insights from the new vaccine surveillance network. Pediatr Infect Dis J. 2004;23:S188–S192. doi: 10.1097/01.inf.0000144660.53024.64. [DOI] [PubMed] [Google Scholar]
- 27.Maertzdorf J, Wang CK, Brown JB, et al. Real-time reverse transcriptase PCR assay for detection of human metapneumoviruses from all known genetic lineages. J Clin Microbiol. 2004;42:981–986. doi: 10.1128/JCM.42.3.981-986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Talbot HK, Crowe JE, Jr, Edwards KM, et al. Coronavirus infection and hospitalizations for acute respiratory illness in young children. J Med Virol. 2009;81:853–856. doi: 10.1002/jmv.21443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinberg GA, Hall CB, Iwane MK, et al. Parainfluenza virus infection of young children: estimates of the population-based burden of hospitalization. J Pediatr. 2009;154:694–699. doi: 10.1016/j.jpeds.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 30.van den Hoogen BG, Herfst S, Sprong L, et al. Antigenic and genetic variability of human metapneumoviruses. Emerg Infect Dis. 2004;10:658–666. doi: 10.3201/eid1004.030393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 32.van den Hoogen BG, Osterhaus DM, Fouchier RA. Clinical impact and diagnosis of human metapneumovirus infection. Pediatr Infect Dis J. 2004;23:S25–S32. doi: 10.1097/01.inf.0000108190.09824.e8. [DOI] [PubMed] [Google Scholar]
- 33.Osterhaus A, Fouchier R. Human metapneumovirus in the community. Lancet. 2003;361:890–891. doi: 10.1016/S0140-6736(03)12785-7. [DOI] [PubMed] [Google Scholar]
- 34.Boivin G, De Serres G, Hamelin ME, et al. An outbreak of severe respiratory tract infection due to human metapneumovirus in a longterm care facility. Clin Infect Dis. 2007;44:1152–1158. doi: 10.1086/513204. [DOI] [PubMed] [Google Scholar]
- 35.Walsh EE, Peterson DR, Falsey AR. Human metapneumovirus infections in adults: another piece of the puzzle. Arch Intern Med. 2008;168:2489–2496. doi: 10.1001/archinte.168.22.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller EK, Lu X, Erdman DD, et al. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195:773–781. doi: 10.1086/511821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peiris JS, Tang WH, Chan KH, et al. Children with respiratory disease associated with metapneumovirus in Hong Kong. Emerg Infect Dis. 2003;9:628–633. doi: 10.3201/eid0906.030009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Principi N, Bosis S, Esposito S. Human metapneumovirus in paediatric patients. Clin Microbiol Infect. 2006;12:301–308. doi: 10.1111/j.1469-0691.2005.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skiadopoulos MH, Biacchesi S, Buchholz UJ, et al. Individual contributions of the human metapneumovirus F, G, and SH surface glycoproteins to the induction of neutralizing antibodies and protective immunity. Virology. 2006;345:492–501. doi: 10.1016/j.virol.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 40.Skiadopoulos MH, Biacchesi S, Buchholz UJ, et al. The two major human metapneumovirus genetic lineages are highly related antigenically, and the fusion (F) protein is a major contributor to this antigenic relatedness. J Virol. 2004;78:6927–6937. doi: 10.1128/JVI.78.13.6927-6937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Hoogen BG, Herfst S, de Graaf M, et al. Experimental infection of macaques with human metapneumovirus induces transient protective immunity. J Gen Virol. 2007;88:1251–1259. doi: 10.1099/vir.0.82663-0. [DOI] [PubMed] [Google Scholar]
- 42.Matsuzaki Y, Itagaki T, Abiko C, Aoki Y, Suto A, Mizuta K. Clinical impact of human metapneumovirus genotypes and genotype-specific seroprevalence in Yamagata, Japan. J Med Virol. 2008;80:1084–1089. doi: 10.1002/jmv.21194. [DOI] [PubMed] [Google Scholar]
- 43.Agapov E, Sumino KC, Gaudreault-Keener M, Storch GA, Holtzman MJ. Genetic variability of human metapneumovirus infection: evidence of a shift in viral genotype without a change in illness. J Infect Dis. 2006;193:396–403. doi: 10.1086/499310. [DOI] [PubMed] [Google Scholar]
- 44.Walsh EE, McConnochie KM, Long CE, Hall CB. Severity of respiratory syncytial virus infection is related to virus strain. J Infect Dis. 1997;175:814–820. doi: 10.1086/513976. [DOI] [PubMed] [Google Scholar]
- 45.Martinello RA, Chen MD, Weibel C, Kahn JS. Correlation between respiratory syncytial virus genotype and severity of illness. J Infect Dis. 2002;186:839–842. doi: 10.1086/342414. [DOI] [PubMed] [Google Scholar]
- 46.Aberle SW, Aberle JH, Sandhofer MJ, Pracher E, Popow-Kraupp T. Biennial spring activity of human metapneumovirus in Austria. Pediatr Infect Dis J. 2008;27:1065–1068. doi: 10.1097/INF.0b013e31817ef4fd. [DOI] [PubMed] [Google Scholar]
- 47.Oliveira DB, Durigon EL, Carvalho AC, et al. Epidemiology and genetic variability of human metapneumovirus during a 4-year-long study in southeastern Brazil. J Med Virol. 2009;81:915–921. doi: 10.1002/jmv.21436. [DOI] [PubMed] [Google Scholar]
- 48.Peltola V, Waris M, Osterback R, Susi P, Hyypia T, Ruuskanen O. Clinical effects of rhinovirus infections. J Clin Virol. 2008;43:411–414. doi: 10.1016/j.jcv.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 49.Mackay IM. Human rhinoviruses: the cold wars resume. J Clin Virol. 2008;42:297–320. doi: 10.1016/j.jcv.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wright PF, Deatly AM, Karron RA, et al. Comparison of results of detection of rhinovirus by PCR and viral culture in human nasal wash specimens from subjects with and without clinical symptoms of respiratory illness. J Clin Microbiol. 2007;45:2126–2129. doi: 10.1128/JCM.02553-06. [DOI] [PMC free article] [PubMed] [Google Scholar]