Abstract
Endocrine signal transduction occurs through cascades that involve the action of both ligand-dependent and ligand-independent nuclear receptors. In insects, two such nuclear receptors are HR3 and E75 that interact to transduce signals initiated by ecdysteroids. We have cloned these nuclear receptors from the crustacean Daphnia pulex to assess their function as regulators of gene transcription in this ecologically and economically important group of organisms. Both nuclear receptors from D. pulex (DappuHR3 (group NR1F) and DappuE75 (group NR1D)) exhibit a high degree of sequence similarity to other NR1F and NR1D group members that is indicative of monomeric binding to the RORE (retinoid orphan receptor element). DappuE75 possesses key amino acid residues required for heme binding to the ligand binding domain. Next, we developed a gene transcription reporter assay containing a luciferase reporter gene driven by the RORE. DappuHR3, but not DappuE75, activated transcription of the luciferase gene in this system. Co-transfection experiments revealed that DappuE75 suppressed DappuHR3-dependent luciferase transcription in a dose-dependent manner. Electrophoretic mobility shift assays confirmed that DappuHR3 bound to the RORE. However, we found no evidence that DappuE75 similarly bound to the response element. These experiments further demonstrated that DappuE75 prevented DappuHR3 from binding to the response element. In conclusion, DappuHR3 functions as a transcriptional activator of genes regulated by the RORE and DappuE75 is a negative regulator of this activity. DappuE75 does not suppress the action of DappuHR3 by occupying the response element but presumably interacts directly with the DappuHR3 protein. Taken together with the previous demonstration that daphnid HR3 is highly induced by 20-hydroxyecdysone, these results support the premise that HR3 is a major component of ecdysteroid signaling in some crustaceans and is under the negative regulatory control of E75.
Keywords: Daphnia pulex, ecdysteroids, endocrine disruptor, nuclear receptors
Introduction
The ecdysteroid signaling pathway is a major component of the endocrine axis controlling development and reproduction in arthropods. Numerous nuclear receptors, transcription factors, coactivators, and corepressors coordinate within this pathway to transmit the ecdysteroid-induced signal. Much is known about the structure, function, and interplay of these signaling components in insects (Smagghe, 2009). Much less is understood about the genomic signaling pathway of ecdysteroid signaling in crustaceans, despite the significant ecological and economic importance of this subphylum and the demonstrated ability of many environmental contaminants to interfere with this pathway in these organisms (LeBlanc, 2007, Tuberty et al., 2005, Zou, 2005). A definitive understanding of ecdysteroid signaling pathways in crustacean is integral to protecting these organisms against endocrine disruptive environmental factors.
Ecdysteroids bind and activate the nuclear receptor heterodimer consisting of the ecdysteroid receptor (EcR) and the retinoid X receptor or ultraspiricle (RXR/USP) (Koelle, et al., 1991, Riddiford et al., 2000, Yao et al., 1992). This ligand-bound heterodimer activates transcription of a cascade of genes that regulate many physiological events including metamorphosis (Bialecki et al. 2002, Thummel, 1996), embryogenesis (Bownes et al., 1988), growth (Bownes et al., 1988), differentiation (Laufer et al., 2002), egg chamber development (Buszczak et al., 1999), ecdysis (Ampleford, 1985), diapause, reproduction, and behavior (Richard et al., 1998). Two important transcriptional regulators in insect ecdysteroid signaling cascades are the nuclear receptors HR3 (group NR1F) and E75 (group NR1D) (King-Jones & Thummel, 2005). HR3 activates target downstream genes in the signaling pathway and E75 is best recognized as a negative regulator of HR3 transcriptional activation (Hiruma & Riddiford, 2004, Swevers et al., 2002, White et al., 1997). In Drosophila melanogaster, the interaction between HR3 and E75 is further regulated by nitric oxide or carbon monoxide, which binds to the heme moiety associated with E75 (Reinking et al., 2005). It is currently unknown whether a similar interplay of receptors operates in the ecdysteroid signaling pathway of crustaceans.
Studies involving receptor signaling in crustaceans of the genus Daphnia have been greatly facilitated by the recent sequencing of the Daphnia pulex genome (http://wFleaBase.org). We identified 25 nuclear receptor genes in the D. pulex genome (Thomson et al., 2009). Many of these receptors are orthologs to insect receptors involved in the ecdysteroid signaling pathway. In particular, we identified sequences for both E75 and HR3. We cloned, sequenced, and characterized expression patterns of these nuclear receptors from the closely related species, Daphnia magna (Hannas & LeBlanc, 2009). The receptor sequences indicate that the proteins may contain structural characteristics similar to those of the Drosophila orthologs, suggesting that they play similar roles to the Drosophila receptors in ecdysteroid signaling.
The goal of the present study was to functionally characterize these receptors by determining: (1) if either HR3 or E75 cloned from D. pulex (DappuHR3 and DappuE75, respectively) activate transcription of a reporter gene under the control of the RORE; and, (2) if any regulatory interactions occur between DappuHR3 and DappuE75.
Materials and methods
Full-length E75 and HR3 derivation
Female daphnids (Daphnia pulex) (clone MP2 (Busey 16)) provided by Dr. Jeffery Dudycha, University of South Carolina, USA), were cultured as described previously (Rider et al., 2005). Adults (>2 weeks after birth) were stored in RNAlater® (Ambion, Austin, TX, USA) at -20°C until sufficient tissue mass was collected for RNA isolation (approximately 30 mg wet weight). Daphnids were homogenized and RNA was isolated using the SV Total RNA Isolation System (Promega, Madison, WI). RNA integrity was confirmed by formaldehyde agarose gel electrophoresis. The concentration of RNA was determined by absorbance at 260 nm and the purity determined by the 260/280 nm absorbance ratio, using a Nanodrop ND-100 Spectrophotometer (NanoDrop Technologies, Montchanin, DE). The ImProm-II™ Reverse Transcription System (Promega) and oligo dT primers were used to reverse transcribe RNA into cDNA.
Primers were designed at the 5’ and 3’ ends of the predicted open reading frame (ORF) for both E75 and HR3 genes derived from the Daphnia pulex genome (Thomson et al., 2009). The primer sequences used were as follows:
DappuE75 F: 5’-GACGACGACAAGATGAGAAGTGAAATTGTTGTG-3’,
DappuE75 R: 5’-GAGGAGAAGCCCGGTTCAGCCCTTCATGATGTTGG-3’,
DappuHR3 F: 5’-GACGACGACAAGATGATGGAAGCTCCGGCCGTT-3’
DappuHR3 R: 5’-GAGGAGAAGCCCGGTTCAACTATCCACGGAAAAGAG-3’.
The bold portion of each primer corresponds to a ligase-independent cloning (LIC) extension sequence for cloning into an Ek/LIC vector (Novagen, EMD Biosciences, San Diego, CA, USA) which can be used for recombinant protein expression in E. coli. The genes were amplified by PCR using 75 ng cDNA, 22.5 μL high fidelity Supermix (Invitrogen, Carlsbad, CA), and 0.4 μM primers in a final volume of 25 μL. Cycling conditions were as follows: denature for 30 seconds at 94°C, anneal primers for 30 seconds at 57°C, and extend products for 3 minutes at 72°C for a total of 40 cycles, followed by a final extension for 7 minutes at 72°C. PCR products were purified from a 1.2% agarose gel using the Wizard® SV Gel and PCR Clean-up System (Promega). Purified products were cloned into the vector pCR®4-TOPO using the TOPO TA cloning kit (Invitrogen) according to the manufacturer's instructions. The gene inserts were subsequently sequenced by primer extension (SeqWright Inc., Houston, TX). The amino acid sequence and molecular weight for both the DappuHR3 and DappuE75 proteins were determined using ExPASy software (http://www.expasy.org/). The DappuHR3 sequence was aligned with the ortholog from Daphnia magna and the D. pulex HR3 sequence predicted from the genome annotation using ClustalW2 software (http://www.ebi.ac.uk/Tools/clustalw2/index.html). The DappuE75 protein sequence was aligned with that of D. magna, the tropical landcrab Gecarcinus lateralis, and the sand shrimp Metapenapeus ensis (Accession numbers are available in Additional File 1). Prediction of the DNA-binding domain and ligand-binding domain locations within the sequence were made based on the DappuE75 and DappuHR3 sequences, using NCBI protein BLAST (Altschul, et al., 1997).
Following release of an update of the Daphnia pulex genome, we discovered that the above cloning of DappuE75 yielded a gene sequence that was missing 403 nucleotides in the C-terminus and this cDNA was used to produce proteins for the functional characterization of the receptors. Therefore, the primers E75 R2 (full length): 5’-GAGGAGAAGCCCGGTTCAGGCGTGAAGGGGAAAAT-3’ and E75 F from above were used in PCR (as above) to obtain the full-length cDNA which was used in comparative functional experiments with the truncated cDNA.
Phylogenetic analysis
Phylogenetic analyses of DappuHR3 and DappuE75 receptors were performed using methods described previously (Thomson et al., 2009) with some modification. First, the DNA binding domain (DBD) and the ligand binding domain (LBD) of each receptor used in the analyses were identified using the conserved domain database (CDD) (Marchler-Bauer et al. 2007); DBD and LBD were joined; and, aligned using the default parameters of ClustalX (Thompson et al., 1997). Alignments and phylogenetic analysis of only the DBD or LBD also were performed and are presented in Additional Files 2-5. Receptor sequences from D. pulex were compared to those of other species available in GenBank (NCBI accession numbers for the receptors used are available in Additional File 1).
Trees were constructed using Bayesian Inference with MrBayes software version 3.1.2 (Ronquist et al, 2003) using two computing clusters: Bioportal (www.bioportal.uio.no) run by the University of Oslo; and, the Computational Biology Service Unit of Cornell University (http://cbsuapps.tc.cornell.edu/mrbayes.aspx). Phylogenetic trees were constructed using the “mixed-model” approach in which the Markov chain Monte Carlo sampler explores nine different fixed-rate amino acid substitution models implemented in MrBayes. We used 4 chains with runs of 5 million generations, chains sampled every 100 generations, a burnin of 10,000 trees with the WAG model (Whelan and Goldman, 2001). Phylogenies were rooted to the C. elegans receptors as the most ancient nuclear receptor on the tree.
Maximum parsimony and neighbor-joining (NJ) distance parameters were used to provide additional phylogenetic support for phylogenetic relationships observed among the nuclear receptors. Unrooted parsimony was constructed using PAUP version 4.0b10 (Swofford, 2001) with heuristic searches, tree-bisection-reconnection, topological constraints not enforced, and multiple tree option in effect with an initial maximum tree setting at 100,000. Branch support was measured by bootstrapping with 5000 replicates. Distance parameters were also measured using PAUP 4.0b10 by NJ with default characteristics (mean character difference and among site rate variation). Branch support was measured by bootstrap analysis with 1000 replicates. Trees were visualized with FigTree, a program freely available at (http://tree.bio.ed.ac.uk/software).
Transcriptional Reporter Assays
DappuHR3 and DappuE75cDNA were amplified using the primers:
pMT-E75 F: 5’-GGTACCGCCATGGGAAGTGAAATTGTTGTG -3’,
pMT-E75 R: 5’-TTCGAACTTCATGATGTTGGCGACGATG -3’, or
pMT-E75 R2 (full length): 5’-CTCGAGGGCGTGAAGGGGAAAATAGTG-3’, and
pMT-HR3 F: 5’-GGTACCGCCATGGAAGCTCCGGCCGTTCCG -3’,
pMT-HR3 R: 5’-CTCGAGATCCACGGAAAAGAGTTCCTTGTG -3’.
Recognition sites for KpnI, BstBI and XhoI are underlined. DappuHR3 and DappuE75 (-403 nucleotides and full length) cDNA were individually inserted into pMT/V5-His vectors (Invitrogen) at the KpnI and BstBI restriction sites and the KpnI and XhoI restriction sites, respectively. The reporter plasmid pEar1-Luc was a generous gift from K. Pardee and H. Krause (Reinking et al., 2005) and contains the luciferase reporter gene, under the control of the RORE (Horner, et al., 1995).
Transcription assays were performed in Drosophila Schneider (S2) cells. Cells were maintained in Schneider's medium (Gibco, Carlsbad, CA, USA) containing 10% heat inactivated fetal bovine serum (Gibco) and incubated at 23°C under ambient air atmosphere. Cells were seeded at a density of 3 ×106 and transfected 16 hours after plating. Transfections were performed by calcium phosphate DNA precipitation with 5 μg pEar1-Luc, and various amounts (indicated in the figures) of pMT-HR3, pMT-E75 (-403 nt) or pMT-E75 (full length), and pPAC-β-gal, which served as a control for transfection efficiency and was a kind contribution from Dr. Robert Tjian (University of California, Berkeley). Cells were transfected for 24 hours at 23°C, washed, and then induced with the addition of CuSO4 at a final concentration of 500 μM. Cells were provided 1.5 μM hemin (Sigma-Aldrich) dissolved in DMSO at the time of induction (Marvin et al., 2009). Cells were harvested and luciferase and ß-galactosidase activities were measured according to manufacturer's protocols for the Luciferase Assay System and the β-galactosidase Enzyme Assay System with Reporter Lysis Buffer (Promega). Luciferase values were normalized with ß-galactosidase activity levels and reported as relative to the untreated control cells (transfected with empty pMT-V5-His vector in lieu of pMT-E75 or pMT-HR3). Differences among transfection groups in the reporter assay were assessed using ANOVA and Tukey-Kramer comparison analysis to compare all pairs of groups. Statistical analyses were performed using JMP statistical software (SAS Institute, Cary, NC, USA).
Immunoblotting
Expression of recombinant protein from the transfected genes was evaluated by immunoblotting against the V5 tag. To prepare lysates, cells were first washed with PBS, and then were lysed using NP-40 buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, pH 8.0), supplemented with protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). Nucleic acids were sheared using a hand-held sonicator probe (Vibra Cell™, Danbury, CT, USA). Proteins were denatured by boiling the lysates at 95°C for 3 minutes. Protein concentrations were determined by the Bradford Assay (Bradford, 1976) using commercially available reagents (Bio-Rad, Hercules, CA) against the standard, bovine serum albumin (Fraction V) (Sigma-Aldrich, St. Louis, MO). Samples were added to 2× SDS loading buffer (100 mM Tris-HCl, pH 6.8, 200 mM DTT, 4% SDS, 0.2% bromophenol blue, 25% glycerol) for electrophoresis. Monoclonal anti-V5-HRP antibody (Invitrogen) was used to detect both DappuHR3 and DappuE75 fusion proteins which each contained a V5 epitope. Proteins were separated by electrophoresis on a 10% NuPAGE Novex Bis-Tris gel and transferred to PVDF membrane using the iBlot™ Dry Blotting System (Invitrogen) according to manufacturer protocol. Protein bands were visualized by chemiluminescence using the ECL Substrate Western blot detection system (Pierce).
Electrophoretic Mobility Shift Assay (EMSA)
DappuHR3 and DappuE75 protein were produced in S2 cells as described for the transcription reporter assays. Cells were harvested and the nuclear proteins were extracted using commercially available reagents and protocol (Pierce). Biotinylated Ear1 oligonucleotide sequence (prepared by IDT, Coralville, Iowa) was used as probe in the EMSA. EMSA reactions were performed using the LightShift Chemiluminescent EMSA kit (Thermo Fisher Scientific, Rockford, IL). Reactions contained 20 fmol of biotinylated probe with amounts of nuclear protein indicated in individual experiments in a total volume of 20 μl. Mobility shifts were generated using 6.0% DNA retardation gels (Invitrogen, Carlsbad, CA). After the electrophoresis, the proteins and oligonucleotides were transferred to Biodyne B nylon membrane (Pierce), molecules were UV cross-linked to the membrane, and biotin-labeled probe was detected by chemiluminescence (Pierce).
Results
DappuHR3 cDNA
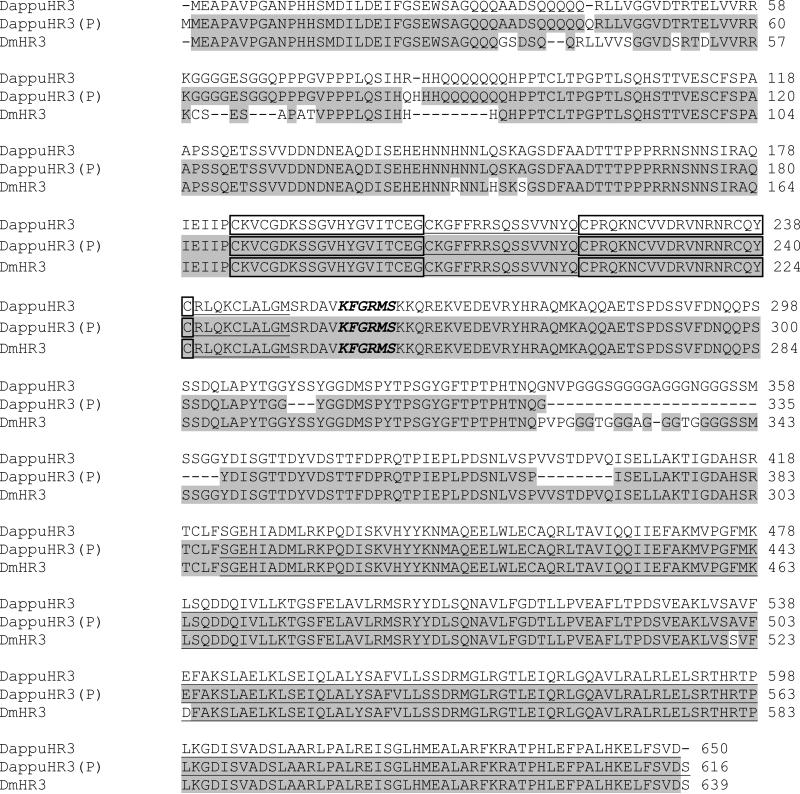
The open reading frame of the nuclear receptor DappuHR3 was sequenced from D. pulex (GenBank accession # FJ755467; Additional file 2). This 1,953 nucleotide cDNA translates to a 649 amino acid protein with an estimated mass of 70,709. DappuHR3 shared 93% identify with the D. magna HR3 (DmHR3) (Fig. 1). DappuHR3 was 94% identical to the predicted DappuHR3 sequence derived from genomic annotation (Thomson et al., 2009). Discrepancies between the predicted and the sequenced cDNA existed largely due to errors in the identification of splice sites resulting in deletions in the predicted sequence in the 309 to 403 amino acid region. Sporadic single amino acid differences between the predicted and actual DappuHR3 sequences may be due to sequencing error or clone differences (clone Log50 was used in the genome sequencing effort) (http://daphnia.cgb.indiana.edu/projects/genome/). The DappuHR3 DBD and LBD were nearly 100% identical to that of D. magna and the predicted D. pulex sequence. The DappuHR3 DBD was equipped with two zinc finger motifs and an adjacent GRIP box (Fig. 1). The DappuHR3 LBD was 100% concordant with the predicted sequence and differed from that of D magna by only two amino acid substitutions. The high degree of similarity in the DBD and LBD suggests functionality is likely conserved between DappuHR3 and DmHR3.
Figure 1.
Comparison of the DappuHR3 amino acid sequence to the sequence predicted from the genomic sequence (DappuHR3(P)), and DmHR3 from Daphnia magna. Shaded areas denote amino acid identity with DappuHR3. The putative DNA-binding C domain and ligand-binding E domain are underlined. Boxed amino acids correspond to the zinc fingers within the C domain. The GRIP-box is indicated by amino acid residues in italics.
DappuE75 cDNA
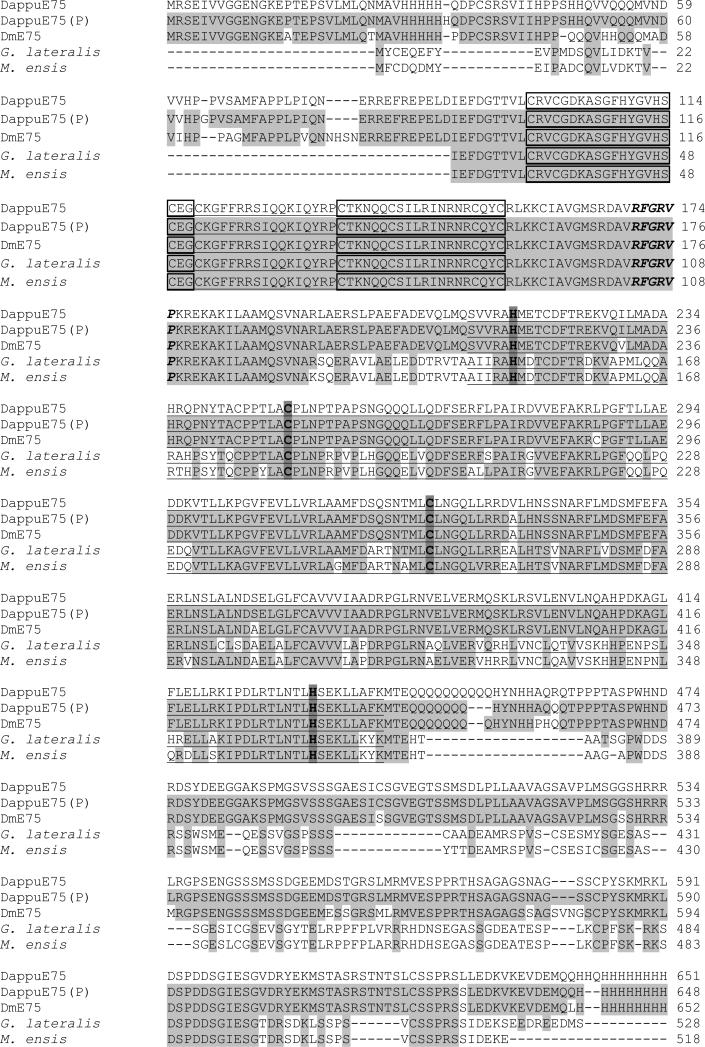
The E75 full-length (2865 nucleotides) open reading frame cDNA was cloned from D. pulex (GenBank accession # FJ946916, Additional file 3). This cDNA encodes a 955 amino acid protein with an estimated molecular mass of 104,136. The full length amino acid sequence for DappuE75 was aligned with that of D. magna (DmE75) and several other species (Fig. 2). DappuE75 shared 90% sequence identity to DmE75 and 95% identity with the DappuHR3 sequence predicted from genome annotation (Thompson et al. 2009). Differences between the predicted and actual amino acid sequences were due to errors in predicted splice sites (region 722-769) and sporadic amino acid differences.
Figure 2.
Comparison of the DappuE75 amino acid sequence to the sequence predicted from the genomic sequence (DappuE75(P)) and the sequences from Daphnia magna (DmE75), the tropical land crab (Gecarcinus lateralis), and the sand shrimp (Metapenapeus ensis). Shaded areas denote amino acid identity with DappuE75. The putative DNA-binding C domain and ligand-binding E domain are underlined. Boxed amino acids correspond to the zinc fingers within the C domain. The GRIP-box is indicated by amino acid residues in italics. Histidines and cysteines required for heme binding are darkly shaded.
The DNA binding C domain (DBD) of the DappuE75 protein contains two zinc fingers and shares 100% identity with E75 orthologs from other crustaceans used in the alignment and D. melanogaster (Fig. 2). The DappuE75 LBD contains histidine and cysteine residues situated in key positions to bind a heme moiety, as described previously (Reinking et al., 2005, de Rosny et al., 2006) (Fig. 2). The LBD of the DappuE75 shared the following conservation with E75 of other species: D. magna (DmE75) 99%, G. lateralis (GE75), 77%, M. ensis (ME75) 70%, D. melanogaster (DE75) 50%.
Phylogenetic analyses
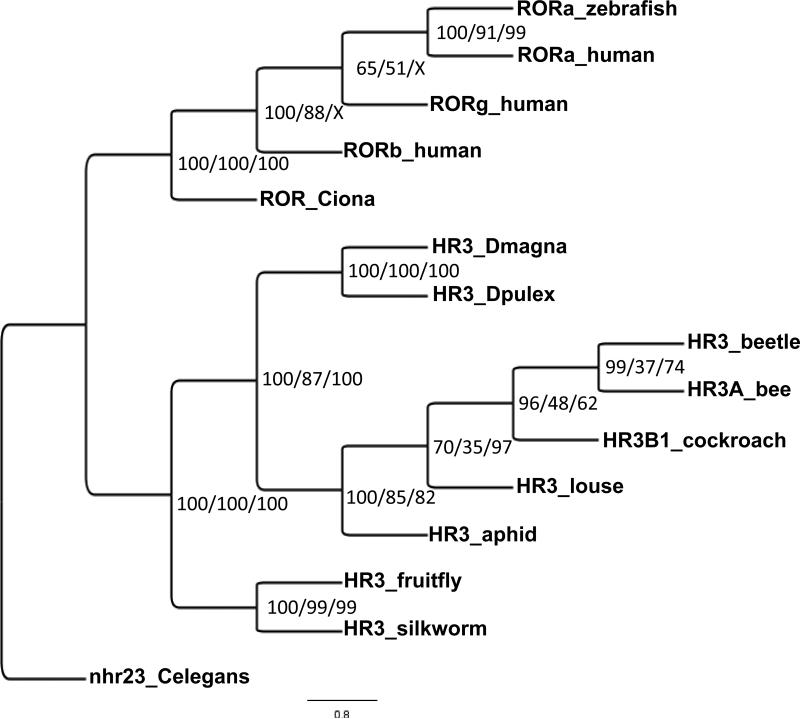
The NR1F (HR3/ROR) phylogenetic tree segregates into two major clades, one consisting of chordates and the other arthropods (Fig. 3). All three methods used to construct trees separated out the arthropod HR3 receptors into two distinct lineages. The fruitfly and silkworm HR3 form one lineage and the remaining arthropods form a second lineage within which the daphnid and insects form separate clades,
Figure 3.
Phylogenetic analysis of the NR1F (HR3/ROR) group of nuclear receptors. The nuclear receptors from several different species including human Rev-Erb, arthropod E75, and the related nematode receptor from C. elegans were subjected to phylogenetic comparisons using Bayesian Inference, Maximum Parsimony, and Neighbor-Joining methods. C. elegans was chosen as the outgroup. The Bayesian tree is shown with posterior probabilities from the Bayesian tree, and bootstrap support values (frequency of occurrence) from the Maximum Parsimony and Neighbor-Joining trees provided in order from left to right, respectively. Posterior probability values are separated by forward slashes at each corresponding node; an X indicates an area of disagreement from the Bayesian tree. Notations on the tree indicate the receptor name followed by an abbreviated common name of the species from which the nuclear receptor was cloned. C. elegans = Caenorhabditis elegans; Dpulex = D. pulex; Dmagna = D. magna; human = Homo sapiens; zebrafish = Danio rerio; Ciona = Ciona intestinalis; fruitfly = Drosophila melanogaster; silkworm = Bombyx mori; bee = Apis mellifera; aphid = Acyrthosiphon pisum; louse = Pediculus humanus corporis; cockroach = Blattella germanica; beetle = Tribolium castaneum. Accession numbers of the analyzed nuclear receptors are provided in Additional File 1.
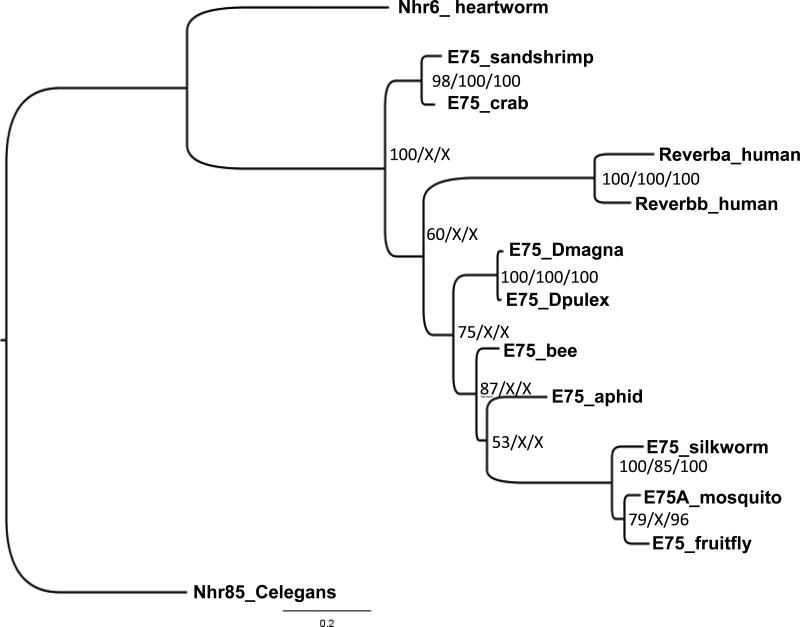
Phylogenetic analysis of the NR1D (E75/Rev-Erb) receptor group, placed the Malacostraca crustaceans, human, and all other arthropods within distinct lineages (Fig. 4). Within the arthropod lineage, four distinct branches exist consisting of Branchiopoda (D. magna and D. pulex), Hymenoptera (honeybee: Apis mellifera), Hemiptera (pea aphid: Acyrthosiphon pisum), and Diptera/Lepidoptera (fruitfly: D. melanogaster; mosquito Aedes aegypti; silkworm: Bombyx mori.
Figure 4.
Phylogenetic relationship of the NR1D (E75/Rev-Erb) group of nuclear receptors. The nuclear receptors from several different species including human Rev-Erb, arthropod E75, and the related nematode receptors from C. elegans and heartworm (D. immitis) were subjected to phylogenetic comparisons using Bayesian Inference, Maximum Parsimony, and Neighbor-Joining methods. C. elegans was chosen as the outgroup. The Bayesian tree is shown with posterior probabilities from the Bayesian tree, and bootstrap support values (frequency of occurrence) from the Maximum Parsimony and Neighbor-Joining trees provided in order from left to right, respectively. Posterior probability values are separated by forward slashes at each corresponding node; an X indicates an area of disagreement from the Bayesian tree. Notations on the tree indicate the receptor name followed by an abbreviated common name of the species from which the nuclear receptor was cloned. C. elegans = Caenorhabditis elegans; heartworm = Dirofilaria immitis; Dpulex = D. pulex; Dmagna = D. magna; human = Homo sapiens; fruitfly = Drosophila melanogaster; mosquito = Aedes aegypti; silkworm = Bombyx mori; bee = Apis mellifera; aphid = Acyrthosiphon pisum. Accession numbers of the analyzed nuclear receptors are provided in Additional File 1.
Some small but significant inconsistencies existed among the three methods of phylogenetic analysis employed. Bayesian Inference (Fig. 4) placed the Malacostraca receptors as more ancestral than the human or remaining arthropod receptors. Maximum Parsimony and Neighbor Joing methods placed the human receptors as more ancestral than the decapods and other arthropod receptors. Bayesian Inference placed the honeybee and aphid receptors into distinct groups (Fig. 4); while, Maximum Parsimony and Neighbor Joining methods placed these receptors in a common clade. Bootstrap values represented in Fig. 4 by an “X” are a consequence of these inconsistencies.
Phylogenetic analyses were also performed with the DNA-binding (DBD) and ligand-binding (LBD) domains, alone, for both nuclear receptors (Additional files 4-7). Trees constructed with the DBDs provided poor resolution of the receptors from different species, likely due to the high level of interspecies similarity among nuclear receptor DBDs. In general, trees constructed from the LBDs agreed well with the trees constructed from the combined DBD-LBD. Indicating that most of the differences among the species used to construct the DBD-LBD tree, exist within the LBD. Among the three constructions for each receptor, trees constructed with the DBD-LBD provided the best resolution and strongest posterior probabilities and were therefore selected for presentation in the manuscript.
Transcription activation by DappuHR3 and DappuE75
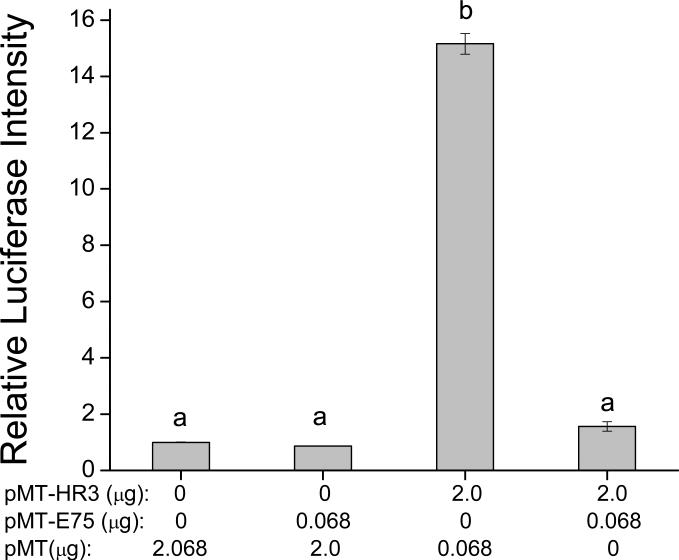
Transient expression luciferase-based reporter assays were performed to determine if DappuHR3 or DappuE75 activates gene transcription driven by the RORE. The DappuHR3 expression vector (pMT-HR3) was cotransfected into Drosophila S2 cells with the luciferase reporter plasmid (pEar1-Luc) which contains six RORE recognition sequences, located upstream of the basal promoter for the luciferase gene (Reinking et al. 2005). Immunoblot blot analysis of cell lysates confirmed that both the DappuHR3 and DappuE75 proteins were expressed in induced cells (Fig. 5). DappuE75 protein accumulated in the transfected cells to a much greater level then did DappuHR3 (Fig. 5). DappuHR3 activated transcription approximately 15-fold over basal activation (Fig. 6). This transcriptional activation was absent when using a basic (pGL3) luciferase reporter (minus the Ear1 sequence) (data not shown). DappuE75 did not activate the luciferase reporter gene when transfected alone (Fig. 6) despite the high level of accumulation in the cells. Cotransfection of DappuHR3 and DappuE75 along with the reporter plasmid suppressed the transcriptional activation seen with DappuHR3 alone (Fig. 6).
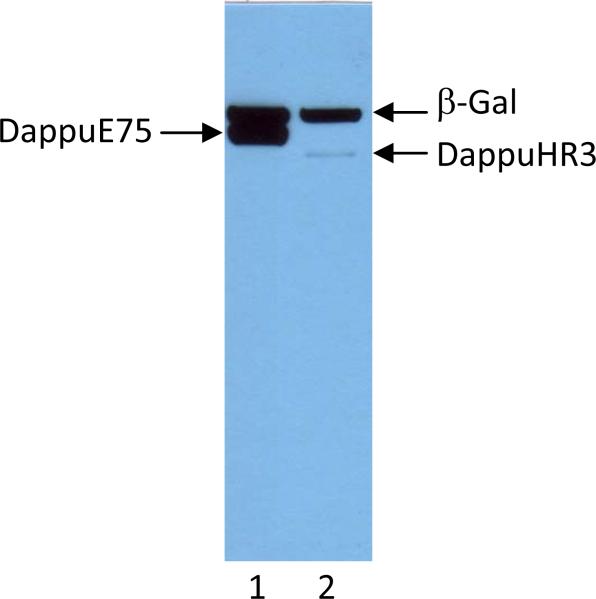
Figure 5.
Expression of DappuE75 in S2 cells transfected with 0.25 μg of pMT-E75 vector (lane 1) or and DappuHR3 in cells transfected with 2.0 μg of pMT-HR3 vector (lane 2). All cells were also transfected with 0.5 μg pMT-lacZ (β-Gal). Proteins were detected with α-V5 tag antibodies.
Figure 6.
DappuHR3 activation and DappuE75 negative regulation of RORE-driven luciferase reporter assay. Amount of expression plasmid DNA transfected for each group was: 2.068 μg empty vector in control, 0.068 μg pMT-E75 in E75 group, 2 μg HR3 in HR3 alone group and 0.068 μg pMT-E75/2 μg HR3 in E75/HR3 group. Data bars represent mean± SEM (n=2) duplicate samples. Transfection groups not connected by the same letter are significantly (p≤0.05) different (ANOVA, Tukey-Kramer comparison).
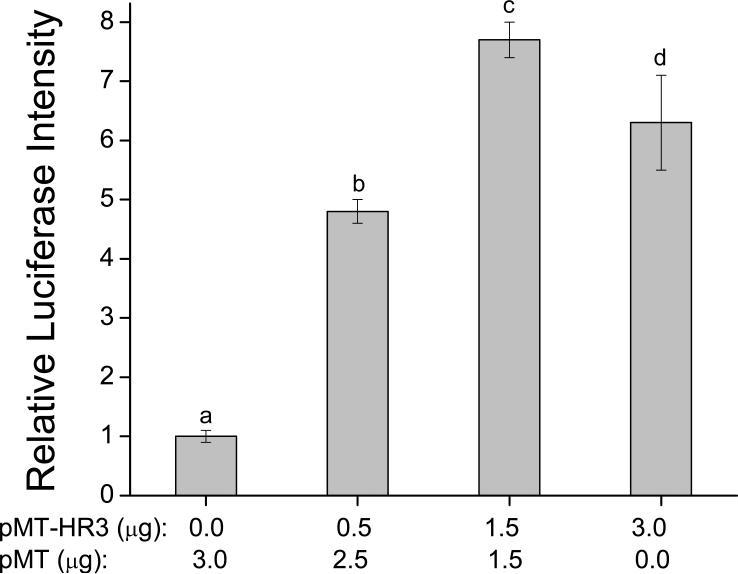
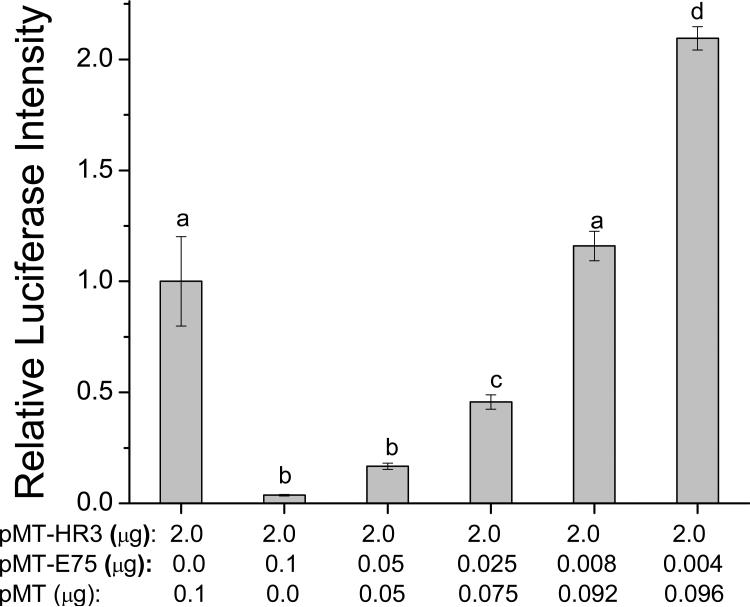
The concentration-response relationship for the transactivation of the luciferase gene by DappuHR3 was evaluated. DappuHR3 increased luciferase activation with an inverted U-shaped concentration response curve (Fig. 7). The decreased transcriptional activity at the high DappuHR3 concentration may reflect toxicity associated with the receptor protein when present in the cells at these levels. Next, a concentration-response analysis was performed to definitively characterize the observed suppression of DappuHR3 activity by DappuE75. Increasing concentrations of the DappuE75 expression plasmid were cotransfected with a constant concentration of both DappuHR3 and reporter plasmid. The resulting relationship was inverse, with reporter activation decreasing as levels of DappuE75 levels increased (Fig. 8). Interestingly, at the lowest concentration of transfected DappuE75 plasmid, reporter activity was significantly greater than that of DappuHR3 transfected alone.
Figure 7.
Concentration-response analyses of the transcriptional activity of DappuHR3. Values under the x-axis represent the amount of each expression construct (pMT-HR3) transfected. Data bars represent mean ± SD (n=3). Transfection groups not connected by the same letter are significantly (p≤0.05) different (ANOVA, Tukey-Kramer comparison).
Figure 8.
Concentration-response analysis of the effect of DappuE75 on DappuHR3 transcriptional activation. Values under the x-axis represent the amount of each expression construct (pMT-E75 or pMT-HR3) transfected. Data bars represent the mean± SD (n=3). Transfection groups not connected by the same letter are significantly (p≤0.05) different (ANOVA, Tukey-Kramer method).
Receptor binding to the Ear1 sequence
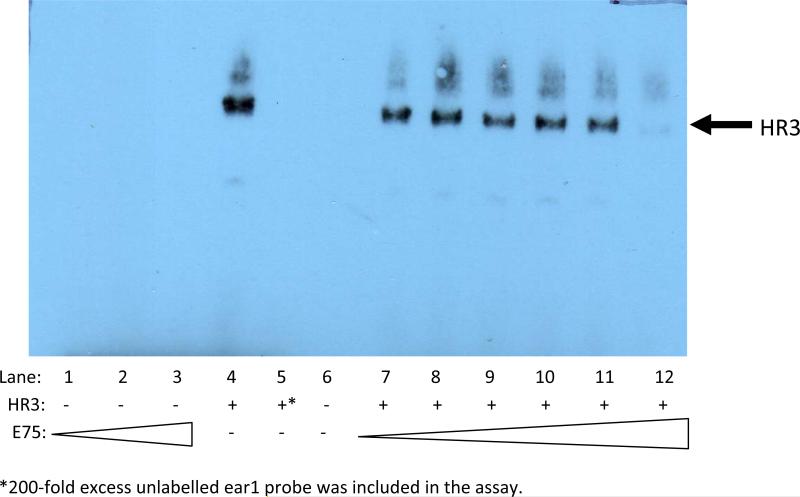
Electrophoretic mobility shift assays were performed to confirm the exclusive interaction of DappuHR3 with the Ear1 response element and to determine whether DappuE75 competed with DappuHR3 for occupancy of the element. DappuHR3 specifically bound to the Ear1 probe; while, DappuE75 exhibited no discernable binding to this probe (Fig. 9). Despite the lack of interaction between DappuE75 and the Ear1 probe, DappuE75 did suppress binding of DappuHR3 to the probe (Fig. 9). These results suggest that DappuE75 interacts directly with DappuHR3, and not the response element, to suppress transcriptional activation.
Figure 9.
EMSA results using a biotinylated ear1 oligonucleotide probe. Lanes 1-3: E75 nuclear extract (0.074, 0.74, and 7.4 μg protein). Lanes 4 and 5: HR3 nuclear extract (6.4 μg protein) in the absence and presence of 200-fold excess unlabelled competitor probe. Lanes 7-12 HR3 nuclear extract (6.4 μg protein) with increasing concentrations of E75 nuclear extract (7.4 ×10-5 to 7.4 μg protein in 10X increments). E75 and HR3 were expressed in Drosophila S2 cells. Immunoblots of cell cytosolic and nuclear extracts demonstrated that both expressed proteins localized to the cell nucleus.
DappuE75 activity: Full-length receptor vs. -403 nucleotides
We used the pMT-E75 (-403 nucleotides) expression plasmid as the source for DappuE75 protein in the reporter assays described above. Therefore, upon discovery of the additional C-terminal nucleotides and cloning of this full sequence, we evaluated the effect of the full-length protein in the reporter assay. Full-length DappuE75 performed identically to the -403 nucleotide receptor in that: a) it did not activate the Ear1-driven reporter gene; and, b) it suppressed transciptional activation of the reporter by DappuHR3 (Additional file 6).
Discussion
The nuclear receptors HR3 and E75 are prominent contributors to ecdysteroid signaling in insects (Hiruma & Riddiford, 2004, Swevers et al., 2002, White et al, 1997). In the present study HR3 and E75 were cloned from the crustacean Daphnia pulex and used to determine whether either of these nuclear receptors activate the transcription of genes regulated by the RORE; and, if DappuHR3 and DappuE75 interact to regulate RORE-mediated gene transcription. We demonstrated that DappuHR3 activates transcription of a reporter gene under the regulatory control of the RORE and DappuE75 negatively regulates this activity of DappuHR3.
Phylogenetic analyses clearly demonstrates the appropriate assignment of the daphnid receptors within the NR1F (DappuHR3) and NR1D (DappuE75) nuclear receptor groupings. Both receptors fit within the arthropod lineage for these receptors forming a distinct clade from that of the insects. Phylogenetic analyses suggested that the Malacostraca E75 receptors are significantly divergent from those of the insects and daphnids. Phylogenetic analyses of three arthropod genes suggests that an arthropod clade consisting of Hexapoda (including insects) and Branchiopoda (including daphnids) diverged from the lineage from which Malacostraca (including decapods) emerged over 550 myr ago (Regier et al., 2005). The phylogeny of E75 is consistent with this premise and further suggests that the Diptera/Lepidoptera receptor subsequently divered from both the Malacostraca and Branchiopoda lineages.
The DBD of DappuHR3, DmHR3, and the Drosophila DHR3 are highly similar (≥95%) (Hannas & LeBlanc, 2009, present study). The DBD of DHR3 binds an 11-bp DNA response element that consists of the half-site AGGTCA flanked on the 5’ end by an A/T rich sequence, referred to as an RORE. DHR3 also contains a highly conserved C-terminal extension (CTE) to the DBD. This CTE contains a “GRIP-box” sequence, which has the consensus sequence (K/R)XGRZ(P/S), where X is any amino acid and Z represents a hydrophobic amino acid (Melvin et al., 2002). The GRIP-box typically provides stability to a monomeric receptor protein when binding DNA (Zhao et al., 1998). DappuHR3 also contains a GRIP-box. The similarities between the structure of the DBD of Daphnia and Drosophila suggest that, like DHR3, DappuHR3 binds the RORE as a monomer.
HR3 is a member of the NR1F group of nuclear receptors, which includes the RORs of vertebrates (Owen & Zelent, 2000). HR3 has been shown in many insects to be ecdysteroid inducible (Eystathioy et al., 2001, Jindra et al., 1994, Palli et al., 1996) and functions in ecdysteroid signaling to regulate aspects of embryogenesis and metamorphosis (Carney et al., 1997, Lam et al., 1999) along with contributions to many other physiological processes (see Introduction). A portion of the HR3 cDNA comprising the LBD was reportedly cloned from the American lobster (Homarus americanus) (El Haj et al., 1997). However, the putative LBD associated with this amino acid sequence exhibited low identity to the HR3 LBD from Drosophila melanogaster (19.6%) and Manduca sexta (22.2%). The sequence cloned from the lobster may represent another receptor or the LBD of lobster HR3 differs significantly from those of insects and daphnids. Aside from D. magna (Hannas & LeBlanc, 2009) and D. pulex (present study), we are not aware of any other crustacean species from which the full HR3 cDNA has been sequenced.
E75 is a member of the NR1D group of nuclear receptors. Vertebrate rev-erbs also are members of this group (Owen & Zelent, 2000). E75 has been cloned from the tropical land crab (G. lateralis) (Kim et al., 2005) and the shrimp (M. ensis) (Chan, 1998). The aligned DBD and LBD of DappuE75 exhibited greater similarity to those of the malacostracan crustaceans as compared to the alignment to the D. melanogaster receptor. This suggests that despite early divergence of the Malacostraca and Branchiopoda lineages, the amino acid sequence of this receptor changed less among these crustaceans as compared to some more rapidly evolving insect lineages.
Functional analyses demonstrated that DappuHR3 activated transcription of a reporter gene driven by the RORE. This result demonstrates that this crustacean representative of the NR1F group of nuclear receptors functions similarly to other group members. In contrast, DappuE75 did not activate RORE-driven gene transcription. Rather, DappuE75 suppressed transcriptional activation mediated by DappuHR3. This suppressor activity of a crustacean E75 is consistent with the suppressive activity demonstrated with insect E75 (Swevers et al., 2002, White et al., 1997), as well as, the vertebrate ortholog REV-ERB (Forman et al., 1994, Ramakrishnan & Muscat, 2006). While equal amounts of plasmid DNA were used in transient transfection experiments, differences in DappuHR3 and DappuE75 protein expression levels were consistently observed in western blot analysis. Both HR3 and E75, like other members of the NRIF and NRID families, are normally rapidly degraded by the proteasome ubiquitin pathway. However, incubation with hemin, as was done in the present experiments, has been shown to stabilize E75 protein (Moraitis et al., 2003, Reinking et al., 2005) providing for its enhanced accumulation relative to HR3.
Two mechanisms have been described through which E75 can suppress the action of HR3. BmE75 from Bombyx mori, equipped with a complete DBD, has been shown to bind the RORE and is capable of competing with BmHR3 for response element occupancy (Swevers et al., 2002). In addition, BmE75 can bind directly to BmHR3 forming a ternary complex with the response element (Swevers et al., 2002), which is presumably inhibitory towards BmHR3. Drosophila DE75B lacks one zinc finger in the DBD but remains capable of inhibiting DHR3 by complexing with DHR3 on the response element (White et al., 1997). E75 of daphnids possesses an intact DBD but does not appear to bind the RORE. DappuE75 may interact directly with DappuHR3 protein to suppress gene transcription.
Co-transfection experiments with the daphnid receptors revealed that, in addition to the suppressive action of DappuE75 on DappuHR3 activity, low levels of DappuE75 augment DappuHR3 transcriptional activation. High levels of DappuHR3 expressed in these assays may have compromised the transcriptional capacity of the cells. The low level of DappuE75 used in the experiment may have protected the cells against this activity while being present at a concentration insufficient to suppress DappuHR3 activity resulting in enhanced transcription of the luciferase gene. Suppressed transcription associated with high levels of DappuHR3 could explain the inverted-U shaped curve of luciferase activity vs. increasing levels of DappuHR3 expression vector observed in the present study. Further investigations are necessary to definitively characterize this interaction. However, these results suggest that an important and potentially unique regulatory interplay occurs between DappuE75 and DappuHR3.
In summary, results from the present study demonstrate that crustaceans express both HR3 and E75 and that these proteins function coordinately to regulate gene transcription via the RORE. DappuHR3 activates transcription of genes driven by this response element and DappuE75 suppresses this activity of DappuHR3. Considering the high similarity in structure and function of these gene products in comparison to those of insects and the rapid induction of daphnid HR3 in response to ecdysteroids (Hannas & LeBlanc, 2009), it is reasonable to conclude that these receptors are integral components of ecdysteroids signaling in crustaceans.
Supplementary Material
Acknowledgements
The authors thank Dr. Jeffery Dudycha, at the University of South Carolina, USA for providing the D. pulex clone used in this study; Dr. Henry Krause at the Charles H. Best Institute, Toronto Canada, Dr. Keith Pardee at the University of Toronto, Canada, and Dr. Robert Tjian at the University of California, Berkeley for the donated plasmids; Dr. Seth Kullman at North Carolina State University for insightful advice related to this work; Ms. Gwijun Kwon and Ms. Sarah Wickman for assistance with cloning and immunoblotting. This research was supported by US Environmental Protection Agency STAR grant RD-83273901 and NSF grant IOS-0744210 to GAL. BRH was supported by an EPA STAR Fellowship and NIEHS training grant #T32 ES007046.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampleford EJ. Ecdysteroids influence the circadian system timing ecdysis in the insect Rodnius prolixus (Hemiptera). J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 1985;157:699–704. [Google Scholar]
- Bialecki M, Shilton A, Fichtenberg C, Segraves WA, Thummel CS. Loss of the ecdysteroid-inducible E75A orphan nuclear receptor uncouples molting from metamorphosis in Drosophila. Dev. Cell. 2002;3:209–220. doi: 10.1016/s1534-5807(02)00204-6. [DOI] [PubMed] [Google Scholar]
- Bownes M, Shirras A, Blair M, Collins J, Coulson A. Evidence that insect embryogenesis is regulated by ecdysteroids released from yolk proteins. Proc. Natl. Acad. Sci. U.S.A. 1988;85:1554–7. doi: 10.1073/pnas.85.5.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buszczak M, Freeman MR, Carlson JR, Bender M, Cooley L, Segraves WA. Ecdysone response genes govern egg chamber development during midoogenesis in Drosophila. Development. 1999;126:4581–4589. doi: 10.1242/dev.126.20.4581. [DOI] [PubMed] [Google Scholar]
- Carney GE, Wade AA, Sapra R, Goldstein ES, Bender M. DHR3, an ecdysone-inducible early-late gene encoding a Drosophila nuclear receptor, is required for embryogenesis. Proc. Natl. Acad. Sci. U.S.A. 1997;94:12024–12029. doi: 10.1073/pnas.94.22.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SM. Cloning of a shrimp (Metapenaeus ensis) cDNA encoding a nuclear receptor superfamily member: an insect homologue of E75 gene. FEBS Lett. 1998;436:395–400. doi: 10.1016/s0014-5793(98)01148-x. [DOI] [PubMed] [Google Scholar]
- de Rosny E, de Groot A, Jullian-Binard C, Gaillard J, Borel F, Pebay-Peyroula E, Fontecilla-Camps JC, Jouve HM. Drosophila nuclear receptor E75 is a thiolate hemoprotein. Biochemistry. 2006;45:9727–9734. doi: 10.1021/bi060537a. [DOI] [PubMed] [Google Scholar]
- El Haj AJ, Tamone SL, Peake M, Reddy S, Chang ES. An ecdysteroid-responsive gene in a lobster - a potential crustacean member of the steroid hormone receptor superfamily. Gene. 1997;201:127–135. doi: 10.1016/s0378-1119(97)00437-x. [DOI] [PubMed] [Google Scholar]
- Eystathioy T, Swevers L, Iatrou K. The orphan nuclear receptor BmHR3A of Bombyx mori: hormonal control, ovarian expression and functional properties. Mech. Develop. 2001;103:107–115. doi: 10.1016/s0925-4773(01)00335-5. [DOI] [PubMed] [Google Scholar]
- Forman BM, Chen J, Blumberg B, Kliewer SA, Henshaw R, Ong ES, Evans RM. Cross talk among ROR-alpha1 and the rev-erb family of orphan nuclear receptors. Mol. Endocrinol. 1994;8:1253–1261. doi: 10.1210/mend.8.9.7838158. [DOI] [PubMed] [Google Scholar]
- Hannas BR, LeBlanc GA. Expression and ecdysteroid responsiveness of the nuclear receptors HR3 and E75 in the crustacean Daphnia magna. Mol. Cell. Endocrinol. 2009;315:208–218. doi: 10.1016/j.mce.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma K, Riddiford LM. Differential control of MHR3 promoter activity by isoforms of the ecdysone receptor and inhibitory effects of E75A and MHR3. Dev. Biol. 2004;272:510–521. doi: 10.1016/j.ydbio.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Horner MA, Chen T, Thummel CS. Ecdysteroid regulation and DNA binding properties of Drosophila nuclear hormone receptor superfamily members. Dev. Biol. 1995;168:490–502. doi: 10.1006/dbio.1995.1097. [DOI] [PubMed] [Google Scholar]
- Jindra M, Sehnal F, Riddiford LM. Isolation and developmental expression of the ecdysteroid-induced GHR3 gene of the wax moth Galleria mellonella. Insect Biochem. Mol. Biol. 1994;24:763–773. doi: 10.1016/0965-1748(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Kim HW, Lee SG, Mykles DL. Ecdysteroid-responsive gene, RXR and E75, in the tropical land crab, Gecarcinus lateralis: Differential tissue expression of multiple RXR isoforms generated at three alternative splicing sites in the hinge and ligand-binding domains. Mol. Cell. Endocrinol. 2005;242:80–95. doi: 10.1016/j.mce.2005.08.001. [DOI] [PubMed] [Google Scholar]
- King-Jones K, Thummel CS. Nuclear receptors: a perspective from Drosophila. Nat. Rev. Genet. 2005;4:311–323. doi: 10.1038/nrg1581. [DOI] [PubMed] [Google Scholar]
- Koelle MR, Talbot WS, Segraves WA, Bender MT, Cherbas P, Hogness DS. The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor family. Cell. 1991;67:59–77. doi: 10.1016/0092-8674(91)90572-g. [DOI] [PubMed] [Google Scholar]
- Lam G, Hall BL, Bender M. DHR3 is required for the prepupal-pupal transition and differentiationof adult structures during Drosophila metamorphosis. Dev. Biol. 1999;212:204–216. doi: 10.1006/dbio.1999.9343. [DOI] [PubMed] [Google Scholar]
- Laufer H, Ahl J, Rotllant G, Baclaski B. Evidence that ecdysteroids and methyl farnesoate control allometric growth and differentiation in a crustacean. Insect Biochem. Mol. Biol. 2002;32:205–210. doi: 10.1016/s0965-1748(01)00104-7. [DOI] [PubMed] [Google Scholar]
- LeBlanc GA. Crustacean endocrine toxicology: a review. Ecotoxicology. 2007;16:61–81. doi: 10.1007/s10646-006-0115-z. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Anderson JB, Derbyshire MK, DeWeese-Scott C, Gonzales NR, Gwadz M, Hao L, He S, Hurwitz DI, Jackson JD, et al. CDD: a conserved domain database for interactive domain family analysis. Nucleic Acids Res. 2007;35:237–240. doi: 10.1093/nar/gkl951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin KA, Reinking JL, Lee AJ, Pardee K, Krause HM, Burstyn JN. Nuclear Receptors Homo sapiens Rev-erbβ and Drosophila melanogaster E75 are thiolate-ligated heme proteins which undergo redox-,ediated ligand switching and bind CO and NO. Biochemistry. 2009;48:7056–7071. doi: 10.1021/bi900697c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melvin VS, Roemer SC, Churchill MEA, Edwards DP. The C-terminal extension (CTE) of the nuclear hormone receptor DNA binding domain determines interactions and functional response to the HMGB-1/-2 co-regulatory proteins. J Biol. Chem. 2002;277:25115–25124. doi: 10.1074/jbc.M110400200. [DOI] [PubMed] [Google Scholar]
- Moratis AN, Giguere V. The co-repressor hairless protects RORalpha orphan nuclear receptor from proteasome-mediated degradation. J. Biol. Chem. 2003;278:52511–52518. doi: 10.1074/jbc.M308152200. [DOI] [PubMed] [Google Scholar]
- Owen GI, Zelent A. Origins and evolutionary diversification of the nuclear receptor superfamily. Cell. Mol. Life Sci. 2000;57:809–827. doi: 10.1007/s000180050043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palli SR, Ladd TR, Sohi SS, Cook BJ, Retnakaran A. Cloning and developmental expression of Choristoneura hormone receptor 3, an ecdysoneinducible gene and a member of the steroid hormone receptor superfamily. Insect Biochem. Mol. Biol. 1996;26:485–499. doi: 10.1016/0965-1748(96)00004-5. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan SN, Muscat EO. The orphan rev-erb nuclear receptors: a link between metabolism, circadian rhythm and inflammation? Nuclear Recept. Sig. 2006;4 doi: 10.1621/nrs.04009. DOI: 10.1621/nrs.04009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinking J, Lam MMS, Pardee K, Sampson HM, Liu SY, Yang P, Williams S, White W, Lajoie G, Edwards A, Krause HM. The Drosophila nuclear receptor E75 contains heme and is gas responsive. Cell. 2005;122:195–207. doi: 10.1016/j.cell.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Regier JC, Shultz JW, Kambic RE. Pancrustacean phylogeny: hexapods are terrestrial crustaceans and maxillopods are not monophyletic. Proc. R. Soc. B. 2005;272:395–401. doi: 10.1098/rspb.2004.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard DS, Watkins NL, Serafin RB, Gilbert LI. Ecdysteroids regulate yolk protein uptake by Drosophila melanogaster oocytes. J. Insect Physiol. 1998;44:637–644. doi: 10.1016/s0022-1910(98)00020-1. [DOI] [PubMed] [Google Scholar]
- Riddiford LM, Cherbas P, Truman JW. Ecdysone receptors and their biological actions. Vitam. Horm. 2000;60:1–73. doi: 10.1016/s0083-6729(00)60016-x. [DOI] [PubMed] [Google Scholar]
- Rider CV, Gorr TA, Olmstead AW, Wasilak BA, LeBlanc GA. Stress signaling: coregulation of hemoglobin and male sex determination through a terpenoid signaling pathway in a crustacean. J. Exp. Biol. 2005;208:15–23. doi: 10.1242/jeb.01343. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Smagghe G. Ecdysone: Structures and Functions. Springer; New York: 2009. p. 590pp. [Google Scholar]
- Swevers L, Ito K, Iatrou K. The BmE75 nuclear receptors function as dominant repressors of the nuclear receptor BmHR3A. J. Biol. Chem. 2002;277:41637–41644. doi: 10.1074/jbc.M203581200. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP*: phylogenetic analysis using parsimony (*and other methods), version 4.0b10. Sinauer Associates; Sunderland, MA: 2001. [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The clustalx windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;24:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson SA, Baldwin WS, Wang YH, Kwon G, LeBlanc GA. Annotation, phylogenetics, and expression of the nuclear receptors in Daphnia pulex. BMC Genomics. 2009;10:500. doi: 10.1186/1471-2164-10-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel CS. Flies on steroids: Drosophila metamorphosis and the mechanisms of steroid hormone action. Trends Genet. 1996;12:306–310. doi: 10.1016/0168-9525(96)10032-9. [DOI] [PubMed] [Google Scholar]
- Tuberty SR, McKenney CL., Jr. Ecdysteroid responses of estuarine crustaceans exposed through complete larval development to juvenile hormone agonist insecticides. Integr. Comp. Biol. 2005;45:106–117. doi: 10.1093/icb/45.1.106. [DOI] [PubMed] [Google Scholar]
- Whelan S, Goldman N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol. Biol. Evol. 2001;18:691–699. doi: 10.1093/oxfordjournals.molbev.a003851. [DOI] [PubMed] [Google Scholar]
- White KP, Hurban P, Watanabe T, Hogness DS. Coordination of Drosophila metamorphosis by two ecdysone-induced nuclear receptors. Science. 1997;276:114–117. doi: 10.1126/science.276.5309.114. [DOI] [PubMed] [Google Scholar]
- Yao T, Segraves WA, Oro AE, McKeown M, Evan RM. Drosophila ultraspiricle modulates ecdysone receptor function via heterodimer formation. Cell. 1992;71:63–72. doi: 10.1016/0092-8674(92)90266-f. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Khorasanizadeh S, Miyoshi Y, Lazar MA, Rastinejad F. Structural elements of an orphan nuclear receptor-DNA complex. Mol. Cell. 1998;1:849–861. doi: 10.1016/s1097-2765(00)80084-2. [DOI] [PubMed] [Google Scholar]
- Zou E. Impacts of xenobiotics on crustacean molting: the invisible endocrine disruption. Integr. Comp. Biol. 2005;45:33–38. doi: 10.1093/icb/45.1.33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.