Abstract
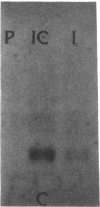
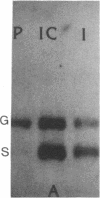
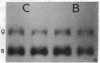
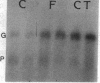
Expression of the gene encoding preproglucagon gives rise to different glucagon-related peptides in the pancreas and intestine. Glucagon gene expression is regulated by a protein kinase C-dependent pathway in rat islet cell lines, whereas activation of the adenylate cyclase pathway in islet cell lines is without effect. To elucidate the factors important for the control of proglucagon biosynthesis in the intestine, we have studied proglucagon gene expression and proglucagon biosynthesis in rat intestine. Analysis of intestinal cDNA clones encoding preproglucagon indicated that pancreatic and intestinal glucagon mRNA transcripts were identical. The regulation of proglucagon gene expression in rat intestine differed markedly from that previously observed in islet cell lines. Phorbol esters increased the secretion of glucagon-like immunoreactive peptides (GLI) but had no effect on proglucagon mRNA levels in rat intestinal cells. Bombesin also increased the secretion of GLI without affecting proglucagon mRNA levels or biosynthesis. In contrast, dibutyryl cyclic AMP, forskolin, and cholera toxin increased both proglucagon mRNA levels and GLI biosynthesis and secretion, suggesting that proglucagon gene expression in the intestine is regulated by a cyclic AMP-dependent pathway. These observations suggest that tissue-specific differences in both the regulation of proglucagon gene expression and the posttranslational processing of proglucagon contribute to the diversity of glucagon gene expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell G. I., Sanchez-Pescador R., Laybourn P. J., Najarian R. C. Exon duplication and divergence in the human preproglucagon gene. 1983 Jul 28-Aug 3Nature. 304(5924):368–371. doi: 10.1038/304368a0. [DOI] [PubMed] [Google Scholar]
- Brubaker P. L. Control of glucagon-like immunoreactive peptide secretion from fetal rat intestinal cultures. Endocrinology. 1988 Jul;123(1):220–226. doi: 10.1210/endo-123-1-220. [DOI] [PubMed] [Google Scholar]
- Brubaker P. L., Vranic M. Fetal rat intestinal cells in monolayer culture: a new in vitro system to study the glucagon-like immunoreactive peptides. Endocrinology. 1987 May;120(5):1976–1985. doi: 10.1210/endo-120-5-1976. [DOI] [PubMed] [Google Scholar]
- Buchan A. M., Barber D. L., Gregor M., Soll A. H. Morphologic and physiologic studies of canine ileal enteroglucagon-containing cells in short-term culture. Gastroenterology. 1987 Oct;93(4):791–800. doi: 10.1016/0016-5085(87)90442-2. [DOI] [PubMed] [Google Scholar]
- Buchan A. M., Polak J. M. The classification of the human gastroenteropancreatic endocrine cells. Invest Cell Pathol. 1980 Jan-Mar;3(1):51–71. [PubMed] [Google Scholar]
- Böttcher G., Alumets J., Håkanson R., Sundler F. Co-existence of glicentin and peptide YY in colorectal L-cells in cat and man. An electron microscopic study. Regul Pept. 1986 Feb;13(3-4):283–291. doi: 10.1016/0167-0115(86)90046-7. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Comb M., Birnberg N. C., Seasholtz A., Herbert E., Goodman H. M. A cyclic AMP- and phorbol ester-inducible DNA element. 1986 Sep 25-Oct 1Nature. 323(6086):353–356. doi: 10.1038/323353a0. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Deschenes R. J., Lorenz L. J., Haun R. S., Roos B. A., Collier K. J., Dixon J. E. Cloning and sequence analysis of a cDNA encoding rat preprocholecystokinin. Proc Natl Acad Sci U S A. 1984 Feb;81(3):726–730. doi: 10.1073/pnas.81.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch P. J., Hoeffler J. P., Jameson J. L., Habener J. F. Cyclic AMP and phorbol ester-stimulated transcription mediated by similar DNA elements that bind distinct proteins. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7922–7926. doi: 10.1073/pnas.85.21.7922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker D. J., Philippe J., Jepeal L., Habener J. F. Glucagon gene 5'-flanking sequences promote islet cell-specific gene transcription. J Biol Chem. 1987 Nov 15;262(32):15659–15665. [PubMed] [Google Scholar]
- Drucker D. J., Philippe J., Mojsov S., Chick W. L., Habener J. F. Glucagon-like peptide I stimulates insulin gene expression and increases cyclic AMP levels in a rat islet cell line. Proc Natl Acad Sci U S A. 1987 May;84(10):3434–3438. doi: 10.1073/pnas.84.10.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker D. J., Philippe J., Mojsov S. Proglucagon gene expression and posttranslational processing in a hamster islet cell line. Endocrinology. 1988 Oct;123(4):1861–1867. doi: 10.1210/endo-123-4-1861. [DOI] [PubMed] [Google Scholar]
- Efrat S., Teitelman G., Anwar M., Ruggiero D., Hanahan D. Glucagon gene regulatory region directs oncoprotein expression to neurons and pancreatic alpha cells. Neuron. 1988 Sep;1(7):605–613. doi: 10.1016/0896-6273(88)90110-9. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- George S. K., Uttenthal L. O., Ghiglione M., Bloom S. R. Molecular forms of glucagon-like peptides in man. FEBS Lett. 1985 Nov 18;192(2):275–278. doi: 10.1016/0014-5793(85)80124-1. [DOI] [PubMed] [Google Scholar]
- Heinrich G., Gros P., Habener J. F. Glucagon gene sequence. Four of six exons encode separate functional domains of rat pre-proglucagon. J Biol Chem. 1984 Nov 25;259(22):14082–14087. [PubMed] [Google Scholar]
- Heinrich G., Gros P., Lund P. K., Bentley R. C., Habener J. F. Pre-proglucagon messenger ribonucleic acid: nucleotide and encoded amino acid sequences of the rat pancreatic complementary deoxyribonucleic acid. Endocrinology. 1984 Dec;115(6):2176–2181. doi: 10.1210/endo-115-6-2176. [DOI] [PubMed] [Google Scholar]
- Herbert E., Uhler M. Biosynthesis of polyprotein precursors to regulatory peptides. Cell. 1982 Aug;30(1):1–2. doi: 10.1016/0092-8674(82)90002-2. [DOI] [PubMed] [Google Scholar]
- Hisatomi A., Maruyama H., Orci L., Vasko M., Unger R. H. Adrenergically mediated intrapancreatic control of the glucagon response to glucopenia in the isolated rat pancreas. J Clin Invest. 1985 Feb;75(2):420–426. doi: 10.1172/JCI111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst J. J. Gut glucagon, enteroglucagon, gut glucagonlike immunoreactivity, glicentin--current status. Gastroenterology. 1983 Jun;84(6):1602–1613. [PubMed] [Google Scholar]
- Holst J. J., Orskov C., Nielsen O. V., Schwartz T. W. Truncated glucagon-like peptide I, an insulin-releasing hormone from the distal gut. FEBS Lett. 1987 Jan 26;211(2):169–174. doi: 10.1016/0014-5793(87)81430-8. [DOI] [PubMed] [Google Scholar]
- Kreymann B., Williams G., Ghatei M. A., Bloom S. R. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987 Dec 5;2(8571):1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- Leiter A. B., Toder A., Wolfe H. J., Taylor I. L., Cooperman S., Mandel G., Goodman R. H. Peptide YY. Structure of the precursor and expression in exocrine pancreas. J Biol Chem. 1987 Sep 25;262(27):12984–12988. [PubMed] [Google Scholar]
- Lickley H. L., Kemmer F. W., Gray D. E., Kovacevic N., Hatton T. W., Perez G., Vranic M. Chromatographic pattern of extrapancreatic glucagon and glucagon-like immunoreactivity before and during stimulation by epinephrine and participation of glucagon in epinephrine-induced hepatic glucose overproduction. Surgery. 1981 Aug;90(2):186–194. [PubMed] [Google Scholar]
- Lopez L. C., Frazier M. L., Su C. J., Kumar A., Saunders G. F. Mammalian pancreatic preproglucagon contains three glucagon-related peptides. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5485–5489. doi: 10.1073/pnas.80.18.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojsov S., Heinrich G., Wilson I. B., Ravazzola M., Orci L., Habener J. F. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986 Sep 5;261(25):11880–11889. [PubMed] [Google Scholar]
- Mojsov S., Weir G. C., Habener J. F. Insulinotropin: glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest. 1987 Feb;79(2):616–619. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak U., Wilks A., Buell G., McEwen S. Identical mRNA for preproglucagon in pancreas and gut. Eur J Biochem. 1987 May 4;164(3):553–558. doi: 10.1111/j.1432-1033.1987.tb11162.x. [DOI] [PubMed] [Google Scholar]
- Orskov C., Holst J. J., Knuhtsen S., Baldissera F. G., Poulsen S. S., Nielsen O. V. Glucagon-like peptides GLP-1 and GLP-2, predicted products of the glucagon gene, are secreted separately from pig small intestine but not pancreas. Endocrinology. 1986 Oct;119(4):1467–1475. doi: 10.1210/endo-119-4-1467. [DOI] [PubMed] [Google Scholar]
- Philippe J., Drucker D. J., Chick W. L., Habener J. F. Transcriptional regulation of genes encoding insulin, glucagon, and angiotensinogen by sodium butyrate in a rat islet cell line. Mol Cell Biol. 1987 Jan;7(1):560–563. doi: 10.1128/mcb.7.1.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippe J., Drucker D. J., Habener J. F. Glucagon gene transcription in an islet cell line is regulated via a protein kinase C-activated pathway. J Biol Chem. 1987 Feb 5;262(4):1823–1828. [PubMed] [Google Scholar]
- Philippe J., Drucker D. J., Knepel W., Jepeal L., Misulovin Z., Habener J. F. Alpha-cell-specific expression of the glucagon gene is conferred to the glucagon promoter element by the interactions of DNA-binding proteins. Mol Cell Biol. 1988 Nov;8(11):4877–4888. doi: 10.1128/mcb.8.11.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Ruoff H. J. Rat gastric mucosal cAMP and cGMP after adrenergic stimulation and blockade. Eur J Pharmacol. 1977 Aug 15;44(4):349–354. doi: 10.1016/0014-2999(77)90309-0. [DOI] [PubMed] [Google Scholar]
- Sagor G. R., Ghatei M. A., O'Shaughnessy D. J., Al-Mukhtar M. Y., Wright N. A., Bloom S. R. Influence of somatostatin and bombesin on plasma enteroglucagon and cell proliferation after intestinal resection in the rat. Gut. 1985 Jan;26(1):89–94. doi: 10.1136/gut.26.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. W., Saunders G. F. Structure of the human glucagon gene. Nucleic Acids Res. 1986 Jun 25;14(12):4719–4730. doi: 10.1093/nar/14.12.4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynshaw-Boris A., Lugo T. G., Short J. M., Fournier R. E., Hanson R. W. Identification of a cAMP regulatory region in the gene for rat cytosolic phosphoenolpyruvate carboxykinase (GTP). Use of chimeric genes transfected into hepatoma cells. J Biol Chem. 1984 Oct 10;259(19):12161–12169. [PubMed] [Google Scholar]