Abstract
RNA pseudoknots are important for function. Three-dimensional structural information is available, insights into factors affecting pseudoknot stability are being reported, and computer programs are available for predicting pseudoknots.
Introduction and context
RNA pseudoknots are important for many functions [1-3]. Pseudoknots are formed by pairing between bases in a loop region and complementary bases outside the loop (Figure 1). That is, for any base pairs i-j and k-l, i<j, k<l, and i<k, there are cases in which i<k<j<l. A two-loop nomenclature was initially used, but 30.9% of pseudoknots now listed in PseudoBase [4] have an additional loop, so the three-loop nomenclature from Brierley et al. [1] is more generally applicable (Figure 1). Loop 2 typically has zero or one nucleotide, whereas loops 1 and 3 and the stems are more variable (Table 1).
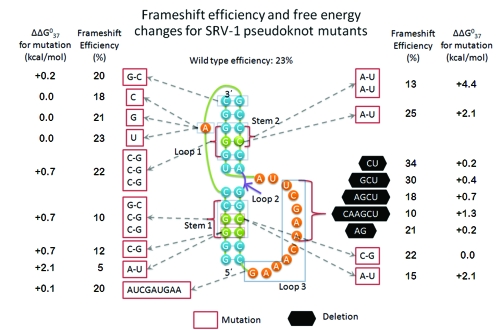
Figure 1. Simian retrovirus-1 (SRV-1) mutants’ frameshift efficiency [49-51] and their predicted free energy changes for the mutations.
A three-loop nomenclature is used for the pseudoknot. The free energy changes are predicted by coupling the individual nearest neighbor model [37] with a statistical polymer model [21,22], neglecting tertiary interactions.
Table 1. Three-dimensional structures of short pseudoknots.
| Type of Organism or RNA | RNA | Function | Method | PDB # | Ref. | Size, nts | Stem 1, bps | Loop 1, nts | Stem 2, bps | Loop 2, nts | Loop 3, nts | Coaxial stack or bend |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mammalian retrovirus | Ribosomal frameshifting | Coaxial stack | ||||||||||
| Simian retrovirus-1 (SRV-1) | NMR | 1E95 | [51] | 36 | 6 | 1 | 6 | 0 | 12 | |||
| Mouse mammary tumor virus (MMTV) | NMR | 1KPD | [56] | 32 | 5 | 2 | 6 | 1 | 8 | |||
| 1KAJ | [57] | |||||||||||
| 1RNK | [58] | |||||||||||
| Plant Luteoviridae viruses | Beet western yellow virus (BWYV) | X-ray | 437D | [5] | 26 | 5 | 2 | 3 | 1 | 7 | Bend | |
| 1L2X | [9] | |||||||||||
| Sugarcane yellow leaf virus (ScYLV) | NMR | 2AP0 | [8] | 28 | 5 | 2 | 3 | 1 | 9 | |||
| 1YG4 | [2] | |||||||||||
| Potato leaf roll virus (PLRV) | X-ray | 2A43 | [7] | 26 | 4 | 2 | 3 | 1 | 9 | |||
| Pea enation mosaic virus (PEMV) | NMR | 1KPY | [6] | 33 | 5 | 2 | 3 | 1 | 7 | |||
| Human | Telomerase RNA | Telomere maintenance | NMR | 1YMO | [3] | 46 | 6 | 8 | 9 | 0 | 8 | Slight bend |
| 2K96 | [43] | |||||||||||
| Plant virus (tymovirus) 3′ NCR | Turnip yellow mosaic virus (TYMV) | t-RNA like | NMR | 3PHP | [59] | 23 | 3 | 4 | 5 | 0 | 3 | Coaxial stack |
| 1A60 | [60] | |||||||||||
| Bacteria | Aquifex aeolicus tmRNA | Trans-translation | NMR | 2G1W | [61] | 21 | 4 | 1 | 3 | 1 | 5 | Bend |
bps, base pairs; NCR, non-coding region; NMR, nuclear magnetic resonance; nts, nucleotides; PDB, Protein Data Bank; Ref., reference.
Major recent advances
Folding of pseudoknots
Quite a few three-dimensional (3D) structures have been determined for isolated pseudoknots of fewer than 50 nucleotides (Table 1). Some tertiary interactions are conserved in particular classes and are essential for biological activity [2,3]. Examples include a quadruple-base interaction in pseudoknots from Luteoviridae viruses [2,5-9] and triplexes in telomerase RNA [3] and in viral mRNA that undergo -1 frameshifting [10]. Structures of natural pseudoknots bound to small molecules are being reported [11-13], and structures of larger RNAs are revealing long-range pseudoknots [14-20].
The stability of RNA pseudoknots is a key factor determining structure-function relationships [10] and is important for predicting RNA structure [21-32]. Thermodynamic measurements have started appearing [3,33-36].
A statistical polymer model for loops [21,22] coupled with the INN-HB (Individual Nearest Neighbor-Hydrogen Bonding) model for stems [37] allows estimates of the stability of small pseudoknots. Coaxial stacking of the two stems can be included, although this is not always observed in 3D structures [5]. Contributions from tertiary interactions between the loops and stems are neglected because little is known about their thermodynamics.
Mechanical unfolding of single molecules by optical tweezers [10,38,39] reveals that frameshifting efficiency is highly related to the mechanical stability of pseudoknots, as suggested from cryoelectron microscopy [40] and prediction by a statistical polymer model [41]. Single-molecule experiments also indicate that pseudoknot folding and unfolding at low forces are stepwise [42] and that the presence of Mg2+ stabilizes the pseudoknot more than hairpins [39]. A better understanding of pseudoknot thermodynamic and mechanical stability and of folding dynamics will help reveal structure-function relationships [43].
Finding pseudoknots
Finding pseudoknots by computationally folding RNA sequences is a difficult problem. Because of computational cost, most of the popular dynamic programming algorithms for predicting the lowest free energy structure do not allow pseudoknots. With state-of-the-art knowledge, finding the lowest free energy structure with pseudoknots takes an exponentially increasing amount of time as the sequence gets longer; that is, the problem is NP-complete [23,24].
To predict low free energy structures with pseudoknots, roughly four different practical approaches are used by available programs. The first approach is to apply stochastic methods either to simulate folding pathways or to sample structures [25,26,44]. With these algorithms, structures are revised according to an element of chance and new pairs that are pseudoknotted with existing pairs can be added. A variation on this theme follows a folding pathway to find low free energy structures but is deterministic in its choices of stems [27]. The second approach is to use a dynamic programming algorithm in which the possible topologies of the predicted structures are limited [28-30]. The possible topologies predicted by a number of different programs have been examined [31]. A third approach is to assemble structures from component base pairs using a graph-theoretic approach [45,46]. A fourth approach is to iteratively build structures using algorithms that cannot predict pseudoknots with a single iteration [47,48]. The Nuclear magnetic resonance (NMR)-Assisted Prediction of RNA Secondary Structure (NAPSS) algorithm is an iterative approach that includes constraints from simple NMR experiments to improve predictions [32].
A number of the programs cited above either require multiple homologous sequences or are capable of using them to find a conserved structure [44-46,48]. These programs should be more accurate at structure prediction than those that use a single sequence because of the additional information available in the multiple sequences. For example, pairs that are not conserved in the set of sequences can be excluded from the final structure.
A recent benchmark of the accuracy of structure prediction using single sequences provides guidance for choosing a program [47]; Table 2 lists programs available for free. For 12 RNA sequences of 210 nucleotides or longer, a pathway folding algorithm [25] had the highest accuracy. The iterative approaches also performed similarly and were time-efficient [47,48].
Table 2. Available programs for secondary structure prediction including pseudoknots.
| Program name | Website |
|---|---|
| HotKnots [47] | http://www.cs.ubc.ca/labs/beta/Software/HotKnots/ |
| hxmatch [46] | http://www.tbi.univie.ac.at/papers/SUPPLEMENTS/HXMATCH/ |
| ILM [48] | http://cs.utsa.edu/~jruan/Software.html |
| KineFold [26] | http://kinefold.curie.fr/ |
| pknotsRG [29] | http://bibiserv.techfak.uni-bielefeld.de/pknotsrg/ |
| SimulFold [44] | http://people.cs.ubc.ca/~irmtraud/simulfold/ |
| vsfold5 [27] | http://www.rna.it-chiba.ac.jp/~vsfold/vsfold5/ |
This table provides a list of programs that are available for free and can make predictions on a desktop computer for sequences that are up to at least 200 nucleotides long. Note that hxmatch is designed to find conserved structures for sequences in a multiple alignment and that ILM can be run with one sequence or with an alignment of multiple sequences. SimulFold works on a set of homologous sequences and infers the sequence alignment. KineFold and vsfold5 are available as web servers. pknotsRG is available for use on a web server or for download.
Future directions
Predictions of the occurrence and stabilities of pseudoknots can be improved. The best-performing program tested by Ren et al. [47] predicted only 57% of known canonical base pairs, and only 39% of the predicted pairs were in the known structures. New insights into the structures and stabilities will suggest computational simplifications for existing strategies and improve approximations of stabilities. New methods to find pseudoknots conserved in multiple sequences and to incorporate more experimental data will restrain folding space. Finding more pseudoknots will likely expand the types of functions known for pseudoknots. Increased understanding of the sequence dependence of pseudoknot stabilities can improve comparisons with functional studies. For example, the relative frameshifting efficiencies of simian retrovirus-1 (SRV-1) pseudoknot and its mutants [49,50] qualitatively correlate with their predicted relative stabilities (Figure 1, revised from [51]). The exception is mutant U11A34, which is predicted to be less stable than the wild-type pseudoknot but shows higher efficiency of frameshifting. This could result from an additional tertiary interaction if a U11A34/A7 base triple is formed. Such comparisons can suggest new experiments.
Future work can also resolve current ambiguities. For example, there is disagreement about the role of pseudoknot stability in human telomerase RNA. The pseudoknot domain may act as a molecular switch [52], in which the pseudoknot and the stem 1 hairpin have nearly equal stability [34], or only the pseudoknot conformation may be important for function [53]. A two-base mutation destabilizing the human telomerase pseudoknot is found in some patients with the inherited disease dyskeratosis congenita [54]. A theoretical calculation suggests that folding kinetics of the pseudoknot may determine activity [55]. There is much more to be revealed about the roles of pseudoknots and their modus operandi.
Acknowledgments
This work was supported by National Institutes of Health grants GM22939 (DHT) and R01GM076485 (DHM).
Abbreviations
- 3D
three-dimensional
- INN-HB
individual nearest neighbor-hydrogen bonding
- NAPSS
nuclear magnetic resonance (NMR)-assisted prediction of RNA secondary structure
- NMR
nuclear magnetic resonance
- SRV-1
simian retrovirus-1
Competing interests
The authors declare that they have no competing interests.
The electronic version of this article is the complete one and can be found at:http://f1000.com/reports/b/2/8
References
- 1.Brierley I, Pennell S, Gilbert RJ. Viral RNA pseudoknots: versatile motifs in gene expression and replication. Nat Rev Microbiol. 2007;5:598–610. doi: 10.1038/nrmicro1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornish PV, Hennig M, Giedroc DP. A loop 2 cytidine-stem 1 minor groove interaction as a positive determinant for pseudoknot-stimulated -1 ribosomal frameshifting. Proc Natl Acad Sci U S A. 2005;102:12694–9. doi: 10.1073/pnas.0506166102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Theimer CA, Blois CA, Feigon J. Structure of the human telomerase RNA pseudoknot reveals conserved tertiary interactions essential for function. Mol Cell. 2005;17:671–82. doi: 10.1016/j.molcel.2005.01.017. [DOI] [PubMed] [Google Scholar]; f1000 Factor 6.0 Must ReadEvaluated by Samuel Butcher 16 Mar 2005
- 4.Taufer M, Licon A, Araiza R, Mireles D, van Batenburg FH, Gultyaev AP, Leung MY. PseudoBase++: an extension of PseudoBase for easy searching, formatting and visualization of pseudoknots. Nucleic Acids Res. 2009;37:D127–35. doi: 10.1093/nar/gkn806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su L, Chen L, Egli M, Berger JM, Rich A. Minor groove RNA triplex in the crystal structure of a ribosomal frameshifting viral pseudoknot. Nat Struct Biol. 1999;6:285–92. doi: 10.1038/6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nixon PL, Rangan A, Kim YG, Rich A, Hoffman DW, Hennig M, Giedroc DP. Solution structure of a luteoviral P1-P2 frameshifting mRNA pseudoknot. J Mol Biol. 2002;322:621–33. doi: 10.1016/S0022-2836(02)00779-9. [DOI] [PubMed] [Google Scholar]
- 7.Pallan PS, Marshall WS, Harp J, Jewett FC 3rd, Wawrzak Z, Brown BA, 2nd, Rich A, Egli M. Crystal structure of a luteoviral RNA pseudoknot and model for a minimal ribosomal frameshifting motif. Biochemistry. 2005;44:11315–22. doi: 10.1021/bi051061i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornish PV, Stammler SN, Giedroc DP. The global structures of a wild-type and poorly functional plant luteoviral mRNA pseudoknot are essentially identical. RNA. 2006;12:1959–69. doi: 10.1261/rna.199006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egli M, Minasov G, Su L, Rich A. Metal ions and flexibility in a viral RNA pseudoknot at atomic resolution. Proc Natl Acad Sci U S A. 2002;99:4302–7. doi: 10.1073/pnas.062055599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen G, Chang KY, Chou MY, Bustamante C, Tinoco I., Jr Triplex structures in an RNA pseudoknot enhance mechanical stability and increase efficiency of -1 ribosomal frameshifting. Proc Natl Acad Sci U S A. 2009;106:12706–11. doi: 10.1073/pnas.0905046106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert SD, Rambo RP, Van Tyne D, Batey RT. Structure of the SAM-II riboswitch bound to S-adenosylmethionine. Nat Struct Mol Biol. 2008;15:177–82. doi: 10.1038/nsmb.1371. [DOI] [PubMed] [Google Scholar]; f1000 Factor 3.0 RecommendedEvaluated by Kathleen Hall 22 Feb 2008
- 12.Klein DJ, Edwards TE, Ferre-D’Amare AR. Cocrystal structure of a class I preQ1 riboswitch reveals a pseudoknot recognizing an essential hypermodified nucleobase. Nat Struct Mol Biol. 2009;16:343–4. doi: 10.1038/nsmb.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spitale RC, Torelli AT, Krucinska J, Bandarian V, Wedekind JE. The structural basis for recognition of the PreQ0 metabolite by an unusually small riboswitch aptamer domain. J Biol Chem. 2009;284:11012–6. doi: 10.1074/jbc.C900024200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein DJ, Ferre-D’Amare AR. Structural basis of glmS ribozyme activation by glucosamine-6-phosphate. Science. 2006;313:1752–6. doi: 10.1126/science.1129666. [DOI] [PubMed] [Google Scholar]; f1000 Factor 10.0 ExceptionalEvaluated by Robert Batey 13 Oct 2006, Günter Mayer 16 Oct 2006, David Waugh 24 Oct 2006, Barry Stoddard 30 Nov 2006
- 15.Toor N, Keating KS, Taylor SD, Pyle AM. Crystal structure of a self-spliced group II intron. Science. 2008;320:77–82. doi: 10.1126/science.1153803. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000 Factor 8.0 ExceptionalEvaluated by Robert Batey 08 Apr 2008, Douglas Turner 14 Jul 2008
- 16.Adams PL, Stahley MR, Kosek AB, Wang J, Strobel SA. Crystal structure of a self-splicing group I intron with both exons. Nature. 2004;430:45–50. doi: 10.1038/nature02642. [DOI] [PubMed] [Google Scholar]; f1000 Factor 9.6 ExceptionalEvaluated by Douglas Turner 09 Jul 2004, David MJ Lilley 12 Jul 2004
- 17.Golden BL, Kim H, Chase E. Crystal structure of a phage Twort group I ribozyme-product complex. Nat Struct Mol Biol. 2005;12:82–9. doi: 10.1038/nsmb868. [DOI] [PubMed] [Google Scholar]
- 18.Pfingsten JS, Costantino DA, Kieft JS. Structural basis for ribosome recruitment and manipulation by a viral IRES RNA. Science. 2006;314:1450–4. doi: 10.1126/science.1133281. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000 Factor 6.5 Must ReadEvaluated by Robert Batey 01 Dec 2006, Douglas Turner 05 Jan 2007, Daniel Gallie 19 Jan 2007
- 19.Ferre-D’Amare AR, Zhou K, Doudna JA. Crystal structure of a hepatitis delta virus ribozyme. Nature. 1998;395:567–74. doi: 10.1038/26912. [DOI] [PubMed] [Google Scholar]
- 20.Guo F, Gooding AR, Cech TR. Structure of the Tetrahymena ribozyme: base triple sandwich and metal ion at the active site. Mol Cell. 2004;16:351–62. doi: 10.1016/j.molcel.2004.10.003. [DOI] [PubMed] [Google Scholar]; f1000 Factor 6.0 Must ReadEvaluated by John Burke 06 Dec 2004
- 21.Cao S, Chen SJ. Predicting RNA pseudoknot folding thermodynamics. Nucleic Acids Res. 2006;34:2634–52. doi: 10.1093/nar/gkl346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao S, Chen SJ. Predicting structures and stabilities for H-type pseudoknots with interhelix loops. RNA. 2009;15:696–706. doi: 10.1261/rna.1429009. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000 Factor 3.2 RecommendedEvaluated by Niles Lehman 05 Jun 2009, Douglas Turner 24 Jun 2009
- 23.Akutsu T. Dynamic programming algorithms for RNA secondary structure prediction with pseudoknots. Discrete Appl Math. 2000;104:45–62. doi: 10.1016/S0166-218X(00)00186-4. [DOI] [Google Scholar]
- 24.Lyngso RB, Pedersen CN. RNA pseudoknot prediction in energy-based models. J Comput Biol. 2000;7:409–27. doi: 10.1089/106652700750050862. [DOI] [PubMed] [Google Scholar]
- 25.van Batenburg FH, Gultyaev AP, Pleij CW. An APL-programmed genetic algorithm for the prediction of RNA secondary structure. J Theor Biol. 1995;174:269–80. doi: 10.1006/jtbi.1995.0098. [DOI] [PubMed] [Google Scholar]
- 26.Isambert H, Siggia ED. Modeling RNA folding paths with pseudoknots: application to hepatitis delta virus ribozyme. Proc Natl Acad Sci U S A. 2000;97:6515–20. doi: 10.1073/pnas.110533697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dawson WK, Fujiwara K, Kawai G. Prediction of RNA pseudoknots using heuristic modeling with mapping and sequential folding. PLoS One. 2007;2:e905. doi: 10.1371/journal.pone.0000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rivas E, Eddy SR. A dynamic programming algorithm for RNA structure prediction including pseudoknots. J Mol Biol. 1999;285:2053–68. doi: 10.1006/jmbi.1998.2436. [DOI] [PubMed] [Google Scholar]
- 29.Reeder J, Giegerich R. Design, implementation and evaluation of a practical pseudoknot folding algorithm based on thermodynamics. BMC Bioinformatics. 2004;5:104. doi: 10.1186/1471-2105-5-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dirks RM, Pierce NA. A partition function algorithm for nucleic acid secondary structure including pseudoknots. J Comput Chem. 2003;24:1664–77. doi: 10.1002/jcc.10296. [DOI] [PubMed] [Google Scholar]
- 31.Condon A, Davy B, Rastegari B, Zhao S, Tarrant F. Classifying RNA pseudoknotted structures. Theor Comput Sci. 2004;320:35–50. doi: 10.1016/j.tcs.2004.03.042. [DOI] [Google Scholar]
- 32.Hart JM, Kennedy SD, Mathews DH, Turner DH. NMR-assisted prediction of RNA secondary structure: identification of a probable pseudoknot in the coding region of an R2 retrotransposon. J Am Chem Soc. 2008;130:10233–9. doi: 10.1021/ja8026696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nixon PL, Giedroc DP. Energetics of a strongly pH dependent RNA tertiary structure in a frameshifting pseudoknot. J Mol Biol. 2000;296:659–71. doi: 10.1006/jmbi.1999.3464. [DOI] [PubMed] [Google Scholar]
- 34.Theimer CA, Finger LD, Trantirek L, Feigon J. Mutations linked to dyskeratosis congenita cause changes in the structural equilibrium in telomerase RNA. Proc Natl Acad Sci U S A. 2003;100:449–54. doi: 10.1073/pnas.242720799. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000 Factor 6.4 Must ReadEvaluated by Samuel Butcher 24 Jan 2003, Douglas Turner 31 Jul 2003
- 35.Soto AM, Misra V, Draper DE. Tertiary structure of an RNA pseudoknot is stabilized by “diffuse” Mg2+ ions. Biochemistry. 2007;46:2973–83. doi: 10.1021/bi0616753. [DOI] [PubMed] [Google Scholar]
- 36.Liu B, Shankar N, Turner DH. Fluorescence competition assay measurements of free energy changes for RNA pseudoknots. Biochemistry. 2009 doi: 10.1021/bi901541j. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia T, SantaLucia J Jr., Burkard ME, Kierzek R, Schroeder SJ, Jiao X, Cox C, Turner DH. Thermodynamic parameters for an expanded nearest-neighbor model for formation of RNA duplexes with Watson-Crick base pairs. Biochemistry. 1998;37:14719–35. doi: 10.1021/bi9809425. [DOI] [PubMed] [Google Scholar]
- 38.Hansen TM, Reihani SN, Oddershede LB, Sorensen MA. Correlation between mechanical strength of messenger RNA pseudoknots and ribosomal frameshifting. Proc Natl Acad Sci U S A. 2007;104:5830–5. doi: 10.1073/pnas.0608668104. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000 Factor 6.0 Must ReadEvaluated by P Shing Ho 05 Apr 2007
- 39.Green L, Kim CH, Bustamante C, Tinoco I., Jr Characterization of the mechanical unfolding of RNA pseudoknots. J Mol Biol. 2008;375:511–28. doi: 10.1016/j.jmb.2007.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Namy O, Moran SJ, Stuart DI, Gilbert RJ, Brierley I. A mechanical explanation of RNA pseudoknot function in programmed ribosomal frameshifting. Nature. 2006;441:244–7. doi: 10.1038/nature04735. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000 Factor 6.0 Must ReadEvaluated by Samuel Butcher 17 May 2006
- 41.Cao S, Chen SJ. Predicting ribosomal frameshifting efficiency. Phys Biol. 2008;5:16002. doi: 10.1088/1478-3975/5/1/016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen G, Wen JD, Tinoco I. Single-molecule mechanical unfolding and folding of a pseudoknot in human telomerase RNA. RNA. 2007;13:2175–88. doi: 10.1261/rna.676707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim NK, Zhang Q, Zhou J, Theimer CA, Peterson RD, Feigon J. Solution structure and dynamics of the wild-type pseudoknot of human telomerase RNA. J Mol Biol. 2008;384:1249–61. doi: 10.1016/j.jmb.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer IM, Miklos I. SimulFold: Simultaneously inferring RNA structures including pseudoknots, alignments, and trees using a Bayesian MCMC framework. PLoS Comput Biol. 2007;3:1441–54. doi: 10.1371/journal.pcbi.0030149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tabaska JE, Cary RB, Gabow HN, Stormo GD. An RNA folding method capable of identifying pseudoknots and base triples. Bioinformatics. 1998;14:691–9. doi: 10.1093/bioinformatics/14.8.691. [DOI] [PubMed] [Google Scholar]
- 46.Witwer C, Hofacker IL, Stadler PF. Prediction of consensus RNA secondary structures including pseudoknots. IEEE/ACM Trans Comput Biol Bioinform. 2004;1:66–77. doi: 10.1109/TCBB.2004.22. [DOI] [PubMed] [Google Scholar]
- 47.Ren J, Rastegari B, Condon A, Hoos HH. HotKnots: heuristic prediction of RNA secondary structures including pseudoknots. RNA. 2005;11:1494–504. doi: 10.1261/rna.7284905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ruan J, Stormo GD, Zhang W. An iterated loop matching approach to the prediction of RNA secondary structures with pseudoknots. Bioinformatics. 2004;20:58–66. doi: 10.1093/bioinformatics/btg373. [DOI] [PubMed] [Google Scholar]
- 49.ten Dam E, Brierley I, Inglis S, Pleij C. Identification and analysis of the pseudoknot-containing gag-pro ribosomal frameshift signal of simian retrovirus-1. Nucleic Acids Res. 1994;22:2304–10. doi: 10.1093/nar/22.12.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ten Dam EB, Verlaan PW, Pleij CW. Analysis of the role of the pseudoknot component in the SRV-1 gag-pro ribosomal frameshift signal: loop lengths and stability of the stem regions. RNA. 1995;1:146–54. [PMC free article] [PubMed] [Google Scholar]
- 51.Michiels PJ, Versleijen AA, Verlaan PW, Pleij CW, Hilbers CW, Heus HA. Solution structure of the pseudoknot of SRV-1 RNA, involved in ribosomal frameshifting. J Mol Biol. 2001;310:1109–23. doi: 10.1006/jmbi.2001.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Comolli LR, Smirnov I, Xu L, Blackburn EH, James TL. A molecular switch underlies a human telomerase disease. Proc Natl Acad Sci U S A. 2002;99:16998–7003. doi: 10.1073/pnas.262663599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen JL, Greider CW. Functional analysis of the pseudoknot structure in human telomerase RNA. Proc Natl Acad Sci U S A. 2005;102:8080–5. doi: 10.1073/pnas.0502259102. discussion 8077-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen JL, Greider CW. Telomerase RNA structure and function: implications for dyskeratosis congenita. Trends Biochem Sci. 2004;29:183–92. doi: 10.1016/j.tibs.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 55.Cao S, Chen SJ. Biphasic folding kinetics of RNA pseudoknots and telomerase RNA activity. J Mol Biol. 2007;367:909–24. doi: 10.1016/j.jmb.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kang H, Tinoco I., Jr A mutant RNA pseudoknot that promotes ribosomal frameshifting in mouse mammary tumor virus. Nucleic Acids Res. 1997;25:1943–9. doi: 10.1093/nar/25.10.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kang H, Hines JV, Tinoco I., Jr Conformation of a non-frameshifting RNA pseudoknot from mouse mammary tumor virus. J Mol Biol. 1996;259:135–47. doi: 10.1006/jmbi.1996.0308. [DOI] [PubMed] [Google Scholar]
- 58.Shen LX, Tinoco I., Jr The structure of an RNA pseudoknot that causes efficient frameshifting in mouse mammary tumor virus. J Mol Biol. 1995;247:963–78. doi: 10.1006/jmbi.1995.0193. [DOI] [PubMed] [Google Scholar]
- 59.Kolk MH, van der Graaf M, Fransen CT, Wijmenga SS, Pleij CW, Heus HA, Hilbers CW. Structure of the 3′-hairpin of the TYMV pseudoknot: preformation in RNA folding. EMBO J. 1998;17:7498–504. doi: 10.1093/emboj/17.24.7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kolk MH, van der Graaf M, Wijmenga SS, Pleij CW, Heus HA, Hilbers CW. NMR structure of a classical pseudoknot: interplay of single- and double-stranded RNA. Science. 1998;280:434–8. doi: 10.1126/science.280.5362.434. [DOI] [PubMed] [Google Scholar]
- 61.Nonin-Lecomte S, Felden B, Dardel F. NMR structure of the Aquifex aeolicus tmRNA pseudoknot PK1: new insights into the recoding event of the ribosomal trans-translation. Nucleic Acids Res. 2006;34:1847–53. doi: 10.1093/nar/gkl111. [DOI] [PMC free article] [PubMed] [Google Scholar]