Abstract
We have characterized the relative efficacies of a number of protein crosslinking agents that have the potential for use in the crosslinking of proteinaceous matrices both in vitro and in vivo. The crosslinkers tested were; l-threose (LT), Genipin (GP), Methylglyoxal (MG), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), proanthrocyanidin (PA) and glutaraldehyde (GA). The relative effectiveness of the crosslinkers with regard to their saturating concentrations was: GA > PA > EDC > MG = GP >> LT. Most of the crosslinkers displayed a pH dependence and were more effective at more alkaline pH. At optimal pH and saturating conditions, the relative reaction rates of the crosslinkers were: PA = GA > EDC > GP > MG >> LT.
1 Introduction
Protein crosslinking has been used in a number of ways to modify the biological and mechanical properties of biomaterials. It has been extensively utilized in vitro to modulate the mechanical properties of various implants [1–9]. Another benefit of such crosslinking is the stabilization of collagenous matrices prior to implantation, rendering them less susceptible to enzymatic degradation in vivo [10–13]. One concern in this area has been the retention of potentially toxic crosslinking reagents such as glutaraldehyde within the implant which might be eluted in vivo [14]. The identification of sufficiently efficacious yet less toxic crosslinking reagents would help to ameliorate this concern.
The approach being taken in our laboratory is to alter the mechanical properties of load-bearing structures by crosslinking native collagenous tissues in vivo by direct injection of crosslinking agents. Degenerative disc disease (DDD) is a chronic pathology of the spinal disc resulting in the gradual deterioration of this largely avascular, immunoisolated tissue [15, 16]. As the tissue degrades its mechanical properties alter to the point where it is unable to withstand the stresses and strains of physiological mechanical loading [17], resulting in bulging, tearing and eventual rupture. DDD provides an ideal candidate indication for the use of protein crosslinking to re-engineer this tissue in vivo and reverse the adverse effects associated with tissue degeneration since it has been shown that crosslinking of the annulus fibrosis has multiple beneficial effects on the mechanical properties of the disc [18–21].
Many molecules have been utilized in the above in vitro applications and our in vivo approach and while some information regarding their reaction kinetics is available [11, 22, 23], little has been reported with regard to their relative reaction requirements or reactivity. In this paper, we report the results of our biochemical analysis of the kinetics of a number of potentially useful crosslinking agents. The crosslinkers studied were; l-Threose (LT), Genipin (GP), Methylglyoxal (MG), 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and proanthrocyanidin (PA). The activities of these reagents were compared to a commonly used and potent, yet toxic, crosslinker, glutaraldehyde (GA).
GA is a potent protein crosslinker that has been commonly used in the field of tissue engineering [1, 4, 5], but its acute toxicity has always been a concern [6, 14], as has the propensity of GA-treated implants to promote calcification in vivo [24]. EDC is a highly efficient “zero-length” crosslinker which is highly efficient at directly crosslinking protein-bound amines and carboxyls on residues such as lysine and aspartate [25], and has been tested as a potential crosslinker of biological implants [8]. PA, a polyphenol that is particularly abundant in grape seed extracts has been shown to be over 120-times less toxic than GA and yet can still be highly effective at stabilizing collagen matrices [11]. LT is a 4-carbon sugar and an optical isomer of the naturally occurring d-threose (DT), which is involved in the formation of advanced glycation end-products (AGEs) [26], carbohydrate-induced crosslinks that accumulate in vivo during chronological aging and which are particularly problematic in individuals suffering from diabetes. Crosslinking of collagen in vitro using DT has been shown to increase stiffness [27]. We chose instead to test LT, rationalizing that it would be more stable than DT in vivo, since the l-isomer should not be susceptible to catabolism. MG is an aldehyde form of pyruvic acid and is also involved in AGE formation [28, 29], downstream of DT, and has been shown to crosslink proteins in vitro [30–32]. GP is the aglycone of the iridoid glycoside, geniposide, a major constituent of the fruit of Gardenia jasminoides. GP has been extensively studied with regard to its ability to crosslink proteins [13, 33], and its effects on the mechanical properties of various tissues such as the pericardium [34], aortic valves [35] and the spinal disc [21, 35, 36], as well as its potential use for the formation of cellular scaffolds for tissue engineering [2] have been widely documented, as has its superior safety profile [37, 38].
We utilized the fact that crosslinking of proteinaceous matrices results in an increasing resistance to proteolysis due to both the masking of potential cleavage sites and to the increased number of scissions required to release soluble peptide fragments. We measured the relative crosslinking efficiencies of crosslinking reagents under various conditions by monitoring the amount of hydroxyproline (an amino acid that is present predominantly only in extracellular matrix proteins and is particularly abundant in collagen) into solution following digestion of tissue treated with Clostridium histolyticum collagenase.
The reaction kinetics of the crosslinkers were analyzed with respect to their concentration and pH dependence as well as their reaction rates under optimal conditions.
2 Materials and methods
Genipin was purchased from Challenge Bioproducts Co., Ltd. (Taiwan) and proanthrocyanidin was purchased from Polyphenolics (Madera, CA). Note that proanthrocyanidin consists of a mixture of varied-length polymers of epicatechin. In order to calculate the molarity of a solution of PA, we used the molecular weight of the epicatechin monomer (290.27). Thus references to the concentration of PA solutions refer to the total monomer content and not necessarily to the concentration of any given polymeric species. All other reagents were purchased from Sigma.
2.1 Tissue homogenization
Three to four bovine lumbar discs (approximately 3–5 g each) were excised from 4 to 6 month old bovine lumbar spines and cut into 1–2 mm2 using a kitchen knife. The annulus tissue was homogenized in batches in a 50 ml Falcon tube in distilled water pre-chilled to 4°C using a homogenizer, run at maximum speed and fitted with a 10 mm stainless steel, saw toothed generator probe. Care was taken to ensure that the temperature of the suspension did not exceed 25°C. After each 1–2 min pulse large particles were allowed to settle by gravity and the suspension carefully decanted into a fresh tube, avoiding the inclusion of any large particles. Tissue was harvested by centrifugation at 4500 rpm for 5 min. The supernatant was decanted and the pellets stored at –20°C until needed.
2.2 Tissue crosslinking
Approximately 20–30 mg aliquots of homogenized annulus tissue were accurately weighed into individual 1.5 ml Eppendorf tubes and the weights noted. Crosslinking agent in buffer (usually 0.5 ml) was added to each sample as required for the experiment. In most cases one sample was treated in buffer alone as a negative control. Samples were then incubated at 37°C while shaking at 1500 rpm for the time required for the experiment
Samples were harvested by centrifugation at 10,000 rpm in a bench-top microfuge for 2 min. The supernatants were removed, the pellets washed with 1 ml of H2O, re-centrifuged and the wash solution discarded. Samples were then treated at 37°C while shaking as described above following addition of 0.5 ml of a 1 mg/ml solution of type I collagenase from Clostridium histolyticum in Collagenase Buffer (100 mM Tris pH7.5, 10 mM CaCl2). The incubation time varied depending on the amount of tissue in the sample and the efficacy of the crosslinker, but never exceeded the amount required for complete dissolution of any of the samples. The incubation time was approximately 20 min since even untreated tissue was not completely solubilized under these conditions. Samples were centrifuged as above and the supernatants removed for analysis of hydroxyproline content.
The buffer chosen for crosslinker titration was phosphate buffered saline (PBS; 10 mM sodium phosphate, pH 7.4, 137 mM NaCl, 2.7 mM KCl). The only exception to this was EDC, where the buffer used was 100 mM MES, pH 6. This buffer was selected based on previous precedents of its use with this reagent [25].
The buffers used for pH titration were selected to lack amines, since these could also potentially react with, and therefore quench, the crosslinkers being tested. Although sodium acetate contains a carboxyl group, we were unable to identify a buffer lacking such a moiety that was capable of buffering at this pH. The buffers chosen were: sodium acetate (pH 4), sodium cacodylate (pH 5), MES (pH 6), MOPS (pH 7), EPPS (pH 8) and sodium borate (pH 9) and the crosslinker concentrations were: 0.7 mM (EDC), 10 mM (MG), 12,5 mM (LT), 0.1 mM (GA), 0.44 mM (GP) and 0.34 mM (PA).
In the case of time-course studies, reactions were conducted in 100 mM EPPS buffer, pH 8, except for EDC where 100 mM MES, pH 6, was used. Crosslinker concentrations used were: 3.5 mM (PA), 8.8 mM (GP), 2 mM (GA), 50 mM (LT), 5 mM (EDC) and 10 mM MG.
In the case of concentration and pH titrations and the time course studies, two experiments were conducted for each crosslinker and the average signal at each point used to report the data.
2.3 Hydroxyproline assay
Hydroxyproline content was determined by an adaptation of several previously described methods [39–43]. A 50 μl aliquot of each collagenase digest was placed into a 2 ml conical bottom screw cap tube fitted with an ethylene propylene rubber gasket. In addition 50 μl of collagenase solution was also placed into a separate tube to act as a background correction sample. Fifty μl of 12 N HCl was added to each sample and then incubated for 1 h at 120°C to hydrolyze the protein. Following hydrolysis, tube caps were removed and the samples evaporated to dryness by further incubation at 120°C for approximately 1 h. Dried samples were dissolved in 0.4 ml of OP buffer. This buffer consisted of 30.4 g of citric acid, 80 g sodium acetate trihydrate, 22.75 g of NaOH, 8 ml glacial acetic acid and 200 ml of n-propanol adjusted to pH6.3 with NaOH in a final volume of 1 liter.
Fifty μl duplicate samples of each hydrolysate were transferred to fresh tubes and 50 μl of H2O was used as a blank for the spectrophotometer followed by addition of 350 μl of OP buffer. One hundred μl of 83.4 mM Chloramine T was then added and the samples incubated at room temperature for 25 min. Next, 0.5 ml of Ehrlich's Solution (0.5 M 4-(dimethylamino) benzaldehyde, 9.1% (v/v) perchloric acid, 30% (v/v) n-propanol) was added and the samples incubated at 60°C for 20 min. Absorbance was measured at 550 nm. The water control was used to blank the machine and the absorbance of the collagenase control was subtracted from all of the sample readings. Absorbances were normalized for variations in tissue weight by dividing by the mass of the individual sample and then further to the signal from the untreated control sample.
3 Results
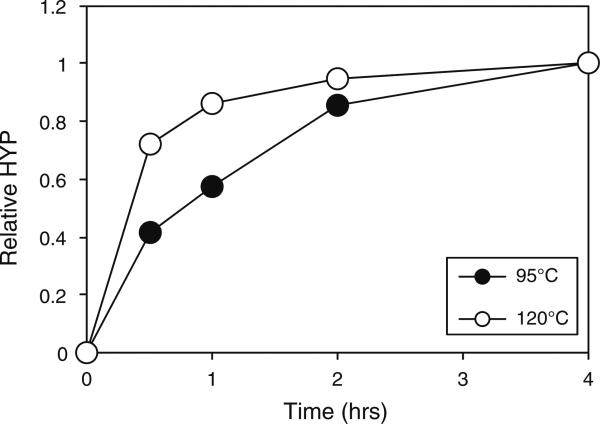
In order to determine the hydroxyproline content of a protein sample, it is first necessary to hydrolyze the proteins to their constituent amino acids. Assay of intact proteins and peptides yields very low signals (data not shown). Total HCl hydrolysis of proteins is usually conducted overnight at 110°C [44]. Such an incubation would greatly reduce the throughput of the hydroxyproline assay and thus, in order to reduce the time taken for the analysis, we sought to determine the extent of hydrolysis that occurred in collagenase-treated annulus samples over time. A sample of annulus tissue was hydrolyzed with collagenase and then aliquots of the supernatant subjected to HCl hydrolysis for varying times at either 95°C or 120°C. Hydrolysis at 95°C was less efficient than that at 120°C (Fig. 1). At 120°C, hydrolysis appeared to be almost complete after 4 h as evidenced by the plateau of the curve. Based on this, hydrolysis was 85% complete after 1 h at 120°C and so, as a compromise between time and signal development, these conditions were selected for future experiments.
Fig. 1.
Effect of different HCl hydrolysis times and temperatures on hydroxyproline detection. A sample of annulus tissue was digested with collagenase and then aliquots of the supernatant subjected to HCl hydrolysis for 0.5, 1, 2 or 4 h at either 95°C or 120°C. Samples were analyzed for hydroxyproline content and the results normalized to the signal obtained after 4 h
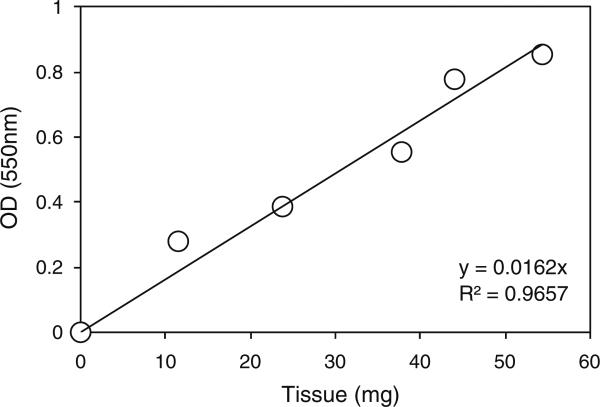
To minimize the differential effects of crosslinker diffusion between samples due to surface-to-area differences and inter-sample heterogeneity we chose to conduct our study on homogenized samples instead of intact biopsies. Due to the difficulty of weighing out exactly equal amounts of such tissue for each sample in a given experiment, we decided to weigh approximately equal amounts and then normalize the results from the experiment to the weight of each sample. This approach assumed that the ratio of tissue to collagenase solution did not affect the efficiency of digestion. In order to confirm that this assumption was valid, we digested increasing amounts of annulus tissue in a constant volume of collagenase solution. This showed that the hydroxyproline signal in these samples displayed a linear relationship to the amount of tissue in the digest (Fig. 2).
Fig. 2.
Effect tissue mass on hydroxyproline signal. Samples of annulus tissue weighing approximately 10, 20, 30, 40 or 50 mg were digested in a constant (0.5 ml) volume of collagenase solution for 20 min at 37°C and then subjected to hydroxyproline analysis
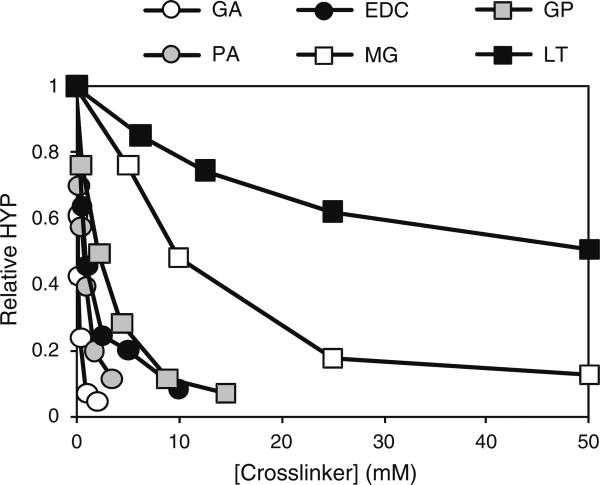
In order to obtain the relative efficacies of the various crosslinking reagents, annulus tissue was treated with increasing concentrations of each crosslinker. Tissue samples were incubated with increasing concentrations of crosslinker and the extent of crosslinking determined by quantifying the amount of HYP released by each sample following digestion with collagenase. The greater the amount of crosslinking in the sample, the less HYP should be released, resulting in a lower signal and allowing us to indirectly measure the extent of crosslinking. The results from these experiments are summarized in Fig. 3 and the approximate saturating concentrations of these reagents under these conditions are summarized in Table 1.
Fig. 3.
Concentration dependence of protein crosslinking reagents. Increasing concentrations of protein crosslinkers were added to homogenized annulus tissue and incubated for either 1 h (EDC, GP, MG, PA and GA) or 6 h (DT and LT). Crosslinking was quantified by determining the relative amount of hydroxyproline (HYP) released from the tissue by collagenase digestion
Table 1.
Summary of optimization results for various crosslinking reagents
| Crosslinker | [Saturating] at pH 7.4 | pH Optimum | t½ |
|---|---|---|---|
| EDC | 2.5–5 mM | 6 | <5 min |
| Genipin | 5–10 mM | 8–9 | 10–18 min |
| l-Threose | 50 mM | 8–9 | 2.5 h |
| Methylglyoxal | 30–50 mMa | 8–9 | 14–20 min |
| Proanthrocyanidin | 0.1% (w/v) | 5–9 | <5 min |
| Glutaraldehyde | 1–2 mM | 8–9 | <5 min |
At pH 8, [Saturating] was 5–10 mM
In most cases reactions reached a point where crosslinking was essentially complete. For example, MG crosslinking appeared to be almost complete at 50 mM, suggesting that at this point the crosslinker was saturating and the rate of the reaction could not be accelerated by the further addition of more reagent. In the cases of LT the length of crosslinking time of the incubations were insufficient to result in complete protection of the collagen from the protease under these digestion conditions
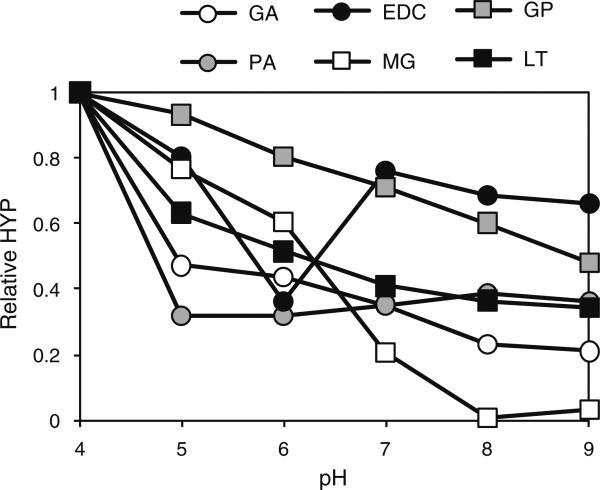
In order to determine the optimal pH for crosslinking by the various reagents, crosslinking was conducted at varying pH. In addition we used sub-saturating crosslinkers concentrations in order to ensure that both positive and negative effects of pH on reaction rate could be detected. These results are summarized in Fig. 4.
Fig. 4.
Dependence of protein crosslinking reagents on pH. Homogenized annulus tissue was treated with sub-saturating concentrations of various crosslinkers at varying pH and incubated for either 1 h (EDC, GP, MG, PA and GA) or 6 h (LT). Crosslinking was quantified by determining the amount of hydroxyproline (HYP) released from the tissue by collagenase
In general, all the crosslinkers were most effective at more alkaline pH. The only exception was EDC, which displayed a strict pH requirement of 6. Proanthrocyanidin was the least sensitive reagent, displaying no substantial differences in activity between pH 5 and 9 inclusive. The pH optima are summarized in Table 1.
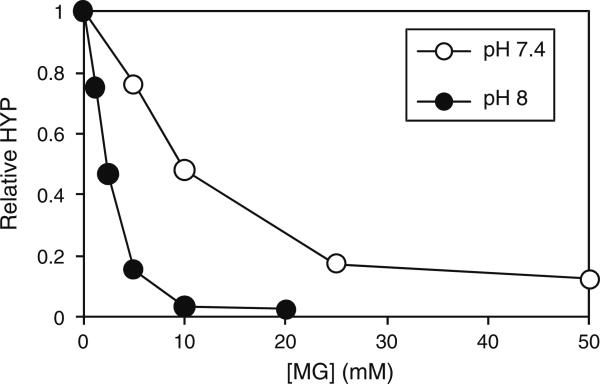
Of the crosslinkers tested, MG showed the strongest pH dependence. At pH 8 and 9, crosslinking was apparently complete (Fig. 4). This observation prompted us to re-titrate this reagent at an elevated pH, i.e. pH 8. MG displayed a marked increase in its crosslinking ability at pH 8 compared to pH 7.4 (Fig. 5). Under these conditions the MG was saturating at a concentration of 10 mM.
Fig. 5.
Effect of pH on the MG dose–response. Samples of annulus were treated with increasing concentrations of MG in either PBS (pH7.4) or in 100 mM EPPS buffer, pH8. Crosslinking was quantified by determining the amount of hydroxyproline (HYP) released from the tissue by collagenase digestion
Having determined the saturating concentrations and pH optima for the various crosslinkers, we sought to directly compare their reaction rates at optimal, but sustainable and physiologically tolerable, pH and at saturating concentrations. Tissue was treated for various periods of time and the extent of crosslinking at each time point determined as before (Fig. 6).
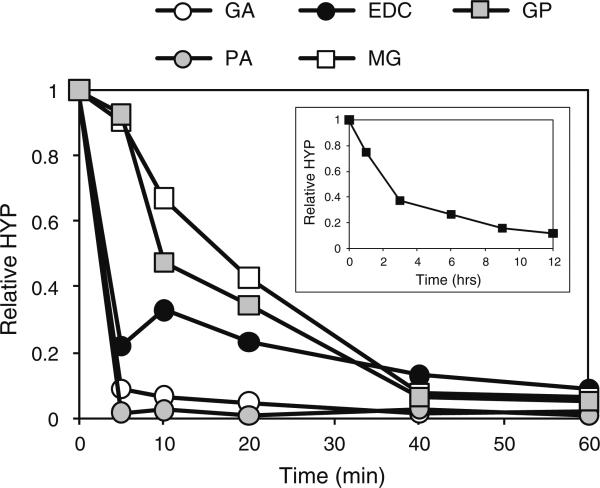
Fig. 6.
Crosslinking reaction rates. Bovine annulus tissue was treated at saturating concentrations with various crosslinkers for various periods of time. The reaction of LT is shown separately (inset). pH was 8.0 in all cases except for EDC (pH = 6.0)
Both PA and GA reacted extremely rapidly with the tissue. The reaction appeared essentially complete by the earliest time point (5 min) in both cases. EDC was almost as fast with almost 80% of the reaction having gone to completion at the first (5 min) time point. GP and MG displayed similar kinetics, taking 40 min to reach completion while LT was the slowest crosslinker, almost reaching completion after 12 h. These data are summarized as t½ values (time taken to reach 50% completion) in Table 1.
4 Discussion
Modulation of the chemical and mechanical properties of proteinaceous matrices by protein crosslinking is a growing theme in the field of tissue engineering. Glutaraldehyde has been commonly used for this purpose in the past, but suffers from the drawback of its acute toxicity. When crosslinking is achieved in vitro, or in situ, such as in an “open” procedure where the reagent can be rinsed and suctioned away after application, this effect can be ameliorated to some extent by thorough washing of the tissue prior to implantation, as in the cases of heart valve replacement and arterial repair, respectively. In applications where crosslinking is to be achieved in situ via injection, however, the poor safety profile of GA may prove to be problematic.
In order to evaluate the relative reactivities of various, potentially less toxic, crosslinking reagents we tested their ability to confer resistance to proteolysis to an insoluble tissue. We tested several important reaction parameters of a number of crosslinking reagents (i.e. concentration, pH and reaction rates) and compared them to GA.
As crosslinks accumulate in a tissue it becomes progressively less susceptible to digestion by proteases as any given scission event is less likely to release a soluble peptide fragment due to its continued attachment to the insoluble protein matrix via the crosslinks. We therefore monitored crosslink formation indirectly using a collagenase protection assay which has been commonly used for this purpose previously [10, 45–48]. This method, however, may underestimate the number of crosslinks formed since it is possible that complete protease protection might be achieved before reaction of every possible crosslinking site. Therefore, while our reaction rate data provide a comparison of the relative reaction velocities of the crosslinkers tested, complete chemical crosslinking may require more time than reported in these studies. This caveat does not apply to the saturating concentrations and pH optima reported here since these parameters are dependent on the concentration and the chemistry of the reactants and not on the analytical method employed. Annulus fibrosis from bovine spinal discs was used as a test substrate since this tissue has relatively little inter-specimen variation, and has a long history of use as a biomechanical model of human discs in our laboratory and elsewhere. Approximately half of the dry weight of annulus fibrosus consists of collagen [49], which is comparable to that of other protein matrices [50–52]. Our results, therefore, should be applicable to crosslinking of various tissue types.
Samples of tissue were treated with various concentrations of crosslinkers and the extent of crosslinking assessed by determining the ability of collagenase to digest each sample. The relative effectiveness of the crosslinkers was: GA > PA > EDC > GP >> MG >> LT.
The pH dependence of the crosslinkers was compared by incubating tissue samples at sub-saturating crosslinker concentrations at various pHs between 4 and 9 (Fig. 4). All of the crosslinkers were least effective at pH 4 and, In general, their reactivity increased with pH. The two exceptions were EDC, which exhibited a distinct pH optimum at pH 6, and PA whose reactivity was unaffected in the range from 5 to 9. This preference for alkaline pH of the remaining crosslinkers is presumably at least partly due to the deprotonation of amine residues on the matrix, allowing them to conduct nucleophilic attacks on the crosslinkers via their free electron pairs.
Of the remaining reagents, GA and LT appeared to possess a similar pH sensitivity (as judged by the slope of the curves within the 5–9 pH range) while GP was slightly more sensitive to its pH environment. MG was the most pH sensitive of the crosslinkers tested and exhibited a sigmoid reactivity curve as pH increased. In summary, the relative pH sensitivities of the crosslinkers tested were EDC > MG > GP > LT = GA > PA. Because of the substantial sensitivity of MG to pH, we retitrated MG at pH 8and found that its dose response curve was very similar to that of GP (Fig. 5).
Under optimal pH conditions, and at saturating crosslinker concentrations, GA and PA reacted most rapidly with bovine annulus tissue (Fig. 6). The reaction in both cases was complete within 5 min. Due to the time taken for manipulating the samples, it was impractical to assess the extent of crosslinking at shorter time points in order to discriminate between these two reagents. EDC also reacted rapidly, but was slower than GA and PA. The time courses for MG and GP were slower than that for EDC, but very similar to each other, while LT was substantially slower than all the other reagents tested (by an order of magnitude compared to GP and MG).
The data presented here provide a basis for the selection of protein crosslinking reagents for use either in vitro or in vivo. Moreover, depending on the intended use, the rate of crosslinking can be modulated by varying parameters such as crosslinker selection, concentration and/or pH.
Acknowledgment
This work was supported by the National Institutes of Health (1R43 AR055014-01).
Contributor Information
Paul Slusarewicz, Orthopeutics, L.P., 111 Cooperative Way, Suite 210, Georgetown, TX 78626, USA.
Keng Zhu, Orthopeutics, L.P., 111 Cooperative Way, Suite 210, Georgetown, TX 78626, USA.
Tom Hedman, Orthopeutics, L.P., 111 Cooperative Way, Suite 210, Georgetown, TX 78626, USA; Department of Biomedical Engineering, Texas A&M University, College Station, TX, USA.
References
- 1.Hoffmann B, Seitz D, Mencke A, Kokott A, Ziegler G. Glutaraldehyde and oxidised dextran as crosslinker reagents for chitosan-based scaffolds for cartilage tissue engineering. J Mater Sci Mater Med. 2009;20:1495–503. doi: 10.1007/s10856-009-3707-3. [DOI] [PubMed] [Google Scholar]
- 2.Mwale F, Iordanova M, Demers CN, Steffen T, Roughley P, Antoniou J. Biological evaluation of chitosan salts cross-linked to genipin as a cell scaffold for disk tissue engineering. Tissue Eng. 2005;11:130–40. doi: 10.1089/ten.2005.11.130. [DOI] [PubMed] [Google Scholar]
- 3.O'Halloran DM, Collighan RJ, Griffin M, Pandit AS. Characterization of a microbial transglutaminase cross-linked type II collagen scaffold. Tissue Eng. 2006;12:1467–74. doi: 10.1089/ten.2006.12.1467. [DOI] [PubMed] [Google Scholar]
- 4.Wu X, Black L, Santacana-Laffitte G, Patrick CW., Jr Preparation and assessment of glutaraldehyde-crosslinked collagen-chitosan hydrogels for adipose tissue engineering. J Biomed Mater Res A. 2007;81:59–65. doi: 10.1002/jbm.a.31003. [DOI] [PubMed] [Google Scholar]
- 5.Yang SH, Hsu CK, Wang KC, Hou SM, Lin FH. Tricalcium phosphate and glutaraldehyde crosslinked gelatin incorporating bone morphogenetic protein—a viable scaffold for bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2005;74:468–75. doi: 10.1002/jbm.b.30200. [DOI] [PubMed] [Google Scholar]
- 6.Kim SS, Lim SH, Cho SW, Gwak SJ, Hong YS, Chang BC, et al. Tissue engineering of heart valves by recellularization of glutaraldehyde-fixed porcine valves using bone marrow-derived cells. Exp Mol Med. 2006;38:273–83. doi: 10.1038/emm.2006.33. [DOI] [PubMed] [Google Scholar]
- 7.Zhai W, Chang J, Lin K, Wang J, Zhao Q, Sun X. Crosslinking of decellularized porcine heart valve matrix by procyanidins. Biomaterials. 2006;27:3684–90. doi: 10.1016/j.biomaterials.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Gratzer PF, Lee JM. Control of pH alters the type of cross-linking produced by 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) treatment of acellular matrix vascular grafts. J Biomed Mater Res. 2001;58:172–9. doi: 10.1002/1097-4636(2001)58:2<172::aid-jbm1004>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 9.Chachra D, Gratzer PF, Pereira CA, Lee JM. Effect of applied uniaxial stress on rate and mechanical effects of cross-linking in tissue-derived biomaterials. Biomaterials. 1996;17:1865–75. doi: 10.1016/0142-9612(95)00305-3. [DOI] [PubMed] [Google Scholar]
- 10.Duan X, Sheardown H. Crosslinking of collagen with dendrimers. J Biomed Mater Res A. 2005;75:510–8. doi: 10.1002/jbm.a.30475. [DOI] [PubMed] [Google Scholar]
- 11.Han B, Jaurequi J, Tang BW, Nimni ME. Proanthocyanidin: a natural crosslinking reagent for stabilizing collagen matrices. J Biomed Mater Res A. 2003;65:118–24. doi: 10.1002/jbm.a.10460. [DOI] [PubMed] [Google Scholar]
- 12.Roe SC, Milthorpe BK, Schindhelm K. Collagen cross-linking and resorption: effect of glutaraldehyde concentration. Artif Organs. 1990;14:443–8. doi: 10.1111/j.1525-1594.1990.tb03001.x. [DOI] [PubMed] [Google Scholar]
- 13.Sung HW, Liang IL, Chen CN, Huang RN, Liang HF. Stability of a biological tissue fixed with a naturally occurring crosslinking agent (genipin). J Biomed Mater Res. 2001;55:538–46. doi: 10.1002/1097-4636(20010615)55:4<538::aid-jbm1047>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Guldner NW, Jasmund I, Zimmermann H, Heinlein M, Girndt B, Meier V, et al. Detoxification and endothelialization of glutaraldehyde-fixed bovine pericardium with titanium coating: a new technology for cardiovascular tissue engineering. Circulation. 2009;119:1653–60. doi: 10.1161/CIRCULATIONAHA.108.823948. [DOI] [PubMed] [Google Scholar]
- 15.Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it? Spine. 2006;31:2151–61. doi: 10.1097/01.brs.0000231761.73859.2c. [DOI] [PubMed] [Google Scholar]
- 16.Martin MD, Boxell CM, Malone DG. Pathophysiology of lumbar disc degeneration: a review of the literature. Neurosurg Focus. 2002;13:1–6. doi: 10.3171/foc.2002.13.2.2. [DOI] [PubMed] [Google Scholar]
- 17.Acaroglu ER, Iatridis JC, Setton LA, Foster RJ, Mow VC, Weidenbaum M. Degeneration and aging affect the tensile behavior of human lumbar anulus fibrosus. Spine. 1995;20:2690–701. doi: 10.1097/00007632-199512150-00010. [DOI] [PubMed] [Google Scholar]
- 18.Chuang SY, Odono RM, Hedman TP. Effects of exogenous crosslinking on in vitro tensile and compressive moduli of lumbar intervertebral discs. Clin Biomech. 2007;22:14–20. doi: 10.1016/j.clinbiomech.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Hedman T, Odono R, Shung KCS-Y. Effects of exogenous crosslinking on compressive load sharing in the intervertebral disc. Orthop Trans. 2006;31:51. [Google Scholar]
- 20.Hedman T, Han B, Loree H. In vivo validation of collagen crosslink augmentation in a rat tail model. Orthop Trans. 2008;33:339. [Google Scholar]
- 21.Hedman TP, Saito H, Vo C, Chuang SY. Exogenous cross-linking increases the stability of spinal motion segments. Spine. 2006;31:480–5. doi: 10.1097/01.brs.0000224531.49174.ea. [DOI] [PubMed] [Google Scholar]
- 22.Murata-Kamiya N, Kamiya H. Methylglyoxal, an endogenous aldehyde, crosslinks DNA polymerase and the substrate DNA. Nucleic Acids Res. 2001;29:3433–8. doi: 10.1093/nar/29.16.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung HW, Chang Y, Liang IL, Chang WH, Chen YC. Fixation of biological tissues with a naturally occurring crosslinking agent: fixation rate and effects of pH, temperature, and initial fixative concentration. J Biomed Mater Res. 2000;52:77–87. doi: 10.1002/1097-4636(200010)52:1<77::aid-jbm10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 24.Kim KM, Herrera GA, Battarbee HD. Role of glutaraldehyde in calcification of porcine aortic valve fibroblasts. Am J Pathol. 1999;154:843–52. doi: 10.1016/S0002-9440(10)65331-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang M, Vogel HJ. Determination of the side chain pKa values of the lysine residues in calmodulin. J Biol Chem. 1993;268:22420–8. [PubMed] [Google Scholar]
- 26.Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Recent Prog Horm Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Verzijl N, De Groot J, Ben ZC, Brau-Benjamin O, Maroudas A, Bank RA, et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114–23. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 28.Lapolla A, Flamini R, Dalla VA, Senesi A, Reitano R, Fedele D, et al. Glyoxal and methylglyoxal levels in diabetic patients: quantitative determination by a new GC/MS method. Clin Chem Lab Med. 2003;41:1166–73. doi: 10.1515/CCLM.2003.180. [DOI] [PubMed] [Google Scholar]
- 29.Mirza MA, Kandhro AJ, Memon SQ, Khuhawar MY, Arain R. Determination of glyoxal and methylglyoxal in the serum of diabetic patients by MEKC using stilbenediamine as derivatizing reagent. Electrophoresis. 2007;28:3940–7. doi: 10.1002/elps.200700129. [DOI] [PubMed] [Google Scholar]
- 30.Chellan P, Nagaraj RH. Protein crosslinking by the Maillard reaction: dicarbonyl-derived imidazolium crosslinks in aging and diabetes. Arch Biochem Biophys. 1999;368:98–104. doi: 10.1006/abbi.1999.1291. [DOI] [PubMed] [Google Scholar]
- 31.Murata-Kamiya N, Kamiya H, Kaji H, Kasai H. Methylglyoxal induces G:C to C:G and G:C to T:A transversions in the supF gene on a shuttle vector plasmid replicated in mammalian cells. Mutat Res. 2000;468:173–82. doi: 10.1016/s1383-5718(00)00044-9. [DOI] [PubMed] [Google Scholar]
- 32.Wagner DR, Reiser KM, Lotz JC. Glycation increases human annulus fibrosus stiffness in both experimental measurements and theoretical predictions. J Biomech. 2006;39:1021–9. doi: 10.1016/j.jbiomech.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Sung HW, Chang WH, Ma CY, Lee MH. Crosslinking of biological tissues using genipin and/or carbodiimide. J Biomed Mater Res A. 2003;64:427–38. doi: 10.1002/jbm.a.10346. [DOI] [PubMed] [Google Scholar]
- 34.Sung HW, Chang Y, Chiu CT, Chen CN, Liang HC. Crosslinking characteristics and mechanical properties of a bovine pericardium fixed with a naturally occurring crosslinking agent. J Biomed Mater Res. 1999;47:116–26. doi: 10.1002/(sici)1097-4636(199911)47:2<116::aid-jbm2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 35.Sung HW, Chang Y, Chiu CT, Chen CN, Liang HC. Mechanical properties of a porcine aortic valve fixed with a naturally occurring crosslinking agent. Biomaterials. 1999;20:1759–72. doi: 10.1016/s0142-9612(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 36.Yerramalli CS, Chou AI, Miller GJ, Nicoll SB, Chin KR, Elliott DM. The effect of nucleus pulposus crosslinking and glycosaminoglycan degradation on disc mechanical function. Biomech Model Mechanobiol. 2007;6:13–20. doi: 10.1007/s10237-006-0043-0. [DOI] [PubMed] [Google Scholar]
- 37.Huang LL, Sung HW, Tsai CC, Huang DM. Biocompatibility study of a biological tissue fixed with a naturally occurring crosslinking reagent. J Biomed Mater Res. 1998;42:568–76. doi: 10.1002/(sici)1097-4636(19981215)42:4<568::aid-jbm13>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 38.Tsai CC, Huang RN, Sung HW, Liang HC. In vitro evaluation of the genotoxicity of a naturally occurring crosslinking agent (genipin) for biologic tissue fixation. J Biomed Mater Res. 2000;52:58–65. doi: 10.1002/1097-4636(200010)52:1<58::aid-jbm8>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 39.Bannister DW, Burns AB. Adaptation of the Bergman and Loxley technique for hydroxyproline determination to the autoanalyzer and its use in determining plasma hydroxyproline in the domestic fowl. Analyst. 1970;95:596–600. doi: 10.1039/an9709500596. [DOI] [PubMed] [Google Scholar]
- 40.Berg RA. Determination of 3- and 4-hydroxyproline. Methods Enzymol. 1982;82(Pt A):372–98. doi: 10.1016/0076-6879(82)82074-0. [DOI] [PubMed] [Google Scholar]
- 41.Prockop DJ, Udenfriend S, Lindstedt S. A simple technique for measuring the specific activity of labeled hydroxyproline in biological materials. J Biol Chem. 1961;236:1395–8. [PubMed] [Google Scholar]
- 42.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–9. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 43.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–73. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 44.Tsugita A, Scheffler JJ. A rapid method for acid hydrolysis of protein with a mixture of trifluoroacetic acid and hydrochloric acid. Eur J Biochem. 1982;124:585–8. doi: 10.1111/j.1432-1033.1982.tb06634.x. [DOI] [PubMed] [Google Scholar]
- 45.Charulatha V, Rajaram A. Influence of different crosslinking treatments on the physical properties of collagen membranes. Biomaterials. 2003;24:759–67. doi: 10.1016/s0142-9612(02)00412-x. [DOI] [PubMed] [Google Scholar]
- 46.Nimni ME, Cheung D, Strates B, Kodama M, Sheikh K. Chemically modified collagen: a natural biomaterial for tissue replacement. J Biomed Mater Res. 1987;21:741–71. doi: 10.1002/jbm.820210606. [DOI] [PubMed] [Google Scholar]
- 47.Petite H, Frei V, Huc A, Herbage D. Use of diphenylphosphorylazide for cross-linking collagen-based biomaterials. J Biomed Mater Res. 1994;28:159–65. doi: 10.1002/jbm.820280204. [DOI] [PubMed] [Google Scholar]
- 48.Vasudev SC, Chandy T. Effect of alternative crosslinking techniques on the enzymatic degradation of bovine pericardia and their calcification. J Biomed Mater Res. 1997;35:357–69. doi: 10.1002/(sici)1097-4636(19970605)35:3<357::aid-jbm10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 49.Eyre DR, Muir H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim Biophys Acta. 1977;492:29–42. doi: 10.1016/0005-2795(77)90211-2. [DOI] [PubMed] [Google Scholar]
- 50.Lowry OH, Rourke Gilligan D, Ketersky EM. The determination of collagen and elastin in tissues, with results obtained in various normal tissues from different species. J Biol Chem. 1941;139:795–804. [Google Scholar]
- 51.Svejcar J, Prerovsky I, Linhart J, Kruml J. Content of collagen, elastin, and water in walls of the internal saphenous vein in man. Circ Res. 1962;11:296–300. doi: 10.1161/01.res.11.2.296. [DOI] [PubMed] [Google Scholar]
- 52.Ignat'eva NYu, Danilov NA, Averkiev SV, Obrezkova MV, Lunin VV, Sobol EN. Determination of hydroxyproline in tissues and the evaluation of the collagen content of the tissues. J Anal Chem. 2007;62:51–7. [Google Scholar]