Abstract
Dental pulp cells (DPCs) can differentiate into osteoblasts and are deemed a promising cell source for bone regeneration. Static magnetic field (SMF) stimulates osteoblast differentiation but the effect in DPCs remains unknown. The aim of this study was to investigate the effect of SMF exposure on the osteogenic differentiation and mineralization of rat DPCs in vitro. Cells were continuously exposed to SMF at 290 mT in the presence/absence of osteogenic induction [dexamethasone (Dex)/β-glycerophosphate (β-GP)]. Results showed that SMF alone did not impair the cell cycle and proliferation. On the other hand, obvious condensation in the metachromatic staining of the extracellular matrix with toluidine blue was observed for SMF-exposed cells as well as the Dex/β-GP treated cells. SMF in combination with Dex/β-GP significantly increased the mRNA expression of osteogenic genes, as well as the ALP activity and extracellular calcium concentration at the early stage, followed by obvious calcium deposits later. Besides, SMF exposure increased the activity of extracellular signal-regulated kinase 1/2 (ERK1/2) at 3 h and accelerated the mRNA expression of osteogenic transcription factor, Cbfa1, advancing its activation time from 168 to 72 h under osteogenic induction. In summary, SMF exposure in combination of Dex/β-GP induction could significantly accelerate the osteogenic differentiation and mineralization of DPCs.
Keywords: Static magnetic field, Dental pulp cells, Dexamethasone, Gene expression, Mineralization
Introduction
Physical force plays a crucial role in the development and maintenance of many tissues including bone. Physical factors such as mechanical, electrical, and magnetic stimulation exhibit anabolic action on bone formation (Bassett 1993; De Mattei et al. 1999; Vander Molen et al. 2000; McLeod and Collazo 2000). Static magnetic field (SMF) has been used advantageously in various orthodontic treatments (Noar and Evans 1999). Studies have demonstrated that SMF increased bone repair (Darendeliler et al. 1997), bone deposition (Darendeliler et al. 1995) and bone formation in vitro and in vivo (Kotani et al. 2002). SMF was also used to prevent the decrease in bone density caused by surgery or implantation (Yan et al. 1998). At the cellular level, SMF was shown to stimulate osteoblastic differentiation by activation of p38 phosphorylation (Yuge et al. 2003). SMF, however, also induced apoptosis of lymphocytes and macrophages (Flipo et al. 1998). Yamamoto et al. (2003) has established that bone formation from rat calvaria cells was enhanced by SMF to similar extent for the magnetic flux density at 160, 280 or 340 mT. The effect of SMF on the differentiation of dental pulp cells (DPCs) remains unknown.
Dental pulp cells are not a homogeneous cell population. They contain progenitor/stem cells that can differentiate into dentin-forming odontoblasts and maintain the homeostasis of dental mineralized tissue by polarization and secretion of predentin-dentin components, in response to the appropriate stimuli, e.g. caries, chemicals or trauma (Tonomura et al. 2007; Gronthos et al. 2002). Researches indicated that DPCs whether from human, pigs or rats were recognized as a source of mesenchymal stem cells (Yang et al. 2007; Zhang et al. 2005; Alliot-Licht et al. 2005). They can be induced and differentiated into odontoblast-like and osteoblast-like cells by a variety of inducing reagents such as dexamethasone (Dex), β-glycerophosphate (β-GP) and 1, 25-dihydroxyvitamin D, and can help to the repair the dentin and bone after injury (Tonomura et al. 2007; Alliot-Licht et al. 2005). Recently, DPCs were deemed as a promising cell source for bone regeneration because of the following advantages (Graziano et al. 2008a, b). The collection site of these cells is easy and produces very low morbidity. The extraction of stem cells from pulp tissue is highly efficient. They have an extensive differentiation ability and can interact with biomaterials, which makes them ideal for tissue reconstruction. Additionally, DPCs possess immunoprivileges as they can be grafted into allogenic tissues and seem to exert anti-inflammatory abilities (Graziano et al. 2008b; de Mendonca et al. 2008; Laino et al. 2005). In bone regeneration, DPCs have been shown to differentiate into osteoblast-like cells in vitro (Papaccio et al. 2006) and to repair the large defect of cranial and fibrous bone in vivo (Laino et al. 2005; Graziano et al. 2008b; de Mendonca et al. 2008). However, not all cell populations existing in DPCs have the potential for osteoblastic differentiation (Papaccio et al. 2006). Thus it may be essential to augment the osteogenic differentiation potential of DPCs.
Dex is one of the common osteogenic inducers. It can induce the osteogenesis of marrow stromal cells and maturity of osteoblasts when used in vitro either alone or in combination with β-GP, ascorbate-2-phosphate and bone morphogenetic proteins (BMPs) (McCabe et al. 1995). The activation of Dex is mediated via glucocorticoid receptors to trigger the early events in cooperation with transcription factors (D’Souza et al. 1999). Differentiation of the mesenchymal tissue is controlled by universally expressed and unique transcriptional factors. The core-binding factor α1, Cbfa1, a transcriptional factor that belongs to the runt domain gene family (Cbfa1/RUNX2), regulates the gene expression of osteocalcein (OCN), a bone-matrix protein involved in controlling the late phase of osteogenesis. Cbfa1 is essential for osteoblastic differentiation, bone formation and tooth development (D’Souza et al. 1999). It can be phosphorylated by the integrin-mediated activation of the extracellular signal-regulated kinase 1/2 (ERK1/2), the subgroups of mitogen-activated protein kinase (MAPK) (MEK-ERK1/2-Cbfa1 signaling), to control transcription of osteogenic gene (Xiao et al. 2000; Kanno et al. 2007). Moreover, mechanical force can increase the differentiation of osteoblasts, by altering the interaction of cell-extracellular matrices (ECM) and inducing the ECM integrin-dependent ERK1/2 to regulate the osteogenic genes (Kanno et al. 2007; Hughes-Fulford 2004).
As mentioned, many previous studies have indicated that SMF accelerates the differentiation of osteoblasts and bone formation in vitro and in vivo. Albeit, there have been no studies examining whether the SMF exposure also influences the proliferation and differentiation of DPCs and its role in the differentiation-related mechanism. In the presented work, the effects of SMF exposure on the cell viability and capacity of osteogenic differentiation in rat DPCs were investigated. The osteogenic marker genes of alkaline phosphatase (ALP), osteopontin (OPN) and osteocalcein (OCN), ALP activity, extracellular calcium concentration, mineralization and ERK-cbfa1 signaling pathway of rat DPCs in the presence/absence of Dex/β-GP induction were examined in vitro.
Materials and methods
Animal treatment
All experimental procedures involving animals were conducted in accordance with the NIH guidelines. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the university.
Primary cultures
Rat DPCs were isolated from incisors by a modification of the method described previously (Yokose et al. 2000). Rat dental pulp was isolated from the mandibular incisors of 2-week-old neonatal Sprague–Dawley rats, placed into calcium and magnesium-free phosphate-buffered saline (PBS) and washed two times. The extracted teeth were cut into several pieces. The tissue fragments were incubated at 37 °C for 60 min with gentle shaking in 3 mL of a sterile enzyme solution containing 0.3% collagenase type 1A (Sigma, St. Louis, MO, USA) and dipase II (Roche, Basel, Switzerland) in PBS. The enzyme medium containing rat DPCs was screened with a 40 μM mesh (BD Falcon, Becton–Dickinson, Heidelberg, Germany) and washed with PBS three times. The cell pellets were resuspended with 1:1 mixture of Dulbecco’s modified Eagle medium (DMEM-LG, Gibco/BRL, Grand Island, NY, USA) and Ham’s F12 (Gibco/BRM, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FCS, Biological Industries, Israel) and 100 U/ml penicillin–streptomycin (Sigma, St. Louis, MO, USA), and cultured in T75 flask (BD Falcon., Becton–Dickinson, Heidelberg, Germany) in an atmosphere of 5% CO2 at 37 °C. The cell passages 2 to 5 were used in the subsequent experiments. After having reached a subconfluent state, the cells were inoculated into 24-/or 6-multiwell plates (BD Falcon, Becton–Dickinson, Heidelberg, Germany) at a density of 2 × 104/cm2 for in vitro osteogenic assays. At the following day (day 0), the cells were grown either in fresh basal medium or in the presence of osteogenic supplements containing 2 mM β-glycerophosphate (β-GP, Sigma, St. Louis, MO, USA), 50 μg/ml L-ascorbic acid (Sigma, St. Louis, MO, USA) and 10−8 M dexamethasone (Dex, Sigma, St. Louis, MO, USA) during osteogenic differentiation. The medium was changed at 2 day intervals.
Exposure system of static magnetic field
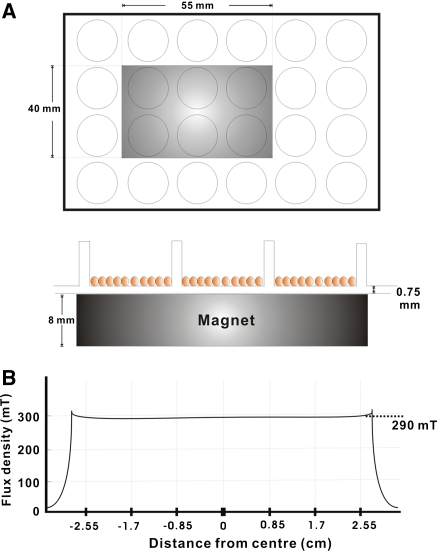
The rat DPCs culture with basal or osteogenic induction medium was divided into unexposed control groups (Basal/Con and Dex/β-GP/Con) and SMF-exposed groups (Basal/SMF and Dex/β-GP/SMF). The exposure system is depicted in Fig. 1, which was a modification of the system described previously (Yamamoto et al. 2003). The magnetic field was generated by a neodymium-iron-boron magnet disk (NEOMAX, Sumitomo, Osaka, Japan). The length, width, and thickness of a disk was 55, 40 and 8 mm. 24-well plastic culture plates (0.75 mm thickness) were used. The magnet was placed below the wells to expose the cultures to north fields as shown in Fig. 1a. A total of six wells of a 24-well plastic culture plate could be exposed simultaneously to the same magnetic field. During the exposure process, two adjacent culture plates were set apart by more than 50 mm as the edge-to-edge distance in order to exclude the mutual interference from the adjacent magnetic fields. In the SMF-exposed groups, the cells with the basal or osteogenic culture medium were subjected to continuous SMF exposure. The magnetic flux density was monitored with a Gauss meter (Series 9900, F.W. Bell, Orlando, FL, USA) at the bottom of each well, where rat pulp cells attached themselves to the culture plates. The magnetic field showed an average flux of 290 mT (Fig. 1b). In the unexposed control groups, non-magnetic disks of neodymium-iron-boron were placed below the culture plates. The culture plate of the unexposed control groups was placed next to that of the experimental groups in the same incubator. The flux density values in the wells of the control culture plates were not greater than 0.05 mT, which is the level of the natural magnetic field of the earth.
Fig. 1.
Diagram showing the top view of magnet (gray color) placement below a 24-well plastic culture plate (a), and the distribution of magnetic flux density (solid line) in the wells of the culture plate (b). A total of 6 wells in the 24-well culture plate could be exposed simultaneously to the same magnetic field. The defined location of wells and magnetic flux density were recorded along the centre position of the magnet
Proliferation assay
To determine cell proliferation, cells were seeded at a density of 2 × 104 cells/cm2 in a 24-well plastic plate in the presence/absence of Dex/β-GP with or without SMF exposure, and cultured for 1, 3, 5, 7 and 9 days. Cells were detached from the culture wells with 0.05% trypsin/EDTA (Gibco/BRL, Grand Island, NY, USA) and counted using a hemocytometer.
Cell cycle analysis by flow cytometry
At 0 and 24 h after SMF exposure, cells were collected (500 g, 10 min) and incubated with ice-cold PBS. Then the cells were fixed with ethanol, collected, and washed with PBS by centrifugation (500 g, 10 min). Cell deposition was added with 1 ml of PBS and 200 μl of RNAase A (100 μg/ml) (Sigma–Aldrich, St. Louis, MO, USA) at 37 °C for 30 min. Cells were stained with 5 μl of propidium iodide (10 μg/ml) (Sigma–Aldrich, St. Louis, MO, USA) in dark. Cells were measured for their DNA content by flow cytometry (BD FACScan, San Jose, CA, USA) after passing through a cell filter.
Alkaline phosphatase (ALP) activity and protein assay
Alkaline phosphatase activity was determined in cell lysates using a Sigma FAST-p-nitrophenyl phosphate (Sigma, St. Louis, MO, USA) kit. Samples of lysates were added to p-nitrophenyl phosphate as substrate and reacted for 30 min. The reaction was stopped with 0.02 N NaOH, and the concentration of the product was determined by means of a UV/VIS spectrophotometer (Hitachi, Japan) at 405 nm. Cell lysates were also analyzed for the protein content by means of a Bio-Rad Protein Assay kit (Mississauga, Ontario, Canada). The ALP activity was normalized to the total protein concentration (O.D./30 min/mg protein).
Histochemical stain and bone nodule formation assay
To assess the ECM and calcium deposition around the cells, toluidine blue stain (metachromatic staining) and von Kossa technique were used. Cells were washed with PBS three times, followed by fixation with 3.7% paraformaldehyde in 3.5% sucrose for 10 min. They were again washed three times with distilled water. The cells were stained with toluidine blue O (0.1%) (Sigma, St. Louis, MO, USA) for 2–3 min and washed with distilled water for another three times. For von Kossa stain, the fixed cells were stained with 5% AgNO3 (Sigma, St. Louis, MO, USA) solution for 1 h. After being stained, they were washed with distilled water for three times and fixed in 3% Na2S2O3 (Sigma, St. Louis, MO, USA) solution for 5 min, and counterstained with Nuclear-fast Red (Sigma, St. Louis, MO, USA) for 5 min.
Extracellular calcium assay
The extracellular calcium ion content per well was determined using a calcium diagnostic kit (Sigma, St. Louis, MO, USA), based on the o-cresolphthalein complexone color development method), according to the manufacturer’s recommendations. A standard curve was generated each time using the standards provided in the kit, and the calcium ion concentration in the samples was calculated. Samples of the culture medium after 7 days of culture were collected and added to the reaction buffer and the staining solution. After reaction for 3 min, the calcium content in the samples was determined by measuring the absorbance at 575 nm using a microtiter plate reader (Immunella, 990 GDV, Italy). The values have been deducted from those obtained for the individual blank culture medium.
Semi-quantitative reverse transcription-polymerase chain reaction (RT–PCR)
Total RNA was extracted from the cultured rat DPCs grown in culture conditions or normal medium with/without SMF for different days, by using the Trizol® reagent (Invitrogen, USA) according to the manufacturer’s instructions. cDNA synthesis and amplification via PCR were performed using RevertAid™ First Strand cDNA Synthesis Kit (Fermentas, USA) and an oligo dT. Furthermore, cDNAs were also amplified using specific primers by RT–PCR to generate products corresponding to mRNA. The cDNA obtained from a 0.5 μg of total RNA was used for one PCR. The PCR reaction mixture was made up in 25 μl and 5 μM of targeted gene oligonucleotide primers: alkaline phosphatase (ALP), F: AGGCAGGATTGACCACGG, R: TGTAGTTCTGCTCATGGA; osteopontin (OPN), F: ATGAGACTGGCAGTGGTT, R: GCTTTCATTGGAGTTGCT; osteocalcein (OCN), F: AGGACCCTCTCTCTGCTCAC, R: AACGGTGGTGCCATAGATGC; core-binding factor α1 (Cbfa1), F: ATGCTTCATTCGCCTCACAAA, R: CTACAACCTTGAAGGCCACG; β-actin, F: TCCTGTGGCATCCACGAAACT, R: GGAGCAATGATCCTGATCTTC. Using the Thermal Cycler (GeneAmp PCR System 2700, Applied Biosystems, Foster City, CA, USA), 35 cycles of PCR consisting of denaturing at 94 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 50 s were performed. β-actin primers were added in all reactions as an internal control. The PCR products were then detected on a 1% agarose gel. The image was recorded by an image analyzer (BioDoc-It™ System, UVP, Upland, CA, USA) and the intensity of the bands was quantified by using LabWorks software (UVP, Upland, CA, USA).
Western blot
The phosphorylation status of ERK1/2 was analyzed at each time period, for groups with/without Dex/β-GP at SMF 290 mT. To obtain a protein sample, the cultured cells were washed with PBS, lysed by adding RIPA buffer (50 mM Tris (pH 7.5), 150 mM NaCl, 10 mM EDTA, 1% NP-40, 0.1% SDS, 1 mM PMSF and 10 μg/ml Aprotinin). After reaction for 30 min on ice, the lysate was centrifuged at 5,000 g for 15 min at 4 °C. After heating to 95 °C for 5 min, the protein samples were separated by 10% SDS–PAGE and blotted onto polyvinylidene difluoride membranes (Hybond-P, Amersham International Plc., Amersham, UK).Western blots were performed by using primary antibodies for phosphorylated ERK1/2 (p44/p42) (Thr202/Tyr204) (rabbit monoclonal IgG) (Cell Signaling, MA, USA) and for the total forms of ERK1/2 (Cell Signaling, MA, USA). The membranes were blocked with the blocking buffer (Tris-buffered saline, pH 7.6, containing 0.1% Tween 20 and 5% non-fat dried milk) for 1 h at room temperature and incubated with the primary antibodies by gentle agitation overnight at 4 °C. After extensive washing, the membranes were incubated for 1 h at room temperature with an anti-rabbit secondary antibody conjugated to horseradish peroxidase (HRP) (Chemicon, Temecula, CA, USA) and the substrate solution (TMB/H, Chemicon, Temecula, CA, USA) was added. The blotting images were scanned with a computer-associated scanner and the densities of the bands were recorded by an image analyzing software (BioDoc-It™ System, UVP, Upland, CA).
Statistical analysis
Numerical values were expressed as the mean ± standard deviation (SD) for 3–5 samples per group. In all studies, at least three independent experiments were performed for each type of experiment to ensure repeatability. Statistical differences among the experimental groups were evaluated by the analysis of variance followed by Student’s t test; p values < 0.05 were considered statistically significant.
Results
The effect of SMF exposure on cell-cycle distribution and cell proliferation
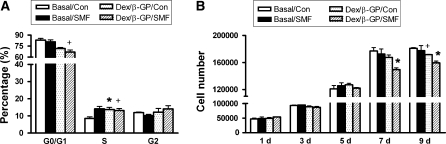
After SMF exposure alone for 24 h (Basal/Con), the portion of cells with cell cycle in the S phase was obviously enhanced (from 8.6 ± 1.37 to 14.1 ± 2.09%) but that in the G2/M phase was not (Fig. 2a) (p < 0.05). For cells cultivated with osteogenic induction (Dex/β-GP/Con) for 24 h, the percentage of G0/G1 phase was obviously reduced (from 83.2 ± 3.28 to 71.7 ± 2.09%), and the expression of S phase was promoted (from 8.6 ± 1.37 to 13.7 ± 1.82%) (p < 0.05). On the other hand, there was no significant difference in cell cycle between the SMF-exposed and unexposed cells cultivated with Dex/β-GP. The effects of osteogenic induction and SMF exposure on cell viability were further examined by cell proliferation assay. After 9 days of culture, Dex/β-GP induction significantly reduced the cell proliferation compared with the basal culture medium (Basal/Con) (Fig. 2b) (p < 0.05). Exposure to SMF alone (Basal/SMF) did not affect cell proliferation. However, when cells were exposed to SMF with the Dex/β-GP (Dex/β-GP/SMF), the cell proliferation was significantly reduced at the 7th and 9th day of culture when compared with the control cells (Dex/β-GP/Con) (Fig. 2b) (p < 0.05).
Fig. 2.
The effect of SMF exposure (290 mT, average flux density) on the cell properties. The cell cycle after 24 h of SMF exposure (a) and cell proliferation at different periods of time were analyzed, respectively in the DPCs culture with the basal or osteogenic medium (Dex/β-GP) (b). +, p < 0.05 (n = 3; compared with the difference between the basal and osteogenic medium culture in SMF-unexposed groups) *, p < 0.05 (n = 3; compared the difference between SMF-exposed and unexposed groups in basal medium or osteogenic medium culture) (Con) control, unexposed group; (SMF) static magnetic field; (d) days; (Basal) basal medium; (Dex/β-GP) dexamethasone/β-glycerophosphate
The matrices condensation and mineralization in SMF-exposed cells
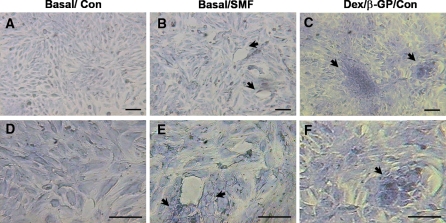
The toluidine blue analysis (metachromatic staining) (Fig. 3) revealed the presence of organized ECM-sulfated proteoglycans around the SMF-exposed cells. Cells were arranged in circular shape after SMF exposure in basal medium for 7 days (Basal/SMF) (Fig. 3b) whereas the unexposed cells did not show such a trend (Basal/Con) (Fig. 3a). As indicated by arrows (Fig. 3b), the matrices were more abundant around the cells in circular pattern. Similar cell morphology and ECM patterns were also observed for the cells cultivated with osteogenic induction medium (Dex/β-GP/Con) (Fig. 3c). Cell images at a higher magnification (Fig. 3d,e,f) clearly revealed that SMF-exposed cells accumulated a greater amount of metachromatic ECM shown as deeper blue color (Fig. 3e, indicated by arrows). The more obvious expression in ECM condensation was also observed when cells were cultivated with osteogenic induction medium (Dex/β-GP/Con) (Fig. 3f). The above results indicated that the reorganization and condensation of ECM was indeed induced during osteogenic induction and a similar phenomenon was found in cells exposed to SMF alone.
Fig. 3.
The cell morphology and the expression of extracellular matrix in cells exposed to SMF (290 mT, average flux density) for 7 days. The metachromatic staining of the extracellular matrix with toluidine blue was observed with different magnification of images for the unexposed (a,d) and SMF-exposed group (b,e) in basal culture medium. The cells cultivated with osteogenic medium served as the positive control (Dex/β-GP/Con) to compare the SMF-mediated effect (c,f). The arrows indicated the special morphology (b,c) and the obvious matrix condensation (e,f) around the circle of cell arrangement to compare with non-exposed cells with basal culture medium. The scale bar represents 50 μm. (Con) control, unexposed group; (SMF) static magnetic field; (Basal) basal medium; (Dex/β-GP) dexamethasone/β-glycerophosphate
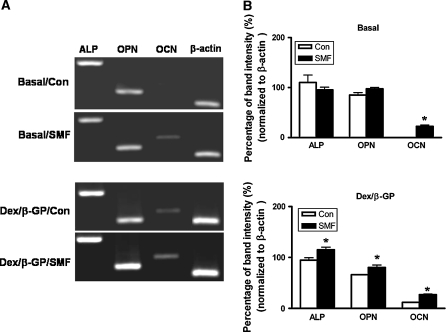
To understand which osteo-specific genes were involved with such a morphological change, the mRNA expressions of ALP, OPN and OCN were examined by RT–PCR at 7 days of culture in the presence/absence of osteogenic induction (Dex/β-GP) (Fig. 4). The ALP and OPN but not OCN genes were found to obviously express in the original rat DPCs (110.3 ± 20.81 and 85.1 ± 7.16%, respectively) (Fig. 4a). SMF exposure alone and Dex/β-GP did not significantly affect gene expression of ALP and OPN. On the other hand, SMF exposure alone as well as the Dex/β-GP induction induced OCN gene expression (22.6 ± 3.42 and 11.7 ± 0.42%, respectively) (p < 0.05) (Fig. 4a, b). Moreover, SMF exposure promoted the expression of OCN induced by the osteogenic medium (from 11.7 ± 0.4 to 26.9 ± 1.44%) (p < 0.05). Not only the expression of OCN but also that of ALP and OPN genes was upregulated by SMF exposure in the presence of Dex/β-GP (from 94.7 ± 6.62 to 115.14 ± 7.3% and 66.1 ± 0.12 to 80.5 ± 6.40%, respectively) (p < 0.05) (Fig. 4b).
Fig. 4.
The expression of osteogenic genes in DPCs with/without SMF exposure (290 mT, average flux density) for 7 days in basal and osteogenic culture medium (Dex/β-GP) culture. The mRNA expressions were analyzed by RT–PCR (a) and the band intensities were further quantified (b). *, p < 0.05 (n = 3; compared the difference between SMF-exposed and unexposed groups in basal or osteogenic culture medium) (Con) control, unexposed group; (SMF) static magnetic field; (Basal) basal medium; (Dex/β-GP) dexamethasone/β-glycerophosphate
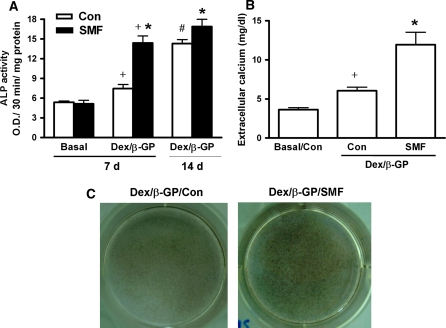
In order to further confirm the effect of SMF exposure on the ability of osteogenic differentiation in rat DPCs, the ALP activity, extracellular calcium concentration and calcium deposition were analyzed (Fig. 5). Dex/β-GP induction (Dex/β-GP/Con) significantly increased the ALP activity (7.4 ± 1.26) by about 39% after 7 days of culture over the nontreated cells (5.4 ± 0.38) (Basal/Con). About a 92% enhancement was observed from the culture period of 7 to 14 days (p < 0.05) (Fig. 5a). SMF exposure alone (Basal/SMF) did not affect the ALP activity within 7 days of culture (Basal/Con) (5.1 ± 1.03 v.s. 5.4 ± 0.38) (Fig. 5a). However, the combination of SMF exposure and Dex/β-GP (Dex/β-GP/SMF) significantly promoted the cellular ALP activity at 7 days of culture (from 7.4 ± 1.26 to 14.4 ± 2.15) and the value was similar to that with Dex/β-GP induction alone (Dex/β-GP/Con) for 14 days (14.3 ± 0.87). The efficiency of enhancement (93%) was obviously higher than that by Dex/β-GP alone (39%) (Dex/β-GP/Con) for 7 days of culture (p < 0.05) (Fig. 5a). After 14 days of Dex/β-GP induction, the expression of ALP in SMF-exposed cells (16.9 ± 1.94) was still higher than that in unexposed cells (14.3 ± 0.87). However, the enhancement by SMF between 7 and 14 days (17%) was lower than that by Dex/β-GP (92%) (Fig. 5a). The extracellular calcium concentration and calcium deposition were analyzed further in the presence of osteogenic induction for 7 and 14 days, respectively (Fig. 5b, c). The results showed that the extracellular calcium concentration (mg/dl) was induced by Dexβ-GP induction (Dex/β-GP/Con) (from 3.9 ± 0.54 to 6.1 ± 1.03) (p < 0.05) (Fig. 5b). The combination of SMF exposure and Dex/β-GP (Dex/β-GP/SMF) further enhanced the extracellular calcium concentration by about 2-fold relative to Dex/β-GP treated cells (Dex/β-GP/Con) at 7 days (from 6.1 ± 1.03 to 12.0 ± 3.56) (p < 0.05) (Fig. 5b). SMF alone affected neither extracellular calcium concentration (Fig. 5b) nor ALP activity (Fig. 5a). An increased calcium deposition (using von Kossa staining) (deep colored black-brown dots) was also observed in the group of Dex/β-GP/SMF after 14 days of culture (Fig. 5c).
Fig. 5.
The efficiency of osteogenic differentiation in DPCs with/without SMF exposure (290 mT, average flux density). The ALP activity of cells cultivated with the basal and osteogenic medium (Dex/β-GP) for 7 days was analyzed (a). The prolonged expression of ALP activity was further analyzed under Dex/β-GP culture of 14 days. The extracellular calcium concentration (b) and calcium deposition of cells cultivated in osteogenic medium (Dex/β-GP) was analyzed at the 7th and 14th day of culture, respectively (c). +, p < 0.05 (n = 5; compared with the difference between basal and osteogenic medium culture in SMF-exposed or unexposed groups) *, p < 0.05 (n = 5; compared the difference between SMF-exposed and unexposed groups in osteogenic medium culture of 7 or 14 days) #, p < 0.05 (n = 5; compared the difference between 7 and 14 days of culture in osteogenic medium) (Con) control, unexposed group; (d) days; (SMF) static magnetic field; (Basal) basal medium; (Dex/β-GP) dexamethasone/β-glycerophosphate
Time tracking in mRNA of Cbfa1 and upstream-regulating MAPK pathway during the osteogenic process
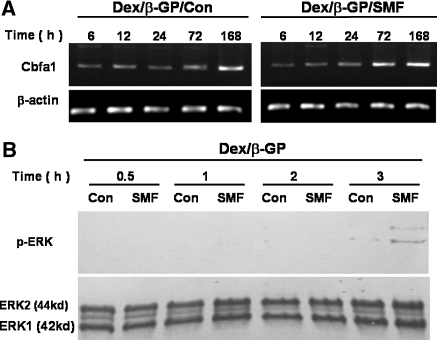
The expression of transcriptional factor Cbfa1 is essential to turn on the transcription of the osteogenic gene, OCN, during the osteogenic differentiation. Therefore, the gene expression of Cbfa1 was further examined from 6 to 168 h with/without SMF exposure during the osteogenic induction (Fig. 6). In the presence of Dex/β-GP, the mRNA expression level of Cbfa1 remained relatively low from 6 to 72 h and was induced at 168 h in the cells without SMF exposure (Dex/β-GP/Con) (Fig. 6a). However, the expression of the Cbfa1 gene was activated by SMF exposure as early as 72 h and kept its expression until 168 h (Fig. 6a). The expression for the SMF exposed group at 72 h was significantly higher (about 3.5 fold in the normalized band intensity to β-actin) compared to the nontreated group (0.95 vs. 0.32, p < 0.05, data not shown). To further confirm the upstream regulating signal in activation of Cbfa1 during the osteogenic process, the ERK1/2 activity was analyzed by Western blot at different time course in the presence of Dex/β-GP and was evaluated by the phosphorylated status of ERK1/2 proteins (p-ERK) (Fig. 6b). The ERK phosphorylation was observed for cells cultivated in osteogenic medium for 3 h, and its expression was obviously enhanced when combined with SMF exposure (Fig. 6b).
Fig. 6.
The effect of SMF exposure (290mT, average flux density) on the mRNA expression of transcriptional factor (core-binding factor 1, Cbfa1), and the related upstream-regulation MAPK pathway (ERK) during the osteogenic process. Time-dependent mRNA expression in Cbfa1 from 6 to 168 h with/without SMF exposure was analyzed by RT–PCR (a). Time tracing of the ERK activity (phosphorylated ERK1/2) from 0.5 to 3 h with/without SMF exposure was examined by Western blot during the early stage of osteogenic differentiation (b). (Con) control, unexposed group; (SMF) static magnetic field; (h) hours; (Dex/β-GP) dexamethasone/β-glycerophosphate; (ERK) extracellular signal-regulated protein kinase; (p-ERK) the phosphorylation of extracellular signal-regulated protein kinase
Discussion
SMF (at a flux density of 100 mT) did not affect bone growth of rat calvaria cells but significantly inhibited thymidine uptake (Camilleri and McDonald 1993). The present study showed that SMF exposure (290 mT) alone increased the double DNA content (the expression of the S phase) but did not activate mitosis (the expression of the G2 phase), leading to cell growth. The cell proliferation results showed that SMF exposure alone affected neither cell dynamics at 24 h nor cell proliferation even in a long-term culture. Therefore, the present results agreed with the previous findings, which demonstrated that SMF exposure did not cause DNA damage or affect cell viability (Lin et al. 2008; Amara et al. 2007). Alliot-Licht et al. (2005), however, indicated that Dex significantly inhibited the proliferation of human DPCs but conversely stimulated ALP activity in a 7-day culture. In the present study, we found a similar result that the Dex/β-GP treatment decreased cell proliferation at 9 days but increased ALP activity when compared with nontreated cells. Moreover, when combined with SMF exposure, Dex/β-GP decreased cell proliferation as early as at 7 days, together with a higher expression of ALP activity. The expression of ALP activity is essential and an early indicator of osteoblastic differentiation in mesenchymal stem cells (Yoshiki and Kurahashi 1971; Osyczka et al. 2004). SMF exposure alone did not induce ALP activity, extracellular calcium concentration, or affect cell proliferation, but it promoted the osteogenic differentiation induced by Dex/β-GP, including the expression of osteogenic genes, as well as the increase in ALP activity and calcium accumulation. Therefore, we proposed that SMF exposure may play a cooperative role to affect the cell properties and osteogenic differentiation in rat DPCs.
Cell condensation is a pivotal stage in skeletal development. During osteogenesis, condensation amplifies the number of committed osteogenic cells. When differentiation is initiated, the cell commitment is via condensation to the onset of osteogenic differentiation (Hall and Miyake 1995). The osteoblast-specific transcription factor, Cbfa1, was activated via ECM–dependent ERK1/2 signal transduction to turn on OCN gene transcription (Xiao et al. 1997; Kanno et al. 2007). Therefore, cell-ECM interactions are very important for osteoblastic differentiation. In the present study, obvious ECM condensation occurred at 7 days in cells with osteogenic induction. Interestingly, a similar phenomenon was also observed in cells exposed to SMF alone. It has been demonstrated that SMF (1 T) exposure can result in the orientation of macromolecules, such as collagen in animal cells (Torbet and Ronziere 1984; Miyakoshi 2005). The fibroblasts were oriented along the magnetic orientation of the collagen resulting from strong SMF (4.0 and 4.7 T) (Guido and Tranquillo 1993). Recent research has also found that SMF exposure (10 and 120 mT) affected cell shape and shrinkage (Xu et al. 2008). Here, we found additionally that SMF exposure alone not only affected the cell morphology but also simultaneously increased gene expression of OCN (bone matrix protein). However, the present results showed that SMF-mediated ECM-transmitted signaling alone was insufficient to induce the occurrence of osteogenic differentiation in rat DPCs. This could be because the regulation of matrix signals in osteogenic differentiation requires a post-translational pathway or accessory factors (Xiao et al. 1998). On the other hand, SMF exposure promoted the osteogenic differentiation in cells treated with Dex/β-GP, but ECM condensation was not increased (data not shown). The mechanism for the effect of SMF in osteogenic medium and that regarding how the change in cell morphology or ECM pattern could affect the osteogenic differentiation of DPCs deserve further exploration.
Alkaline phosphatase activity is widely detected in pre-osteoblasts and osteoblasts in the bone tissue and is an early marker of osteoblast differentiation. A high level of ALP activity has also been found in dental papilla tissue, and even in dental pulp cells (Goggins and Fullmer 1967; Spoto et al. 2001). In this study, we isolated rat DPCs that had obvious colony-forming morphology, similar to rat bone marrow stromal cells (data not shown). Moreover, we also noticed that not only the ALP gene but also its protein expression in rat DPCs was higher than human gingival fibroblasts (1.48 ± 0.29) and was similar to human dental pulp cells (5.46 ± 0.24) (data not shown). Dental pulp cells are the common progenitors of odontoblasts and osteoblasts (Batouli et al. 2003) and they proceed with differentiation/mineralization from the total population of cells (Kasugai et al. 1993; Gronthos et al. 2000). Accordingly, we thought that DPCs still had the potential to differentiate into osteoblast-like cells from the limited population of dental progenitors/stem cells, inspite of a possibly lower efficiency of differentiation. On the other hand, bone and dentin are similar in their matrix protein composition, including OPN, collagen type I, and bone sialoprotein, though their crystal structures are different (Batouli et al. 2003; Shimazu et al. 2002). One of the functions of those matrices seemed to support cell differentiation, whether in the osteogenesis or detinogenesis (Batouli et al. 2003). We found that DPCs had the original expression of ALP and OPN genes. The bone matrix of OCN was not expressed in undifferentiated DPCs in our findings, consistent with earlier reports (Laino et al. 2005). OCN expression is restricted to mature osteoblasts and is a late marker for osteogenic differentiation (Frendo et al. 1998). Therefore, the gene expression of OCN certainly helped to clarify the effect of Dex/β-GP induction, SMF exposure or their interplay on osteogenic differentiation in the present study. Relative to the OCN gene, the variations in the gene expressions of ALP and OPN seemed to have interfered with the original expression, leading to the unapparent results in the current study. Nevertheless, a significant difference was still shown by the quantification of the band intensity. Because of the above observations, analyses by ALP activity and calcium accumulation became crucial to provide more direct evidence regarding the effect of Dex/β-GP and SMF exposure on the osteogenic differentiation of DPCs. In addition, mineralization is an essential process, both in osteogenesis and detinogenesis (Batouli et al. 2003). We suggested that SMF exposure also assisted in the dentinogenesis based on the present findings in this study, though we did not study the differentiation of odontoblasts.
Dex/β-GP induced significant ALP activity after 7 days, but more notable induction was not observed until the 14th day. When it was combined with SMF exposure for 7 days, the Dex/β-GP induction was activated in advance, and the value was similar to the Dex/β-GP induction for 14 days. Therefore, we believe that SMF exposure accelerated the process of osteogenic differentiation in the presence of Dex/β-GP. Further evidence was obtained from an analysis of the matrices, osteospecific genes, and extracellular calcium. As expected, SMF exposure promoted osteogenic differentiation at an early stage and obvious mineralization was demonstrated after 14 days of culturing. Moreover, the related evidences in cell signaling also showed that the combination of SMF exposure advanced the activation time of Cbfa1 mRNA caused by Dex/β-GP from 168 to 72 h. Furthermore, the SMF exposure increased the amount of ERK1/2 phosphorylation (Cbfa1-upstream signaling transduction) induced by Dex/β-GP after 3 h. The increase in OCN gene transcription was accompanied by an increase in the binding of Cbfa1 to the promoter of the OCN gene (Kanno et al. 2007). The ERK1/2 activation initiated by ECM-integrin interaction can subsequently stimulate the expression of down-stream gene, OCN, by phosphorylating Cbfa1 to advance the early stage of differentiation (Yuge et al. 2003; Kanno et al. 2007). The advance of ERK phosphorylation observed after SMF stimulation for 3 h was not surprising because MAPK/ERK kinase activities had been reported to be rapidly regulated by Dex treatment within 2 h (Engelbrecht et al. 2003). Besides, the ECM-induced activation of ERK during the osteogenic process of stem cells was observed within 2 h, which represented one of the earliest decision making events controlling their commitment to the osteogenic phenotype (Salasznyk et al. 2007). Thus, the expression pattern of ERK activity at different time points during the osteogenic process remained to be elucidated in the future. In summary, the above results supported our assumption that the cooperative influence from SMF in osteogenic differentiation was possibly via reorganizing the interaction of cell-ECM to accelerate the activation and maturation of the DPCs-derived osteoblast-like cells that produced obvious mineralization. On the other hand, the regulation of ERK1/2 phosphorylation by SMF exposure seemed to depend on the difference in cell type, as well as on the magnetic flux intensity selected (Prina-Mello et al. 2006; Yuge et al. 2003). Despite the positive findings on the effect of SMF on the osteogenic differentiation of DPCs in this study, it needs to be confirmed whether SMF effect is mediated by ERK1/2-dependent activation or by another mechanism.
The repair of human mandible bone defect by grafting of DPCs and collagen sponge biocomplexes has been demonstrated recently (d’Aquino et al. 2009). The advantage of this procedure is efficient, exhibits low morbidity of the collection site, and is free from diseases incurred by transmission of pathogens. Thus, the autologous DPCs technique represents a new tool for bone tissue engineering. Here, our in vitro study further showed that SMF (290 mT) combined with osteogenic induction could enhance early osteogenic gene expressions, ALP activity, and extracellular calcium and bone mineralization of DPCs. SMF exposure alone was not sufficient to induce the occurrence of osteogenic differentiation, though it was able to change the reorganization and pattern of ECM and induce the mRNA expression of the OCN gene. Therefore, we speculated that it may play a cooperative role to accelerate osteogenic differentiation, the maturation of osteoblast-like cells, and mineralization, via the ECM-mediated activation of ERK1/2-Cbfa1 signaling. It has been demonstrated that a magnetic field accelerates the differentiation of osteoblasts. This study revealed for the first time that SMF exposure can increase the potential for progenitors/stem cells in dental pulp to differentiate into osteoblast-like cells, which may contribute to increase the efficacy of bone defect repair via DPCs transplantation in the bone tissue engineering.
Acknowledgments
This work was supported by the National Health Research Institutes and the National Science Council, Taiwan, Republic of China, and conducted in the Center of Tissue Engineering and Stem Cells Research of this university.
References
- Alliot-Licht B, Bluteau G, Magne D, Lopez-Cazaux S, Lieubeau B, et al. Dexamethasone stimulates differentiation of odontoblast-like cells in human dental pulp cultures. Cell Tissue Res. 2005;321:391–400. doi: 10.1007/s00441-005-1115-7. [DOI] [PubMed] [Google Scholar]
- Amara S, Douki T, Ravanat JL, Garrel C, Guiraud P, et al. Influence of a static magnetic field (250 mT) on the antioxidant response and DNA integrity in THP1 cells. Phys Med Biol. 2007;52:889–898. doi: 10.1088/0031-9155/52/4/002. [DOI] [PubMed] [Google Scholar]
- Bassett CA. Beneficial effects of electromagnetic fields. J Cell Biochem. 1993;51:387–393. doi: 10.1002/jcb.2400510402. [DOI] [PubMed] [Google Scholar]
- Batouli S, Miura M, Brahim J, Tsutsui TW, Fisher LW, et al. Comparison of stem-cell-mediated osteogenesis and dentinogenesis. J Dent Res. 2003;82:976–981. doi: 10.1177/154405910308201208. [DOI] [PubMed] [Google Scholar]
- Camilleri S, McDonald F. Static magnetic field effects on the sagittal suture in Rattus norvegicus. Am J Orthod Dentofacial Orthop. 1993;103:240–246. doi: 10.1016/0889-5406(93)70004-8. [DOI] [PubMed] [Google Scholar]
- d’Aquino R, De RA, Lanza V, Tirino V, Laino L, et al. Human mandible bone defect repair by the grafting of dental pulp stem/progenitor cells and collagen sponge biocomplexes. Eur Cell Mater. 2009;18:75–83. doi: 10.22203/ecm.v018a07. [DOI] [PubMed] [Google Scholar]
- D’Souza RN, Aberg T, Gaikwad J, Cavender A, Owen M, et al. Cbfa1 is required for epithelial-mesenchymal interactions regulating tooth development in mice. Development. 1999;126:2911–2920. doi: 10.1242/dev.126.13.2911. [DOI] [PubMed] [Google Scholar]
- Darendeliler MA, Sinclair PM, Kusy RP. The effects of samarium-cobalt magnets and pulsed electromagnetic fields on tooth movement. Am J Orthod Dentofacial Orthop. 1995;107:578–588. doi: 10.1016/S0889-5406(95)70100-1. [DOI] [PubMed] [Google Scholar]
- Darendeliler MA, Darendeliler A, Sinclair PM. Effects of static magnetic and pulsed electromagnetic fields on bone healing. Int J Adult Orthodon Orthognath Surg. 1997;12:43–53. [PubMed] [Google Scholar]
- Mattei M, Caruso A, Traina GC, Pezzetti F, Baroni T, et al. Correlation between pulsed electromagnetic fields exposure time and cell proliferation increase in human osteosarcoma cell lines and human normal osteoblast cells in vitro. Bioelectromagnetics. 1999;20:177–182. doi: 10.1002/(SICI)1521-186X(1999)20:3<177::AID-BEM4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Mendonca CA, Bueno DF, Martins MT, Kerkis I, Kerkis A, et al. Reconstruction of large cranial defects in nonimmunosuppressed experimental design with human dental pulp stem cells. J Craniofac Surg. 2008;19:204–210. doi: 10.1097/scs.0b013e31815c8a54. [DOI] [PubMed] [Google Scholar]
- Engelbrecht Y, de WH, Horsch K, Langeveldt CR, Hough FS, et al. Glucocorticoids induce rapid up-regulation of mitogen-activated protein kinase phosphatase-1 and dephosphorylation of extracellular signal-regulated kinase and impair proliferation in human and mouse osteoblast cell lines. Endocrinology. 2003;144:412–422. doi: 10.1210/en.2002-220769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flipo D, Fournier M, Benquet C, Roux P, Le BC, et al. Increased apoptosis, changes in intracellular Ca2 + , and functional alterations in lymphocytes and macrophages after in vitro exposure to static magnetic field. J Toxicol Environ Health A. 1998;54:63–76. doi: 10.1080/009841098159033. [DOI] [PubMed] [Google Scholar]
- Frendo JL, Xiao G, Fuchs S, Franceschi RT, Karsenty G, et al. Functional hierarchy between two OSE2 elements in the control of osteocalcin gene expression in vivo. J Biol Chem. 1998;273:30509–30516. doi: 10.1074/jbc.273.46.30509. [DOI] [PubMed] [Google Scholar]
- Goggins JF, Fullmer HM. Hydrolytic enzyme histochemistry of the rat molar pulp. Arch Oral Biol. 1967;12:639–644. doi: 10.1016/0003-9969(67)90082-9. [DOI] [PubMed] [Google Scholar]
- Graziano A, d’Aquino R, Cusella-De Angelis MG, De FF, Giordano A, et al. Scaffold’s surface geometry significantly affects human stem cell bone tissue engineering. J Cell Physiol. 2008;214:166–172. doi: 10.1002/jcp.21175. [DOI] [PubMed] [Google Scholar]
- Graziano A, d’Aquino R, Laino G, Papaccio G. Dental pulp stem cells: a promising tool for bone regeneration. Stem Cell Rev. 2008;4:21–26. doi: 10.1007/s12015-008-9015-3. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000;97:13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, et al. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531–535. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- Guido S, Tranquillo RT. A methodology for the systematic and quantitative study of cell contact guidance in oriented collagen gels. Correlation of fibroblast orientation and gel birefringence. J Cell Sci. 1993;105(Pt 2):317–331. doi: 10.1242/jcs.105.2.317. [DOI] [PubMed] [Google Scholar]
- Hall BK, Miyake T. Divide, accumulate, differentiate: cell condensation in skeletal development revisited. Int J Dev Biol. 1995;39:881–893. [PubMed] [Google Scholar]
- Hughes-Fulford M (2004) Signal transduction and mechanical stress. Sci STKE 2004:RE12 [DOI] [PubMed]
- Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T. Mechanical stress-mediated Runx2 activation is dependent on Ras/ERK1/2 MAPK signaling in osteoblasts. J Cell Biochem. 2007;101:1266–1277. doi: 10.1002/jcb.21249. [DOI] [PubMed] [Google Scholar]
- Kasugai S, Shibata S, Suzuki S, Susami T, Ogura H. Characterization of a system of mineralized-tissue formation by rat dental pulp cells in culture. Arch Oral Biol. 1993;38:769–777. doi: 10.1016/0003-9969(93)90073-U. [DOI] [PubMed] [Google Scholar]
- Kotani H, Kawaguchi H, Shimoaka T, Iwasaka M, Ueno S, et al. Strong static magnetic field stimulates bone formation to a definite orientation in vitro and in vivo. J Bone Miner Res. 2002;17:1814–1821. doi: 10.1359/jbmr.2002.17.10.1814. [DOI] [PubMed] [Google Scholar]
- Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, et al. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB) J Bone Miner Res. 2005;20:1394–1402. doi: 10.1359/JBMR.050325. [DOI] [PubMed] [Google Scholar]
- Lin SL, Chang WJ, Chiu KH, Hsieh SC, Lee SY, et al. Mechanobiology of MG63 osteoblast-like cells adaptation to static magnetic forces. Electromagn Biol Med. 2008;27:55–64. doi: 10.1080/15368370701878960. [DOI] [PubMed] [Google Scholar]
- McCabe LR, Kockx M, Lian J, Stein J, Stein G. Selective expression of fos- and jun-related genes during osteoblast proliferation and differentiation. Exp Cell Res. 1995;218:255–262. doi: 10.1006/excr.1995.1154. [DOI] [PubMed] [Google Scholar]
- McLeod KJ, Collazo L. Suppression of a differentiation response in MC-3T3–E1 osteoblast-like cells by sustained, low-level, 30 Hz magnetic-field exposure. Radiat Res. 2000;153:706–714. doi: 10.1667/0033-7587(2000)153[0706:SOADRI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Miyakoshi J. Effects of static magnetic fields at the cellular level. Prog Biophys Mol Biol. 2005;87:213–223. doi: 10.1016/j.pbiomolbio.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Noar JH, Evans RD. Rare earth magnets in orthodontics: an overview. Br J Orthod. 1999;26:29–37. doi: 10.1093/ortho/26.1.29. [DOI] [PubMed] [Google Scholar]
- Osyczka AM, Diefenderfer DL, Bhargave G, Leboy PS. Different effects of BMP-2 on marrow stromal cells from human and rat bone. Cells Tissues Organs. 2004;176:109–119. doi: 10.1159/000075032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaccio G, Graziano A, d’Aquino R, Graziano MF, Pirozzi G, et al. Long-term cryopreservation of dental pulp stem cells (SBP-DPSCs) and their differentiated osteoblasts: a cell source for tissue repair. J Cell Physiol. 2006;208:319–325. doi: 10.1002/jcp.20667. [DOI] [PubMed] [Google Scholar]
- Prina-Mello A, Farrell E, Prendergast PJ, Campbell V, Coey JM. Influence of strong static magnetic fields on primary cortical neurons. Bioelectromagnetics. 2006;27:35–42. doi: 10.1002/bem.20173. [DOI] [PubMed] [Google Scholar]
- Salasznyk RM, Klees RF, Williams WA, Boskey A, Plopper GE. Focal adhesion kinase signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells. Exp Cell Res. 2007;313:22–37. doi: 10.1016/j.yexcr.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazu Y, Nanci A, Aoba T. Immunodetection of osteopontin at sites of resorption in the pulp of rat molars. J Histochem Cytochem. 2002;50:911–921. doi: 10.1177/002215540205000705. [DOI] [PubMed] [Google Scholar]
- Spoto G, Fioroni M, Rubini C, Tripodi D, Di SM, et al. Alkaline phosphatase activity in normal and inflamed dental pulps. J Endod. 2001;27:180–182. doi: 10.1097/00004770-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Tonomura A, Sumita Y, Ando Y, Iejima D, Kagami H, et al. Differential inducibility of human and porcine dental pulp-derived cells into odontoblasts. Connect Tissue Res. 2007;48:229–238. doi: 10.1080/03008200701507909. [DOI] [PubMed] [Google Scholar]
- Torbet J, Ronziere MC. Magnetic alignment of collagen during self-assembly. Biochem J. 1984;219:1057–1059. doi: 10.1042/bj2191057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Molen MA, Donahue HJ, Rubin CT, McLeod KJ. Osteoblastic networks with deficient coupling: differential effects of magnetic and electric field exposure. Bone. 2000;27:227–231. doi: 10.1016/S8756-3282(00)00315-X. [DOI] [PubMed] [Google Scholar]
- Xiao G, Cui Y, Ducy P, Karsenty G, Franceschi RT. Ascorbic acid-dependent activation of the osteocalcin promoter in MC3T3–E1 preosteoblasts: requirement for collagen matrix synthesis and the presence of an intact OSE2 sequence. Mol Endocrinol. 1997;11:1103–1113. doi: 10.1210/me.11.8.1103. [DOI] [PubMed] [Google Scholar]
- Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RT. Role of the alpha2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J Biol Chem. 1998;273:32988–32994. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- Xiao G, Jiang D, Thomas P, Benson MD, Guan K, et al. MAPK pathways activate and phosphorylate the osteoblast-specific transcription factor, Cbfa1. J Biol Chem. 2000;275:4453–4459. doi: 10.1074/jbc.275.6.4453. [DOI] [PubMed] [Google Scholar]
- Xu C, Fan Z, Chao YL, Du L, Zhang FQ. Magnetic fields of 10 and 120 mT change cell shape and structure of F-actins of periodontal ligament cells. Bioelectrochemistry. 2008;72:41–46. doi: 10.1016/j.bioelechem.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Ohsaki Y, Goto T, Nakasima A, Iijima T. Effects of static magnetic fields on bone formation in rat osteoblast cultures. J Dent Res. 2003;82:962–966. doi: 10.1177/154405910308201205. [DOI] [PubMed] [Google Scholar]
- Yan QC, Tomita N, Ikada Y. Effects of static magnetic field on bone formation of rat femurs. Med Eng Phys. 1998;20:397–402. doi: 10.1016/S1350-4533(98)00051-4. [DOI] [PubMed] [Google Scholar]
- Yang X, van den DJ, Walboomers XF, Zhang W, Bian Z, et al. The odontogenic potential of STRO-1 sorted rat dental pulp stem cells in vitro. J Tissue Eng Regen Med. 2007;1:66–73. doi: 10.1002/term.16. [DOI] [PubMed] [Google Scholar]
- Yokose S, Kadokura H, Tajima Y, Fujieda K, Katayama I, et al. Establishment and characterization of a culture system for enzymatically released rat dental pulp cells. Calcif Tissue Int. 2000;66:139–144. doi: 10.1007/s002230010028. [DOI] [PubMed] [Google Scholar]
- Yoshiki S, Kurahashi Y. A light and electron microscopic study of alkaline phosphatase activity in the early stage of dentinogenesis in the young rat. Arch Oral Biol. 1971;16:1143–1154. doi: 10.1016/0003-9969(71)90043-4. [DOI] [PubMed] [Google Scholar]
- Yuge L, Okubo A, Miyashita T, Kumagai T, Nikawa T, et al. Physical stress by magnetic force accelerates differentiation of human osteoblasts. Biochem Biophys Res Commun. 2003;311:32–38. doi: 10.1016/j.bbrc.2003.09.156. [DOI] [PubMed] [Google Scholar]
- Zhang W, Walboomers XF, Wolke JG, Bian Z, Fan MW, et al. Differentiation ability of rat postnatal dental pulp cells in vitro. Tissue Eng. 2005;11:357–368. doi: 10.1089/ten.2005.11.357. [DOI] [PubMed] [Google Scholar]