Abstract
Objective
To determine whether intranasal corticosteroids are superior to oral H1 receptor antagonists (antihistamines) in the treatment of allergic rhinitis.
Design
Meta-analysis of randomised controlled trials comparing intranasal corticosteroids with oral antihistamines.
Setting
Randomised controlled trials conducted worldwide and published between 1966 and 1997.
Subjects
2267 subjects with allergic rhinitis in 16 randomised controlled trials.
Main outcome measures
Nasal blockage, nasal discharge, sneezing, nasal itch, postnasal drip, nasal discomfort, total nasal symptoms, nasal resistance, and eye symptoms and global ratings. Outcomes measured on different scales were combined to determine pooled odds ratios (categorical outcomes) or standardised mean differences (continuous outcomes). Assessment of heterogeneity between studies, and subgroup analyses of eye symptoms, were undertaken.
Results
Intranasal corticosteroids produced significantly greater relief than oral antihistamines of nasal blockage (standardised mean difference −0.63, 95% confidence interval −0.73 to −0.53), nasal discharge (−0.5, −0.6 to −0.4), sneezing (−0.49, −0.59 to −0.39), nasal itch (−0.38, −0.49 to −0.21), postnasal drip (−0.24, −0.42 to −0.06), and total nasal symptoms (−0.42, −0.53 to −0.32), and global ratings gave an odds ratio for deterioration of symptoms of 0.26 (0.08 to 0.8). There were no significant differences between treatments for nasal discomfort, nasal resistance, or eye symptoms. The effects on sneezing, total nasal symptoms, and eye symptoms were significantly heterogeneous between studies. Other combined outcomes were homogeneous between studies. Subgroup analysis of the outcome of eye symptoms suggested that the duration of assessment (averaged mean score over the study period versus mean score at end of study period) might have accounted for the heterogeneity.
Conclusion
The results of this systematic review, together with data on safety and cost effectiveness, support the use of intranasal corticosteroids over oral antihistamines as first line treatment for allergic rhinitis.
Key messages
Allergic rhinitis is increasing in prevalence and is a common cause of morbidity
Intranasal corticosteroids produce greater relief from most nasal symptoms than do oral H1 receptor antagonists (antihistamines)
Intranasal corticosteroids and oral antihistamines show no difference in the relief of the eye symptoms of allergic rhinitis
Intranasal corticosteroids are more cost effective than oral antihistamines
Intranasal corticosteroids are recommended for first line treatment of allergic rhinitis
Introduction
Allergic rhinitis is a common disease characterised by nasal itch, sneezing, watery and mucous rhinorrhoea, and nasal obstruction.1 The condition is often accompanied by allergic conjunctivitis. In the past 30 years there has been a dramatic increase in the prevalence of allergic rhinitis, and studies from England, Sweden, and Australia have confirmed a doubling of prevalence over this time.2–4 Studies from Australia showed that in Tasmania the prevalence of hay fever is 41%,4 and that hay fever is the second most frequently self reported condition in Australia.5
Apart from local disease, allergic rhinitis can cause considerable morbidity including chronic sinusitis and otitis. The condition can also cause irritability and impaired sleep which can affect quality of life by leading to poor performance at school or work, absenteeism from school or work, and chronic tiredness. It can also have detrimental effects on emotional and social wellbeing.1,6
Treatment of allergic rhinitis includes avoiding allergens (when possible), intranasal corticosteroids, short term decongestants, oral or topical H1 receptor antagonists (antihistamines), intranasal cromoglycate, anticholinergic agents, and allergen immunotherapy.1
Topical intranasal corticosteroids are said to be more effective than oral antihistamines in controlling nasal blockage and discharge.1,7 Furthermore, oral antihistamines are said to be better at treating nasal itch, sneezing, and eye symptoms.1,7 There is also a perception, especially in popular reviews on allergic rhinitis, that intranasal corticosteroids do not improve eye symptoms.8
To address these issues we reviewed published randomised controlled trials comparing intranasal corticosteroids with oral antihistamines, and performed a meta-analysis on the efficacy of these interventions on relevant clinical outcomes.
Selection criteria
We restricted our review to randomised controlled trials, which provide the strongest evidence for the efficacy of any medical treatment. We included only studies that focused on allergic rhinitis, and we did not consider studies on the treatment of nasal polyps.
For the purposes of our review, intranasal corticosteroids included beclomethasone dipropionate, budesonide, flunisolide, fluocortin, fluticasone propionate, mometasone, and triamcinolone acetonide. All forms of delivery vehicle (aqueous and non-aqueous) were considered. We included studies of comparisons with any form of oral antihistamine, but excluded studies that used topical antihistamines or topical mast cell stabilisers. Studies were also excluded if they were not randomised or not double blinded.
For a study to be included in our review at least one of the following clinical outcomes had to be reported: nasal symptoms (including total nasal symptom scores), eye symptoms, global symptoms, drug requirements for treating the rhinitis, nasal function (including measurements of nasal resistance), and assessment of quality of life.
We excluded studies that reported only nasal challenge with specific allergens or non-clinical outcomes, such as in vitro results of inflammatory mediators. We considered studies published in languages other than English if the translated abstract indicated that the study was a randomised controlled trial of intranasal corticosteroids for rhinitis, and a translator was sought.
Search strategy
We conducted Medline and Embase searches for randomised controlled trials of topical corticosteroids and rhinitis published between 1966 and 1997. Specifically, studies were retrieved that were indexed as randomised controlled trials with treatments comprising the following intranasal corticosteroids: beclomethasone dipropionate, budesonide, flunisolide, fluocortin, fluticasone propionate, mometasone, and triamcinolone acetonide. Review articles identified in this process were surveyed for additional and earlier citations. We also used Healthgate and Winspirs software to search Medline for more recently published studies. Where relevant abstracts were identified in conference proceedings, Medline searches were conducted and inquiries made of the authors or sponsoring companies to identify any subsequent full publications.
Methods
Inclusion of studies in the review was decided by a simple majority of all three reviewers, who independently read the methods sections of papers identified by the search strategy and applied the stated criteria. Quality assessment was performed by two reviewers (RMP and JMW), who independently assessed the concealment of allocation following the guidelines of the Cochrane Collaboration.9 We calculated the mean daily cost of intranasal corticosteroids and of non-sedating oral antihistamines available in Australia.
Statistical considerations
We compared the effectiveness of intranasal corticosteroids versus oral antihistamines on nasal symptoms, eye symptoms, and nasal resistance, whenever the results were reported.
For the purpose of statistical analysis outcome data were extracted and entered into RevMan 3.1 (Update Software, Oxford). Categorical outcomes (global ratings) were analysed as odds ratios and 95% confidence intervals, calculated by Peto’s method for individual studies. The odds ratio was calculated by expressing the odds for deterioration or no change in the treatment group divided by the odds for deterioration or no change in the control group. The convention of the Cochrane Collaboration is to consider odds ratios >1.0 as indicating clinically undesirable outcomes.
Continuous outcomes (symptom scores, nasal resistance) were also extracted from tables. Continuous outcomes were analysed as standardised mean differences. The standardised mean difference is a statistic which expresses the difference in means between corticosteroid groups and control groups after treatment in units of the pooled SD. In individual studies, scores for nasal itch and other symptoms were self reported by patients. Standardised mean differences allow the scores from different assessment scales to be combined. It was thus possible for us to combine symptom scores measured on ordinal scales and visual analogue scales.
Fixed effects models were used to obtain summary statistics for the overall efficacy of intranasal corticosteroids on both categorical and continuous outcomes, and χ2 tests were performed to assess heterogeneity between studies. In this context, a P value of <0.05 indicates significant differences between studies, and raises doubts whether the results can be meaningfully combined. Sensitivity and subgroup analyses were undertaken to identify the sources of such heterogeneity.
Description of studies
We identified 16 studies that complied with the inclusion criteria, and from which we were able to obtain sufficient data either directly, or after corresponding with the sponsoring companies or authors.10–25 These studies totalled 2267 subjects (mean age 32 years, range 12 to 75 years), of whom 1247 (55%) were men. Two further studies met the inclusion criteria but they contained insufficient data to allow meta-analysis, and we were unable to obtain further information despite several attempts.26,27 The table summarises the characteristics of the included studies.
We excluded 16 studies from the meta-analysis. Reasons for exclusion were use of: non-random allocation,28,29 a single blind protocol,30,31 combined intranasal corticosteroid and oral antihistamines in the comparison arm,32,33 topical antihistamines in the comparison arm,34,35 decongestant in the comparison arm,36–38 non-clinical challenge or outcome,39,40 and the publication of an abstract only without reporting detailed results.41–43
Methodological quality
All included studies were of high calibre incorporating the features of clearly stated objectives, defined diagnostic criteria, stated source of subjects, randomisation, double blindedness, well defined treatments, and a description of withdrawals and dropouts. Methods of concealing allocation to the treatment arms were identified and classified according to the criteria of the Cochrane Collaboration9: A, adequate; B, allocation method unclear; and C, inadequate. Two studies were classified as A13,19 and the remainder were classified as B.
Results
Nasal symptoms
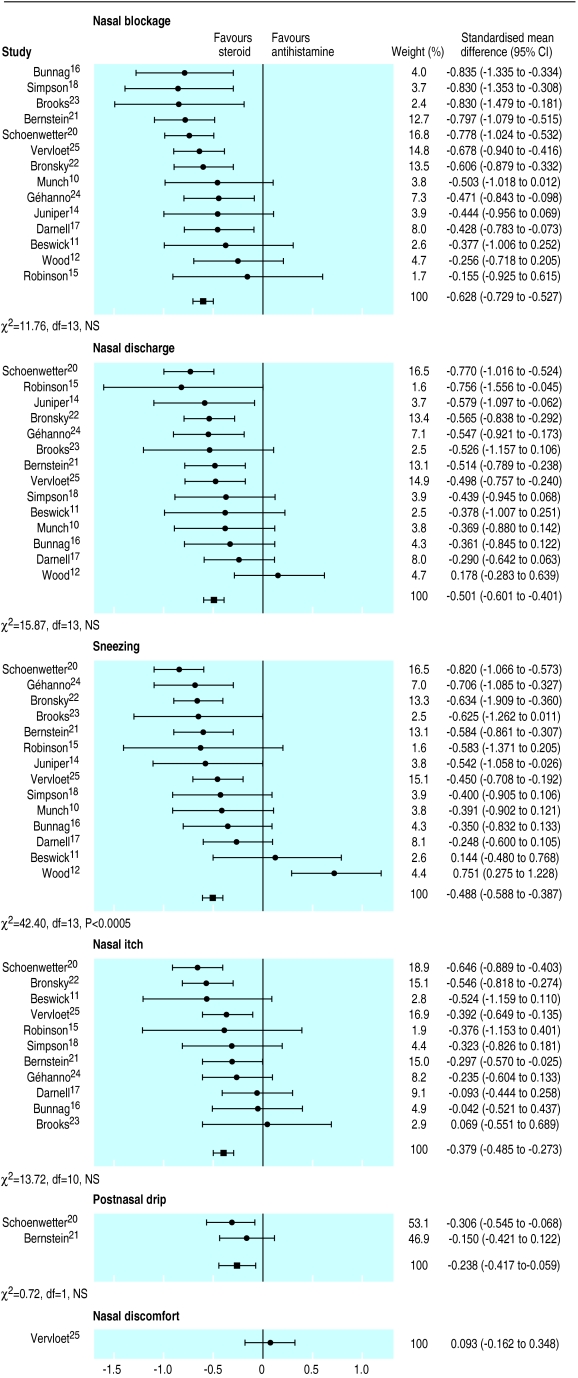
Figure 1 gives an overview of the results for nasal symptoms. Scores for nasal blockage, nasal discharge, and sneezing were reported by 14 studies each. Intranasal corticosteroids produced significantly greater relief of nasal blockage than did oral antihistamines (combined standardised mean difference −0.63 (95% confidence interval −0.73 to −0.53)). Intranasal corticosteroids produced significantly greater relief of nasal discharge (−0.5, −0.6 to −0.4) than did oral antihistamines. These effects were homogeneous between studies (χ2=11.8, 15.9 NS). Intranasal corticosteroids were also more effective in relieving sneezing (−0.49, −0.59 to −0.39). However, there was significant heterogeneity (χ2=42.4, P<0.0005) with one study showing that oral antihistamines produced greater relief of sneezing than did intranasal corticosteroids.12
Figure 1.
Comparison of effectiveness of intranasal corticosteroids and oral H1 receptor antagonists (antihistamines) on nasal symptoms
Nasal itch scores were reported by 11 studies. Intranasal corticosteroids produced significantly greater relief of itch than did oral antihistamines (combined standardised mean difference −0.38, −0.49 to −0.21). In two studies there was a modest but still significant effect of intranasal corticosteroids on postnasal drip (−0.24, −0.42 to −0.06). Both of these effects were homogeneous. Only one study reported nasal discomfort, and there was no significant difference between the two treatments.25
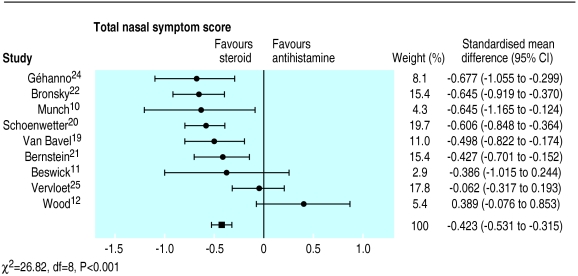
Total nasal symptom scores were reported by nine studies (fig 2). Intranasal corticosteroids produced significantly greater relief of total nasal symptoms than did oral antihistamines (−0.42, −0.53 to −0.32). However, there was significant heterogeneity (χ2=26.8, P<0.001), with Wood12 showing greater (albeit not significantly) relief of symptoms with oral antihistamines than with intranasal corticosteroids.
Figure 2.
Comparison of effects of intranasal corticosteroids and oral H1 receptor antagonists (antihistamines) on total nasal symptom scores
Eye symptoms
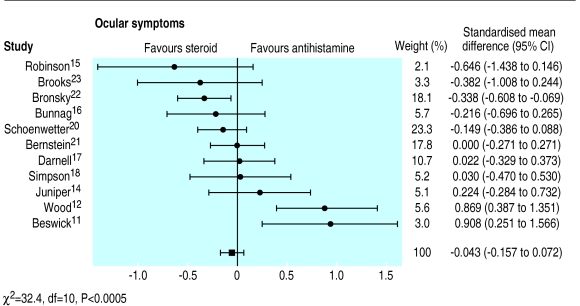
Eye symptoms were reported by 11 studies (fig 3). There was no significant difference between intranasal corticosteroids and oral antihistamines on eye symptoms. There was significant heterogeneity (χ2=32.4, P<0.0005), and both Beswick11 and Wood12 showed that oral antihistamines were more efficacious than intranasal corticosteroids. When these two outliers were removed, homogeneity was achieved.
Figure 3.
Comparison of effects of intranasal corticosteroids and oral H1 receptor antagonists (antihistamines) on eye symptoms
To further identify the source of the heterogeneity, subgroup analyses were conducted. Stratification by intranasal corticosteroids showed that most of the heterogeneity (χ2=18.7, P<0.001) occurred in trials with beclomethasone. Trials that used intranasal fluticasone, triamcinolone, or budesonide all showed no difference from oral antihistamines. This was a homogeneous finding. Stratification by antihistamine showed significant heterogeneity in trials that used terfenadine (χ2=14.6, P<0.01) or astemizole (χ2=12, P<0.01). Stratification by the period of data extraction showed a small homogeneous benefit from intranasal corticosteroids (−0.17, −0.35 to −0.05) in those trials reporting eye symptoms as a single end point. There was, however, significant heterogeneity (χ2=20.2, P<0.0005) when eye symptoms had been averaged over the duration of the trial.
Other outcomes
Global ratings were reported by only two studies.13,23 Patients randomised to receive intranasal corticosteroids were 0.26 (95% confidence interval 0.08 to 0.8) times more likely to deteriorate than those patients randomised to receive oral antihistamines. There was thus a significant and homogeneous benefit from intranasal corticosteroids. Nasal resistance was only reported by one trial, which did not find any difference between treatments.13 None of the studies included in this review separately reported drug scores or quality of life.
Discussion
Our systematic review of the effectiveness of intranasal corticosteroids versus oral H1 receptor antagonists (antihistamines) for allergic rhinitis identified 18 randomised controlled trials that met the inclusion criteria. The meta-analysis of 16 evaluable trials confirmed that intranasal corticosteroids were significantly more effective at relieving nasal blockage, discharge, and itch, and postnasal drip than were oral antihistamines. Furthermore, all these results were homogeneous between studies. This indicates that an analysis of pooled data from clinical trials strongly supports the clinical suspicion that intranasal corticosteroids are more effective than oral antihistamines for such nasal symptoms.1
Intranasal corticosteroids were also more effective at relieving sneezing and at reducing total nasal symptoms than oral antihistamines, but there was significant heterogeneity between studies. Some heterogeneity could be accounted for by differences in scoring symptoms, although only one of the 13 studies showed that oral antihistamines produced greater relief of sneezing than did intranasal corticosteroids, and none of the nine studies showed that oral antihistamines significantly improved total nasal symptom scores. Despite the heterogeneity, we suggest that the pooled data favour the use of intranasal corticosteroids for relieving nasal symptoms.
Two studies met the inclusion criteria, but we were unable to obtain sufficient data for analysis.26,27 Both of these studies favoured intranasal corticosteroids for treating allergic rhinitis, and inclusion of these studies was unlikely to have altered the combined outcomes.
The various studies, however, measured symptom scores on different scales. For example in the study by Géhanno and Desfougeres the benefit from intranasal corticosteroids was equivalent to an additional 1.8 days symptom free per week.24 We believe that effects of this magnitude are clinically important.
The results of the pooled data on eye symptoms are surprising as there was no difference between the effectiveness of intranasal corticosteroids and oral antihistamines, although there was significant heterogeneity. Subgroup analysis suggested that this heterogeneity was not due to the use of different intranasal corticosteroids and oral antihistamines in the various trials. In some trials, however, the effect of each treatment was expressed as a mean score over the whole study period of 6 to 8 weeks and in others as the mean score in the last 2 weeks of an intervention period of 8 weeks. The stratified analysis indicated that much of the heterogeneity resulted from those studies where eye symptoms had been averaged over the entire duration of treatment. A possible explanation for this observation is the difference in the onset of action between intranasal corticosteroids and oral antihistamines. However, the difference in onset may be much smaller than commonly believed.44 Although the effect of oral antihistamines on the suppression of histamine induced wheal and flare reactions is rapid, the clinical onset in seasonal allergic rhinitis may take up to 5 hours.45 Furthermore, although intranasal corticosteroids were previously thought to take 3-10 days before a beneficial effect was observed, recent studies have shown significant relief of nasal symptoms in 12-24 hours.46,47 In addition, continuing treatment with intranasal corticosteroids may lead to a significant inhibition of the early nasal response as well as almost total inhibition of the late nasal response.48 Briefly, we believe that differences in onset between the intranasal corticosteroids and oral antihistamines might explain the observed heterogeneity of the subgroup analysis, but we are not convinced that these differences in onset of action translate into important clinical differences, for the reasons outlined.
Despite these reservations the results do not support the widely held view that oral antihistamines are superior to intranasal corticosteroids for controlling eye symptoms in allergic rhinitis.7 We calculated that there was no difference between these treatment modalities when eye symptoms were measured. Intranasal corticosteroids may improve eye symptoms by increasing nasolacrimal drainage, or there may be an effect from absorption of the corticosteroid.
Intranasal corticosteroids are considered safe. Local adverse effects are usually mild (mucosal irritation, epistaxis), and nasal septal perforation is exceptionally rare.49 Clinical and histopathological examination of nasal mucosa up to 5.5 years of continuous budesonide use have failed to show significant changes.50 Intranasal corticosteroids can result in systemic bioavailability,51 but studies have failed to show significant effects on serum markers of bone metabolism,52 short term bone growth,53 or cortisol concentrations after stimulation by adrenocorticotrophic hormone.54
First generation oral antihistamines are safe, but sedative and anticholinergic effects may be troublesome.55 Second generation (non-sedating or low-sedating) oral antihistamines do not have these effects and are well tolerated. Near fatal and fatal arrhythmias have been reported with terfenadine and astemizole,55 and these drugs are contraindicated in patients with heart or liver disease or when there is concomitant treatment with drugs that inhibit the hepatic cytochrome P-450 system.
The cost effectiveness of intranasal corticosteroids versus oral antihistamines was assessed in three randomised controlled trials on the treatment of allergic rhinitis. An American study showed that if a patient used terfenadine for more than 11 to 22 days, then fluticasone was a more cost effective choice.56 Two cost effectiveness analyses performed in Canada produced cost effective ratios of 1:2.5 and 1:5.7 in favour of fluticasone versus terfenadine and loratadine respectively.57 We were not able to perform such an analysis on our data, but we did compare the mean daily cost of oral antihistamines in Australia (by asking pharmacists in four Australian states) with the mean daily cost of intranasal corticosteroids (based on Australian pharmaceutical benefits schedule). The mean daily cost of oral antihistamines was 4.5 times that of intranasal corticosteroids. We believe that the results of the North American studies and our data suggest that intranasal corticosteroids are more cost effective than oral antihistamines in the first line treatment of allergic rhinitis. There may be a role for oral antihistamines as ancillary treatment, particularly if eye symptoms or nasal itch are not controlled by intranasal corticosteroids.
Table.
Characteristics of included studies. All studies were double blind, double dummy,* parallel group, randomised controlled trials except where indicated
| Study | No of participants with seasonal allergic rhinitis | Age range (mean age) | Interventions | Outcomes |
|---|---|---|---|---|
| Munch10 | 61 | 16-56 (29) | Budesonide 400 μg | Nasal symptoms |
| Dexchlorpheniramine 12 mg | Eye symptoms | |||
| Beswick11 | 49 | 14-64 (28) | Beclomethasone 400 μg | Nasal symptoms |
| Terfenadine 120 mg | Eye symptoms | |||
| Global rating | ||||
| Wood12 | 74 | 12-75 (28) | Beclomethasone 400 μg | Nasal symptoms |
| Astemizole 10 mg | Eye symptoms | |||
| Lancer13 | 18 | Not reported | Beclomethasone 400 μg | Global rating |
| Terfenadine 120 mg | Nasal resistance | |||
| Juniper14 | 90 | 18-70 (41) | Beclomethasone 400 μg | Nasal symptoms |
| Astemizole 10 mg | Eye symptoms | |||
| Robinson†15 | 20 | 15-58 (31) | Beclomethasone 400 μg | Nasal symptoms |
| Terfenadine 120 mg | Eye symptoms | |||
| Bunnag16 | 69‡ | 16-70 (30) | Budesonide 200 μg | Nasal symptoms |
| Astemizole 10 mg | ||||
| Darnell17 | 214 | 13-69 (28) | Fluticasone 200 μg | Nasal symptoms |
| Terfenadine 120 mg | Eye symptoms | |||
| Global rating | ||||
| Simpson18 | 143 | >15 (27) | Budesonide 400 μg | Nasal symptoms |
| Terfenadine 120 mg | Eye symptoms | |||
| Van Bavel19 | 232 | >12 (40) | Fluticasone 200 μg | Nasal symptoms |
| Terfenadine 120 mg | Global rating | |||
| Schoenwetter20 | 298 | >12 (31) | Triamcinolone 220 μg | Nasal symptoms |
| Loratadine 10 mg | Eye symptoms | |||
| Bernstein21 | 239 | Adults (36) | Triamcinolone 220 μg | Nasal symptoms |
| Astemizole 10 mg | Eye symptoms | |||
| Global rating | ||||
| Bronsky22 | 348 | >12 (30) | Fluticasone 200 μg | Nasal symptoms |
| Terfenadine 120 mg | Global rating | |||
| Nasal resistance | ||||
| Brooks23 | 60 | Not reported | Beclomethasone 336 μg | Nasal symptoms |
| Loratadine 10 mg | Eye symptoms | |||
| Global rating | ||||
| Géhanno24 | 114 | 13-80 (39) | Fluticasone 200 μg | Nasal symptoms |
| Loratadine 10 mg | Global rating | |||
| Vervloet25 | 238 | 12-75 (29) | Fluticasone 200 μg | Nasal symptoms |
| Cetirizine 10 mg |
All patients took tablets and used nasal sprays, but only one preparation was active.
Double blind double dummy crossover randomised controlled trial.
Participants had perennial allergic rhinitis.
Acknowledgments
We thank the following people who provided unpublished data from clinical trials: Eric Lehl (Astra Australia), Margaret Ward Curran (Glaxo-Wellcome), and Katy Williams-Day and Steven Francom (Pharmacia and Upjohn). Michael Bailey prepared the illustrations.
Footnotes
Funding: Astra Pharmaceuticals (Australia) provided library and research assistance and support for presentation at a scientific meeting. Astra did not provide any other direct financial support.
Conflict of interest: Each of the authors is involved in clinical practice and prescribes both intranasal corticosteroids and oral antihistamines for patients. While the support of Astra Pharmaceuticals (manufacturers of budesonide brand nasal spray) is acknowledged, the authors produced this review independently and it was not subject to any editorial review or changes by Astra. The authors believe that no conflict of interest arose during the production of this paper.
References
- 1.International Rhinitis Management Working Group. International consensus report on the diagnosis and management of rhinitis. Allergy. 1994;49(suppl 19):1–34. [PubMed] [Google Scholar]
- 2.Fleming DM, Crombie LD. Prevalence of hay fever in England and Wales. BMJ. 1987;294:279–283. doi: 10.1136/bmj.294.6567.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aberg N. Asthma and allergic rhinitis in Swedish conscripts. Clin Exp Allergy. 1989;19:59–63. doi: 10.1111/j.1365-2222.1989.tb02345.x. [DOI] [PubMed] [Google Scholar]
- 4.Hopper T, Jenkin M, Carlin C, Giles G. Increase in the self-reported prevalence of asthma and hay fever in adults over the past generation. Aust J Pub Health. 1995;19:120–124. doi: 10.1111/j.1753-6405.1995.tb00359.x. [DOI] [PubMed] [Google Scholar]
- 5.Australian Bureau of Statistics, 1989-90. National health survey. Asthma and other respiratory conditions. Australia: Commonwealth Government Printer; 1991. [Google Scholar]
- 6.Ziering RW. Immediate and late effects of hay fever. Postgrad Med. 1989;85:183–190. doi: 10.1080/00325481.1989.11700699. [DOI] [PubMed] [Google Scholar]
- 7.Calderon-Zapata MA, Davies RJ. Treatment and management of allergic rhinitis. In: Kay AB, editor. Allergy and allergic diseases. Oxford: Blackwell; 1997. [Google Scholar]
- 8.Prenner BM. Allergies for all seasons! Allergy Asthma. 1997;6:8–9. [Google Scholar]
- 9.Sackett D, Oxman A, editors. The Cochrane Collaboration handbook. Oxford: Update Software; 1996. [Google Scholar]
- 10.Munch EP, Soborg M, Norreslet TT, Mygind N. A comparative study of dexchlorpheniramine maleate sustained release tablets and budesonide nasal spray in seasonal allergic rhinitis. Allergy. 1983;38:517–524. doi: 10.1111/j.1398-9995.1983.tb02361.x. [DOI] [PubMed] [Google Scholar]
- 11.Beswick KB, Kenyon GS, Cherry JR. A comparative study of beclomethasone dipropionate aqueous nasal spray with terfenadine tablets in seasonal allergic rhinitis. Curr Med Res Opin. 1985;9:560–567. doi: 10.1185/03007998509109635. [DOI] [PubMed] [Google Scholar]
- 12.Wood SF. Oral antihistamine or nasal steroid in hay fever; a double-blind double-dummy comparative study of once daily oral astemizole vs twice daily nasal beclomethasone dipropionate. Clin Allergy. 1986;16:195–201. doi: 10.1111/j.1365-2222.1986.tb00766.x. [DOI] [PubMed] [Google Scholar]
- 13.Lancer JM, Jones AS, Stevens JC, Beckingham E. A comparison by rhinomanometry of beclomethasone and terfenadine in the treatment of seasonal rhinitis. J Laryngol Otol. 1987;101:350–354. [Google Scholar]
- 14.Juniper EF, Kline PA, Hargreave FE, Dolovich J. Comparison of beclomethasone dipropionate aqueous nasal spray, astemizole, and the combination in the prophylactic treatment of ragweed pollen-induced rhinoconjunctivitis. J Allergy Clin Immunol. 1989;83:627–633. doi: 10.1016/0091-6749(89)90075-4. [DOI] [PubMed] [Google Scholar]
- 15.Robinson AC, Cherry JR, Daly S. Double-blind cross-over trial comparing beclomethasone dipropionate and terfenadine in perennial rhinitis. Clin Exp Allergy. 1989;19:569–573. doi: 10.1111/j.1365-2222.1989.tb02437.x. [DOI] [PubMed] [Google Scholar]
- 16.Bunnag C, Jareoncharsri P, Wong EC. A double-blind comparison of nasal budesonide and oral astemizole for the treatment of perennial rhinitis. Allergy. 1992;47:313–317. doi: 10.1111/j.1398-9995.1992.tb02060.x. [DOI] [PubMed] [Google Scholar]
- 17.Darnell R, Pecoud A, Richards DH. A double-blind comparison of fluticasone propionate aqueous nasal spray, terfenadine tablets and placebo in the treatment of patients with seasonal allergic rhinitis to grass pollen. Clin Exp Allergy. 1994;24:1144–1150. doi: 10.1111/j.1365-2222.1994.tb03320.x. [DOI] [PubMed] [Google Scholar]
- 18.Simpson RJ. Budesonide and terfenadine, separately and in combination, in the treatment of hay fever. Ann Allergy. 1994;73:497–502. [PubMed] [Google Scholar]
- 19.Van Bavel J, Findlay SR, Hampel FC, Jr, Martin BG, Ratner P, Field E. Intranasal fluticasone propionate is more effective than terfenadine tablets for seasonal allergic rhinitis. Arch Intern Med. 1994;154:2699–2704. doi: 10.1001/archinte.1994.00420230086010. [DOI] [PubMed] [Google Scholar]
- 20.Schoenwetter W, Lim J. Comparison of intranasal triamcinolone acetonide with oral loratadine for the treatment of patients with seasonal allergic rhinitis. Clin Ther. 1995;17:479–492. doi: 10.1016/0149-2918(95)80113-8. [DOI] [PubMed] [Google Scholar]
- 21.Bernstein DI, Creticos PS, Busse WW, Cohen R, Graft DF, Howland WC, et al. Comparison of triamcinolone acetonide nasal inhaler with astemizole in the treatment of ragweed-induced allergic rhinitis. J Allergy Clin Immunol. 1996;97:749–755. doi: 10.1016/s0091-6749(96)80151-5. [DOI] [PubMed] [Google Scholar]
- 22.Bronsky EA, Dockhorn RJ, Meltzer EO, Shapiro G, Boltansky H, LaForce C, et al. Fluticasone propionate aqueous nasal spray compared with terfenadine tablets in the treatment of seasonal allergic rhinitis. J Allergy Clin Immunol. 1996;97:915–921. doi: 10.1016/s0091-6749(96)80065-0. [DOI] [PubMed] [Google Scholar]
- 23.Brooks CD, Francom SF, Peel BG, Chene BL, Klott KA. Spectrum of seasonal allergic rhinitis symptom relief with topical corticoid and oral antihistamine given singly or in combination. Am J Rhinol. 1996;10:193–199. [Google Scholar]
- 24.Géhanno P, Desfougeres JL. Fluticasone propionate aqueous nasal spray compared with oral loratadine in patients with seasonal allergic rhinitis. Allergy. 1997;52:445–450. doi: 10.1111/j.1398-9995.1997.tb01027.x. [DOI] [PubMed] [Google Scholar]
- 25.Vervloet D, Charpin D, Desfougeres JL. Intranasal fluticasone once daily compared with once daily cetirizine in the treatment of seasonal allergic rhinitis. Clin Drug Invest. 1997;13:291–298. doi: 10.2165/00044011-199713060-00001. [DOI] [PubMed] [Google Scholar]
- 26.Frolund L. Efficacy of an oral antihistamine, loratadine, as compared with a nasal steroid spray, beclomethasone dipropionate, in seasonal allergic rhinitis. Clin Otolaryngol. 1991;16:527–531. doi: 10.1111/j.1365-2273.1991.tb00965.x. [DOI] [PubMed] [Google Scholar]
- 27.Jordana G, Dolovich J, Briscoe MP, Day JH, Drouin MA, Gold M, et al. Intranasal fluticasone propionate versus loratadine in the treatment of adolescent patients with seasonal allergic rhinitis. J Allergy Clin Immunol. 1996;97:588–595. doi: 10.1016/s0091-6749(96)70303-2. [DOI] [PubMed] [Google Scholar]
- 28.Sibbald B, Hilton S, D’Souza M. An open cross-over trial comparing two doses of astemizole and beclomethasone dipropionate in the treatment of perennial rhinitis. Clin Allergy. 1986;16:203–211. doi: 10.1111/j.1365-2222.1986.tb00767.x. [DOI] [PubMed] [Google Scholar]
- 29.Andersson M, Lindqvist N, Svensson C, Ek L, Pipkorn U. Dry powder inhalation of budesonide in allergic rhinitis. Clin Otolaryngol. 1993;18:30–33. doi: 10.1111/j.1365-2273.1993.tb00805.x. [DOI] [PubMed] [Google Scholar]
- 30.Dickson DJ, Cruickshank JM. Comparison of flunisolide nasal spray and terfenadine tablets in hay fever. Br J Clin Pract. 1984;38:416–420. [PubMed] [Google Scholar]
- 31.Backhouse CI, Finnamore VP, Gosden CW. Treatment of seasonal allergic rhinitis with flunisolide and terfenadine. J Int Med Res. 1986;14:35–41. [PubMed] [Google Scholar]
- 32.Benincasa C, Lloyd RS. Evaluation of fluticasone propionate aqueous nasal spray taken alone and in combination with cetirizine in the prophylactic treatment of seasonal allergic rhinitis. Drug Invest. 1994;8:225–233. [Google Scholar]
- 33.Drouin MA, Yang WH, Horak F, van de Heyning PH, Kunkel GH, Backhouse CI, et al. Adding loratadine to topical nasal steroid therapy improves moderately severe seasonal allergic rhinoconjunctivitis. Adv Ther. 1995;12:340–349. [Google Scholar]
- 34.Davies RJ, Lund VJ, Harten-Ash VJ. The effect of intranasal azelastine and beclomethasone on the symptoms and signs of nasal allergy in patients with perennial allergic rhinitis. Rhinology. 1993;31:159–164. [PubMed] [Google Scholar]
- 35.Pelucchi A, Chiapparino A, Mastropasqua B, Marazzini L, Hernandez A, Foresi A. Effect of intranasal azelastine and beclomethasone dipropionate on nasal symptoms, nasal cytology, and bronchial responsiveness to methacholine in allergic rhinitis in response to grass pollens. J Allergy Clin Immunol. 1995;95:515–523. doi: 10.1016/s0091-6749(95)70313-6. [DOI] [PubMed] [Google Scholar]
- 36.Harding SM, Heath H. Intranasal steroid aerosol in perennial rhinitis; comparison with an antihistamine compound. Clin Allergy. 1976;6:369–372. doi: 10.1111/j.1365-2222.1976.tb01918.x. [DOI] [PubMed] [Google Scholar]
- 37.Lau SK, Wei WI, van Hasselt CA, Sham CL, Woo J, Choa D, et al. A clinical comparison of budesonide nasal aerosol, terfenadine and a combination therapy of budesonide and oxymetazoline in adult patients with perennial rhinitis. Asian Pac J Allergy Immunol. 1990;8:109–115. [PubMed] [Google Scholar]
- 38.Negrini AC, Troise C, Voltolini S, Horak F, Bachert C, Janssens M. Oral antihistamine/decongestant treatment compared with intranasal corticosteroids in seasonal allergic rhinitis. Clin Exp Allergy. 1995;25:60–65. doi: 10.1111/j.1365-2222.1995.tb01003.x. [DOI] [PubMed] [Google Scholar]
- 39.Hilberg O. Effect of terfenadine and budesonide on nasal symptoms, olfaction, and nasal airways patency following allergen challenge. Allergy. 1995;50:683–688. doi: 10.1111/j.1398-9995.1995.tb02586.x. [DOI] [PubMed] [Google Scholar]
- 40.Meltzer EO. Nasal cytological changes following pharmacological intervention. Allergy. 1995;50(suppl 23):15–20. doi: 10.1111/j.1398-9995.1995.tb02736.x. [DOI] [PubMed] [Google Scholar]
- 41.Ramsdale EH, Kline PA. Comparison of an intranasal steroid (budesonide) with terfenadine in the treatment of ragweed-induced rhinoconjunctivitis. J Allergy Clin Immunol. 1990;85:244. doi: 10.1016/0091-6749(90)90100-i. . [Abstract.] [DOI] [PubMed] [Google Scholar]
- 42.Ramsdale EH, Kline PA. Comparison of once daily treatment with an antihistamine (loratadine) and topical steroid (fluticasone propionate) during the ragweed pollen season. J Allergy Clin Immunol. 1991;87:298. . [Abstract.] [Google Scholar]
- 43.Stricker W, Klimas J, Mendelson L, Morris RJ, Reed CE, Shapiro G, et al. Intranasal fluticasone propionate is more effective than astemizole for seasonal allergic rhinitis. Ann Allergy. 1994;72:86. . [Abstract.] [Google Scholar]
- 44.Nathan RA. Changing strategies in the treatment of allergic rhinitis. Ann Allergy Asthma Immunol. 1996;77:255–259. doi: 10.1016/S1081-1206(10)63315-7. [DOI] [PubMed] [Google Scholar]
- 45.Meltzer EO, Weiler JM, Widlitz MD. Comparative outdoor study of the efficacy, onset and duration of action, and safety of cetirizine, loratadine, and placebo for seasonal allergic rhinitis. J Allergy Clin Immunol. 1996;97:617–626. doi: 10.1016/s0091-6749(96)70307-x. [DOI] [PubMed] [Google Scholar]
- 46.Munk ZM, LaForce C, Furst JA, Simpson B, Feiss G, Smith JA, et al. Efficacy and safety of triamcinolone acetonide aqueous nasal spray in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 1996;77:277–281. doi: 10.1016/S1081-1206(10)63320-0. [DOI] [PubMed] [Google Scholar]
- 47.Selner JC, Weber RW, Richmond GW, Stricker WE, Norton JD. Onset of action of aqueous beclomethasone dipropionate nasal spray in seasonal allergic rhinitis. Clin Ther. 1995;17:1099–1109. doi: 10.1016/0149-2918(95)80088-3. [DOI] [PubMed] [Google Scholar]
- 48.Rak S, Jacobson MR, Sudderick RM, Masuyama K, Juliusson S, Kay AB, et al. Influence of prolonged treatment with topical corticosteroid (fluticasone propionate) on early and late phase nasal responses and cellular infiltration in the nasal mucosa after allergen challenge. Clin Exp Allergy. 1994;24:930–939. doi: 10.1111/j.1365-2222.1994.tb02724.x. [DOI] [PubMed] [Google Scholar]
- 49.Gawchick SM, Saccar CL. The use of nasal corticosteroids in allergic rhinitis. Pediatr Asthma Allergy Immunol. 1995;9:25–38. [Google Scholar]
- 50.Pipkorn U, Pukander J, Suonpaa J, Makinen J, Lindqvist N. Long-term safety of budesonide nasal aerosol: a 5.5-year follow-up study. Clin Allergy. 1988;18:253–259. doi: 10.1111/j.1365-2222.1988.tb02867.x. [DOI] [PubMed] [Google Scholar]
- 51.Lipworth BJ, Seckl JR. Measures for detecting systemic bioactivity with inhaled and intranasal corticosteroids. Thorax. 1997;52:476–482. doi: 10.1136/thx.52.5.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martinati LC, Sette L, Chiocca E, Zaninotto M, Plebani M, Boner AL. Effect of beclomethasone dipropionate nasal aerosol on serum markers of bone metabolism in children with seasonal allergic rhinitis. Clin Exp Allergy. 1993;23:986–991. doi: 10.1111/j.1365-2222.1993.tb00288.x. [DOI] [PubMed] [Google Scholar]
- 53.Wolthers OD, Pederson S. Knemometric assessment of systemic activity of once daily intranasal dry-powder budesonide in children. Allergy. 1994;49:96–99. doi: 10.1111/j.1398-9995.1994.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 54.Brannan MD, Herron JM, Reidenberg P, Affrime MB. Lack of hypothalamic-pituitary-adrenal axis suppression with once-daily or twice-daily beclomethasone dipropionate aqueous nasal spray administered to patients with allergic rhinitis. Clin Ther. 1995;17:637–647. doi: 10.1016/0149-2918(95)80040-9. [DOI] [PubMed] [Google Scholar]
- 55.Simons FER, Simons KJ. The pharmacology and use of H1-receptor-antagonist drugs. N Engl J Med. 1994;330:1663–1670. doi: 10.1056/NEJM199406093302307. [DOI] [PubMed] [Google Scholar]
- 56.Kozma CM, Schulz RM, Sclar DA, Kral KM, Mackowiak JI. A comparison of costs and efficacy of intranasal fluticasone and terfenadine tablets for seasonal allergic rhinitis. Clin Ther. 1996;18:334–346. doi: 10.1016/s0149-2918(96)80014-2. [DOI] [PubMed] [Google Scholar]
- 57.Berka C, Chin W. Fluticasone propionate intranasal steroid: cost-effectiveness vs antihistamines. J Allergy Clin Immunol. 1994;93:165. . [Abstract.] [Google Scholar]