Abstract
Introduction
Although consensus guidelines recommend checking serum B12 in patients with dementia, clinicians are often faced with various questions: (1) Which patients should be tested? (2) What test should be ordered? (3) How are inferences made from such testing? (4) In addition to serum B12, should other tests be ordered? (5) Is B12 deficiency compatible with dementia of the Alzheimer’s type? (6) What is to be expected from treatment? (7) How is B12 deficiency treated?
Methods
On January 31st, 2009, a Medline search was performed revealing 1,627 citations related to cobalamin deficiency, hyperhomocysteinemia, and dementia. After limiting the search terms, all abstracts and/or articles and other references were categorized into six major groups (general, biochemistry, manifestations, associations and risks, evaluation, and treatment) and then reviewed in answering the above questions.
Results
The six major groups above are described in detail. Seventy-five key studies, series, and clinical trials were identified. Evidence-based suggestions for patient management were developed.
Discussion
Evidence is convincing that hyperhomocysteinemia, with or without hypovitaminosis B12, is a risk factor for dementia. In the absence of hyperhomocysteinemia, evidence is less convincing that hypovitaminosis B12 is a risk factor for dementia. B12 deficiency manifestations are variable and include abnormal psychiatric, neurological, gastrointestinal, and hematological findings. Radiological images of individuals with hyperhomocysteinemia frequently demonstrate leukoaraiosis. Assessing serum B12 and treatment of B12 deficiency is crucial for those cases in which pernicious anemia is suspected and may be useful for mild cognitive impairment and mild to moderate dementia. The serum B12 level is the standard initial test: 200 picograms per milliliter or less is low, and 201 to 350 picograms per milliliter is borderline low. Other tests may be indicated, including plasma homocysteine, serum methylmalonic acid, antiparietal cell and anti-intrinsic factor antibodies, and serum gastrin level. In B12 deficiency dementia with versus without pernicious anemia, there appear to be different manifestations, need for further workup, and responses to treatment. Dementia of the Alzheimer’s type is a compatible diagnosis when B12 deficiency is found, unless it is caused by pernicious anemia. Patients with pernicious anemia generally respond favorably to supplemental B12 treatment, especially if pernicious anemia is diagnosed early in the course of the disease. Some patients without pernicious anemia, but with B12 deficiency and either mild cognitive impairment or mild to moderate dementia, might show some degree of cognitive improvement with supplemental B12 treatment. Evidence that supplemental B12 treatment is beneficial for patients without pernicious anemia, but with B12 deficiency and moderately-severe to severe dementia is scarce. Oral cyanocobalamin is generally favored over intramuscular cyanocobalamin.
Keywords: Alzheimer, dementia, cognitive impairment, cognitive dysfunction, cobalamin, cyanocobalamin, B12, homocysteine, hyperhomocysteinemia, homocystinuria
Introduction
The notion of vitamin B12 deficiency (ie, B12 hypovitaminosis), its psychiatric and neurological manifestations, and its treatment is an age-old issue that has generated much controversy over many years. Although consensus guidelines1–4 recommend checking serum B12 levels in patients with dementia, clinicians are often faced with questions regarding how to interpret and what to do with the results.5 In order to help in clarifying these uncertainties, six questions were posed and then answered: 1) In which patients should vitamin B12 be routinely assessed? 2) What test or tests should be ordered, and how are inferences made from such testing? 3) Does the finding of low serum B12 or elevated homocysteine (Hcy) require evaluation for other medical conditions? 4) When vitamin B12 deficiency is found in dementia, is dementia of the Alzheimer’s type (DAT) a compatible diagnosis? 5) Based on the benefit-to-risk ratio in treatment of dementia, if vitamin B12 deficiency is determined, should supplemental B12 be initiated? 6) How is vitamin B12 deficiency treated?
By assimilating data from in vitro, in vivo animal, and in vivo human studies and epidemiological studies, this article clarifies these and other issues regarding hypovitaminosis B12, hyperhomocysteinemia (HHcy), and dementia.
Methods/results
The study design is a qualitative and quantitative review of the literature. The problem discussed is that in clinicians’ geriatric practices, patients with dementia and low vitamin B12 were not showing significant improvement with supplemental B12 therapy. Our hypothesis is that patients with dementia and low vitamin B12 improve with supplemental B12 therapy. The null hypothesis is that patients with dementia and low vitamin B12 do not improve with supplemental B12 therapy.
On January 31st, 2009 a Medline search was performed using the search terms: (Alzheimer OR Alzheimer’s OR dementia OR cognitive impairment OR cognitive dysfunction) AND (cobalamin OR cyanocobalamin OR B12 OR B-12 OR B 12 OR homocysteine OR hyperhomocysteinemia OR homocystinuria), which revealed 1,627 citations. “Title/Abstract” field limits decreased the search to 1,095 citations, which included 230 review articles. Using a Boolean operation, the review articles were removed, reducing the search to 865 citations. Subsequently, the search was limited to only citations with abstracts, so as to exclude publications such as ‘Letters to the Editor’ and case reports, which revealed 824 citations. Furthermore, in order to not miss any positive findings of individual case reports or case series, on September 6th, 2009 another Medline search was performed using the search terms: pernicious anemia AND dementia AND (case report OR case series) revealing 20 citations. Of the 844 articles, all abstracts were reviewed and, when useful, relevant articles were obtained and reviewed. Bibliographies from relevant articles were reviewed and, when applicable, review articles, ‘Letters to the Editor,’ and case reports were included in the overall review. Data from (1) other Medline searches, (2) Internet searches, (3) basic and clinical science textbooks, and (4) personal communications were added for clarification of technical issues. All abstracts, articles, and other references were categorized, allowing duplications, into six major categories: (1) general information, (2) biochemical evidence suggesting that hypovitaminosis B12 or HHcy are causal factors in dementia, (3) clinical and radiological manifestations, (4) associations between hypovitaminosis B12 or HHcy and cognitive impairment, (5) evaluation, and (6) treatment. Endnote version X.0.2 (Thomson Reuters, Philadelphia, PA) was used to maintain the reference library, which contained 839 citations. Evidenced-based medicine was used to develop suggestions for vitamin B12 workup and treatment in patients with suspected mild cognitive impairment (MCI) or dementia.
There are 511 articles and other references relevant to the six questions posed in the introduction and the six major categories listed above. Question 5 in the Introduction considers treatment benefits and risks. In terms of treatment benefits, the study objective was to determine whether or not vitamin B12 is beneficial for B12-deficient dementia. Letting the null hypothesis be, “Patients with dementia and low vitamin B12 do not improve with supplemental B12 therapy,” not rejecting the null hypothesis when it is not true would be a type 2 error (false negative). In order to avoid a type 2 error, thus concluding B12 treatment is not beneficial, when in truth it is beneficial, all published studies and reports contained in Medline (Box 1), including case series, in which supplemental B12 was an exposure and cognitive change was an outcome are included in the discussion and tables (N = 38), regardless of the quality of the study or number of subjects. Also included are all published cohort and longitudinal studies in Medline, where exposure pertains to metabolic or serum B12 deficiency and outcomes pertain to change in cognitive function or development or prevention of dementia (N = 37). Also included are the majority of the retrospective and cross-sectional studies in Medline that examine similar outcomes and exposures. Articles pertaining to genetics, biochemistry, pathophysiology, clinical manifestations, and radiological manifestations illuminating our understanding on relationships between HHcy with and without cobalamin deficiency and dementia are included in the review, as are articles relevant to evaluation, prognosis, and treatment.
Box 1. Studies and reports in which supplemental B12 is an exposure and cognitive change is an outcome
| Abyad, 2002 | Fox, 1975 | McMahon, 2006 |
| Aisen, 2008 | Healton, 1991 | Nilsson, 2001 |
| Bolaman, 2003 | Hvas 2004 | Osimani, 2005 |
| Bryan, 2002 | Ikeda, 1992 | Remington, 2009 |
| Carmel, 1995 | Kalita, 2008 | Seal, 2002 |
| Clarke, 2003 | Kwok, 1998 | Stott, 2005 |
| Crystal, 1994 | Kwok, 2008 | Sun, 2007 |
| Cunha, 1990 | La Rue, 1997 | Teunisse, 1996 |
| Cunha, 1995 | Lehmann, 2003 | van Asselt, 2001 |
| Eastley, 2000 | Levine 2006 | van Dyck, 2008 |
| El Otmani, 2008 | Lin, 2008 | van Uffelen, 2008 |
| Eussen, 2006 | Lindenbaum, 1988 | Wolters, 2005 |
| Fourniere, 1997 | Martin, 1992 |
Discussion
General information
Vitamin B12 is composed of a central cobalt atom, attached to a dimethylbenzimidazole group, four nitrogen atoms, each pertaining to four pyrrole rings, and an R group (-CN, -OH, -CH3, or adenosyl group), denoting the specific type of cobalamin.6–8 The definition of vitamin B12 deficiency is a quantitative lack of vitamin B12 in the diet, body fluids, or cells or a qualitative lack of intracellular B12 utilization.
B12 deficiency occurs in roughly 10% of general9–23 and 17% of demented10,24–26 elderly populations. B12 deficiency in demented individuals ranges from one10,27,28 to five25,29 times that of controls. Serum B12 levels and cerebral spinal fluid (CSF) folate levels decrease with advancing age,9–12,30–34 whereas serum folate levels may either increase or decrease with age.32 Hcy is a nonessential thiol amino acid.35,36 HHcy is defined as an abnormally high level of total Hcy in the plasma. HHcy occurs in 6% to 81% of individuals, depending on the population studied.37–44 Causes of HHcy and B12 deficiency are listed in Table 1.7,18,19,39,45–62 Further details regarding differential diagnoses are available on the Internet.62
Table 1.
Causes of hyperhomocysteinemia and B12 deficiency
| Causes of hyperhomocysteinemia |
|---|
|
Causes of B12 deficiency45,47,50,55,56,62
|
Biochemical evidence suggesting that hypovitaminosis B12 or hyperhomocysteinemia are causal factors in dementia
The means by which HHcy is involved in dementia may possibly be explained by aging and reduced vitamin B12 or folate supply versus demand ratio,63,64 with functional, structural, genetic, and nutritional determinants. Possible functional determinants include Hcy agonism of N-methyl-D-aspartic acid (NMDA) receptors, which causes excessive intracellular calcium influx and neuronal death65,66 and HHcy creating a state of hypomethylation in the pathogenesis of Alzheimer’s disease (AD),21,58,67 causing deoxyribonucleic acid (DNA) damage and apoptosis.68–71 HHcy inhibits adult mammal hippocampal neurogenesis. 72 Hcy may compete with gamma-amino butyric acid (GABA) at the GABA receptor and may affect its inhibitory function.73,74
Potential structural determinants include HHcy causing blood–brain barrier (BBB) dysfunction75–78 and endothelial cell toxicity,47,64 whereby Hcy changes endothelial cell surface properties from anticoagulant to procoagulant.47,52,73,79 Genetic determinants include various polymorphisms and homozygous monogenic deficiencies, involving methylenetetrahydrofolate reductase (MTHFR), trans-cobalamin (TC) II, methionine synthetase (MS), and cystathione beta-synthetase (CBS). These may contribute to hypovitaminosis B12, HHcy, and dementia.7,47,52,64,73,80–82 Meta-analyses83–89 of the MTHFR polymorphism show that those with 677 TT alleles compared to 677 CC alleles have elevated Hcy and increased risk for myocardial infarction (MI), transient ischemic attack, and stroke. Hyperhomocysteinemic individuals with certain butyrylcholinesterase-K (BuChE-K) alleles cognitively decline more rapidly than those with wild-type BuChE alleles.90 Nutritional determinants include decreased vitamin B12 ingestion9,45,47 and food-cobalamin malabsorption.9,15,19,45,49,91–93
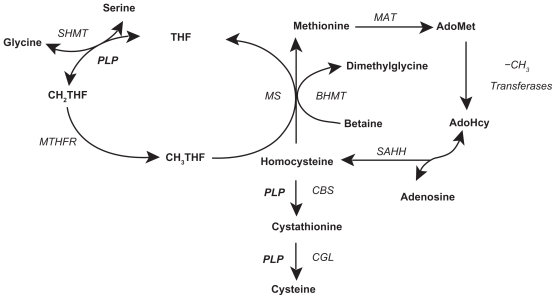
In vitro, in vivo animal, and in vivo human studies suggest AD pathophysiology conceivably involves a hypomethylation state,67,94–97 reactive oxygen species (ROS) generation,27,98–101 immune activation,102–110 and anomalous protein development.111 Abnormal Hcy metabolism is involved in each of these four processes. Hcy is metabolized by the methionine cycle and trans-sulfuration pathway (Figure 1),112 where it has one of three possible fates: methylation to methionine (Met), transsulfuration to cystathionine, or adenosylation to S-adenosylhomocysteine (SAH).113,114 With normal Hcy levels, the first two reactions are maintained, the first occurring in the brain and body and the second predominately occurring in the body, promoting homeostatic methylation reactions and maintaining healthy cells. With Hcy elevation the third reaction ensues, promoting a hypomethylation state leading to disease.115
Figure 1.
Folate cycle, methionine cycle, and transsulfuration pathway. Copyright © 2005. Adapted with permission from Davis SR, Quinlivan EP, Shelnutt KP, et al. Homocysteine synthesis is elevated but total remethylation is unchanged by the methylenetetrahydrofolate reductase 677C->T polymorphism and by dietary folate restriction in young women. J Nutr. 2005;135(5):1045–1050.
Notes: This schematic is of the folate cycle (left), methionine cycle (right), and transsulfuration pathway (bottom), with homocysteine being the common substance to all three. Folic acid (synthetic) is converted by DHFR to dihydrofolic acid (dietary), which is converted by DHFR to THF, which enters the folate cycle: THF →N5,N10-methylene THF → N5-methyl THF → THF. In this last step, vitamin B12 is required as a cofactor for MS. With low or absent vitamin B12, this last step is hindered leading to the methylfolate trap with elevated CH3-THF. Homocysteine metabolism: homocysteine is produced in the methionine cycle by the deadenosylation/hydration of AdoHcy, and is either remethylated to methionine, by the methionine cycle or catabolized to cysteine, by the transsulfuration pathway. Note the AdoHcy deadenosylation/hydration to Hcy is a reversible reaction favoring homocysteine adenosylation/hydration to AdoHcy. Methyl groups produced by AdoMet demethylation to AdoHcy are used for nucleic acid, protein, lipid, and neurotransmitter biosynthesis. Cysteine is a nonessential amino acid used in the biosynthesis of proteins, glutathione, coenzyme A, taurine, and inorganic sulfur. Glutathione is an antioxidant that protects cells from ROS.
Abbreviations: AdoHcy, S-adenosylhomocysteine; AdoMet, S-adenosylmethionine; BHMT, betaine-homocysteine methyltransferase; CBS, cystathionine β-synthase; CGL, cystathionine gamma-lyase; CH2THF, methylenetetrahydrofolate; -CH3, methyl group; CH3THF methyl tetrahydrofolate; DHFR, dihydrofolate reductase; MAT, methionine adenosyltransferases; MS, methionine synthase; MTHFR, methylenetetrahydrofolate reductase; PLP, pyridoxal phosphate (the active form of vitamin B6, pyridoxine); ROS, reactive oxygen species; SAHH, S-adenosylhomocysteine hydrolase; SHMT, serine hydroxymethyltransferase; THF, tetrahydrofolate.
On the one hand, without oxidative chemical reactions, as the basis for cellular respiration, we would not have life, at least as we know it. On the other hand, without these reactions, we would not have ROS.116 There are thousands of publications related to ROS and aging, the greatest known risk factor for sporadic AD.114,116,117 Oxidative metabolism generates a very small fraction of ROS,117 which can be beneficial or detrimental to the central nervous system. Oxidative stress occurs when ROS generation exceeds ROS defense,118 leading to potential molecular and cellular damage.117 Aberrant mitochondrial enzymes may facilitate this process, thereby contributing to the pathophysiology of AD.116
Hcy is rapidly auto-oxidized to homocysteine thiolactone, homocystine, and mixed disulfides,7,119 producing ROS,18,21,47,52,68,120 including singlet oxygen, superoxide anions, hydroxyl radicals, and hydrogen peroxide.21,42,52,68,117,118 Hcy elevation is associated with microglia activation and proliferation71 and immune activation and deposition,108 which is associated with choroid plexus dysfunction,121–123 possibly impeding vitamin B12123–125 and folate122,125 influx to, and amyloid beta (Aβ) peptide123,126,127 clearance from, brain tissue in AD. In certain systems, Hcy elevation leads to amyloid precursor128 and tau128–132 protein hyperphosphorylation, and Hcy oxidation produces products that crosslink with Aβ and tau proteins, causing their precipitation.119,128,133 Hcy elevation is associated with increased Aβ peptide, in both brain134 and plasma.51,135,136
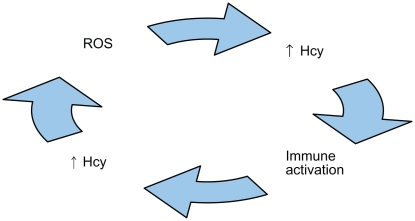
A deleterious cycle may occur between ROS generation and immune activation, where the former may cause the latter137–139 and vice versa.63,64,140–144
Hcy may promote a means for such a cycle,64,106,120 because ROS generation is associated with Hcy elevation,120,142,145–147 which is associated with immune activation,64,71,79,148–150 and immune activation is associated with Hcy elevation,64,142,151,152 which is associated with ROS generation (Figure 2).47,64,71,153–155 Additional evidence verifies ROS,145,151,156 hypomethylation,21,58,67,94,95,128,136,157 immunological components,64,79,106,109,110,142 and Aβ and tau proteins58,65,66,120,136,158 interact with one another, producing a neurodegeneration cascade in AD. ROS generation precedes both Aβ peptide deposition159–162 and tau-associated neurodegeneration.163 ROS generation deregulates tau protein phosphorylation,164,165 and is associated with decreased S-adenosylmethionine (SAM),120 increased SAH, a decreased SAM/SAH ratio,115 and hyperconsumption and depletion of antioxidants98,151,156 and tetrahydrofolate (THF).64,120,151,156,166
Figure 2.
Illustration of a biologically plausible deleterious cycle of reactive oxygen species (ROS), homocysteine (Hcy), and immune activation that possibly may be involved in the pathogenesis of Alzheimer’s disease.
With absolutely or relatively low folate or vitamin B12, MS-mediated Hcy clearance is impeded,52,120 resulting in a hypomethylation state. Also, Hcy elevation may lead to hyperconsumption and depletion of vitamin B12 in various cases of AD and vascular dementia (VaD).146 Hcy potentiates Aβ peptide-induced ROS generation and apoptosis.69,154,155,167 Aβ and tau proteins are concentrated sources for further ROS generation, Hcy elevation, and immune activation, thereby perpetuating the deleterious cycle.120,143,168–170 Also, in vivo human studies show that free cobalt is elevated in individuals with AD compared to controls.8 In vitro studies show free cobalt generates oxidative stress, as measured by reduced glutathione, increases Aβ peptide secretion, and produces neuroblastoma cytotoxicity.8,120
Within the central nervous system, a balance may occur between endogenous neurotoxic agents on one hand and endogenous neurotrophic agents on the other hand.171 Examples of potential neurotoxic agents include tumor necrosis factor-alpha (TNF-α), nerve growth factor (NGF), and the soluble CD40-soluble CD40 ligand dyad (sCD40-sCD40). Examples of potential neurotrophic agents include interleukin-6 (IL-6), epidermal growth factor (EGF), and transforming growth factor-beta1 (TGF-β1). In animals, if the balance is tilted in favor of TNF-α, NGF, and sCD40-sCD40 (eg, by the administration of exogenous TNF-α),172 as opposed to IL-6, NGF, and TGF-β1, then the morphological changes of subacute combined degeneration (SCD) are observed: white matter interstitial edema, intra-myelinic edema, spongy vacuolation, and astrogliosis.172–175 Vitamin B12-depleted animals exhibit increased levels of TNF-α, NGF, and sCD40-sCD40172,176 and decreased levels of IL-6 and EGF,176 thereby tilting the balance and developing the myelopathic changes of SCD. Not only does treatment with vitamin B12 reduce or reverse these changes,172,175 but treatment with anti-TNF-α antibodies, IL-6, EGF, and TGF-β1 does so as well.172,176 Interestingly, a similar observation has been observed in humans. Serum TNF-α is higher and serum EGF is lower in subjects with severe B12 deficiency compared to controls, where a direct correlation is found between plasma Hcy and serum TNF-α.173 The association is translative into the CSF, where CSF B12 and EGF are lower and CSF Hcy and TNF-α are higher in subjects with SCD compared to non-B12 deficient controls.177 B12-repletion lowers serum TNF-α and raises serum EGF, thereby normalizing the imbalance, which occurs concomitantly with clinical and hematological disease remission.173 Hence, in addition to well-known enzymatic roles for vitamin B12, it is thought to also have nonenzymatic roles, where it is associated with downgrading synthesis and release of TNF-α and upgrading synthesis and release of EGF.173,177,178
Clinical and radiological manifestations
Clinical manifestations of low vitamin B12 include abnormal psychiatric, neurological, and gastrointestinal findings. Psychiatric manifestations consist of psychoses, including paranoia, delusions, and hallucinations,23,58,179–184 cognitive dysfunction, including memory impairment, delirium, and dementia,7,21,23,49,58,93,180,181,185–188 and affective syndromes, including mania and depression,7,21,23,58,93,180,181,186,189–191 which also occurs with elevated Hcy192–194 and low SAM.67,192 Associations exist between HHcy and cognitive dysfunction in bipolar disorder195–197 and perhaps schizophrenia.198 Neurological manifestations include myelopathy and peripheral, autonomic, and optic neuropathies.199 Paresthesia is caused by a sensory lesion anywhere between the peripheral nerve and brain and is often the initial symptom.23,58,92,180,181,188 SCD refers to myelopathy affecting posterior and lateral columns, characterized by a pernicious sequence of vacuolar demyelination, axonal degeneration, and neuronal death.18,58,200–202 Posterior column myelopathy affects afferent pathways, causing the most common neurological signs: ataxia, diminished proprioception and vibratory senses, and presence of Romberg’s sign.18,49,58,180,185,188,200,201,203 Lateral column myelopathy affects efferent pathways, causing the second most common neurological signs: extremity muscular weakness, spasticity, hyperactive reflexes, and Babinski’s sign.18,49,58,180,181,200,201 Peripheral and autonomic neuropathies cause hypoactive reflexes, sensory loss, orthostatic hypotension, fecal and urinary incontinence, and impotence. 18,23,49,58,92,180,181,185,188,200,203 Although optic neuropathy is uncommon,23,58,180,203 visual impairment may occasionally be the earliest or sole manifestation of the disease.203 Gastrointestinal manifestations include epithelial atrophy of the tongue, referred to as atrophic glossitis, which causes the tongue to be sore and beefy red,18,23 and epithelial atrophy of the stomach.15
Computerized axial tomographic (CAT) and magnetic resonance imaging (MRI) scans of nondemented elderly brains may show age-related cerebral atrophy and various grades of periventricular white matter disease consistent with chronic microvascular ischemia.204–208 Although linear and volume measurement methods, evaluating ventricle-to-brain ratios and medial temporal lobe atrophy, reveal significant differences in group means, between those with AD and controls,205,207,209–217 such strategies are not recommended for the purpose of diagnosing AD.3,218 Assuming normal distributions of both nondemented and demented groups, a certain degree of overlap may exist,205,207,216 unless specified by scan angle-adjusted temporal lobe neuroimaging.207,214 Even so, such imaging may not distinguish non-Alzheimer’s dementia from controls.217 Although CAT scans of nondemented elderly commonly show age-related cerebral atrophy, those of demented elderly often show cerebral atrophy more than expected for age,210 and are read as variably judged atrophy and differently interpreted white matter changes.218,219 Whether or not such findings relate to dementia with low vitamin B12 and/or elevated Hcy is often unclear.
Accordingly, a literature review finds radiological manifestations of low vitamin B12 and/or elevated Hcy to include leukoaraiosis, brain atrophy, and silent brain infarcts.199,220–237 Leukoaraiosis is a radiological term that refers to brain white matter hypodensity on CAT scans or hyperintensity on T2-weighted magnetic resonance imaging (MRI).238 It is associated with aging,221,230,239–244 chronic microvascular hypo-perfusion, 245 BBB dysfunction,246 hypertension,221,230,240,241,242,244 stroke,230,239,240–242,244,247 and death.239 Leukoaraiosis is described pathologically as periventricular leukoencephalopathy or subcortical arteriosclerotic encephalopathy; it occurs in brains of individuals with AD243 and dementia of the Binswanger type (DBT),248 which is a relatively rarer type of dementia and is associated with other findings. HHcy increases the risk for leukoaraiosis.220,221,223–226,228,230,232,236 Independently from plasma Hcy levels, one cross-sectional study249 found an inverse association between the concentration of normal range serum B12 levels and the degree of leukoaraiosis; nonetheless, in the absence of HHcy, two studies227,231 found low serum B12 does not increase the risk for leukoaraiosis. Parenthetically, one prospective study250 found an inverse association between serum folate levels and the degree of leukoaraiosis. Although leukoaraiosis is more prevalent in demented than nondemented individuals225,247 and it increases the risk for developing dementia,247,248,251 results are mixed in terms of whether or not the link between HHcy and cognitive dysfunction is specifically mediated by leukoaraiosis.228,232,240,243,252–255 In both cross-sectional and prospective evaluations, hypovitaminosis B12 and HHcy are associated with brain atrophy.199,222,229,232,234,237 HHcy is associated with silent brain infarcts.232
VaD,61 DBT,256 and AD,61,222,243,257–259 represent various spectra of vascular pathology. To illustrate, cerebral amyloid angiopathy occurs in many cases of DBT248 and in most cases of AD,46,260,261 where increased vessel atrophy,262 decreased microvascular density,262 reduced temporal lobe blood flow,263,264 and spontaneous cerebral emboli265,266 are other significant findings. Hcy permeates through the BBB,267,268 causing BBB dysfunction,74,76–78 which is observed in AD,247,269 DBT,220,223,256,270 and VaD,75 allowing easy influx for a wide variety of proteins to cerebral interstitial fluid271,272 and vice versa.273 With BBB dysfunction, brain parenchyma likely becomes less protected from toxic effects of systemic HHcy,229 which increases risk for acute macrovascular disease, or strokes,7,21,70,84,85,87,151,223,274–279 causing loss of volume, and chronic microvascular disease, or ischemia,135,220,280 causing loss of cortical-to-subcortical connections,248 occasionally with findings as those in DBT.220,223,281,282
Associations between hypovitaminosis B12 or HHcy and cognitive impairment
HHcy, with hypovitaminosis B12, are common findings in the evaluation of MCI,59,283–288 AD,43,60,61,82,111,222,276,284,289–291 VaD,43,61,145,276,284,292 and other dementia subtypes.90,282,293,294 This raises the question of whether or not these findings are the cause of, result of, or unrelated to the disease process. Although multiple retrospective and cross-sectional studies find associations between hypovitaminosis B12 and HHcy and cognitive impairment with or without dementia,28,39,43,59–61,82,90,111,145,186,196,222,276,283–306 cohort studies are required to provide evidence that hypovitaminosis B12 or HHcy are risk factors for cognitive dysfunction. Eighteen106,136,252,258,301,307–320 of 22 cohort studies demonstrate HHcy either increases the risk for cognitive impairment or development of dementia. Depending upon whether plasma Hcy levels increase or decrease over time, the degree of change either increases the risk for developing dementia313 or decreases the risk for poorer memory performance,316 respectively. The greater the baseline plasma Hcy level, the faster the rate of cognitive decline.320 However, one study found that the elevated plasma Hcy risk for developing dementia diminished when controlling for low serum folic acid.301 Four321–324 of 22 cohort studies conclude Hcy is not a factor in the development of MCI or dementia, although outcome assessment may have been a methodological weakness in one of these.324 One cohort study325 concludes HHcy is a consequence of the development of cognitive impairment. Most,222,276,316,326–328 but not all,329 studies find HHcy is associated with intensity, rather than duration, of illness in AD. Thus, evidence that HHcy increases the risk for cognitive impairment and dementia usually is consistent and reproducible, 233 with HHcy predictably increasing the risk that subjects with MCI will progress to dementia.233 With respect to the involvement of low vitamin B12, seven cohort studies308,312,313,330–333 show low vitamin B12 either increases the risk for cognitive impairment or development of dementia, while eight13,325,334–339 show no increased risk. Paradoxical findings in these studies include modest increases in serum B12 levels over time may increase the risk for dementia313 and individuals with normal serum B12 levels having a higher incidence of AD compared to those with low serum B12 levels. 13 Thus, evidence that low vitamin B12 increases the risk for cognitive impairment or dementia is inconclusive. Then again, the increased risk may occur in the opposite direction. AD may increase the risk for B12 deficiency.340
Evaluation
In which patients should vitamin B12 be routinely assessed?
Guidelines for the workup of dementia are listed in Figure 3.4,341 Although practices may range from checking serum B12 in all elderly to only particular patients with dementia, an evidence-based approach warrants checking serum B12 in all patients with MCI and mild to moderate dementia of two years or less duration. It is especially useful to assess serum B12 levels in all patients with MCI, because many will ultimately advance to dementia, where there may be a window of opportunity when vitamin B12 treatment of B12 deficiency-related cognitive dysfunction is potentially beneficial. Additionally, serum B12 should be checked in all patients with (1) history of gastric bypass surgery, partial or total gastrectomy, terminal ileum disease or resection, or pancreatic insufficiency,14,20,33,200 (2) chronic use of levodopa, histamine type-2 (H2) receptor blockers, or protein pump inhibitors (PPIs),22,33,51,54,342–345 or (3) findings suggestive of (a) behavioral and psychological symptoms of dementia (BPSD), (b) SCD, including paresthesias, ataxia, or loss of position or vibratory senses, or (c) pernicious anemia (PA), including low hemoglobin (Hgb), elevated mean corpuscular volume (MCV), or corpuscular changes on peripheral smear.62 If such findings are absent, and patients have moderately severe to severe dementia of longer than two years duration, then universal recommendations1–4 of assessing for and, when found, treating B12 deficiency may not be supported by reliable evidence.13,16,24,26,92,187,326,346–364 H2 receptor blockers and PPIs impair cobalamin absorption; 18,22,33,365,366 their use is associated with supplemental B12 initiation.367 Individuals who have had gastric surgery have a high prevalence of B12 deficiency,20 and those who have had gastrectomies, who are B12-deficient, have a high prevalence of cognitive dysfunction and electroencephalographic (EEG) abnormalities.332 Therefore, patients prescribed H2 receptor blockers or PPIs, and those who have had gastrectomies or gastric bypass surgery, require monitoring for hypovitaminosis B12.368
Figure 3.
Guidelines for the workup of dementia.
Note: Excellent guidelines for the diagnosis of dementia also are available at http://www.cmaj.ca/cgi/content/full/178/7/825.
Abbreviations: AD, Alzheimer’s disease; APOE, apolipoprotein E; CAT, computerized axial tomography; CJD, Creutzfeldt-Jakob Disease; CNS, central nervous system; DAT, Dementia of the Alzheimer Type; DLB, Dementia with Lewy Bodies; DSM-IIR, Diagnostic and Statistical Manual of Mental Disorders-III-Revised; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders-IV; EEG, electroencephalogram; FTD, frontotemporal dementia; MRI, magnetic resonance imaging; NINCDS-ADRDA, National Institute of Neurologic, Communicative Disorders and Stroke–Alzheimer’s Disease and Related Disorders Association; PET, positron emission tomography; SPECT, single photon emission computerized tomography; V aD, vascular dementia.
What test or tests should be ordered, and how are inferences made from such testing?
Common tests include the deoxyuridine suppression test (dUST), serum B12, serum TC II, plasma Hcy, serum or urinary methylmalonic acid (MMA), and the post-methionine load test. For convenience, conversion of vitamin B12 units (nanograms per liter to picograms per milliliter (pg/mL) and pg/mL to picomoles per liter) are available at http://www.cdc.gov/ncbddd/b12/index.html.
The dUST is probably the most sensitive and specific test for assessing functional folate or B12 deficiency.92,369 Although the test is not fully understood and probably more complex than a simple explanation,369 if incubated bone marrow cells or peripheral blood lymphocytes have sufficient folate and cobalamin, nonradioactive deoxyuridine is believed to suppress the thymidylate synthetase conversion of radioactive thymidine into thymidylate, which is a normal response, but if cells have insufficient folate or cobalamin, nonradioactive deoxyuridine does not suppress the thymidylate synthetase conversion of radioactive thymidine into thymidylate, which is an abnormal response.
The most common test is the serum B12 level,15 but often it is difficult to determine which serum B12 levels represent deficiency states and which do not. When using a specific serum B12 cutoff point, for example 200 pg/mL or less,22,181,200,368 in determining which individuals do and do not have B12 deficiency, problems encountered include false positives and false negatives.7,15,19,30,33,57,188,370–376 One of the reasons for this is because most serum B12 assays measure total B12, including free B12 and that bound to B12 binding proteins: TC I, also called haptocorrin, TC II, simply referred to as transcobalamin, and TC III, which is produced by neutrophils.7,371,374 TC I and III are R-proteins (R for rapid movement on electrophoresis).7,23,200,377 TC I is a storage protein and does not participate in cellular uptake.200,377 TC II participates in all cellular uptake.7,58,200,371,372,377 TC III participates in hepatocyte uptake only.200 TC I and II comprise about 80% and 20% of total B12, respectively. Falsely low serum B12 occurs in congenital TC I deficiency, where TC I is low or absent, but TC II, intracellular B12, and hematopoiesis are normal.7,56,200,377 Other causes of falsely low serum B12 include folate deficiency, multiple myeloma, oral contraceptive use, and pregnancy.7,56 The mechanism by which low serum folate causes falsely low serum B12 is poorly understood.7,368 Alternatively, low serum cobalamin causes methyltetrahydrofolate (CH3-THF) trapping, elevating serum CH3-THF, which causes falsely high serum folic acid (Figure 1).58,200,372,378 Falsely normal serum B12 occurs in congenital TC II deficiency, where TC I is normal, but TC II is low or absent, and intracellular B12 is insufficient, causing severe megaloblastic, macrocytic anemia.7,56,200,377,379 Other causes of falsely normal serum B12 include liver disease, myelopro-liferative disorders, and intestinal bacterial overgrowth.7,56 Since serum TC II levels decrease with advancing age, a given serum B12 level in an elder may represent a deficiency, compared with the same level in a younger adult.30,380 Directly measuring serum TC II may be helpful in these cases and others.17,38,308,335,351,372,374,380–383
Studies show 10%56,203 to 50%19,368 of truly vitamin B12 deficient individuals have serum B12 between 200 and 350 pg/mL, presenting clinicians with a diagnostic challenge. Based upon known biochemistry, specific tests can be ordered to help with this challenge. Cytoplasmic methylcobalamin is needed for MS-catalyzed Hcy methylation to Met, and mitochondrial adenosylcobalamin is needed for L-methylmalonyl-CoA mutase-catalyzed L-methylmalonyl-CoA conversion to succinyl-CoA.7,18,19,20,52,57,58,203 With low cellular B12, these reactions are impeded, elevating Hcy in the former and L-methylmalonyl-CoA, D-methylmalonyl-CoA, and MMA in the latter.18,20,285 Thus, HHcy and hypermethylmalonic acidemia (HMMA) are surrogate markers for low vitamin B12 cellular levels (ie, metabolic B12 deficiency).7,11,15,19–22,30,33,44,59,60,188,203,285,294,351,353,368,370,372,373,376,380,384,385 HHcy occurs with deficiency of pyridoxine, folate, or vitamin B12;19,306,355,370,386 usually, only deficiency of vitamin B12 causes HMMA.19,20,370 Hcy may be the more sensitive and MMA the more specific surrogate marker.17,20,38,40,151,156,285,387–389 Either surrogate marker is more sensitive than serum B12 in evaluating PA,7,19,373,390 and many individuals with HHcy have normal serum folate and B12 levels.38,39,42 When used alone, B12,371,375 TC II,371 Hcy,38 or MMA389 may be insufficient as screening tests, but in combination, normal plasma Hcy and serum MMA rule out B12 deficiency in virtually all cases.19,390 Although the post-methionine load test is twice as sensitive as basal Hcy in detecting HHcy in individuals with AD,391 it is not recommended as an initial test.17
Most laboratory assays measure total Hcy, including reduced and oxidized forms: Hcy representing the former and homocystine and mixed disulfides (protein-bound Hcy and cysteine-Hcy) the latter.52 Although the upper reference limit for plasma Hcy varies according to different conditions,17,33 plasma Hcy greater than 14 μmol/L in patients with vitamin B12 levels between 201 and 350 pg/mL suggests cellular B12 deficiency.23,41,200,222,252,258,357,387,392 Serum B12 greater than 350 pg/mL rules out B12 deficiency in almost all individuals.56,200,340 Since tissues store vitamin B12 for up to five years18,368,393 and plasma Hcy increases only minimally after protein-rich meals,17 serum B12 and plasma Hcy may be obtained fasting or nonfasting. Immediate centrifugation, or keeping samples refrigerated or ice cooled until centrifugation, prevents spuriously elevated Hcy results.17,394,395 Suspected B12 deficiency can be confirmed when individuals having characteristic findings, including anemia, macrocytosis, corpuscular changes on peripheral smear, or signs and symptoms of SCD or peripheral neuropathy, improve with vitamin B12 treatment or when elevated Hcy or MMA are lowered with vitamin B12 treatment.19,20,92,135,188,347,362,370,392,396,397 Although not intended to replace clinical judgment, suggestions for vitamin B12 workup and treatment in patients with suspected MCI or dementia are presented (Figure 4).
Figure 4.
Suggestions for vitamin B12 workup and treatment in patients with suspected neuropathology.
Notes: *Studies have reliably shown that PO cyanocobalamin therapy is another option.
Abbreviations: μmol/L, micromoles per liter; CBC, complete blood count; Hcy, homocysteine; Hgb, hemoglobin; IM, intramuscular; MCV, mean corpuscular volume; PA, pernicious anemia; pg/mL, picograms per milliliter; PO, oral; SC, subcutaneous.
Does the finding of low serum B12 or elevated Hcy require evaluation for other medical conditions?
Some authors recommend establishing the etiology of B12 deficiency as part of the diagnostic approach.5 It is beyond the scope of this paper to cover all diagnostic considerations in Table 1, but levodopa treatment, folate deficiency, and PA are noteworthy.
In Parkinson’s disease (PD) it is the treatment rather than the disease that causes HHcy.37,54,294 In such cases, HHcy may293,294 or may not398 be associated with cognitive impairment. Recall from Figure 1, Met is converted to SAM, which is converted to SAH, which is converted to Hcy. SAM demethylation to SAH serves as a methyl group donor for the biosynthesis of nucleic acids, proteins, phospholipids, and catecholamines and the metabolism of drugs and toxins. Catechol-O-methyl transferase (COMT) catalyzes levodopa methylation to 3-O-methyldopa.344 Increased methyl group demand, required for the methylation of levodopa to 3-O-methyldopa, favors the conversion of Met to SAM, demethylation to SAH, conversion to Hcy, and development of HHcy.51,54,342,344 In a cross-sectional study,342 individuals on levodopa alone, compared to those on combined levodopa and COMT inhibitor therapy, had higher plasma Hcy levels. However, only one344 of three54,343,344 prospective studies showed addition of a COMT inhibitor to those on levodopa prevents dopamine-associated plasma Hcy elevation or serum B12 reduction.
Since the relationship between folate and vitamin B12 is biochemically and, in deficiency states, pathologically united,285 there are caveats to keep in mind when working up and treating folate and B12 deficiencies. When folate and B12 deficiencies occur together, monotherapy with either folate or vitamin B12 can worsen the manifestations of the other vitamin deficiency.7,23,180,285,303,355,371,399,400 Thus, it is beneficial to assess serum levels of both vitamins and treat whichever deficiency occurs. Although PA and SCD share the common etiology, type A (autoimmune) chronic atrophic gastritis, with vitamin B12 malabsorption, they have distinct pathophysiologies.18,201 In the methionine cycle, folate- and vitamin B12-dependent MS catalyzes Hcy methylation to Met, as CH3-THF, serving as the methyl group donor, is demethylated to THF.47,52,58,151,200 Impairment of this reaction causes defective DNA synthesis, leading to megaloblastic, macrocytic anemia (ie, PA).21,58 Supplemental folic acid can override the impairment, restoring DNA synthesis and normalizing erythropoiesis.58,401 Furthermore, in the methionine cycle, Met is adenosylated to SAM, which is demethylated to SAH, which is deadenosylated to Hcy.58 Impairment of SAM demethylation to SAH impedes methylation reactions, leading to vacuolar demyelination, axonal degeneration, and neuronal death (ie, SCD).21,58 Supplemental folic acid cannot override the methylation impairment, resulting in progressive neuropathology and neuronal death.58 Treating a combined folate and B12 deficiency with folic acid alone may correct hematological abnormalities, but not neurological abnormalities, and can aggressively worsen B12-deficient neurological sequelae.7,18,19,23,200,203,355 Thus, B12 deficiency should be ruled out before correcting folate deficiency.
When folate and vitamin B12 are in balance, increased serum folate levels are associated with decreased plasma Hcy and serum MMA levels, but when vitamin B12 is underrepresented, increased serum folate levels are associated with increased plasma Hcy and serum MMA levels.285 Also, higher folate states require relatively higher vitamin B12 levels than normal folate states to protect against metabolic B12 deficiency.285 Therefore, the borderline (201–350 pg/mL) serum B12 range may be higher in the presence of high folate states.
In what was described as the most severe neuropathic epidemic of modern times,402 between 1991 and 1993 more than 50,000 Cubans developed peripheral neuropathy, associated with reduced nutrient intake of group B vitamins,403–409 in the setting of strict embargos and economic deterioration.404,405,407,408 Although widespread distribution of group B vitamins404,406,407,409 and government-mandated folic acid fortification404,410 curbed the epidemic,404,406 some speculate that endemic subclinical group B vitamin deficiencies, coupled with Helicobacter pylori (H. pylori) infections, are responsible for the higher prevalence of dementia in Cuba compared with other Caribbean countries.404 Before and after the Cuban epidemic, in 1976 and 1995, researchers411,412 discovered that folate deficiency and relatively high, compared with relatively low, Hcy levels in pregnant women are associated with neural tube defects (NTDs) in infants born to such women, findings that have since been replicated.7,18,52,355,393,413,414 Since supplemental folic acid during pregnancy decreases the risk for such abnormalities, 415–420 in 1998 the United States Food and Drug Administration (FDA) and Health Canada required wheat and other grain products to be fortified with folic acid.35,58,421–423 To date, more than 50 countries have mandated folic acid fortification.410,420 It is largely accepted that high-dose folic acid may mask B12 deficiency. It is less clear whether or not low dose folic acid masks B12 deficiency.285,401 Although the United States FDA requires 140 μg of folic acid per 100 g of grain or flour,424,425 an amount chosen because it was considered high enough to prevent NTDs, but low enough to not mask B12 deficiency, some have advocated increasing this amount.426 Due to insufficient sensitivity, neither Hgb nor MCV are useful in ruling out B12-deficient dementia or SCD.7,13,33,58,124,289,300,371,373,378,385,427 Although the complete blood count (CBC) is part of routine diagnostic testing for dementia,368 additional vigilance is needed,20,58,381,428 especially in the era of folic acid fortification. In B12 deficiency, supplemental folic acid may protect against anemia, but not neurodegeneration;19,52,371 thus, the CBC alone may become even less sensitive in evaluating B12-deficient psychiatric and neurological abnormalities.
Further evaluation to rule out type A chronic atrophic gastritis, or PA,181,429 might be warranted because of increased risk for gastric cancer, where radiographic or endoscopic screening is useful, especially in cases of MCI and early dementia.15,18,33,200,201 Thus, it is useful to obtain additional testing when B12-deficient dementia occurs with anemia, macrocytosis, corpuscular changes on peripheral smear, or signs and symptoms of SCD or peripheral neuropathy.7 When B12-deficient dementia occurs in the absence of such findings, the decision to rule out type A chronic atrophic gastritis may be made on a case by case basis.
Although PA, or type A chronic atrophic gastritis,181,429 is often cited as the most common cause of low serum B12,7,18,19,33,49,56,58,180,201,390 emerging evidence suggests dissociation between PA and B12-deficient dementia.10,13,15,17–19,21,30,32,33,45,47–49,56,58,91–93,146,180,190,200,201,203,290,350,368,371,373,376,393,427–434 Thus, B12-deficient dementia may be subdivided into B12-deficient dementia with PA and B12-deficient dementia without PA. Studies support the postulate that these two disease states are different.10,13–15,17,19,21,25,30,32,33,45,47–49,58,91–93,146,190,201,203,289,290,350,368,427,429,431–435 They appear to have different etiologies, pathophysiological findings, prevalences, and responses to treatment.
B12-deficient dementia with PA and B12-deficient dementia without PA have separate etiologies, whereby the former is caused by type A chronic atrophic gastritis181,429 and the latter is generally caused by type B (nonautoimmune) chronic atrophic gastritis, age-associated decrease in hydrochloric acid production, or decreased vitamin B12 ingestion.9,10,14,17,21,22,30,45,47,49,58,91,92,203,368 Type B chronic atrophic gastritis and age-associated decrease in hydrochloric acid production cause food-cobalamin malabsorption.30 Less established causes of relative B12 deficiency include impairment of vitamin B12 transfer, from capillaries to CSF436 and from CSF to neurons,382 and vitamin B12 hyperconsumption,54,63,340,342 inactivation,146 or destruction.290 Type A chronic atrophic gastritis is due to antiparietal cell and anti-intrinsic factor antibodies,15,33,58,181,201,203,429 which occur in 90% and 55% of cases, respectively,7,203 causing parietal cell insufficiency and intrinsic factor dysfunction, sparing the antrum.33,58,201 Type B chronic atrophic gastritis is usually caused by H. pylori infection, involving the antrum.15,58,201,203 This is especially worth mentioning, because gastritis involving both body and antrum437 and H. pylori infection, confirmed by serological,437–439 histological,437,438,440 and rapid urease438 tests, is significantly more prevalent in individuals with MCI437 and AD438–440 compared to controls. In those with MCI, there is a correlation between serum anti- H. pylori immunoglobulin G concentration and degree of cognitive impairment,437 which may be mediated by elevated Hcy levels.437 One study438 showed H. pylori eradication improved both cognitive and functional measures in patients with AD. Since gastrin is secreted by antral G-cells, serum gastrin is elevated with type A,378 but low with type B,21 chronic atrophic gastritis.
B12-deficient dementia with and without PA have separate pathophysiological findings, where myeloneuropathy is characteristic and macrocytosis or anemia are present in the former,181,190,429 but myeloneuropathy may be rare19 and macrocytosis and anemia are often absent13,19,25,30,92,146,289,290,350,427,431–433 in the latter. Neuropathy associated with PA classically involves disease progression, beginning in the cervicothoracic region of the spine,202,441 with upper extremity findings classically occurring before lower extremity findings, followed by peripheral nerve involvement, and lastly, if at all, brain involvement.23,200,203 Dementia is believed to be a late and rare finding of neuropathy associated with PA.19,25,48,203,290 Alternatively, dementia with B12 deficiency often occurs independently from PA.13,25,146,290,427,432,433
B12-deficient dementia without PA is relatively common,25,146,290,427,432,433 but B12-deficient dementia with PA is considered rare,14,19,25,30,32,48,93,203,290,427,432 where it causes only a small fraction of B12 deficiency in demented individuals.14,25,30,32,93,290,427,432 PA incidence peaks late in midlife,181,429 while dementia incidence increases with age.233 Both chronic atrophic gastritis type A and B are characterized by low pepsinogen I/pepsinogen II ratios and achlorhydria;7,15,21,22,33,378 however, low pepsinogen I/pepsinogen II ratios occur in roughly 30% of elderly populations378 and PA occurs in only about 2%,23,428 suggesting the majority of cases with low ratios are caused by factors other than PA.
Meta-analyses or systematic reviews confirm that HHcy is a risk factor for AD,111,233 but they also confirm that vitamin B12 treatment of B12-deficient dementia is ineffective for improving cognitive function.16,348,355 Thus, B12-deficient dementia without PA and B12-deficient dementia with PA have distinct responses to supplemental B12 treatment, where it appears that moderately severe to severe dementia does not improve in the former,13,15,16,24,26,33,92,187,326,349,350,355,356,358,359,362 but may improve in the latter.185,190,199,429,434,435 The apparent dissociation of hematological findings and neurocognitive impairment provides additional support to the postulate that B12-deficient dementia with or without PA represent two separate disease states. Progressive neurocognitive decline often occurs in the presence of normal Hgb and MCV, where one or both values are normal in many individuals with B12-deficient dementia13,33,124,188,200,433 or neurological abnormalities.18,20,124,180,188,373 When anemia is absent, Hgb is indirectly proportional to cognitive function,18,19,200 and when present, it is an inconsistent risk factor for cognitive dysfunction.92,430 Neurological impairment occurring concomitantly with anemia is more likely to improve with supplemental B12 therapy in those cases where the anemia is more severe and less likely to improve where the anemia is less severe.180,393 With supplemental B12 therapy, increased reticulocytosis occurs within one week,376,393 but neurological improvement may require six months or longer.180,393 Vitamin B12 supplementation reverses hematological abnormalities in almost all patients,15,33,185,362 may improve neurological abnormalities in roughly one-half,15,33,185 and arrests or reverses dementia in only very few.13,15,16,24,26,33,92,187,326,347,349,350,355,361,362
In B12 deficiency dementia with versus without PA, there appear to be different manifestations, need for further workup, and responses to treatment. Therefore, when findings include low serum B12 or elevated Hcy, it is useful to rule out PA. In the workup for type A chronic atrophic gastritis, assessment of antiparietal cell and anti-intrinsic factor antibodies is an initial option.32,181,368,429 Since the former are sensitive, occurring in up to 10% of elderly populations,56 and the latter are specific, the absence of antiparietal cell antibodies suggests low likelihood, 7 while the presence of anti-intrinsic factor antibodies suggests high likelihood,7 for type A chronic atrophic gastritis. If antiparietal cell antibodies are present and anti-intrinsic factor antibodies are absent, assessment of serum gastrin levels is a further option.7,33 Type A and B chronic atrophic gastritis also can be distinguished by endoscopy with mucosal surface biopsy.7,181,429 Although Schilling’s test has been largely supplanted by the aforementioned tests,203,368 it may be useful if such tests are unremarkable or equivocal.434 However, decreased vitamin B12 ingestion9,47 and food-cobalamin malabsorption9,45,49 are common causes of low serum B12 in elderly populations, and Schilling’s test is most often negative (ie, step one results are normal) in these conditions.25,33,93,203
When vitamin B12 deficiency is found in dementia, is dementia of the Alzheimer’s type a compatible diagnosis?
AD is diagnosed by tissue pathology. According to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR), DAT is essentially a diagnosis of exclusion, after other causes of dementia, including B12 deficiency, have been ruled out, thus dichotomizing dementia due to B12 deficiency and DAT. Although two studies359,442 support this dichotomy, most studies13,26,82,222,333,336,382 suggest DAT occurs with or without B12 deficiency. When MCI and various subtypes of dementia are compared with one another, similar concentrations of serum B12 and Hcy are observed.276,284,443 When B12-deficient dementia is compared to other subtypes of dementia, similar rates of decline are observed across almost all measured outcomes.336,350,444 Nonetheless, there is likely a DAT subgroup, having low serum B12427,445 and perhaps elevated platelet monoamine oxidase (MAO) activity.29 Since B12 deficiency dementia with PA may arrest or reverse with treatment190,429,434 and B12 deficiency dementia without PA is usually progressively neurodegenerative despite treatment, the presence of PA would suggest dementia due to B12 deficiency, while its absence would suggest DAT.446
Treatment
Based on the benefit-to-risk ratio in treatment of dementia, if vitamin B12 deficiency is determined, should supplemental B12 be initiated?
Vitamin B12 treatment is considered one of the safest medical treatments available.447 Although severe adverse events are very rare, those reported include anaphylactic shock and death with administration of parenteral vitamin B12, hypokalemia with sudden death in conditions of severe anemia, and severe and sudden optic atrophy in those with Leber’s hereditary optic neuropathy.448 Other adverse events include polycythemia, thrombosis, pruritus, rash, skin eruptions449 congestive heart failure, diarrhea, edema, and swelling. One randomized controlled trial (RCT)347 found a greater prevalence of depression in the treatment group, who received high-dose pyridoxine, folate, and vitamin B12, than in the placebo group.
Although no overdosage has been reported, in treating hypovitaminosis B12 with supplemental B12, there is a fine line between help and harm. The serum cobalamin-plasma Hcy concentration–response curve appears curvilinear.17 As serum cobalamin increases from the lowest detectable level to 950 pg/mL, plasma Hcy decreases, but as serum cobalamin further increases from 950 pg/mL to 1,350 pg/mL, plasma Hcy begins to rise. One cohort study,386 found a dose-dependent association between increasing serum B12 levels and incidence of coronary artery disease (CAD) and mortality, where each 100 pg/mL increase in serum cobalamin was associated with a 10% increased incidence for such events. One RCT450 found that low-dose pyridoxine, folic acid, and vitamin B12 treatment decreased the risk for stroke, CAD events, and death, while moderately high dose pyridoxine, folic acid, and vitamin B12 treatment did not. Although the earliest two451,452 of eight other RCTs showed combined pyridoxine, folic acid, and vitamin B12 treatment decreased morbidity in individuals with or at risk for CAD, the later six453–458 failed to validate these findings, and four454,456–458 of the six showed treatment potentially increased the risk for harm. One of these RCTs455 was prematurely terminated due to potential risk for harm demonstrated by group B vitamin treatment.454 Therefore, some authors386,454 suggest supplemental B12 should not be administered unless B12 deficiency is present, and when present, only enough supplemental B12 should be administered to correct the deficiency. Parenthetically, meta-analyses of folic acid treatment, aimed at lowering HHcy, show mixed results in terms of whether or not treatment decreases the risk for stroke.459,460 Since most prospective studies show supplemental B12 does not improve cognitive function or prevent dementia in cognitively intact individuals,306,354,360 including those with hypovitaminosis B12351,353 or HHcy,357 it is not recommended401 for these purposes.
HHcy increases the risks for birth defects, cognitive dysfunction, dementia, cerebrovascular disease, stroke, CAD, MI, peripheral vascular disease, venous thrombosis, osteoporosis, hip fractures, and death.21,39,279,386,461–468 Benefits of treating B12 deficiency include lowering Hcy levels,19,21,47,135,198,326,347,351,357,360–362,397,469 lowering Aβ peptide levels,470 decreasing tau hyperphosphorylation,132 anti-inflammation, 471 neuroprotection,471 reduction of MAO activity,29 and causing the BBB to be less leaky.469
It may not be feasible to perform a meta-analysis on studies that have ascertained whether or not vitamin B12 treatment is associated with improved cognitive function or prevention of dementia due to the heterogeneity of these studies (Table 2). Published studies include multiple designs (eg, case-control, other retrospective studies, RCTs, and other prospective studies), subjects (eg, those who are healthy, ill, communitydwelling, residents of tertiary care facilities, with and without cognitive dysfunction, and with and without vitamin B12 deficiency), interventions, including vitamin B12 alone or in combination with other drugs or dietary supplements, type of vitamin B12 administered, and route of administration.
Table 2.
Examples of multiple binary variables in studies examining efficacy of B12 treatment on cognition
| No | Time course | Type of study | Absence/presence of cognitive dysfunction | B12 status* | Study results; author, year |
|---|---|---|---|---|---|
| 1 | Retrospective and cross-sectional | Not case control | Absent | Unknown | Positive; La Rue, 1997 |
| 2 | Not case control | Absent | Low | Positive; Healton, 19911 | |
| 3 | Not case control | Present | Low | Positive; Lindenbaum, 1988 El Otmani, 20082 Fox, 19753 Healton, 19911 |
|
| 4 | Case control | Absent | Normal | Negative; Lin, 20084 | |
| 5 | Case control | Present | Low | Negative; Eastley, 20005 | |
| 6 | Case control | Present | Low | Positive; Eastley, 20005 Osimani, 20056 | |
| 7 | Not randomized controlled trial | Absent | Low | Positive; van Asselt, 2001 | |
| 8 | Prospective | Not randomized controlled trial | Present | Unknown | Positive; Ikeda, 19927 |
| 9 | Not randomized controlled trial | Present | Normal | Positive; Lehmann, 20038 | |
| 10 | Not randomized controlled trial | Present | Low | Negative; Carmel,1995 Crystal, 19949 Cunha, 199010 Kwok, 200811 Teunisse, 199612 van Dyck, 200813 |
|
| 11 | Not randomized controlled trial | Present | Low | Positive; Abyad, 200214 Cunha, 199515 Kalita, 2008 Kwok, 200811 Martin, 199216 Nilsson, 200117 |
|
| 12 | Randomized controlled trial | Absent | Unknown | Negative; Stott, 200518 Wolters, 2005 |
|
| 13 | Randomized controlled trial | Absent | Unknown | Positive; Bryan, 2002 | |
| 14 | Randomized controlled trial | Absent | Normal | Positive; Levine, 200619 | |
| 15 | Randomized controlled trial | Absent | Low | Negative; Eussen, 2006 Kwok, 1998 McMahon, 2006 |
|
| 16 | Randomized controlled trial | Present | Unknown | Negative; van Uffelen, 2008 | |
| 17 | Randomized controlled trial | Present | Unknown | Positive; Remington, 200920 | |
| 18 | Randomized controlled trial | Present | Normal | Negative; Aisen, 2008 Clarke, 200321 Sun, 2007 |
|
| 19 | Randomized controlled trial | Present | Low | Negative; Clarke, 200321 Fourniere, 1997 Hvas, 2004 Seal, 2002 |
|
| 20 | Randomized controlled trial | Present | Low | Positive; Bolaman, 200322 |
Notes:
Subjects had nervous system involvement;
N = 1;
N = 1;
sample consisted of asymptomatic nursing home males;
results were negative for subjects with dementia, and positive for those with mild cognitive impairment;
baseline neuropsychiatric tests determined those who did not improve from those who improved;
B12 status was not a major factor mentioned in the methods or results sections, treatment consisted of intravenous mecobalamin;
subjects had normal vitamin B12, hyperhomocysteinemia, and mild cognitive impairment at baseline, and there were no subjects who progressed to dementia;
N = 3;
N = 13;
delirium improved, but dementia did not improve;
N = 26;
N = 28;
shorter duration of cognitive dysfunction was associated with greater response to B12 treatment;
outcome was obtained in 19/46 patients, 16/19 worsened, and 3/16 improved with B12 therapy, those who improved had mild dementia of less than two years duration;
individuals who were symptomatic less than 12 months improved with B12 therapy;
those with mild-moderate dementia and those with hyperhomocysteinemia improved with supplemental B12 therapy;
subjects were elders with ischemic vascular disease;
subjects had schizophrenia;
the dietary supplement also contained N-acetylcysteine, S-adenosylmethionine, and multiple other ingredients;
the sample presumably included those with normal and low serum B12;
all subjects had pernicious anemia, oral was compared with intramuscular cyanocobalamin.
Binary variables include B12 status: known versus unknown, and known-normal versus known-low.
Traditional recommendations of assessing for and, when found, treating B12-deficient dementia are supported by case-control studies,350,359 retrospective series,49,180,188,435,472 and case reports,190,429,434 where B12 deficiency was specifically caused by PA. Evidence supporting these recommendations appears to diminish with many RCTs,16,185,198,298,306,326,347,348,351–353,355,357,360,361,364,473,474 clinical trials (CTs),13,24,185,187,199,346,356,362,397,469 and other prospective studies26,92,349,358,475 (Tables 3a and b). Twelve16,306,326,347,351–353,355,357,360,361,364 of 16 RCTs found supplemental B12 therapy was of no benefit for improving cognitive function. Four185,198,473,474 of 16 RCTs found supplemental B12 therapy was beneficial for improving cognitive function; however, subjects in these studies included nondemented, healthy adult women,473 individuals with schizophrenia and HHcy,198 and individuals with dementia, irrespective of serum B12 levels, treated with multiple vitamins and other dietary supplements.474 The design of one of these studies185 did not include a separate control group. In all other RCTs of individuals with hypovitaminosis B12,16,351,353,355 HHcy,357 HMMA,352 dementia,16,326,347,355,361 or at risk for dementia,326,348,352,360,364 vitamin B12 treatment group results were no better than placebo group results. Nonetheless, the results from the RCTs may not generalize to all patients. Five of these306,351,353,357,360 did not examine subjects with MCI or dementia, three16,326,355 may have included subjects in various dementia stages, and six appear to have enrolled subjects, with either normal347,361 or irrespective306,326,360,364 of baseline folate and vitamin B12 statuses.
Table 3a.
Studies showing vitamin B12 treatment is not associated with improved cognitive function or prevention of dementia
| No | Principal author, Year | Study design | Type of sample | N | Duration | Major measures | Exposures/treatments | Major results/outcomes |
|---|---|---|---|---|---|---|---|---|
| 1 | Aisen, 2008 | A multicenter, randomized, doubleblind, controlled clinical trial | Patients with mild to moderate AD and normal folic acid, vitamin b12, and Hcy levels | 409 | 18 months | ADAS-cog | High-dose folic acid, vitamin B6, and vitamin B12 treatment | Vitamin treatment was not beneficial on the rate of change in the ADAS-cog score |
| 2 | Carmel, 1995 | Prospective investigation | Demented and nondemented patients with low serum B12 | 16 | NA | Neurological examination, EEG, visual evoked potentials, somatosensory potentials, dUST, plasma Hcy, serum MMA | Vitamin B12 treatment | 50% of patients had abnormal dUST, 44% had increased plasma Hcy and/or MMA, 73% had mild neurological abnormalities (primarily neuropathies), 75% had EE G abnormalities, 77% had abnormal visual evoked potentials, 33% had abnormal somatosensory potentials Although B12 treatment improved most abnormalities, it did not improve cognitive function in the 13 demented patients |
| 3 | Clarke, 2003 | Randomized, double-blind, placebo-controlled trial | Individuals with MCI or dementia, irrespective of baseline serum B12 and plasma Hcy levels | 149 | 3 months | Cognitive function assessment, plasma Hcy, serum folate, serum B12, urine 11-dehydrothromboxane B2 (a marker of platelet activation) and 8-epiprostaglandin F2alpha (a marker of reactive oxygen species) | Low-dose aspirin treatment, folic acid and B12 treatment, vitamin E and vitamin C treatment, placebo | Treatment did not affect cognitive function |
| 4 | Crystal, 1994 | Cohort | Ambulatory, nondemented, healthy elderly | 79 of 388 individuals developed dementia | 60 months | Specific neuropsychological tests, serum B12 | Vitamin B12 treatment | 22 of 388 patients had low serum B12 levels; 3 of these became demented. 57 of 388 individuals had higher B12 levels. AD incidence among the low B12 group was 4.5% compared with 7.5% in the higher B12 group. Mean B12 level at time of diagnosis in subjects who developed AD was 551 pg/mL. There was no evidence of hematologic abnormalities among the 22 subjects with low B12. Of the 3 low B12 patients who became demented, none responded to IM B12 |
| 5 | Cunha, 1990 | Prospective investigation | Demented elderly outpatients | 13 of 110 patients had low serum B12 | NA | Mental status examination | Vitamin B12 treatment | Mental status examination did not improve, and most cases demonstrated persistent cognitive deterioration |
| 6 | Eastley, 2000* | Case-control | Mildly cognitive impaired or demented outpatients with low serum B12 | 88 | NA | Specific neuropsychological tests, Hgb, MCV, serum B12 | Vitamin B12 treatment | There were no cases of arrestable or reversible dementia. Demented patients with low serum B12 did no better with B12 treatment than demented controls without low serum B12 |
| 7 | Eussen, 2006 | Randomized, double-blind, placebo-controlled trial | Elders with mild vitamin B12 deficiency | 195 | 6 months | Specific neuropsychological tests | Vitamin B12 treatment, folic acid and vitamin B12 treatment, or placebo | Neither treatment group demonstrated cognitive improvement. The placebo group demonstrated greater memory improvement than the vitamin B12-alone group |
| 8 | Fourniere, 1997 | Randomized, placebo-controlled trial | Demented individuals with low serum B12 | NA | NA | NA | Vitamin B12 treatment | No improvement in cognitive function |
| 9 | Hvas, 2004 | Randomized, placebo-controlled | Individuals with elevated plasma MMA, not previously treated with vitamin B12, many of whom had cognitive impairment at baseline | 140 | 3 months | Cognitive function assessed by the CAMCOG, MMSE, and a 12-words learning test, depressive symptoms evaluated by the MDI | Vitamin B12 | Treatment group did no better than placebo group on any of the measured outcomes of cognitive function or depressive symptoms |
| 10 | Kwok, 1998 | Randomized clinical trial | Apparently healthy elders with low serum B12 | 50 | 4 months | MMSE, specific neuropsychological tests, serum B12, serum MMA | Vitamin B12 treatment or no intervention | Individuals treated with IM B12 performed more poorly on motor function scores compared with the control group Individuals treated with intramuscular B12 demonstrated improvement in performance IQ, but the improvement was not statistically significant compared with the control group Overall, individuals treated with intramuscular B12 showed no improvement in cognitive function compared with the control group |
| 11 | Kwok, 2008* | Clinical trial | Mild to moderately demented patients with low serum B12 | 30 | 9 months | MMSE, specific neuropsychological tests, serum B12 | Vitamin B12 treatment | There were no significant changes in cognitive function or behavioral symptoms |
| 12 | Lin, 2008 | Case-control | Asymptomatic nursing home elderly males, excluding those with renal insufficiency or low serum B12 | 419 | Vitamin B12 supplementation status, MMSE, GDS, serum B12 | Vitamin B12 supplementation | Cognitive impairment and depression prevalences were similar in those taking and not taking B12 supplements. MMSE and GDS were similar between those taking and not taking B12 supplements |
|
| 13 | McMahon, 2006 | Randomized, double-blind, placebo-controlled trial | Healthy elders with plasma Hcy at least 13 μmol per liter | 276 | 24 months | Plasma Hcy | Folate, vitamin B6, and vitamin B12 treatment | Hcy lowering therapy did not improve cognitive function in elders with Hcy |
| 14 | Seal, 2002 | Randomized, placebo-controlled trial | Demented individuals with low serum b12 | NA | NA | NA | Vitamin B12 treatment | No improvement in cognitive function |
| 15 | Stott, 2005 | Randomized, double-blind, placebo-controlled trial | Elders with ischemic vascular disease | 185 | 12 months | Specific neuropsychological tests, plasma Hcy | Folic acid and vitamin B12 treatment, riboflavin treatment, or vitamin B6 treatment | Folic acid and vitamin B12 did not improve cognitive function in elders with ischemic vascular disease |
| 16 | Sun, 2007 | Randomized, double-blind, placebo-controlled trial | Elders with mild to moderate AD and normal serum folate and B12 | 89 | 6 months | ADAS-cog, ADL evaluation, serum folate, serum B12, plasma Hcy | AChE I and mecobalamin plus multivitamin containing vitamin B6, folic acid, other vitamins, and iron or AChE I and placebo | Supplemental mecobalamin plus multivitamin was no different than placebo in ADAS-cog scores and ADL evaluations |
| 17 | Teunisse, 1996 | Prospective investigation | Outpatients with dementia | 26 of 170 had low serum B12 | 6 months | Cognitive function, dementia severity, ADL’s, behavioral disturbances, caregiver burden, serum B12 | Vitamin B12 treatment | B12 treatment did not improve functioning in demented patients with low serum B12. The rate of dementia deterioration in patients with low serum B12 was the same as those with AD |
| 18 | van Dyck, 2008 | Clinical trial with blinded raters and a comparison group | Elderly, demented, nursing home residents with low serum B12 | 28 in the low serum B12 group, 28 in the comparison group with normal serum B12 | 2 months | DRS, BPRS, GDS, serum B12 | Vitamin B12 treatment | B12 treatment did not produce significant effects on cognitive or psychiatric variables B12 treatment produced significant improvement in hematologic and metabolic parameters |
| 19 | van Uffelen, 2008 | Randomized, placebo-controlled trial | Community-dwelling elders with MCI | 152 | 12 months | Specific neuropsychological tests, quality of life measurements | Moderate intensity walking program versus a low intensity placebo activity; combined vitamin B6, folic acid, and vitamin B12 treatment versus placebo | Those in the moderate intensity walking group showed small improvements or trends in a few of the subscales of neuropsychological tests. Those in the vitamin treatment group were no different than placebo across cognitive function measurements |
| 20 | Wolters, 2005 | Randomized, double-blind, placebo-controlled trial | Healthy, free-living, well-nourished, elderly women without dementia | 220 | 6 months | Specific neuropsychological tests, RBC folate, RBC functional B6 activity, serum folate, serum B12, plasma Hcy, serum MMA | Multivitamin supplementation | Multivitamin supplementation did not improve cognitive function |
Although few included control groups,198,199,473,474 CTs and other prospective studies with positive results24,185,187,198,199,346,356,358,397,469,473–475 show cognitive improvement may be associated with five factors: Hcy state,37,57,200,358,397 disease duration,18,24,30,180,346,356 disease intensity,24,180,350,358,359 and treatment type,474,475 as shown in Table 4.17,24,37,58,146,180,290,306,346,350,351,353,356–359,361,473–495 The fifth factor, and perhaps the most striking, is whether or not the B12 deficiency-associated cognitive dysfunction is due to PA. If so, then these individuals may respond remarkably well to supplemental B12 treatment, regardless of the severity of the dementia.185,190,429,434 Parenthetically, all RCTs show supplemental folic acid is no better than placebo at improving cognitive function in individuals with dementia.348,355,361,496
Table 4.
Factors associated with cognitive improvement in B12 supplementation of B12-deficient dementia
Etiology
|
| Homocysteine state |
| Disease duration |
Disease intensity
|
Treatment type
|
BPSD include symptoms and signs of disturbed behavior, mood, psychomotor activity, and thought content (eg, delusions and hallucinations) in patients with dementia. Exogenous SAM improves depression in patients with PD481 and other conditions.494,495 In individuals with AD, those with HHcy have increased EEG slow wave activity (ie, increased theta and delta waves) compared to those without HHcy.184,257 In nondemented individuals, lowering plasma Hcy levels with vitamin B12 treatment reverses such EEG findings.184,397 Accordingly, prospective studies of individuals with B12-deficient dementia show patients with delirium187 or psychoses359 improve with vitamin B12 supplementation. In demented individuals, prospective studies show vitamin B12 supplementation enhances vigilance when combined with bright light therapy,497 improves mood disturbances,475 but may worsen motor performance.353 Although not a consistent finding, 187,362 supplemental B12 in B12-deficient dementia may be beneficial for some patients with BPSD.359,475 Independent from serum folic acid and B12 levels, an association was found between HHcy and schizophrenia;498 in spite of the HHcy association being independent from group B vitamins in that study, another study198 showed combined pyridoxine, folic acid and vitamin B12 treatment in individuals with HHcy and schizophrenia improved positive symptoms, negative symptoms, and cognitive function. Depression, mania, psychoses, and delirium associated with PA or hypovitaminosis B12 may improve with supplemental B12 treatment.181–184,190,191
Considering the potential risks for adverse events, added cost, and added administration, and the potential benefit for arresting or reversing dementia, if B12 deficiency is determined, it should be treated. Vitamin B12 treatment should be initiated in those cases where serum B12 levels are 200 pg/mL or less and those where serum B12 levels are 201–350 pg/mL with plasma Hcy greater than 14 μmol/L.320 Duration of therapy is based on etiology and other factors, with an optimum serum B12 not being more than 950 pg/mL. In cases of PA, treatment is continued for life. In cases of dietary deficiency, low vitamin B12 in dementia normalizes with dietary correction.14 Possible explanations on why dementia does not reverse or arrest with supplemental B12 include treatment being an ineffective form,146,290 initiation outside of a time course window,30,346,356 or dementia not solely being caused by PA or low serum B12.13,26,82,222,333,336,359,442
How is vitamin B12 deficiency treated?
Since bacteria produce natural forms of vitamin B12, which humans ingest by consuming animal products,19,58,201 strict vegetarians may require oral (PO) vitamin B12 supplementation.58 The recommended daily allowance (RDA) is 2.4 μg daily. 499 Supplemental forms of vitamin B12 include cyanocobalamin, used in the United States, hydroxocobalamin, used in Europe, and mecobalamin, used in Asia.7,361,475 Mecobalamin is a synthetic form of methylcobalamin, which is the type of cobalamin utilized intracellularly. Although it may be advantageous in cognitive improvement or protection,475 it has not been compared with cyanocobalamin or hydroxocobalamin in clinical trials, and it may not be readily available in many western countries. Routes for cyanocobalamin administration include PO, sublingual (SL), intranasal (IN), intramuscular (IM), and subcutaneous (SC). Although the intravenous (IV) route has been used in patients with renal failure,500,501 this route is not recommended for general use.448 Compared to PO cyanocobalamin, IM and IV cyanocobalamin potentially pose greater risks for anaphylaxis. Up to 98% of an IM dose is lost in the urine, and even more is lost with an IV dose.387,448 Alternatively, 1% of PO cyanocobalamin is absorbed, throughout the gastrointestinal tract, by passive diffusion, independent of intrinsic factor.18,52,201,368,387 Thus, 10 μg will be absorbed from a 1,000 μg dose, well above the RDA;18,201,502 this effect is seen even in those with PA, prior gastrectomies, or diseases of the terminal ileum.52,368,387,503 Most physicians favor IM over PO routes,20,23,31,203,504 while most patients favor PO over IM routes.503,505 Patient preference is supported by a CT91 and RCTs31,34,185,392 showing PO cyanocobalamin provides equal, if not greater, serum B12 therapeutic levels. Since PO cyanocobalamin is as effective as IM cyanocobalamin in treating B12 deficiency, and its complications, it may be the preferred route of administration, unless there is concern regarding PO cyanocobalamin adherence, such as dysphagia.19,200,376,505 Variance exists in recommendations for dose and administration frequency: common practices include cyanocobalamin 1,000 μg PO daily203,503,505 or cyanocobalamin 1,000 μg IM daily for one week, then weekly for one month, then monthly thereafter.23,185,200,203,346,356,387,393 Alternative regimens are recommended for those with manifestations of severe PA.448
Conclusions
Although this paper represents a synopsis of our current understanding between HHcy and hypovitaminosis B12 and MCI or dementia, some investigations and reviews reveal contrary results or recommendations.27,41,62,188,340,398,401,506–508 Possible errors from this review and its conclusions are that Medline was the primary database, searches may not have captured all relevant studies and case reports, and it is presumed that there are unpublished vitamin B12 treatment responses. In order to attempt to minimize erroneous conclusions, results were crosschecked with Internet searches and basic and clinical science textbooks, other Medline searches were performed using various search terms, and communication took place with experts who care for demented patients. Owing to the latter, it is crucial that PA be ruled out in all demented patients. Also, the trials that have been reported to date may have been of insufficient size or duration to determine a beneficial effect.233
Biological data support the notion that ROS generation may lead to elevated Hcy, but this may be, at least in part, an adaptive mechanism to generate cysteine, which is used in the biosynthesis of glutathione,120,509 an antioxidant that protects cells from oxidative stress. Biochemical and epidemiological evidence is convincing that HHcy, with or without hypovitaminosis B12, is a risk factor for dementia. Evidence is less convincing that hypovitaminosis B12 is a risk factor for dementia in the absence of HHcy. B12 deficiency manifestations are variable and include abnormal psychiatric, neurological, gastrointestinal, and hematological findings. Radiological images of nondemented and demented individuals with HHcy frequently demonstrate leukoaraiosis, potentially related to BBB dysfunction. Thus, the finding of leukoaraiosis on CAT or MRI scan is an indication for checking plasma Hcy and serum B12 levels.
Although historically neuropathy related to PA was described according to a classical disease progression, patient samples now show that symptoms and signs secondary to the neuropathy can be variable.180,188 Nonetheless, dementia secondary to PA, occurring separately from other manifestations of SCD or peripheral neuropathy, and in the absence of macrocystosis and anemia, may still be considered sufficiently rare to be the basis of case reports.190,199,434
On the one hand, literature suggests assessing serum B12 and treatment of B12 deficiency is useful early in the course of cognitive dysfunction, for instance, MCI199,350,469 and mild to moderate dementia of less than two years duration.24,180,346,356,358,359 If low serum B12 is determined, it may be worthwhile to check for antiparietal cell and anti-intrinsic factor antibodies and obtain testing for H. pylori, for management and prognostic purposes. On the other hand, literature suggests assessing serum B12 and treatment of B12 deficiency may not be useful late in the course of cognitive dysfunction, for instance, moderately severe to severe dementia, if symptoms and signs of SCD and peripheral neuropathy, hematological manifestations of PA, and other factors listed on Figure 4 are absent. However, if one has not inquired about neurological symptoms, examined the mouth and tongue, performed a neurological examination, and obtained a CBC on a moderately-severe to severely demented patient, then it could be a ‘medical-legal pitfall’ not to check the serum B12 level.62 The suggestions for vitamin B12 workup and treatment in patients with suspected MCI or dementia are evidence-based. However, since the rate of dementia progression is poorly measured and variable and there are no specific criteria for vitamin B12 testing, the suggestions are not intended to replace care based upon clinical decisions. The serum B12 level remains the standard initial test.181 DAT is a compatible diagnosis when B12 deficiency is found, unless it is caused by PA. Cyanocobalamin PO is favorable over IM, in terms of both therapeutic efficacy and patient preference, unless there is concern regarding PO adherence.
Since the original observations by Biermer in 1872, to the more recent epidemic neuropathy in Cuba, it is evident that adequate folate and vitamin B12 function is required for physical and mental health. Issues surrounding folate, vitamin B12, Hcy, and MMA as they relate to health, especially microvascular disease and cognitive dysfunction, are high interest among researchers and clinicians. Puzzling questions remain. Since B12 deficiency can lead to depression, mania, and psychoses, should clinicians routinely check serum B12 levels in patients with such findings? Among those individuals who are B12 deficient, what are the prevalences of antiparietal, anti-intrinsic factor, and anti-H. pylori anti-bodies? Is HHcy a risk factor for other types of dementia, such as DBT, dementia due to PD, or dementia with Lewy bodies?193,510,511 Since HHcy has been prospectively shown to be an ‘independent’ risk factor for dementia, then why are Hcy-lowering therapies ineffective, or at best modestly effective, at preventing or treating dementia? Answers to questions such as these may one day unleash knowledge necessary for more effective treatment. The notions of age-related, and perhaps disease-related, degradation of the BBB and oxidative chemical reactions, generating ROS and elevating Hcy, coupled with immune activation, both without and within the brain, remain novel areas for further work.120
Table 3b.
Studies showing vitamin B12 treatment is associated with improved cognitive function or prevention of dementia
| No | Principal Author, Year | Study design | Type of sample | N | Duration | Major measures | Exposures/treatments | Major results/outcomes | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Abyad, 2002 | Single-blind clinical trial | Cognitively impaired nursing home residents and geriatric outpatients with low serum B12 | 62 | 12 months | MMSE, clock drawing test, caregiver interviews | Vitamin B12 treatment | 40 of 56 patients completing the study showed cognitive improvement. The shorter the duration of cognitive dysfunction, the greater the response to parenteral B12 therapy |
|
| 2 | Bolaman, 2003 | Randomized clinical trial | Patients 16 years of age or older with megaloblastic anemia due to B12 deficiency | 60 | 3 months | WBC count, reticulocyte count, Hgb, MCV, platelet count, serum B12, neurological evaluation, including MMSE and assessment of vibration, light touch, and pain Baseline neurological findings included recall and/or concentration deficit in 7 patients, loss of the sense of light touch and pain in 9 patients, and loss of the sense of vibration in 5 patients | PO versus IM vitamin B12 | In both groups, serum B12 increased. In both groups, Hgb, WBC count, and platelet count increased, MCV decreased, and reticulocytosis was observed. Neurologic improvement was detected in 7 of 9 patients in the PO B12 group and 9 of 12 patients in the IM B12 group | |
| 3 | Bryan, 2002 | Randomized clinical trial | Nondemented, healthy adult women | 211 | 1 month | Food frequency questionnaire, specific neuropsychological tests, subjective mood assessment | Usual dietary intake of B-complex vitamins, combined vitamin 6, folate, and vitamin B12 treatment, or placebo | Dietary intake status was associated with speed of processing, recall, recognition, and verbal ability. Supplemental B12 slightly improved memory, but had no effect on mood |
|
| 4 | Cunha, 1995 | Clinical trial | Elderly demented outpatients | 46 of 181 individuals had low serum B12 | 3 to 24 months | MMSE, serum B12 | Vitamin B12 treatment | Outcome was obtained in 19 of the 46 patients with low serum B12. 16 of the 19 cognitively declined; 3 of the 19 cognitively improved. The 3 that improved had mild dementia of less than 2 years duration | |
| 5 | Eastley, 2000* | Case-control | Mildly cognitive impaired or demented outpatients with low serum B12 | 88 | NA | Specific neuropsychological tests, Hgb, MCV, serum B12 | Vitamin B12 treatment | Patients with low serum B12 and MCI, treated with B12, improved in verbal fluency compared to controls | |
| 6 | El Otmani, 2008 | Case series | Patients with B12 deficiency involving the nervous system | 27 | NA | Neurological examination, serum B12 | Vitamin B12 treatment | Of 27 cases, 1 had dementia, which improved with B12 treatment Of the remaining cases, 12 improved neurologically with B12 treatment |
|
| 7 | Fox, 1975 | Case series | Patients with dementia | 40 | NA | Various laboratory and radiological tests, including computerized axial tomography | Vitamin B12 treatment | A patient with dementia with PA demonstrated marked improvement with B12 treatment | |
| 8 | Healton, 1991 | Case series | Patients with B12 deficiency involving the nervous system | 143 | 204 months | Neurologic impairment quantitative severity score, Hct, MCV, neutrophil count, platelet count, serum B12 | Vitamin B12 treatment | Of 121 episodes of low serum B12, 64 had a partial response and 57 had a complete response | |
| 9 | Ikeda, 1992 | Prospective investigation | Individuals with AD | 10 | NA | Intellectual function rating scales, serum B12, serum B12 unsaturated binding capacities, CSF B12 | Vitamin B12 treatment | IV mecobalamin improved memory, emotional function, and interpersonal communication. Improvement of interpersonal communication and memory occurred after a serum B12 threshold was maintained for sufficient time and at relatively higher CSF B12 levels | |
| 10 | Kalita, 2008 | Clinical trial of patients, compared with a representative control group | Patients with low serum B12 and/or megaloblastic bone marrow | 36 | 3 months | Neurological examination, MMSE, craniospinal MRI, cognitive evoked potentials, WBC count, RBC count, RBC indices, Hgb, serum chemistry, HIV titer, thyroid profile, antiparietal cell antibodies | Vitamin B12 treatment | The presenting syndrome was myelopathy in 8, cognitive in 1, myeloneuropathy in 10, myelocognitive in 8, and myeloneurocognitive in 9 patients. MMSE was abnormal in 17 patients. Cranial MRI demonstrated cortical atrophy in 1 and multiple white matter hyperintensities in 3 patients. P3 latency was either prolonged or unrecordable in 15 patients compared to controls. Both MMSE and P3 latency improved |
|
| 11 | Kwok, 2008* | Clinical trial | Mild to moderately demented patients with low serum B12 | 30 | 9 months | MMSE, specific neuropsychological test, serum B12 | Vitamin B12 treatment | DRS significantly decreased between baseline and 9 months | |
| 12 | La Rue, 1997 | Retrospective analysis | Community-dwelling elders without MCI or dementia | 137 | 72 months | Specific neuropsychological tests, current and past intake of dietary proteins, dietary vitamins, and supplements | Dietary and supplemental vitamin B12 intake | Higher past B12 intake was related to better performance on recall, visuospatial, or abstraction testing | |
| 13 | Lehmann, 2003 | Clinical trial | Mildly cognitive impaired patients with HHcy, normal serum B12 and normal serum folate | 30 | 9 months | Plasma albumin, plasma Hcy, serum B12, serum folate, CSF albumin, CSF tau protein, Baseline CSF-to-plasma albumin ratio was higher in patients compared to a control group. | Combined vitamin B6, folic acid, and vitamin B12 treatment | Reduction of the CSF-to-plasma albumin ratio CSF tau protein levels did not significantly change, although there was a numeric decline. None of the patients progressed to dementia during the treatment period | |
| 14 | Levine, 2006 | Randomized, double-blind, placebo controlled | Individuals with HHcy and schizophrenia | 42 | 3 months | Neuropsychological testing, including the WCST, PANSS | Vitamin B12 treatment | Neuropsychological testing, in general, and the WCST, in particular, improved in the treatment group. PANSS declined in the treatment group |
|
| 15 | Lindenbaum, 1988 | Case series | Patients with B12 deficiency involving the nervous system | 141 | NA | Neurologic impairment quantitative severity score, Hct, MCV, neutrophil count, platelet count, serum B12, plasma Hcy, serum MMA | Vitamin B12 treatment | Of 121 episodes of low serum B12, all patients responded to B12 treatment, except one patient who died All patients with neuropsychiatric findings associated with low serum B12 with HHcy or HMMA responded to B12 treatment |
|
| 16 | Martin, 1992 | Clinical trial | Cognitively impaired elderly with low serum B12 | 18 | 6 months | MDRS | Vitamin B12 treatment | 11 patients improved cognitively with IM cyanocobalamin. Patients symptomatic for less than 12 months gained an average of 20 points on the MDRS; patients symptomatic greater than 12 months lost an average of three points |
|
| 17 | Nilsson, 2001 | Prospective investigation | Demented elderly | 33 | NA | MMSE, cognitive testing for memory and attention, serum B12, blood folate, plasma Hcy, serum MMA | Folic acid and vitamin B12 treatment | Patients with mild-moderate dementia and elevated plasma Hcy clinically improved, with increased test scores, while severely demented patients and those with normal plasma Hcy levels did not clinically improve | |
| 18 | Osimani, 2005 | Case-control | Demented patients with low serum B12 | 19 | 12 months | Specific neuropsychological tests serum B12 | Vitamin B12 treatment | Twelve patients improved with treatment; seven patients did not improve with treatment. Baseline neuropsychological tests distinguished those who improved from those who did not improve | |
| 19 | Remington, 2009 | Randomized, placebo-controlled | Individuals institutionalized with moderate to severe AD | 12 | 9 months | Cognitive and functional rating scales, including DRS, CDT, NPI, and ADCS-ADL | A dietary supplement containing folic acid, vitamin B12, alpha-tocopherol, SAM, N-acetyl cysteine, and acetyl-L-carnitine | Treatment group demonstrated a delay in decline in the DRS and CDT, 30% improvement in the NPI, and maintenance of performance in the ADCS-ADL | |
| 20 | van Asselt, 2001 | Single-blind, placebo-controlled clinical trial | Healthy, cognitively intact, communitydwelling elders with low serum B12 | 16 | 6 months | Serum B12, plasma Hcy, serum MMA, quantitative EEG, specific neuropsychological tests | 1 month placebo treatment, followed by 5 months vitamin B12 treatment | After B12 treatment, serum B12 increased and plasma Hcy and serum MMA decreased. Quantitative EEG showed more fast activity and less slow activity, which was associated with improved performance on the Verbal Word Learning Test, Verbal Fluency, and Similarities. Psychometric test performance improved on the Verbal Word Learning Test, Verbal Fluency, and Similarities. Lower plasma Hcy concentrations were related to increased fast activity on quantitative EEG and improved performance on the Verbal Word Learning Test and Similarities. |
Abbreviations: Abeta40, amyloid beta-protein ending in Val-40; Abeta42, amyloid beta-protein ending in Ala-42; AChE I, acetylcholinesterase inhibitor; AD, Alzheimer’s disease; ADAS-cog, Alzheimer Disease Assessment Scale – cognitive subscale; ADCS-ADL, Alzheimer Disease Cooperative Study-Activities of Daily Living; ADL, activity of daily living; BPRS, Brief Psychiatric Rating Scale; CAMCOG, Cambridge Cognitive Examination; CDT, Clock Drawing Test; CSF, cerebrospinal fluid; DRS, Dementia Rating Scale; DUST, deoxyuridine suppression test; EEG, electroencephalogram; GDS, Geriatric Depression Scale; Hct, hematocrit; Hcy, homocysteine; Hgb, hemoglobin; HHcy, hyperhomocysteinemia; HIV, Human Immunodeficiency Virus; HMMA, hypermethylmalonic acidemia; IM, intramuscular; IV, intravenous; MCI, mild cognitive impairment; MCV, mean corpuscular volume; MDI, Major Depression Inventory; MDRS, Mattis Dementia Rating Scale; MMA, methylmalonic acid; MMSE, Mini-Mental State Examination; MRI, magnetic resonance image; NA, not applicable or available; NPI, Neuropsychiatric Inventory; PA, pernicious anemia; PANSS, Positive and Negative Syndrome Scale; PO, oral; RBC, red blood cell; WCST, Wisconsin Card Sorting Test;
*Study appears in two areas of table based on outcomes.
Acknowledgments
The author wishes to thank the reviewers of this manuscript, for their thoughtful work and kind suggestions, which were highly valuable in the final writing of this publication. The author reports no conflicts of interest in this work.
References
- 1.Arnold SE, Kumar A. Reversible dementias. Med Clin North Am. 1993;77(1):215–230. doi: 10.1016/s0025-7125(16)30280-2. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Practice guideline for psychiatric evaluation of adults. Am J Psychiatry. 1995;152(11 Suppl):63–80. doi: 10.1176/ajp.152.11.63. [DOI] [PubMed] [Google Scholar]
- 3.Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review) Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56(9):1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 4.Feldman HH, Jacova C, Robillard A, et al. Diagnosis and treatment of dementia: 2. Diagnosis. CMAJ. 2008;178(7):825–836. doi: 10.1503/cmaj.070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneede J, Ueland PM. Novel and estabslished markers of cobalamin deficiency: complementary or exclusive diagnostic strategies. Semin Vasc Med. 2005;5(2):140–155. doi: 10.1055/s-2005-872399. [DOI] [PubMed] [Google Scholar]
- 6.Vanderjagt DJ, McCarthy DM. Pernicious Anemia. In: Glew RH, Ninomiya Y, editors. Clinical Studies in Medical Biochemistry. 2nd Ed. New York, NY: Oxford University Press; 2006. pp. 122–133. [Google Scholar]
- 7.Klee GG. Cobalamin and folate evaluation: measurement of methylmalonic acid and homocysteine vs vitamin B(12) and folate. Clin Chem. 2000;46(8 Pt 2):1277–1283. [PubMed] [Google Scholar]
- 8.Olivieri G, Hess C, Savaskan E, et al. Melatonin protects SHSY5Y neuroblastoma cells from cobalt-induced oxidative stress, neurotoxicity and increased beta-amyloid secretion. J Pineal Res. 2001;31(4):320–325. doi: 10.1034/j.1600-079x.2001.310406.x. [DOI] [PubMed] [Google Scholar]
- 9.Allen LH. How common is vitamin B-12 deficiency? Am J Clin Nutr. 2009;89(2):693S–696S. doi: 10.3945/ajcn.2008.26947A. [DOI] [PubMed] [Google Scholar]
- 10.Basun H, Fratiglioni L, Winblad B. Cobalamin levels are not reduced in Alzheimer’s disease: results from a population-based study. J Am Geriatr Soc. 1994;42(2):132–136. doi: 10.1111/j.1532-5415.1994.tb04939.x. [DOI] [PubMed] [Google Scholar]
- 11.Clarke R, Refsum H, Birks J, et al. Screening for vitamin B-12 and folate deficiency in older persons. Am J Clin Nutr. 2003;77(5):1241–1247. doi: 10.1093/ajcn/77.5.1241. [DOI] [PubMed] [Google Scholar]
- 12.Clarke R, Grimley Evans J, Schneede J, et al. Vitamin B12 and folate deficiency in later life. Age Ageing. 2004;33(1):34–41. doi: 10.1093/ageing/afg109. [DOI] [PubMed] [Google Scholar]
- 13.Crystal HA, Ortof E, Frishman WH, Gruber A, Hershman D, Aronson M. Serum vitamin B12 levels and incidence of dementia in a healthy elderly population: a report from the Bronx Longitudinal Aging Study. J Am Geriatr Soc. 1994;42(9):933–936. doi: 10.1111/j.1532-5415.1994.tb06583.x. [DOI] [PubMed] [Google Scholar]
- 14.Elsborg L, Hansen T, Rafaelsen OJ. Vitamin B12 concentrations in psychiatric patients. Acta Psychiatr Scand. 1979;59(2):145–152. doi: 10.1111/j.1600-0447.1979.tb06956.x. [DOI] [PubMed] [Google Scholar]
- 15.Lechner K, Fodinger M, Grisold W, Puspok A, Sillaber C. Vitamin B12 deficiency. New data on an old theme. Wien Klin Wochenschr. 2005;117(17):579–591. doi: 10.1007/s00508-005-0406-z. [DOI] [PubMed] [Google Scholar]
- 16.Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326. doi: 10.1002/14651858.CD004326. [DOI] [PubMed] [Google Scholar]
- 17.Refsum H, Smith AD, Ueland PM, et al. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem. 2004;50(1):3–32. doi: 10.1373/clinchem.2003.021634. [DOI] [PubMed] [Google Scholar]
- 18.Singh NN, Thomas FP, Diamond AL, Diamond R. Vitamin B-12 associated neurological diseases. 2006. [Accessed on Nov 3, 2009]. Available from: http://emedicine.medscape.com/article/1152670-overview.
- 19.Stabler SP, Lindenbaum J, Allen RH. Vitamin B-12 deficiency in the elderly: current dilemmas. Am J Clin Nutr. 1997;66(4):741–749. doi: 10.1093/ajcn/66.4.741. [DOI] [PubMed] [Google Scholar]
- 20.Sumner AE, Chin MM, Abrahm JL, et al. Elevated methylmalonic acid and total homocysteine levels show high prevalence of vitamin B12 deficiency after gastric surgery. Ann Intern Med. 1996;124(5):469–476. doi: 10.7326/0003-4819-124-5-199603010-00002. [DOI] [PubMed] [Google Scholar]
- 21.Wolters M, Strohle A, Hahn A. Age-associated changes in the metabolism of vitamin B(12) and folic acid: prevalence, aetiopathogenesis and pathophysiological consequences. Z Gerontol Geriatr. 2004;37(2):109–135. doi: 10.1007/s00391-004-0169-6. [DOI] [PubMed] [Google Scholar]
- 22.Wolters M, Strohle A, Hahn A. Cobalamin: a critical vitamin in the elderly. Prev Med. 2004;39(6):1256–1266. doi: 10.1016/j.ypmed.2004.04.047. [DOI] [PubMed] [Google Scholar]
- 23.Worrall B, Rowland L. Nutritional disorders: Malnutrition, malabsorption, and B12 and other vitamin deficiencies. In: Rowland L, editor. Merritt’s Neurology. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. pp. 1091–1098. [Google Scholar]
- 24.Cunha UG, Rocha FL, Peixoto JM, Motta MF, Barbosa MT. Vitamin B12 deficiency and dementia. Int Psychogeriatr. 1995;7(1):85–88. doi: 10.1017/s1041610295001876. [DOI] [PubMed] [Google Scholar]
- 25.Karnaze DS, Carmel R. Low serum cobalamin levels in primary degenerative dementia. Do some patients harbor atypical cobalamin deficiency states? Arch Intern Med. 1987;147(3):429–431. [PubMed] [Google Scholar]
- 26.Teunisse S, Bollen AE, van Gool WA, Walstra GJ. Dementia and subnormal levels of vitamin B12: effects of replacement therapy on dementia. J Neurol. 1996;243(7):522–529. doi: 10.1007/BF00886874. [DOI] [PubMed] [Google Scholar]
- 27.Greilberger J, Koidl C, Greilberger M, et al. Malondialdehyde, carbonyl proteins and albumin-disulphide as useful oxidative markers in mild cognitive impairment and Alzheimer’s disease. Free Radic Res. 2008;42(7):633–638. doi: 10.1080/10715760802255764. [DOI] [PubMed] [Google Scholar]
- 28.Whyte EM, Mulsant BH, Butters MA, et al. Cognitive and behavioral correlates of low vitamin B12 levels in elderly patients with progressive dementia. Am J Geriatr Psychiatry. 2002;10(3):321–327. [PubMed] [Google Scholar]
- 29.Regland B, Gottfries CG, Oreland L. Vitamin B12-induced reduction of platelet monoamine oxidase activity in patients with dementia and pernicious anaemia. Eur Arch Psychiatry Clin Neurosci. 1991;240:4–5. 288–291. doi: 10.1007/BF02189542. [DOI] [PubMed] [Google Scholar]
- 30.Bopp-Kistler I, Ruegger-Frey B, Grob D, Six P. Vitamin B12 deficiency in geriatrics. Schweiz Rundsch Med Prax. 1999;88(45):1867–1875. [PubMed] [Google Scholar]
- 31.Butler CC, Vidal-Alaball J, Cannings-John R, et al. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency: a systematic review of randomized controlled trials. Fam Pract. 2006;23(3):279–285. doi: 10.1093/fampra/cml008. [DOI] [PubMed] [Google Scholar]
- 32.Nagga K, Rajani R, Mardh E, Borch K, Mardh S, Marcusson J. Cobalamin, folate, methylmalonic acid, homocysteine, and gastritis markers in dementia. Dement Geriatr Cogn Disord. 2003;16(4):269–275. doi: 10.1159/000072812. [DOI] [PubMed] [Google Scholar]
- 33.Nilsson-Ehle H. Age-related changes in cobalamin (vitamin B12) handling. Implications for therapy. Drugs Aging. 1998;12(4):277–292. doi: 10.2165/00002512-199812040-00003. [DOI] [PubMed] [Google Scholar]
- 34.Vidal-Alaball J, Butler CC, Cannings-John R, et al. Oral vitamin B12 versus intramuscular vitamin B12 for vitamin B12 deficiency. Cochrane Database Syst Rev. 2005;(3):CD004655. doi: 10.1002/14651858.CD004655.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diaz-Arrastia R. Homocysteine and neurologic disease. Arch Neurol. 2000;57(10):1422–1427. doi: 10.1001/archneur.57.10.1422. [DOI] [PubMed] [Google Scholar]
- 36.Brosnan JT, Brosnan ME. The sulfur-containing amino acids: an overview. J Nutr. 2006;136(6 Suppl):1636S–1640S. doi: 10.1093/jn/136.6.1636S. [DOI] [PubMed] [Google Scholar]
- 37.Barone P, Burn DJ, van Laar T, Hsu C, Poewe W, Lane RM. Rivastigmine versus placebo in hyperhomocysteinemic Parkinson’s disease dementia patients. Mov Disord. 2008;23(11):1532–1540. doi: 10.1002/mds.21997. [DOI] [PubMed] [Google Scholar]
- 38.Gonzalez-Gross M, Sola R, Albers U, et al. B-vitamins and homocysteine in Spanish institutionalized elderly. Int J Vitam Nutr Res. 2007;77(1):22–33. doi: 10.1024/0300-9831.77.1.22. [DOI] [PubMed] [Google Scholar]
- 39.Marengoni A, Cossi S, De Martinis M, Calabrese PA, Orini S, Grassi V. Homocysteine and disability in hospitalized geriatric patients. Metabolism. 2004;53(8):1016–1020. doi: 10.1016/j.metabol.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Nilsson K, Gustafson L, Hultberg B. The plasma homocysteine concentration is better than that of serum methylmalonic acid as a marker for sociopsychological performance in a psychogeriatric population. Clin Chem. 2000;46(5):691–696. [PubMed] [Google Scholar]
- 41.Ravaglia G, Forti P, Maioli F, et al. Blood homocysteine and vitamin B levels are not associated with cognitive skills in healthy normally ageing subjects. J Nutr Health Aging. 2000;4(4):218–222. [PubMed] [Google Scholar]
- 42.Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338(15):1042–1050. doi: 10.1056/NEJM199804093381507. [DOI] [PubMed] [Google Scholar]
- 43.Briani C, Cagnin A, Gallo L, et al. Anti-heparan sulphate antibodies and homocysteine in dementia: markers of vascular pathology. J Neurol Sci. 2005:229–230. 215–218. doi: 10.1016/j.jns.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 44.Nilsson K, Gustafson L, Hultberg B. Plasma homocysteine is a sensitive marker for tissue deficiency of both cobalamines and folates in a psychogeriatric population. Dement Geriatr Cogn Disord. 1999;10(6):476–482. doi: 10.1159/000017193. [DOI] [PubMed] [Google Scholar]
- 45.Andres E, Loukili NH, Noel E, et al. Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ. 2004;171(3):251–259. doi: 10.1503/cmaj.1031155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bergeron C, Ranalli PJ, Miceli PN. Amyloid angiopathy in Alzheimer’s disease. Can J Neurol Sci. 1987;14(4):564–569. [PubMed] [Google Scholar]
- 47.Cook S, Hess OM. Homocysteine and B vitamins. Handb Exp Pharmacol. 2005;(170):325–338. doi: 10.1007/3-540-27661-0_11. [DOI] [PubMed] [Google Scholar]
- 48.El Otmani H, El Moutawakil B, Moutaouakil F, Gam I, Rafai MA, Slassi I. Postoperative dementia: toxicity of nitrous oxide. Encephale. 2007;33(1):95–97. doi: 10.1016/s0013-7006(07)91563-8. [DOI] [PubMed] [Google Scholar]
- 49.El Otmani H, Moutaouakil F, Midafi N, et al. Cobalamin deficiency: Neurological aspects in 27 cases. Rev Neurol (Paris) 2009;165(3):263–267. doi: 10.1016/j.neurol.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 50.Hadithi M, Mulder CJ, Stam F, et al. Effect of B vitamin supplementation on plasma homocysteine levels in celiac disease. World J Gastroenterol. 2009;15(8):955–960. doi: 10.3748/wjg.15.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Irizarry MC, Gurol ME, Raju S, et al. Association of homocysteine with plasma amyloid beta protein in aging and neurodegenerative disease. Neurology. 2005;65(9):1402–1408. doi: 10.1212/01.wnl.0000183063.99107.5c. [DOI] [PubMed] [Google Scholar]
- 52.Jacobsen DW, Gatautis VJ, Green R, et al. Rapid HPLC determination of total homocysteine and other thiols in serum and plasma: sex differences and correlation with cobalamin and folate concentrations in healthy subjects. Clin Chem. 1994;40(6):873–881. [PubMed] [Google Scholar]
- 53.Myles PS, Chan MT, Leslie K, Peyton P, Paech M, Forbes A. Effect of nitrous oxide on plasma homocysteine and folate in patients undergoing major surgery. Br J Anaesth. 2008;100(6):780–786. doi: 10.1093/bja/aen085. [DOI] [PubMed] [Google Scholar]
- 54.O’Suilleabhain PE, Bottiglieri T, Dewey RB, Jr, Sharma S, Diaz-Arrastia R. Modest increase in plasma homocysteine follows levodopa initiation in Parkinson’s disease. Mov Disord. 2004;19(12):1403–1408. doi: 10.1002/mds.20253. [DOI] [PubMed] [Google Scholar]
- 55.Saibeni S, Lecchi A, Meucci G, et al. Prevalence of hyperhomocysteinemia in adult gluten-sensitive enteropathy at diagnosis: role of B12, folate, and genetics. Clin Gastroenterol Hepatol. 2005;3(6):574–580. doi: 10.1016/s1542-3565(05)00022-4. [DOI] [PubMed] [Google Scholar]
- 56.Snow CF. Laboratory diagnosis of vitamin B12 and folate deficiency: a guide for the primary care physician. Arch Intern Med. 1999;159(12):1289–1298. doi: 10.1001/archinte.159.12.1289. [DOI] [PubMed] [Google Scholar]
- 57.Stabler SP, Lindenbaum J, Allen RH. The use of homocysteine and other metabolites in the specific diagnosis of vitamin B-12 deficiency. J Nutr. 1996;126(4 Suppl):1266S–1272S. doi: 10.1093/jn/126.suppl_4.1266S. [DOI] [PubMed] [Google Scholar]
- 58.Weir DG, Scott JM. Brain function in the elderly: role of vitamin B12 and folate. Br Med Bull. 1999;55(3):669–682. doi: 10.1258/0007142991902547. [DOI] [PubMed] [Google Scholar]
- 59.Koike T, Kuzuya M, Kanda S, et al. Raised homocysteine and low folate and vitamin B-12 concentrations predict cognitive decline in community-dwelling older Japanese adults. Clin Nutr. 2008;27(6):865–871. doi: 10.1016/j.clnu.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 60.Li L, Cao D, Desmond R, et al. Cognitive performance and plasma levels of homocysteine, vitamin B12, folate and lipids in patients with Alzheimer disease. Dement Geriatr Cogn Disord. 2008;26(4):384–390. doi: 10.1159/000164271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nilsson K, Gustafson L, Hultberg B. Plasma homocysteine levels and different forms of vascular disease in patients with dementia and other psychogeriatric diseases. Dement Geriatr Cogn Disord. 2009;27(1):88–95. doi: 10.1159/000193628. [DOI] [PubMed] [Google Scholar]
- 62.Conrad ME. Pernicious anemia. [Accessed September 5, 2009]. Available from: http://emedicine.medscape.com/article/204930-overview.
- 63.Schroecksnadel K, Frick B, Winkler C, Leblhuber F, Wirleitner B, Fuchs D. Hyperhomocysteinemia and immune activation. Clin Chem Lab Med. 2003;41(11):1438–1443. doi: 10.1515/CCLM.2003.221. [DOI] [PubMed] [Google Scholar]
- 64.Schroecksnadel K, Frick B, Wirleitner B, Winkler C, Schennach H, Fuchs D. Moderate hyperhomocysteinemia and immune activation. Curr Pharm Biotechnol. 2004;5(1):107–118. doi: 10.2174/1389201043489657. [DOI] [PubMed] [Google Scholar]
- 65.Lehmann J, Tsai C, Wood PL. Homocysteic acid as a putative excitatory amino acid neurotransmitter: I. Postsynaptic characteristics at N-methyl-D-aspartate-type receptors on striatal cholinergic interneurons. J Neurochem. 1988;51(6):1765–1770. doi: 10.1111/j.1471-4159.1988.tb01157.x. [DOI] [PubMed] [Google Scholar]
- 66.Lipton SA, Kim WK, Choi YB, et al. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 1997;94(11):5923–5928. doi: 10.1073/pnas.94.11.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bottiglieri T, Godfrey P, Flynn T, Carney MW, Toone BK, Reynolds EH. Cerebrospinal fluid S-adenosylmethionine in depression and dementia: effects of treatment with parenteral and oral S-adenosylmethionine. J Neurol Neurosurg Psychiatry. 1990;53(12):1096–1098. doi: 10.1136/jnnp.53.12.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang RF, Huang SM, Lin BS, Wei JS, Liu TZ. Homocysteine thiolactone induces apoptotic DNA damage mediated by increased intracellular hydrogen peroxide and caspase 3 activation in HL-60 cells. Life Sci. 2001;68(25):2799–2811. doi: 10.1016/s0024-3205(01)01066-9. [DOI] [PubMed] [Google Scholar]
- 69.Kim HJ, Cho HK, Kwon YH. Synergistic induction of ER stress by homocysteine and beta-amyloid in SH-SY5Y cells. J Nutr Biochem. 2008;19(11):754–761. doi: 10.1016/j.jnutbio.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 70.Kruman II, Culmsee C, Chan SL, et al. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci. 2000;20(18):6920–6926. doi: 10.1523/JNEUROSCI.20-18-06920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zou CG, Zhao YS, Gao SY, et al. Homocysteine promotes proliferation and activation of microglia. Neurobiol Aging. 2009 Jan 6; doi: 10.1016/j.neurobiolaging.2008.11.007. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 72.Rabaneda LG, Carrasco M, Lopez-Toledano MA, et al. Homocysteine inhibits proliferation of neuronal precursors in the mouse adult brain by impairing the basic fibroblast growth factor signaling cascade and reducing extracellular regulated kinase 1/2-dependent cyclin E expression. FASEB J. 2008;22(11):3823–3835. doi: 10.1096/fj.08-109306. [DOI] [PubMed] [Google Scholar]
- 73.Kumar M, Tyagi N, Moshal KS, et al. Homocysteine decreases blood flow to the brain due to vascular resistance in carotid artery. Neurochem Int. 2008;53:6–8. 214–219. doi: 10.1016/j.neuint.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kumar M, Tyagi N, Moshal KS, et al. GABAA receptor agonist mitigates homocysteine-induced cerebrovascular remodeling in knockout mice. Brain Res. 2008;1221:147–153. doi: 10.1016/j.brainres.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herrmann W, Obeid R. Biomarkers of folate and vitamin B(12) status in cerebrospinal fluid. Clin Chem Lab Med. 2007;45(12):1614–1620. doi: 10.1515/CCLM.2007.310. [DOI] [PubMed] [Google Scholar]
- 76.Kamath AF, Chauhan AK, Kisucka J, et al. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood. 2006;107(2):591–593. doi: 10.1182/blood-2005-06-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lominadze D, Roberts AM, Tyagi N, Moshal KS, Tyagi SC. Homocysteine causes cerebrovascular leakage in mice. Am J Physiol Heart Circ Physiol. 2006;290(3):H1206–1213. doi: 10.1152/ajpheart.00376.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tyagi SC, Lominadze D, Roberts AM. Homocysteine in microvascular endothelial cell barrier permeability. Cell Biochem Biophys. 2005;43(1):37–44. doi: 10.1385/CBB:43:1:037. [DOI] [PubMed] [Google Scholar]
- 79.Poddar R, Sivasubramanian N, DiBello PM, Robinson K, Jacobsen DW. Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: implications for vascular disease. Circulation. 2001;103(22):2717–2723. doi: 10.1161/01.cir.103.22.2717. [DOI] [PubMed] [Google Scholar]
- 80.Bi XH, Zhao HL, Zhang ZX, Zhang JW. Association of RFC1 A80G and MTHFR C677T polymorphisms with Alzheimer’s disease. Neurobiol Aging. 2009;30(10):1601–1607. doi: 10.1016/j.neurobiolaging.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 81.Deshmukh A, Rodrigue KM, Kennedy KM, Land S, Jacobs BS, Raz N. Synergistic effects of the MTHFR C677T polymorphism and hypertension on spatial navigation. Biol Psychol. 2008;80(2):240–245. doi: 10.1016/j.biopsycho.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim JM, Stewart R, Kim SW, et al. Methylenetetrahydrofolate reductase gene and risk of Alzheimer’s disease in Koreans. Int J Geriatr Psychiatry. 2008;23(5):454–459. doi: 10.1002/gps.1903. [DOI] [PubMed] [Google Scholar]
- 83.Weih M, Junge-Hulsing J, Mehraein S, Ziemer S, Einhaupl KM. Hereditary thrombophilia with ischemiC stroke and sinus thrombosis. Diagnosis, therapy and meta-analysis. Nervenarzt. 2000;71(12):936–945. doi: 10.1007/s001150050690. [DOI] [PubMed] [Google Scholar]
- 84.Kelly PJ, Rosand J, Kistler JP, et al. Homocysteine, MTHFR 677C-->T polymorphism, and risk of ischemic stroke: results of a meta-analysis. Neurology. 2002;59(4):529–536. doi: 10.1212/wnl.59.4.529. [DOI] [PubMed] [Google Scholar]
- 85.Clarke R, Lewington S, Landray M. Homocysteine, renal function, and risk of cardiovascular disease. Kidney Int Suppl. 2003;(84):S131–133. doi: 10.1046/j.1523-1755.63.s84.7.x. [DOI] [PubMed] [Google Scholar]
- 86.Kim RJ, Becker RC. Association between factor V Leiden, prothrombin G20210A, and methylenetetrahydrofolate reductase C677T mutations and events of the arterial circulatory system: a meta-analysis of published studies. Am Heart J. 2003;146(6):948–957. doi: 10.1016/S0002-8703(03)00519-2. [DOI] [PubMed] [Google Scholar]
- 87.Casas JP, Bautista LE, Smeeth L, Sharma P, Hingorani AD. Homocysteine and stroke: evidence on a causal link from mendelian randomisation. Lancet. 2005;365(9455):224–232. doi: 10.1016/S0140-6736(05)17742-3. [DOI] [PubMed] [Google Scholar]
- 88.Haywood S, Liesner R, Pindora S, Ganesan V. Thrombophilia and first arterial ischaemic stroke: a systematic review. Arch Dis Child. 2005;90(4):402–405. doi: 10.1136/adc.2004.049163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cronin S, Furie KL, Kelly PJ. Dose-related association of MTHFR 677T allele with risk of ischemic stroke: evidence from a cumulative meta-analysis. Stroke. 2005;36(7):1581–1587. doi: 10.1161/01.STR.0000169946.31639.af. [DOI] [PubMed] [Google Scholar]
- 90.Lane R, He Y, Morris C, Leverenz JB, Emre M, Ballard C. BuChE-K and APOE epsilon4 allele frequencies in Lewy body dementias, and influence of genotype and hyperhomocysteinemia on cognitive decline. Mov Disord. 2009;24(3):392–400. doi: 10.1002/mds.22357. [DOI] [PubMed] [Google Scholar]
- 91.Andres E, Kaltenbach G, Noblet-Dick M, et al. Hematological response to short-term oral cyanocobalamin therapy for the treatment of cobalamin deficiencies in elderly patients. J Nutr Health Aging. 2006;10(1):3–6. [PubMed] [Google Scholar]
- 92.Carmel R, Gott PS, Waters CH, et al. The frequently low cobalamin levels in dementia usually signify treatable metabolic, neurologic and electrophysiologic abnormalities. Eur J Haematol. 1995;54(4):245–253. doi: 10.1111/j.1600-0609.1995.tb00679.x. [DOI] [PubMed] [Google Scholar]
- 93.Karnaze DS, Carmel R. Neurologic and evoked potential abnormalities in subtle cobalamin deficiency states, including deficiency without anemia and with normal absorption of free cobalamin. Arch Neurol. 1990;47(9):1008–1012. doi: 10.1001/archneur.1990.00530090082017. [DOI] [PubMed] [Google Scholar]
- 94.Fuso A, Cavallaro RA, Zampelli A, et al. gamma-Secretase is differentially modulated by alterations of homocysteine cycle in neuroblastoma and glioblastoma cells. J Alzheimers Dis. 2007;11(3):275–290. doi: 10.3233/jad-2007-11303. [DOI] [PubMed] [Google Scholar]
- 95.Fuso A, Nicolia V, Cavallaro RA, et al. B-vitamin deprivation induces hyperhomocysteinemia and brain S-adenosylhomocysteine, depletes brain S-adenosylmethionine, and enhances PS1 and BACE expression and amyloid-beta deposition in mice. Mol Cell Neurosci. 2008;37(4):731–746. doi: 10.1016/j.mcn.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 96.Herrmann W. Significance of hyperhomocysteinemia. Clin Lab. 2006;52:7–8. 367–374. [PubMed] [Google Scholar]
- 97.West RL, Lee JM, Maroun LE. Hypomethylation of the amyloid precursor protein gene in the brain of an Alzheimer’s disease patient. J Mol Neurosci. 1995;6(2):141–146. doi: 10.1007/BF02736773. [DOI] [PubMed] [Google Scholar]
- 98.Foy CJ, Passmore AP, Vahidassr MD, Young IS, Lawson JT. Plasma chain-breaking antioxidants in Alzheimer’s disease, vascular dementia and Parkinson’s disease. QJM. 1999;92(1):39–45. doi: 10.1093/qjmed/92.1.39. [DOI] [PubMed] [Google Scholar]
- 99.Harris ME, Hensley K, Butterfield DA, Leedle RA, Carney JM. Direct evidence of oxidative injury produced by the Alzheimer’s beta-amyloid peptide (1–40) in cultured hippocampal neurons. Exp Neurol. 1995;131(2):193–202. doi: 10.1016/0014-4886(95)90041-1. [DOI] [PubMed] [Google Scholar]
- 100.Nourhashemi F, Gillette-Guyonnet S, Andrieu S, et al. Alzheimer disease: protective factors. Am J Clin Nutr. 2000;71(2):643S–649S. doi: 10.1093/ajcn/71.2.643s. [DOI] [PubMed] [Google Scholar]
- 101.Pratico D, Clark CM, Liun F, Rokach J, Lee VY, Trojanowski JQ. Increase of brain oxidative stress in mild cognitive impairment: a possible predictor of Alzheimer disease. Arch Neurol. 2002;59(6):972–976. doi: 10.1001/archneur.59.6.972. [DOI] [PubMed] [Google Scholar]
- 102.Blasko I, Stampfer-Kountchev M, Robatscher P, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B. How chronic inflammation can affect the brain and support the development of Alzheimer’s disease in old age: the role of microglia and astrocytes. Aging Cell. 2004;3(4):169–176. doi: 10.1111/j.1474-9728.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- 103.Davis GK, Baboolal NS, Seales D, Ramchandani J, McKell S, McRae A. Potential biomarkers for dementia in Trinidad and Tobago. Neurosci Lett. 2007;424(1):27–30. doi: 10.1016/j.neulet.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 104.Mrak RE, Griffin WS. Potential inflammatory biomarkers in Alzheimer’s disease. J Alzheimers Dis. 2005;8(4):369–375. doi: 10.3233/jad-2005-8406. [DOI] [PubMed] [Google Scholar]
- 105.Streit WJ. Microglia and Alzheimer’s disease pathogenesis. J Neurosci Res. 2004;77(1):1–8. doi: 10.1002/jnr.20093. [DOI] [PubMed] [Google Scholar]
- 106.van den Kommer TN, Dik MG, Comijs HC, Jonker C, Deeg DJ. Homocysteine and inflammation: Predictors of cognitive decline in older persons. Neurobiol Aging. 2008 Nov 10; doi: 10.1016/j.neurobiolaging.2008.09.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 107.Viel TA, Lima Caetano A, Nasello AG, et al. Increases of kinin B1 and B2 receptors binding sites after brain infusion of amyloid-beta 1–40 peptide in rats. Neurobiol Aging. 2008;29(12):1805–1814. doi: 10.1016/j.neurobiolaging.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 108.Frick B, Gruber B, Schroecksnadel K, Leblhuber F, Fuchs D. Homocysteine but not neopterin declines in demented patients on B vitamins. J Neural Transm. 2006;113(11):1815–1819. doi: 10.1007/s00702-006-0539-x. [DOI] [PubMed] [Google Scholar]
- 109.Teunissen CE, Lutjohann D, von Bergmann K, et al. Combination of serum markers related to several mechanisms in Alzheimer’s disease. Neurobiol Aging. 2003;24(7):893–902. doi: 10.1016/s0197-4580(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 110.Teunissen CE, van Boxtel MP, Bosma H, et al. Serum markers in relation to cognitive functioning in an aging population: results of the Maastricht Aging Study (MAAS) Tijdschr Gerontol Geriatr. 2003;34(1):6–12. [PubMed] [Google Scholar]
- 111.Van Dam F, Van Gool WA. Hyperhomocysteinemia and Alzheimer’s disease: A systematic review. Arch Gerontol Geriatr. 2009;48(3):425–430. doi: 10.1016/j.archger.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 112.Davis SR, Quinlivan EP, Shelnutt KP, et al. Homocysteine synthesis is elevated but total remethylation is unchanged by the methylenetetrahydrofolate reductase 677C -->T polymorphism and by dietary folate restriction in young women. J Nutr. 2005;135(5):1045–1050. doi: 10.1093/jn/135.5.1045. [DOI] [PubMed] [Google Scholar]
- 113.De La Haba G, Cantoni G. The enzymatic synthesis of S-adenosyl-L-homocysteine from adenosine and homocysteine. J Biol Chem. 1959;234(3):603–608. [PubMed] [Google Scholar]
- 114.Garcia A, Zanibbi K. Homocysteine and cognitive function in elderly people. CMAJ. 2004;171(8):897–904. doi: 10.1503/cmaj.1031586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tchantchou F, Graves M, Falcone D, Shea TB. S-adenosylmethionine mediates glutathione efficacy by increasing glutathione S-transferase activity: implications for S-adenosyl methionine as a neuroprotective dietary supplement. J Alzheimers Dis. 2008;14(3):323–328. doi: 10.3233/jad-2008-14306. [DOI] [PubMed] [Google Scholar]
- 116.Gibson GE, Sheu KF, Blass JP. Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm. 1998;105:8–9. 855–870. doi: 10.1007/s007020050099. [DOI] [PubMed] [Google Scholar]
- 117.Markesbery WR. The role of oxidative stress in Alzheimer disease. Arch Neurol. 1999;56(12):1449–1452. doi: 10.1001/archneur.56.12.1449. [DOI] [PubMed] [Google Scholar]
- 118.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552(Pt 2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Toohey JI. Mercaptopropionaldehyde from homocysteine: implications for Alzheimer’s disease. J Alzheimers Dis. 2007;12(3):241–243. doi: 10.3233/jad-2007-12305. [DOI] [PubMed] [Google Scholar]
- 120.McCaddon A, Hudson PR. Alzheimer’s disease, oxidative stress and B-vitamin depletion. Future Neurology. 2007;2(5):537–547. [Google Scholar]
- 121.Serot JM, Bene MC, Faure GC. Comparative immunohistochemical characteristics of human choroid plexus in vascular and Alzheimer’s dementia. Hum Pathol. 1994;25(11):1185–1190. doi: 10.1016/0046-8177(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 122.Serot JM, Christmann D, Dubost T, Bene MC, Faure GC. CSF-folate levels are decreased in late-onset AD patients. J Neural Transm. 2001;108(1):93–99. doi: 10.1007/s007020170100. [DOI] [PubMed] [Google Scholar]
- 123.Serot JM, Bene MC, Faure GC. Choroid plexus, aging of the brain, and Alzheimer’s disease. Front Biosci. 2003;8:s515–s521. doi: 10.2741/1085. [DOI] [PubMed] [Google Scholar]
- 124.Nijst TQ, Wevers RA, Schoonderwaldt HC, Hommes OR, de Haan AF. Vitamin B12 and folate concentrations in serum and cerebrospinal fluid of neurological patients with special reference to multiple sclerosis and dementia. J Neurol Neurosurg Psychiatry. 1990;53(11):951–954. doi: 10.1136/jnnp.53.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Spector R, Cancilla P, Damasio A. Is idiopathic dementia a regional vitamin deficiency state? Med Hypotheses. 1979;5(7):763–767. doi: 10.1016/0306-9877(79)90038-0. [DOI] [PubMed] [Google Scholar]
- 126.Crossgrove JS, Li GJ, Zheng W. The choroid plexus removes betaamyloid from brain cerebrospinal fluid. Exp Biol Med (Maywood) 2005;230(10):771–776. doi: 10.1177/153537020523001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Johanson CE, Duncan JA, 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 2008;5(1):10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sontag E, Nunbhakdi-Craig V, Sontag JM, et al. Protein phosphatase 2A methyltransferase links homocysteine metabolism with tau and amyloid precursor protein regulation. J Neurosci. 2007;27(11):2751–2759. doi: 10.1523/JNEUROSCI.3316-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chan AY, Alsaraby A, Shea TB. Folate deprivation increases tau phosphorylation by homocysteine-induced calcium influx and by inhibition of phosphatase activity: Alleviation by S-adenosyl methionine. Brain Res. 2008;1199:133–137. doi: 10.1016/j.brainres.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 130.Luo Y, Zhou X, Yang X, Wang J. Homocysteine induces tau hyperphosphorylation in rats. Neuroreport. 2007;18(18):2005–2008. doi: 10.1097/WNR.0b013e3282f29100. [DOI] [PubMed] [Google Scholar]
- 131.Obeid R, Kasoha M, Knapp JP, et al. Folate and methylation status in relation to phosphorylated tau protein(181P) and beta-amyloid(1–42) in cerebrospinal fluid. Clin Chem. 2007;53(6):1129–1136. doi: 10.1373/clinchem.2006.085241. [DOI] [PubMed] [Google Scholar]
- 132.Zhang CE, Tian Q, Wei W, et al. Homocysteine induces tau phosphorylation by inactivating protein phosphatase 2A in rat hippocampus. Neurobiol Aging. 2008;29(11):1654–1665. doi: 10.1016/j.neurobiolaging.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 133.Agnati LF, Genedani S, Leo G, et al. Abeta peptides as one of the crucial volume transmission signals in the trophic units and their interactions with homocysteine. Physiological implications and relevance for Alzheimer’s disease. J Neural Transm. 2007;114(1):21–31. doi: 10.1007/s00702-006-0564-9. [DOI] [PubMed] [Google Scholar]
- 134.Pacheco-Quinto J, Rodriguez de Turco EB, DeRosa S, et al. Hyperhomocysteinemic Alzheimer’s mouse model of amyloidosis shows increased brain amyloid beta peptide levels. Neurobiol Dis. 2006;22(3):651–656. doi: 10.1016/j.nbd.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 135.Viswanathan A, Raj S, Greenberg SM, et al. Plasma Abeta, homocysteine, and cognition: the Vitamin Intervention for Stroke Prevention (VISP) trial. Neurology. 2009;72(3):268–272. doi: 10.1212/01.wnl.0000339486.63862.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Blasko I, Jellinger K, Kemmler G, et al. Conversion from cognitive health to mild cognitive impairment and Alzheimer’s disease: prediction by plasma amyloid beta 42, medial temporal lobe atrophy and homocysteine. Neurobiol Aging. 2008;29(1):1–11. doi: 10.1016/j.neurobiolaging.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 137.Kang J, Park EJ, Jou I, Kim JH, Joe EH. Reactive oxygen species mediate A beta(25–35)-induced activation of BV-2 microglia. Neuroreport. 2001;12(7):1449–1452. doi: 10.1097/00001756-200105250-00030. [DOI] [PubMed] [Google Scholar]
- 138.Wang JY, Wen LL, Huang YN, Chen YT, Ku MC. Dual effects of antioxidants in neurodegeneration: direct neuroprotection against oxidative stress and indirect protection via suppression of glia-mediated inflammation. Curr Pharm Des. 2006;12(27):3521–3533. doi: 10.2174/138161206778343109. [DOI] [PubMed] [Google Scholar]
- 139.Harman D. Alzheimer’s disease: role of aging in pathogenesis. Ann N Y Acad Sci. 2002;959:384–395. doi: 10.1111/j.1749-6632.2002.tb02109.x. discussion 463–385. [DOI] [PubMed] [Google Scholar]
- 140.Hwang J, Zheng LT, Ock J, Lee MG, Suk K. Anti-inflammatory effects of m-chlorophenylpiperazine in brain glia cells. Int Immunopharmacol. 2008;8(12):1686–1694. doi: 10.1016/j.intimp.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 141.Liu B, Gao HM, Wang JY, Jeohn GH, Cooper CL, Hong JS. Role of nitric oxide in inflammation-mediated neurodegeneration. Ann N Y Acad Sci. 2002;962:318–331. doi: 10.1111/j.1749-6632.2002.tb04077.x. [DOI] [PubMed] [Google Scholar]
- 142.Schroecksnadel K, Leblhuber F, Frick B, Wirleitner B, Fuchs D. Association of hyperhomocysteinemia in Alzheimer disease with elevated neopterin levels. Alzheimer Dis Assoc Disord. 2004;18(3):129–133. doi: 10.1097/01.wad.0000127443.23312.31. [DOI] [PubMed] [Google Scholar]
- 143.Wilkinson BL, Landreth GE. The microglial NADPH oxidase complex as a source of oxidative stress in Alzheimer’s disease. J Neuroinflammation. 2006;3:30. doi: 10.1186/1742-2094-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yamasaki R, Zhang J, Koshiishi I, et al. Involvement of lysosomal storage-induced p38 MAP kinase activation in the overproduction of nitric oxide by microglia in cathepsin D-deficient mice. Mol Cell Neurosci. 2007;35(4):573–584. doi: 10.1016/j.mcn.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 145.Koseoglu E, Karaman Y. Relations between homocysteine, folate and vitamin B12 in vascular dementia and in Alzheimer disease. Clin Biochem. 2007;40(12):859–863. doi: 10.1016/j.clinbiochem.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 146.McCaddon A, Regland B, Hudson P, Davies G. Functional vitamin B(12) deficiency and Alzheimer disease. Neurology. 2002;58(9):1395–1399. doi: 10.1212/wnl.58.9.1395. [DOI] [PubMed] [Google Scholar]
- 147.Shea TB. Effects of dietary supplementation with N-acetyl cysteine, acetyl-L-carnitine and S-adenosyl methionine on cognitive performance and aggression in normal mice and mice expressing human ApoE4. Neuromolecular Med. 2007;9(3):264–269. doi: 10.1007/s12017-007-8005-y. [DOI] [PubMed] [Google Scholar]
- 148.Dawson H, Collins G, Pyle R, Deep-Dixit V, Taub DD. The immunoregulatory effects of homocysteine and its intermediates on T-lymphocyte function. Mech Ageing Dev. 2004;125(2):107–110. doi: 10.1016/j.mad.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 149.Siow YL, Au-Yeung KK, Woo CWOK. Homocysteine stimulates phosphorylation of NADPH oxidase p47phox and p67phox subunits in monocytes via protein kinase Cbeta activation. Biochem J. 2006;398(1):73–82. doi: 10.1042/BJ20051810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang G, OK Homocysteine stimulates the expression of monocyte chemoattractant protein-1 receptor (CCR2) in human monocytes: possible involvement of oxygen free radicals. Biochem J. 2001;357(Pt 1):233–240. doi: 10.1042/0264-6021:3570233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Widner B, Enzinger C, Laich A, Wirleitner B, Fuchs D. Hyperhomocysteinemia, pteridines and oxidative stress. Curr Drug Metab. 2002;3(2):225–232. doi: 10.2174/1389200024605091. [DOI] [PubMed] [Google Scholar]
- 152.Widner B, Leblhuber F, Frick B, Laich A, Artner-Dworzak E, Fuchs D. Moderate hyperhomocysteinaemia and immune activation in Parkinson’s disease. J Neural Transm. 2002;109(12):1445–1452. doi: 10.1007/s00702-002-0758-8. [DOI] [PubMed] [Google Scholar]
- 153.Guidi I, Galimberti D, Lonati S, et al. Oxidative imbalance in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging. 2006;27(2):262–269. doi: 10.1016/j.neurobiolaging.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 154.Ho PI, Collins SC, Dhitavat S, et al. Homocysteine potentiates beta-amyloid neurotoxicity: role of oxidative stress. J Neurochem. 2001;78(2):249–253. doi: 10.1046/j.1471-4159.2001.00384.x. [DOI] [PubMed] [Google Scholar]
- 155.White AR, Huang X, Jobling MF, et al. Homocysteine potentiates copper- and amyloid beta peptide-mediated toxicity in primary neuronal cultures: possible risk factors in the Alzheimer’s-type neurodegenerative pathways. J Neurochem. 2001;76(5):1509–1520. doi: 10.1046/j.1471-4159.2001.00178.x. [DOI] [PubMed] [Google Scholar]
- 156.Fuchs D, Jaeger M, Widner B, Wirleitner B, Artner-Dworzak E, Leblhuber F. Is hyperhomocysteinemia due to the oxidative depletion of folate rather than to insufficient dietary intake? Clin Chem Lab Med. 2001;39(8):691–694. doi: 10.1515/CCLM.2001.113. [DOI] [PubMed] [Google Scholar]
- 157.Fuso A, Seminara L, Cavallaro RA, D’Anselmi F, Scarpa S. S-adenosylmethionine/homocysteine cycle alterations modify DNA methylation status with consequent deregulation of PS1 and BACE and beta-amyloid production. Mol Cell Neurosci. 2005;28(1):195–204. doi: 10.1016/j.mcn.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 158.Jin Y, Brennan L. Effects of homocysteine on metabolic pathways in cultured astrocytes. Neurochem Int. 2008;52(8):1410–1415. doi: 10.1016/j.neuint.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 159.Perry G, Cash AD, Smith MA. Alzheimer Disease and Oxidative Stress. J Biomed Biotechnol. 2002;2(3):120–123. doi: 10.1155/S1110724302203010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pratico D, Uryu K, Leight S, Trojanoswki JQ, Lee VM. Increased lipid peroxidation precedes amyloid plaque formation in an animal model of Alzheimer amyloidosis. J Neurosci. 2001;21(12):4183–4187. doi: 10.1523/JNEUROSCI.21-12-04183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pratico D, Sung S. Lipid peroxidation and oxidative imbalance: early functional events in Alzheimer’s disease. J Alzheimers Dis. 2004;6(2):171–175. doi: 10.3233/jad-2004-6209. [DOI] [PubMed] [Google Scholar]
- 162.Swerdlow RH, Khan SM. A “mitochondrial cascade hypothesis” for sporadic Alzheimer’s disease. Med Hypotheses. 2004;63(1):8–20. doi: 10.1016/j.mehy.2003.12.045. [DOI] [PubMed] [Google Scholar]
- 163.Dias-Santagata D, Fulga TA, Duttaroy A, Feany MB. Oxidative stress mediates tau-induced neurodegeneration in Drosophila. J Clin Invest. 2007;117(1):236–245. doi: 10.1172/JCI28769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gamblin TC, King ME, Kuret J, Berry RW, Binder LI. Oxidative regulation of fatty acid-induced tau polymerization. Biochemistry. 2000;39(46):14203–14210. doi: 10.1021/bi001876l. [DOI] [PubMed] [Google Scholar]
- 165.Liu Q, Smith MA, Avila J, et al. Alzheimer-specific epitopes of tau represent lipid peroxidation-induced conformations. Free Radic Biol Med. 2005;38(6):746–754. doi: 10.1016/j.freeradbiomed.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 166.Percy M, Moalem S, Garcia A, et al. Involvement of ApoE E4 and H63D in sporadic Alzheimer’s disease in a folate-supplemented Ontario population. J Alzheimers Dis. 2008;14(1):69–84. doi: 10.3233/jad-2008-14107. [DOI] [PubMed] [Google Scholar]
- 167.Leo G, Genedani S, Filaferro M, et al. Hyper-homocysteinemia alters amyloid peptide-clusterin interactions and neuroglial network morphology and function in the caudate after intrastriatal injection of amyloid peptides. Curr Alzheimer Res. 2007;4(3):305–313. doi: 10.2174/156720507781077223. [DOI] [PubMed] [Google Scholar]
- 168.Akama KT, Albanese C, Pestell RG, Van Eldik LJ. Amyloid betapeptide stimulates nitric oxide production in astrocytes through an NFkappaB-dependent mechanism. Proc Natl Acad Sci U S A. 1998;95(10):5795–5800. doi: 10.1073/pnas.95.10.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Butterfield DA, Lauderback CM. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radic Biol Med. 2002;32(11):1050–1060. doi: 10.1016/s0891-5849(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 170.Cente M, Filipcik P, Pevalova M, Novak M. Expression of a truncated tau protein induces oxidative stress in a rodent model of tauopathy. Eur J Neurosci. 2006;24(4):1085–1090. doi: 10.1111/j.1460-9568.2006.04986.x. [DOI] [PubMed] [Google Scholar]
- 171.Scalabrino G, Veber D, Mutti E. Experimental and clinical evidence of the role of cytokines and growth factors in the pathogenesis of acquired cobalamin-deficient leukoneuropathy. Brain Res Rev. 2008;59(1):42–54. doi: 10.1016/j.brainresrev.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 172.Buccellato FR, Miloso M, Braga M, et al. Myelinolytic lesions in spinal cord of cobalamin-deficient rats are TNF-alpha-mediated. FASEB J. 1999;13(2):297–304. doi: 10.1096/fasebj.13.2.297. [DOI] [PubMed] [Google Scholar]
- 173.Peracchi M, Bamonti Catena F, Pomati M, De Franceschi M, Scalabrino G. Human cobalamin deficiency: alterations in serum tumour necrosis factor-alpha and epidermal growth factor. Eur J Haematol. 2001;67(2):123–127. doi: 10.1034/j.1600-0609.2001.t01-1-00507.x. [DOI] [PubMed] [Google Scholar]
- 174.Scalabrino G. Cobalamin (vitamin B(12)) in subacute combined degeneration and beyond: traditional interpretations and novel theories. Exp Neurol. 2005;192(2):463–479. doi: 10.1016/j.expneurol.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 175.Scalabrino G, Veber D, Mutti E. New pathogenesis of the cobalamin-deficient neuropathy. Med Secoli. 2007;19(1):9–18. [PubMed] [Google Scholar]
- 176.Scalabrino G. The multi-faceted basis of vitamin B12 (cobalamin) neurotrophism in adult central nervous system: Lessons learned from its deficiency. Prog Neurobiol. 2009;88(3):203–220. doi: 10.1016/j.pneurobio.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 177.Scalabrino G, Carpo M, Bamonti F, et al. High tumor necrosis factor-alpha [corrected] levels in cerebrospinal fluid of cobalamin-deficient patients. Ann Neurol. 2004;56(6):886–890. doi: 10.1002/ana.20325. [DOI] [PubMed] [Google Scholar]
- 178.Scalabrino G, Peracchi M. New insights into the pathophysiology of cobalamin deficiency. Trends Mol Med. 2006;12(6):247–254. doi: 10.1016/j.molmed.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 179.Dogan M, Ozdemir O, Sal EA, et al. Psychotic disorder and extrapyramidal symptoms associated with vitamin B12 and folate deficiency. J Trop Pediatr. 2009;55(3):205–207. doi: 10.1093/tropej/fmn112. [DOI] [PubMed] [Google Scholar]
- 180.Healton EB, Savage DG, Brust JC, Garrett TJ, Lindenbaum J. Neurologic aspects of cobalamin deficiency. Medicine (Baltimore) 1991;70(4):229–245. doi: 10.1097/00005792-199107000-00001. [DOI] [PubMed] [Google Scholar]
- 181.Durand C, Mary S, Brazo P, Dollfus S. Psychiatric manifestations of vitamin B12 deficiency: a case report. Encephale. 2003;29(6):560–565. [PubMed] [Google Scholar]
- 182.Ko SM, Liu TC. Psychiatric syndromes in pernicious anaemia – a case report. Singapore Med J. 1992;33(1):92–94. [PubMed] [Google Scholar]
- 183.Lassen E, Ewald H. Acute organic psychosis caused by thyrotoxicosis and vitamin B12 def iciency: case report. J Clin Psychiatry. 1985;46(3):106–107. [PubMed] [Google Scholar]
- 184.Evans DL, Edelsohn GA, Golden RN. Organic psychosis without anemia or spinal cord symptoms in patients with vitamin B12 deficiency. Am J Psychiatry. 1983;140(2):218–221. doi: 10.1176/ajp.140.2.218. [DOI] [PubMed] [Google Scholar]
- 185.Bolaman Z, Kadikoylu G, Yukselen V, Yavasoglu I, Barutca S, Senturk T. Oral versus intramuscular cobalamin treatment in megaloblastic anemia: a single-center, prospective, randomized, open-label study. Clin Ther. 2003;25(12):3124–3134. doi: 10.1016/s0149-2918(03)90096-8. [DOI] [PubMed] [Google Scholar]
- 186.Cubero L, Mayor J, Almirall P, Valdes M. Memory disorders in the epidemic neuropathy. Rev Neurol. 1999;28(4):343–348. [PubMed] [Google Scholar]
- 187.Kwok T, Lee J, Lam L, Woo J. Vitamin B(12) supplementation did not improve cognition but reduced delirium in demented patients with vitamin B(12) deficiency. Arch Gerontol Geriatr. 2008;46(3):273–282. doi: 10.1016/j.archger.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 188.Lindenbaum J, Healton EB, Savage DG, et al. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318(26):1720–1728. doi: 10.1056/NEJM198806303182604. [DOI] [PubMed] [Google Scholar]
- 189.Heok KE, Ho R. The many faces of geriatric depression. Curr Opin Psychiatry. 2008;21(6):540–545. doi: 10.1097/YCO.0b013e328311cdae. [DOI] [PubMed] [Google Scholar]
- 190.Saracaceanu E, Tramoni AV, Henry JM. An association between subcortical dementia and pernicious anemia – a psychiatric mask. Compr Psychiatry. 1997;38(6):349–351. doi: 10.1016/s0010-440x(97)90932-9. [DOI] [PubMed] [Google Scholar]
- 191.Goggans FC. A case of mania secondary to vitamin B12 deficiency. Am J Psychiatry. 1984;141(2):300–301. doi: 10.1176/ajp.141.2.300. [DOI] [PubMed] [Google Scholar]
- 192.Bottiglieri T, Laundy M, Crellin R, Toone BK, Carney MW, Reynolds EH. Homocysteine, folate, methylation, and monoamine metabolism in depression. J Neurol Neurosurg Psychiatry. 2000;69(2):228–232. doi: 10.1136/jnnp.69.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Meeks TW, Ropacki SA, Jeste DV. The neurobiology of neuropsychiatric syndromes in dementia. Curr Opin Psychiatry. 2006;19(6):581–586. doi: 10.1097/01.yco.0000245746.45384.0e. [DOI] [PubMed] [Google Scholar]
- 194.Reif A, Pfuhlmann B, Lesch KP. Homocysteinemia as well as methylenetetrahydrofolate reductase polymorphism are associated with affective psychoses. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(7):1162–1168. doi: 10.1016/j.pnpbp.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 195.Dittmann S, Seemuller F, Schwarz MJ, et al. Association of cognitive deficits with elevated homocysteine levels in euthymic bipolar patients and its impact on psychosocial functioning: preliminary results. Bipolar Disord. 2007;9:1–2. 63–70. doi: 10.1111/j.1399-5618.2007.00412.x. [DOI] [PubMed] [Google Scholar]
- 196.Dittmann S, Seemuller F, Grunze HC, et al. The impact of homocysteine levels on cognition in euthymic bipolar patients: A cross-sectional study. J Clin Psychiatry. 2008:e1–e8. doi: 10.4088/jcp.v69n0603. [DOI] [PubMed] [Google Scholar]
- 197.Osher Y, Bersudsky Y, Silver H, Sela BA, Belmaker RH. Neuropsychological correlates of homocysteine levels in euthymic bipolar patients. J Affect Disord. 2008;105:1–3. 229–233. doi: 10.1016/j.jad.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 198.Levine J, Stahl Z, Sela BA, et al. Homocysteine-reducing strategies improve symptoms in chronic schizophrenic patients with hyperhomocysteinemia. Biol Psychiatry. 2006;60(3):265–269. doi: 10.1016/j.biopsych.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 199.Kalita J, Misra UK. Vitamin B12 deficiency neurological syndromes: correlation of clinical, MRI and cognitive evoked potential. J Neurol. 2008;255(3):353–359. doi: 10.1007/s00415-008-0660-x. [DOI] [PubMed] [Google Scholar]
- 200.Goldman LAA, editor. Cecil Textbook of Medicine. 22nd ed. Philadelphia, PA: Saunders, Elsevier Inc; 2004. [Google Scholar]
- 201.Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997;337(20):1441–1448. doi: 10.1056/NEJM199711133372007. [DOI] [PubMed] [Google Scholar]
- 202.Puntambekar P, Basha MM, Zak IT, Madhavan R. Rare sensory and autonomic disturbances associated with vitamin B12 deficiency. J Neurol Sci. 2009;287:1–2. 285–287. doi: 10.1016/j.jns.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 203.Ropper A, Brown R. Diseases of the nervous system due to nutritional deficiency. In: Ropper A, Brown R, editors. Adams and Victor’s Principles of Neurology. 8th ed. New York, NY: McGraw-Hill; 2005. pp. 983–1003. [Google Scholar]
- 204.Huckman MS, Fox J, Topel J. The validity of criteria for the evaluation of cerebral atrophy by computed tomography. Radiology. 1975;116(1):85–92. doi: 10.1148/116.1.85. [DOI] [PubMed] [Google Scholar]
- 205.de Leon MJ, George AE, Reisberg B, et al. Alzheimer’s disease: longitudinal CT studies of ventricular change. AJR Am J Roentgenol. 1989;152(6):1257–1262. doi: 10.2214/ajr.152.6.1257. [DOI] [PubMed] [Google Scholar]
- 206.Takeda S, Matsuzawa T. Age-related brain atrophy: a study with computed tomography. J Gerontol. 1985;40(2):159–163. doi: 10.1093/geronj/40.2.159. [DOI] [PubMed] [Google Scholar]
- 207.LeMay M. Radiologic changes of the aging brain and skull. AJR Am J Roentgenol. 1984;143(2):383–389. doi: 10.2214/ajr.143.2.383. [DOI] [PubMed] [Google Scholar]
- 208.Ito M, Hatazawa J, Yamaura H, Matsuzawa T. Age-related brain atrophy and mental deterioration – a study with computed tomography. Br J Radiol. 1981;54(641):384–390. doi: 10.1259/0007-1285-54-641-384. [DOI] [PubMed] [Google Scholar]
- 209.Creasey H, Schwartz M, Frederickson H, Haxby JV, Rapoport SI. Quantitative computed tomography in dementia of the Alzheimer type. Neurology. 1986;36(12):1563–1568. doi: 10.1212/wnl.36.12.1563. [DOI] [PubMed] [Google Scholar]
- 210.DeCarli C, Kaye JA, Horwitz B, Rapoport SI. Critical analysis of the use of computer-assisted transverse axial tomography to study human brain in aging and dementia of the Alzheimer type. Neurology. 1990;40(6):872–883. doi: 10.1212/wnl.40.6.872. [DOI] [PubMed] [Google Scholar]
- 211.DeCarli C, Murphy DG, McIntosh AR, Teichberg D, Schapiro MB, Horwitz B. Discriminant analysis of MRI measures as a method to determine the presence of dementia of the Alzheimer type. Psychiatry Res. 1995;57(2):119–130. doi: 10.1016/0165-1781(95)02651-c. [DOI] [PubMed] [Google Scholar]
- 212.Frisoni GB, Beltramello A, Weiss C, Geroldi C, Bianchetti A, Trabucchi M. Linear measures of atrophy in mild Alzheimer disease. AJNR Am J Neuroradiol. 1996;17(5):913–923. [PMC free article] [PubMed] [Google Scholar]
- 213.Horn R, Ostertun B, Fric M, Solymosi L, Steudel A, Moller HJ. Atrophy of hippocampus in patients with Alzheimer’s disease and other diseases with memory impairment. Dementia. 1996;7(4):182–186. doi: 10.1159/000106876. [DOI] [PubMed] [Google Scholar]
- 214.Jobst KA, Smith AD, Szatmari M, et al. Detection in life of confirmed Alzheimer’s disease using a simple measurement of medial temporal lobe atrophy by computed tomography. Lancet. 1992;340(8829):1179–1183. doi: 10.1016/0140-6736(92)92890-r. [DOI] [PubMed] [Google Scholar]
- 215.Kawamura J, Meyer JS, Ichijo M, Kobari M, Terayama Y, Weathers S. Correlations of leuko-araiosis with cerebral atrophy and perfusion in elderly normal subjects and demented patients. J Neurol Neurosurg Psychiatry. 1993;56(2):182–187. doi: 10.1136/jnnp.56.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Luxenberg JS, Friedland RP, Rapoport SI. Quantitative X-ray computed tomography (CT) in dementia of the Alzheimer type (DAT) Can J Neurol Sci. 1986;13(4 Suppl):570–572. doi: 10.1017/s031716710003732x. [DOI] [PubMed] [Google Scholar]
- 217.Philpot M, Burns A, Jobst, et al. Detection in life of confirmed Alzheimer’s disease using a simple measurement of medial temporal lobe atrophy by computed tomography. Br J Psychiatry. 1993;163:809–812. doi: 10.1192/bjp.163.6.809. [DOI] [PubMed] [Google Scholar]
- 218.Whitehouse PJ. Clinical Practice Guidelines: Guidelines Abstracted from the American Academy of Neurology’s Dementia Guidelines for Early Detection, Diagnosis, and Management of Dementia. The American Geriatrics Society; 2008. [Accessed on August 21, 2009]. Available from: http://www.americangeriatrics.org/products/positionpapers/aan_dementia.shtml. [DOI] [PubMed] [Google Scholar]
- 219.Burns A, Jacoby R, Levy R. Computed tomography in Alzheimer’s disease: a longitudinal study. Biol Psychiatry. 1991;29(4):383–390. doi: 10.1016/0006-3223(91)90224-a. [DOI] [PubMed] [Google Scholar]
- 220.Bertsch T, Mielke O, Holy S, et al. Homocysteine in cerebrovascular disease: an independent risk factor for subcortical vascular encephalopathy. Clin Chem Lab Med. 2001;39(8):721–724. doi: 10.1515/CCLM.2001.120. [DOI] [PubMed] [Google Scholar]
- 221.Censori B, Partziguian T, Manara O, Poloni M. Plasma homocysteine and severe white matter disease. Neurol Sci. 2007;28(5):259–263. doi: 10.1007/s10072-007-0832-y. [DOI] [PubMed] [Google Scholar]
- 222.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55(11):1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 223.Evers S, Koch HG, Grotemeyer KH, Lange B, Deufel T, Ringelstein EB. Features, symptoms, and neurophysiological findings in stroke associated with hyperhomocysteinemia. Arch Neurol. 1997;54(10):1276–1282. doi: 10.1001/archneur.1997.00550220074017. [DOI] [PubMed] [Google Scholar]
- 224.Hassan A, Hunt BJ, O’Sullivan M, et al. Homocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunction. Brain. 2004;127(Pt 1):212–219. doi: 10.1093/brain/awh023. [DOI] [PubMed] [Google Scholar]
- 225.Hogervorst E, Ribeiro HM, Molyneux A, Budge M, Smith AD. Plasma homocysteine levels, cerebrovascular risk factors, and cerebral white matter changes (leukoaraiosis) in patients with Alzheimer disease. Arch Neurol. 2002;59(5):787–793. doi: 10.1001/archneur.59.5.787. [DOI] [PubMed] [Google Scholar]
- 226.Naka H, Nomura E, Takahashi T, et al. Plasma total homocysteine levels are associated with advanced leukoaraiosis but not with asymptomatic microbleeds on T2*-weighted MRI in patients with stroke. Eur J Neurol. 2006;13(3):261–265. doi: 10.1111/j.1468-1331.2006.01205.x. [DOI] [PubMed] [Google Scholar]
- 227.Quadri P, Fragiacomo C, Pezzati R, et al. Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer disease, and vascular dementia. Am J Clin Nutr. 2004;80(1):114–122. doi: 10.1093/ajcn/80.1.114. [DOI] [PubMed] [Google Scholar]
- 228.Sachdev P, Parslow R, Salonikas C, et al. Homocysteine and the brain in midadult life: evidence for an increased risk of leukoaraiosis in men. Arch Neurol. 2004;61(9):1369–1376. doi: 10.1001/archneur.61.9.1369. [DOI] [PubMed] [Google Scholar]
- 229.Sachdev PS. Homocysteine and brain atrophy. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(7):1152–1161. doi: 10.1016/j.pnpbp.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 230.Schwartz GL, Bailey KR, Mosley T, et al. Association of ambulatory blood pressure with ischemic brain injury. Hypertension. 2007;49(6):1228–1234. doi: 10.1161/HYPERTENSIONAHA.106.078691. [DOI] [PubMed] [Google Scholar]
- 231.Scott TM, Tucker KL, Bhadelia A, et al. Homocysteine and B vitamins relate to brain volume and white-matter changes in geriatric patients with psychiatric disorders. Am J Geriatr Psychiatry. 2004;12(6):631–638. doi: 10.1176/appi.ajgp.12.6.631. [DOI] [PubMed] [Google Scholar]
- 232.Seshadri S, Wolf PA, Beiser AS, et al. Association of plasma total homocysteine levels with subclinical brain injury: cerebral volumes, white matter hyperintensity, and silent brain infarcts at volumetric magnetic resonance imaging in the Framingham Offspring Study. Arch Neurol. 2008;65(5):642–649. doi: 10.1001/archneur.65.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Smith AD. The worldwide challenge of the dementias: a role for B vitamins and homocysteine? Food Nutr Bull. 2008;29(2 Suppl):S143–172. doi: 10.1177/15648265080292S119. [DOI] [PubMed] [Google Scholar]
- 234.Vogiatzoglou A, Refsum H, Johnston C, et al. Vitamin B12 status and rate of brain volume loss in community-dwelling elderly. Neurology. 2008;71(11):826–832. doi: 10.1212/01.wnl.0000325581.26991.f2. [DOI] [PubMed] [Google Scholar]
- 235.Williams JH, Pereira EA, Budge MM, Bradley KM. Minimal hippocampal width relates to plasma homocysteine in community-dwelling older people. Age Ageing. 2002;31(6):440–444. doi: 10.1093/ageing/31.6.440. [DOI] [PubMed] [Google Scholar]
- 236.Wright CB, Paik MC, Brown TR, et al. Total homocysteine is associated with white matter hyperintensity volume: the Northern Manhattan Study. Stroke. 2005;36(6):1207–1211. doi: 10.1161/01.STR.0000165923.02318.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yang LK, Wong KC, Wu MY, Liao SL, Kuo CS, Huang RF. Correlations between folate, B12, homocysteine levels, and radiological markers of neuropathology in elderly post-stroke patients. J Am Coll Nutr. 2007;26(3):272–278. doi: 10.1080/07315724.2007.10719611. [DOI] [PubMed] [Google Scholar]
- 238.Jimenez I, Agulla J, Pouso M, et al. Cognitive impairment associated to leukoaraiosis: its pathophysiology, clinical manifestations and treatment. Rev Neurol. 2008;47(10):536–544. [PubMed] [Google Scholar]
- 239.Fu JH, Lu CZ, Hong Z, Dong Q, Luo Y, Wong KS. Extent of white matter lesions is related to acute subcortical infarcts and predicts further stroke risk in patients with first ever ischaemic stroke. J Neurol Neurosurg Psychiatry. 2005;76(6):793–796. doi: 10.1136/jnnp.2003.032771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Longstreth WT, Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27(8):1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 241.Streifler JY, Eliasziw M, Benavente OR, et al. Prognostic importance of leukoaraiosis in patients with symptomatic internal carotid artery stenosis. Stroke. 2002;33(6):1651–1655. doi: 10.1161/01.str.0000018010.38749.08. [DOI] [PubMed] [Google Scholar]
- 242.Streifler JY, Eliasziw M, Benavente OR, et al. Development and progression of leukoaraiosis in patients with brain ischemia and carotid artery disease. Stroke. 2003;34(8):1913–1916. doi: 10.1161/01.STR.0000080939.39414.83. [DOI] [PubMed] [Google Scholar]
- 243.George AE, de Leon MJ, Gentes CI, et al. Leukoencephalopathy in normal and pathologic aging: 1. CT of brain lucencies. AJNR Am J Neuroradiol. 1986;7(4):561–566. [PMC free article] [PubMed] [Google Scholar]
- 244.Basile AM, Pantoni L, Pracucci G, et al. Age, hypertension, and lacunar stroke are the major determinants of the severity of age-related white matter changes. The LADIS (Leukoaraiosis and Disability in the Elderly) Study. Cerebrovasc Dis. 2006;21:5–6. 315–322. doi: 10.1159/000091536. [DOI] [PubMed] [Google Scholar]
- 245.Bastos-Leite AJ, Kuijer JP, Rombouts SA, et al. Cerebral blood flow by using pulsed arterial spin-labeling in elderly subjects with white matter hyperintensities. AJNR Am J Neuroradiol. 2008;29(7):1296–1301. doi: 10.3174/ajnr.A1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Farrall AJ, Wardlaw JM. Blood-brain barrier: Ageing and microvascular disease – systematic review and meta-analysis. Neurobiol Aging. 2009;30(3):337–352. doi: 10.1016/j.neurobiolaging.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 247.Schwartz GL, Fornage M, Mosley T, Turner ST. Treatment of leukoaraiosis. Curr Treat Options Cardiovasc Med. 2005;7(3):173–177. doi: 10.1007/s11936-005-0045-8. [DOI] [PubMed] [Google Scholar]
- 248.Roman GC. Senile dementia of the Binswanger type. A vascular form of dementia in the elderly. JAMA. 1987;258(13):1782–1788. doi: 10.1001/jama.1987.03400130096040. [DOI] [PubMed] [Google Scholar]
- 249.de Lau LM, Refsum H, Smith AD, Johnston C, Breteler MM. Plasma folate concentration and cognitive performance: Rotterdam Scan Study. Am J Clin Nutr. 2007;86(3):728–734. doi: 10.1093/ajcn/86.3.728. [DOI] [PubMed] [Google Scholar]
- 250.de Lau LM, Smith AD, Refsum H, Johnston C, Breteler MM. Plasma vitamin B12 status and cerebral white-matter lesions. J Neurol Neurosurg Psychiatry. 2009;80(2):149–157. doi: 10.1136/jnnp.2008.149286. [DOI] [PubMed] [Google Scholar]
- 251.Inzitari D, Simoni M, Pracucci G, et al. Risk of rapid global functional decline in elderly patients with severe cerebral age-related white matter changes: the LADIS study. Arch Intern Med. 2007;167(1):81–88. doi: 10.1001/archinte.167.1.81. [DOI] [PubMed] [Google Scholar]
- 252.Dufouil C, Alperovitch A, Ducros V, Tzourio C. Homocysteine, white matter hyperintensities, and cognition in healthy elderly people. Ann Neurol. 2003;53(2):214–221. doi: 10.1002/ana.10440. [DOI] [PubMed] [Google Scholar]
- 253.Polyak Z, Stern F, Berner YN, et al. Hyperhomocysteinemia and vitamin score: correlations with silent brain ischemic lesions and brain atrophy. Dement Geriatr Cogn Disord. 2003;16(1):39–45. doi: 10.1159/000069992. [DOI] [PubMed] [Google Scholar]
- 254.Stenset V, Hofoss D, Johnsen L, et al. White matter lesion severity is associated with reduced cognitive performances in patients with normal CSF Abeta42 levels. Acta Neurol Scand. 2008;118(6):373–378. doi: 10.1111/j.1600-0404.2008.01045.x. [DOI] [PubMed] [Google Scholar]
- 255.Wong A, Mok V, Fan YH, Lam WW, Liang KS, Wong KS. Hyperhomocysteinemia is associated with volumetric white matter change in patients with small vessel disease. J Neurol. 2006;253(4):441–447. doi: 10.1007/s00415-005-0022-x. [DOI] [PubMed] [Google Scholar]
- 256.Wallin A, Sjogren M. Cerebrospinal fluid cytoskeleton proteins in patients with subcortical white-matter dementia. Mech Ageing Dev. 2001;122(16):1937–1949. doi: 10.1016/s0047-6374(01)00306-2. [DOI] [PubMed] [Google Scholar]
- 257.Babiloni C, Bosco P, Ghidoni R, et al. Homocysteine and electroencephalographic rhythms in Alzheimer disease: a multicentric study. Neuroscience. 2007;145(3):942–954. doi: 10.1016/j.neuroscience.2006.12.065. [DOI] [PubMed] [Google Scholar]
- 258.Seshadri S, Beiser A, Selhub J, et al. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med. 2002;346(7):476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 259.Miller JW, Green R, Mungas DM, Reed BR, Jagust WJ. Homocysteine, vitamin B6, and vascular disease in AD patients. Neurology. 2002;58(10):1471–1475. doi: 10.1212/wnl.58.10.1471. [DOI] [PubMed] [Google Scholar]
- 260.Ando S, Nakayama H. Cerebral amyloid angiopathy in Alzheimer’s disease: with special reference to its incidence and distribution. No To Shinkei. 1984;36(11):1109–1116. [PubMed] [Google Scholar]
- 261.Coria F, Castano E, Prelli F, et al. Isolation and characterization of amyloid P component from Alzheimer’s disease and other types of cerebral amyloidosis. Lab Invest. 1988;58(4):454–458. [PubMed] [Google Scholar]
- 262.Buee L, Hof PR, Bouras C, et al. Pathological alterations of the cerebral microvasculature in Alzheimer’s disease and related dementing disorders. Acta Neuropathol (Berl) 1994;87(5):469–480. doi: 10.1007/BF00294173. [DOI] [PubMed] [Google Scholar]
- 263.Eberling JL, Jagust WJ, Reed BR, Baker MG. Reduced temporal lobe blood flow in Alzheimer’s disease. Neurobiol Aging. 1992;13(4):483–491. doi: 10.1016/0197-4580(92)90076-a. [DOI] [PubMed] [Google Scholar]
- 264.Jobst KA, Smith AD, Barker CS, et al. Association of atrophy of the medial temporal lobe with reduced blood flow in the posterior parietotemporal cortex in patients with a clinical and pathological diagnosis of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1992;55(3):190–194. doi: 10.1136/jnnp.55.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Purandare N, Burns A, Daly KJ, et al. Cerebral emboli as a potential cause of Alzheimer’s disease and vascular dementia: case-control study. BMJ. 2006;332(7550):1119–1124. doi: 10.1136/bmj.38814.696493.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Purandare N, Voshaar RC, Morris J, et al. Asymptomatic spontaneous cerebral emboli predict cognitive and functional decline in dementia. Biol Psychiatry. 2007;62(4):339–344. doi: 10.1016/j.biopsych.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 267.Agnati LF, Genedani S, Rasio G, et al. Studies on homocysteine plasma levels in Alzheimer’s patients. Relevance for neurodegeneration. J Neural Transm. 2005;112(1):163–169. doi: 10.1007/s00702-004-0154-7. [DOI] [PubMed] [Google Scholar]
- 268.Lee ES, Chen H, Soliman KF, Charlton CG. Effects of homocysteine on the dopaminergic system and behavior in rodents. Neurotoxicology. 2005;26(3):361–371. doi: 10.1016/j.neuro.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 269.Choi BH, Kim RC, Vaughan PJ, et al. Decreases in protease nexins in Alzheimer’s disease brain. Neurobiol Aging. 1995;16(4):557–562. doi: 10.1016/0197-4580(95)00060-r. [DOI] [PubMed] [Google Scholar]
- 270.Hanyu H, Asano T, Tanaka Y, Iwamoto T, Takasaki M, Abe K. Increased blood-brain barrier permeability in white matter lesions of Binswanger’s disease evaluated by contrast-enhanced MRI. Dement Geriatr Cogn Disord. 2002;14(1):1–6. doi: 10.1159/000058326. [DOI] [PubMed] [Google Scholar]
- 271.Arshavsky YI. Alzheimer’s disease, brain immune privilege and memory: a hypothesis. J Neural Transm. 2006;113(11):1697–1707. doi: 10.1007/s00702-006-0524-4. [DOI] [PubMed] [Google Scholar]
- 272.D’Andrea MR. Add Alzheimer’s disease to the list of autoimmune diseases. Med Hypotheses. 2005;64(3):458–463. doi: 10.1016/j.mehy.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 273.Groppa SA, Chekhonin VP. Specific brain antigens as the indicators of the blood-brain barrier permeability in Alzheimer’s disease. Zh Nevropatol Psikhiatr Im S S Korsakova. 1991;91(9):50–52. [PubMed] [Google Scholar]
- 274.Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288(16):2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 275.Bautista LE, Arenas IA, Penuela A, Martinez LX. Total plasma homocysteine level and risk of cardiovascular disease: a meta-analysis of prospective cohort studies. J Clin Epidemiol. 2002;55(9):882–887. doi: 10.1016/s0895-4356(02)00434-1. [DOI] [PubMed] [Google Scholar]
- 276.Liu XQ, Jiang P, Huang H, Yan Y. Plasma levels of endogenous hydrogen sulfide and homocysteine in patients with Alzheimer’s disease and vascular dementia and the significance thereof. Zhonghua Yi Xue Za Zhi. 2008;88(32):2246–2249. [PubMed] [Google Scholar]
- 277.Moller J, Nielsen GM, Tvedegaard KC, Andersen NT, Jorgensen PE. A meta-analysis of cerebrovascular disease and hyperhomocysteinaemia. Scand J Clin Lab Invest. 2000;60(6):491–499. doi: 10.1080/003655100448473. [DOI] [PubMed] [Google Scholar]
- 278.Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence on causality from a meta-analysis. BMJ. 2002;325(7374):1202. doi: 10.1136/bmj.325.7374.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274(13):1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 280.Troen AM, Shea-Budgell M, Shukitt-Hale B, Smith DE, Selhub J, Rosenberg IH. B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. Proc Natl Acad Sci U S A. 2008;105(34):12474–12479. doi: 10.1073/pnas.0805350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Yao H, Sadoshima S, Kuwabara Y, Ichiya Y, Fujishima M. Cerebral blood flow and oxygen metabolism in patients with vascular dementia of the Binswanger type. Stroke. 1990;21(12):1694–1699. doi: 10.1161/01.str.21.12.1694. [DOI] [PubMed] [Google Scholar]
- 282.Picardi A, Navajas F, Spoto S, Palma Modoni A, De Galasso L, Costantino S. A 74-year-old woman with macrocytic anemia. Clin Ter. 2002;153(1):65–67. [PubMed] [Google Scholar]
- 283.Chin AV, Robinson DJ, O’Connell H, et al. Vascular biomarkers of cognitive performance in a community-based elderly population: the Dublin Healthy Ageing study. Age Ageing. 2008;37(5):559–564. doi: 10.1093/ageing/afn144. [DOI] [PubMed] [Google Scholar]
- 284.Sala I, Belen Sanchez-Saudinos M, Molina-Porcel L, et al. Homocysteine and cognitive impairment. Relation with diagnosis and neuropsychological performance. Dement Geriatr Cogn Disord. 2008;26(6):506–512. doi: 10.1159/000173710. [DOI] [PubMed] [Google Scholar]
- 285.Selhub J, Morris MS, Jacques PF, Rosenberg IH. Folate-vitamin B-12 interaction in relation to cognitive impairment, anemia, and biochemical indicators of vitamin B-12 deficiency. Am J Clin Nutr. 2009;89(2):702S–706S. doi: 10.3945/ajcn.2008.26947C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Stewart R, Asonganyi B, Sherwood R. Plasma homocysteine and cognitive impairment in an older British African-Caribbean population. J Am Geriatr Soc. 2002;50(7):1227–1232. doi: 10.1046/j.1532-5415.2002.50309.x. [DOI] [PubMed] [Google Scholar]
- 287.Ravaglia G, Forti P, Maioli F, et al. Homocysteine and cognitive function in healthy elderly community dwellers in Italy. Am J Clin Nutr. 2003;77(3):668–673. doi: 10.1093/ajcn/77.3.668. [DOI] [PubMed] [Google Scholar]
- 288.Prins ND, Den Heijer T, Hofman A, et al. Homocysteine and cognitive function in the elderly: the Rotterdam Scan Study. Neurology. 2002;59(9):1375–1380. doi: 10.1212/01.wnl.0000032494.05619.93. [DOI] [PubMed] [Google Scholar]
- 289.Cole MG, Prchal JF. Low serum vitamin B12 in Alzheimer-type dementia. Age Ageing. 1984;13(2):101–105. doi: 10.1093/ageing/13.2.101. [DOI] [PubMed] [Google Scholar]
- 290.Malaguarnera M, Ferri R, Bella R, Alagona G, Carnemolla A, Pennisi G. Homocysteine, vitamin B12 and folate in vascular dementia and in Alzheimer disease. Clin Chem Lab Med. 2004;42(9):1032–1035. doi: 10.1515/CCLM.2004.208. [DOI] [PubMed] [Google Scholar]
- 291.McCaddon A, Davies G, Hudson P, Tandy S, Cattell H. Total serum homocysteine in senile dementia of Alzheimer type. Int J Geriatr Psychiatry. 1998;13(4):235–239. doi: 10.1002/(sici)1099-1166(199804)13:4<235::aid-gps761>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 292.Hagnelius NO, Wahlund LO, Nilsson TK. CSF/serum folate gradient: physiology and determinants with special reference to dementia. Dement Geriatr Cogn Disord. 2008;25(6):516–523. doi: 10.1159/000129696. [DOI] [PubMed] [Google Scholar]
- 293.Zoccolella S, Lamberti P, Iliceto G, et al. Plasma homocysteine levels in L-dopa-treated Parkinson’s disease patients with cognitive dysfunctions. Clin Chem Lab Med. 2005;43(10):1107–1110. doi: 10.1515/CCLM.2005.193. [DOI] [PubMed] [Google Scholar]
- 294.Ozer F, Meral H, Hanoglu L, et al. Plasma homocysteine levels in patients treated with levodopa: motor and cognitive associations. Neurol Res. 2006;28(8):853–858. doi: 10.1179/016164106X110445. [DOI] [PubMed] [Google Scholar]
- 295.Budge MM, de Jager C, Hogervorst E, Smith AD. Total plasma homocysteine, age, systolic blood pressure, and cognitive performance in older people. J Am Geriatr Soc. 2002;50(12):2014–2018. doi: 10.1046/j.1532-5415.2002.50614.x. [DOI] [PubMed] [Google Scholar]
- 296.Duthie SJ, Whalley LJ, Collins AR, Leaper S, Berger K, Deary IJ. Homocysteine, B vitamin status, and cognitive function in the elderly. Am J Clin Nutr. 2002;75(5):908–913. doi: 10.1093/ajcn/75.5.908. [DOI] [PubMed] [Google Scholar]
- 297.Elias MF, Robbins MA, Budge MM, et al. Homocysteine and cognitive performance: modification by the ApoE genotype. Neurosci Lett. 2008;430(1):64–69. doi: 10.1016/j.neulet.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 298.Eussen SJ, Ueland PM, Clarke R, et al. The association of betaine, homocysteine and related metabolites with cognitive function in Dutch elderly people. Br J Nutr. 2007;98(5):960–968. doi: 10.1017/S0007114507750912. [DOI] [PubMed] [Google Scholar]
- 299.Garcia AA, Haron Y, Evans LR, Smith MG, Freedman M, Roman GC. Metabolic markers of cobalamin deficiency and cognitive function in normal older adults. J Am Geriatr Soc. 2004;52(1):66–71. doi: 10.1111/j.1532-5415.2004.52012.x. [DOI] [PubMed] [Google Scholar]
- 300.Hin H, Clarke R, Sherliker P, et al. Clinical relevance of low serum vitamin B12 concentrations in older people: the Banbury B12 study. Age Ageing. 2006;35(4):416–422. doi: 10.1093/ageing/afl033. [DOI] [PubMed] [Google Scholar]
- 301.Kado DM, Karlamangla AS, Huang MH, et al. Homocysteine versus the vitamins folate, B6, and B12 as predictors of cognitive function and decline in older high-functioning adults: MacArthur Studies of Successful Aging. Am J Med. 2005;118(2):161–167. doi: 10.1016/j.amjmed.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 302.Miller JW, Green R, Ramos MI, et al. Homocysteine and cognitive function in the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2003;78(3):441–447. doi: 10.1093/ajcn/78.3.441. [DOI] [PubMed] [Google Scholar]
- 303.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr. 2007;85(1):193–200. doi: 10.1093/ajcn/85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Riggs KM, Spiro A, 3rd, Tucker K, Rush D. Relations of vitamin B-12, vitamin B-6, folate, and homocysteine to cognitive performance in the Normative Aging Study. Am J Clin Nutr. 1996;63(3):306–314. doi: 10.1093/ajcn/63.3.306. [DOI] [PubMed] [Google Scholar]
- 305.Styczynska M, Strosznajder JB, Religa D, et al. Association between genetic and environmental factors and the risk of Alzheimer’s disease. Folia Neuropathol. 2008;46(4):249–254. [PubMed] [Google Scholar]
- 306.Wolters M, Hickstein M, Flintermann A, Tewes U, Hahn A. Cognitive performance in relation to vitamin status in healthy elderly German women-the effect of 6-month multivitamin supplementation. Prev Med. 2005;41(1):253–259. doi: 10.1016/j.ypmed.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 307.Annerbo S, Wahlund LO, Lokk J. The relation between homocysteine levels and development of Alzheimer’s disease in mild cognitive impairment patients. Dement Geriatr Cogn Disord. 2005;20(4):209–214. doi: 10.1159/000087297. [DOI] [PubMed] [Google Scholar]
- 308.Clarke R, Birks J, Nexo E, et al. Low vitamin B-12 status and risk of cognitive decline in older adults. Am J Clin Nutr. 2007;86(5):1384–1391. doi: 10.1093/ajcn/86.5.1384. [DOI] [PubMed] [Google Scholar]
- 309.Elias MF, Sullivan LM, D’Agostino RB, et al. Homocysteine and cognitive performance in the Framingham offspring study: age is important. Am J Epidemiol. 2005;162(7):644–653. doi: 10.1093/aje/kwi259. [DOI] [PubMed] [Google Scholar]
- 310.Gabryelewicz T, Styczynska M, Luczywek E, et al. The rate of conversion of mild cognitive impairment to dementia: predictive role of depression. Int J Geriatr Psychiatry. 2007;22(6):563–567. doi: 10.1002/gps.1716. [DOI] [PubMed] [Google Scholar]
- 311.Garcia A, Haron Y, Pulman K, Hua L, Freedman M. Increases in homocysteine are related to worsening of stroop scores in healthy elderly persons: a prospective follow-up study. J Gerontol A Biol Sci Med Sci. 2004;59(12):1323–1327. doi: 10.1093/gerona/59.12.1323. [DOI] [PubMed] [Google Scholar]
- 312.Haan MN, Miller JW, Aiello AE, et al. Homocysteine, B vitamins, and the incidence of dementia and cognitive impairment: results from the Sacramento Area Latino Study on Aging. Am J Clin Nutr. 2007;85(2):511–517. doi: 10.1093/ajcn/85.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Kim JM, Stewart R, Kim SW, et al. Changes in folate, vitamin B12, and homocysteine associated with incident dementia. J Neurol Neurosurg Psychiatry. 2008;79(8):864–868. doi: 10.1136/jnnp.2007.131482. [DOI] [PubMed] [Google Scholar]
- 314.Ravaglia G, Forti P, Maioli F, et al. Blood inflammatory markers and risk of dementia: The Conselice Study of Brain Aging. Neurobiol Aging. 2007;28(12):1810–1820. doi: 10.1016/j.neurobiolaging.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 315.Wright CB, Lee HS, Paik MC, Stabler SP, Allen RH, Sacco RL. Total homocysteine and cognition in a tri-ethnic cohort: the Northern Manhattan Study. Neurology. 2004;63(2):254–260. doi: 10.1212/01.wnl.0000129986.19019.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Nurk E, Refsum H, Tell GS, et al. Plasma total homocysteine and memory in the elderly: the Hordaland Homocysteine Study. Ann Neurol. 2005;58(6):847–857. doi: 10.1002/ana.20645. [DOI] [PubMed] [Google Scholar]
- 317.Nurk E, Drevon CA, Refsum H, et al. Cognitive performance among the elderly and dietary fish intake: the Hordaland Health Study. Am J Clin Nutr. 2007;86(5):1470–1478. doi: 10.1093/ajcn/86.5.1470. [DOI] [PubMed] [Google Scholar]
- 318.McCaddon A, Hudson P, Davies G, Hughes A, Williams JH, Wilkinson C. Homocysteine and cognitive decline in healthy elderly. Dement Geriatr Cogn Disord. 2001;12(5):309–313. doi: 10.1159/000051275. [DOI] [PubMed] [Google Scholar]
- 319.Teunissen CE, Blom AH, Van Boxtel MP, et al. Homocysteine: a marker for cognitive performance? A longitudinal follow-up study. J Nutr Health Aging. 2003;7(3):153–159. [PubMed] [Google Scholar]
- 320.Oulhaj A, Refsum H, Beaumont H, et al. Homocysteine as a predictor of cognitive decline in Alzheimer’s disease. Int J Geriatr Psychiatry. 2009 May 29; doi: 10.1002/gps.2303. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 321.Campbell AK, Jagust WJ, Mungas DM, et al. Low erythrocyte folate, but not plasma vitamin B-12 or homocysteine, is associated with dementia in elderly Latinos. J Nutr Health Aging. 2005;9(1):39–43. [PubMed] [Google Scholar]
- 322.Luchsinger JA, Tang MX, Shea S, Miller J, Green R, Mayeux R. Plasma homocysteine levels and risk of Alzheimer disease. Neurology. 2004;62(11):1972–1976. doi: 10.1212/01.wnl.0000129504.60409.88. [DOI] [PubMed] [Google Scholar]
- 323.Reitz C, Tang MX, Miller J, Green R, Luchsinger JA. Plasma homocysteine and risk of mild cognitive impairment. Dement Geriatr Cogn Disord. 2008;27(1):11–17. doi: 10.1159/000182421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Kalmijn S, Launer LJ, Lindemans J, Bots ML, Hofman A, Breteler MM. Total homocysteine and cognitive decline in a community-based sample of elderly subjects: the Rotterdam Study. Am J Epidemiol. 1999;150(3):283–289. doi: 10.1093/oxfordjournals.aje.a010000. [DOI] [PubMed] [Google Scholar]
- 325.Mooijaart SP, Gussekloo J, Frolich M, et al. Homocysteine, vitamin B-12, and folic acid and the risk of cognitive decline in old age: the Leiden 85-Plus study. Am J Clin Nutr. 2005;82(4):866–871. doi: 10.1093/ajcn/82.4.866. [DOI] [PubMed] [Google Scholar]
- 326.Clarke R, Harrison G, Richards S. Effect of vitamins and aspirin on markers of platelet activation, oxidative stress and homocysteine in people at high risk of dementia. J Intern Med. 2003;254(1):67–75. doi: 10.1046/j.1365-2796.2003.01154.x. [DOI] [PubMed] [Google Scholar]
- 327.Nilsson K, Gustafson L, Hultberg B. Plasma homocysteine concentration relates to the severity but not to the duration of Alzheimer’s disease. Int J Geriatr Psychiatry. 2004;19(7):666–672. doi: 10.1002/gps.1140. [DOI] [PubMed] [Google Scholar]
- 328.Postiglione A, Milan G, Ruocco A, Gallotta G, Guiotto G, Di Minno G. Plasma folate, vitamin B(12), and total homocysteine and homozygosity for the C677T mutation of the 5,10-methylene tetrahydrofolate reductase gene in patients with Alzheimer’s dementia. A case-control study. Gerontology. 2001;47(6):324–329. doi: 10.1159/000052822. [DOI] [PubMed] [Google Scholar]
- 329.Locascio JJ, Fukumoto H, Yap L, et al. Plasma amyloid beta-protein and C-reactive protein in relation to the rate of progression of Alzheimer disease. Arch Neurol. 2008;65(6):776–785. doi: 10.1001/archneur.65.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Corder EH, Beaumont H. Susceptibility groups for Alzheimer’s disease (OPTIMA cohort): integration of gene variants and biochemical factors. Mech Ageing Dev. 2007;128(1):76–82. doi: 10.1016/j.mad.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 331.Engelborghs S, Vloeberghs E, Maertens K, et al. Correlations between cognitive, behavioural and psychological findings and levels of vitamin B12 and folate in patients with dementia. Int J Geriatr Psychiatry. 2004;19(4):365–370. doi: 10.1002/gps.1092. [DOI] [PubMed] [Google Scholar]
- 332.Roos D, Willanger R. Various degrees of dementia in a selected group of gastrectomized patients with low serum B12. Acta Neurol Scand. 1977;55(5):363–376. doi: 10.1111/j.1600-0404.1977.tb05655.x. [DOI] [PubMed] [Google Scholar]
- 333.Wang HX, Wahlin A, Basun H, Fastbom J, Winblad B, Fratiglioni L. Vitamin B(12) and folate in relation to the development of Alzheimer’s disease. Neurology. 2001;56(9):1188–1194. doi: 10.1212/wnl.56.9.1188. [DOI] [PubMed] [Google Scholar]
- 334.Eussen SJ, Ferry M, Hininger I, Haller J, Matthys C, Dirren H. Five year changes in mental health and associations with vitamin B12/folate status of elderly Europeans. J Nutr Health Aging. 2002;6(1):43–50. [PubMed] [Google Scholar]
- 335.Garrod MG, Green R, Allen LH, et al. Fraction of total plasma vitamin B12 bound to transcobalamin correlates with cognitive function in elderly Latinos with depressive symptoms. Clin Chem. 2008;54(7):1210–1217. doi: 10.1373/clinchem.2007.102632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Lopez OL, Becker JT, Klunk W, et al. Research evaluation and diagnosis of possible Alzheimer’s disease over the last two decades: II. Neurology. 2000;55(12):1863–1869. doi: 10.1212/wnl.55.12.1863. [DOI] [PubMed] [Google Scholar]
- 337.Luchsinger JA, Tang MX, Miller J, Green R, Mayeux R. Relation of higher folate intake to lower risk of Alzheimer disease in the elderly. Arch Neurol. 2007;64(1):86–92. doi: 10.1001/archneur.64.1.86. [DOI] [PubMed] [Google Scholar]
- 338.Small BJ, Viitanen M, Winblad B, Backman L. Cognitive changes in very old persons with dementia: the influence of demographic, psychometric, and biological variables. J Clin Exp Neuropsychol. 1997;19(2):245–260. doi: 10.1080/01688639708403855. [DOI] [PubMed] [Google Scholar]
- 339.Small BJ, Backman L. Predictors of longitudinal changes in memory, visuospatial, and verbal functioning in very old demented adults. Dement Geriatr Cogn Disord. 1998;9(5):258–266. doi: 10.1159/000017070. [DOI] [PubMed] [Google Scholar]
- 340.Prodan CI, Cowan LD, Stoner JA, Ross ED. Cumulative incidence of vitamin B12 deficiency in patients with Alzheimer disease. J Neurol Sci. 2009;284:1–2. 144–148. doi: 10.1016/j.jns.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 341.American Academy of Neurology. Practice Parameter: Diagnosis of Dementia. American Academy of Neurology Guideline Summary for Clinicians. Detection, Diagnosis, and Management of Dementia: American Academy of Neurology. [Accessed on September 7, 2009]. Current guideline – reaffirmed February 13, 2004. Available from: http://www.aan.com/professionals/practice/pdfs/dementia_guideline.pdf.
- 342.Valkovic P, Benetin J, Blazicek P, Valkovicova L, Gmitterova K, Kukumberg P. Reduced plasma homocysteine levels in levodopa/entacapone treated Parkinson patients. Parkinsonism Relat Disord. 2005;11(4):253–256. doi: 10.1016/j.parkreldis.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 343.Postuma RB, Espay AJ, Zadikoff C, et al. Vitamins and entacapone in levodopa-induced hyperhomocysteinemia: a randomized controlled study. Neurology. 2006;66(12):1941–1943. doi: 10.1212/01.wnl.0000219815.83681.f7. [DOI] [PubMed] [Google Scholar]
- 344.Lamberti P, Zoccolella S, Iliceto G, et al. Effects of levodopa and COMT inhibitors on plasma homocysteine in Parkinson’s disease patients. Mov Disord. 2005;20(1):69–72. doi: 10.1002/mds.20261. [DOI] [PubMed] [Google Scholar]
- 345.Lamberti P, Zoccolella S, Armenise E, et al. Hyperhomocysteinemia in L-dopa treated Parkinson’s disease patients: effect of cobalamin and folate administration. Eur J Neurol. 2005;12(5):365–368. doi: 10.1111/j.1468-1331.2004.00973.x. [DOI] [PubMed] [Google Scholar]
- 346.Abyad A. Prevalence of vitamin B12 deficiency among demented patients and cognitive recovery with cobalamin replacement. J Nutr Health Aging. 2002;6(4):254–260. [PubMed] [Google Scholar]
- 347.Aisen PS, Schneider LS, Sano M, et al. High-dose B vitamin supplementation and cognitive decline in Alzheimer disease: a randomized controlled trial. JAMA. 2008;300(15):1774–1783. doi: 10.1001/jama.300.15.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Balk EM, Raman G, Tatsioni A, Chung M, Lau J, Rosenberg IH. Vitamin B6, B12, and folic acid supplementation and cognitive function: a systematic review of randomized trials. Arch Intern Med. 2007;167(1):21–30. doi: 10.1001/archinte.167.1.21. [DOI] [PubMed] [Google Scholar]
- 349.Cunha UG. An investigation of dementia among elderly outpatients. Acta Psychiatr Scand. 1990;82(3):261–263. doi: 10.1111/j.1600-0447.1990.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 350.Eastley R, Wilcock GK, Bucks RS. Vitamin B12 deficiency in dementia and cognitive impairment: the effects of treatment on neuropsychological function. Int J Geriatr Psychiatry. 2000;15(3):226–233. doi: 10.1002/(sici)1099-1166(200003)15:3<226::aid-gps98>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 351.Eussen SJ, de Groot LC, Joosten LW, et al. Effect of oral vitamin B12 with or without folic acid on cognitive function in older people with mild vitamin B12 deficiency: a randomized, placebo-controlled trial. Am J Clin Nutr. 2006;84(2):361–370. doi: 10.1093/ajcn/84.1.361. [DOI] [PubMed] [Google Scholar]
- 352.Hvas AM, Juul S, Lauritzen L, Nexo E, Ellegaard J. No effect of vitamin B-12 treatment on cognitive function and depression: a randomized placebo controlled study. J Affect Disord. 2004;81(3):269–273. doi: 10.1016/S0165-0327(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 353.Kwok T, Tang C, Woo J, Lai WK, Law LK, Pang CP. Randomized trial of the effect of supplementation on the cognitive function of older people with subnormal cobalamin levels. Int J Geriatr Psychiatry. 1998;13(9):611–616. doi: 10.1002/(sici)1099-1166(199809)13:9<611::aid-gps832>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 354.Lin YT, Lin MH, Lai HY, Chen LK, Hwang SJ, Lan CF. Regular vitamin B(12) supplementation among older Chinese men in a veterans care home in Taiwan. Arch Gerontol Geriatr. 2009;49(1):186–189. doi: 10.1016/j.archger.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 355.Malouf M, Grimley EJ, Areosa SA. Folic acid with or without vitamin B12 for cognition and dementia. Cochrane Database Syst Rev. 2003;(4):CD004514. doi: 10.1002/14651858.CD004514. [DOI] [PubMed] [Google Scholar]
- 356.Martin DC, Francis J, Protetch J, Huff FJ. Time dependency of cognitive recovery with cobalamin replacement: report of a pilot study. J Am Geriatr Soc. 1992;40(2):168–172. doi: 10.1111/j.1532-5415.1992.tb01939.x. [DOI] [PubMed] [Google Scholar]
- 357.McMahon JA, Green TJ, Skeaff CM, Knight RG, Mann JI, Williams SM. A controlled trial of homocysteine lowering and cognitive performance. N Engl J Med. 2006;354(26):2764–2772. doi: 10.1056/NEJMoa054025. [DOI] [PubMed] [Google Scholar]
- 358.Nilsson K, Gustafson L, Hultberg B. Improvement of cognitive functions after cobalamin/folate supplementation in elderly patients with dementia and elevated plasma homocysteine. Int J Geriatr Psychiatry. 2001;16(6):609–614. doi: 10.1002/gps.388. [DOI] [PubMed] [Google Scholar]
- 359.Osimani A, Berger A, Friedman J, Porat-Katz BS, Abarbanel JM. Neuropsychology of vitamin B12 deficiency in elderly dementia patients and control subjects. J Geriatr Psychiatry Neurol. 2005;18(1):33–38. doi: 10.1177/0891988704272308. [DOI] [PubMed] [Google Scholar]
- 360.Stott DJ, MacIntosh G, Lowe GD, et al. Randomized controlled trial of homocysteine-lowering vitamin treatment in elderly patients with vascular disease. Am J Clin Nutr. 2005;82(6):1320–1326. doi: 10.1093/ajcn/82.6.1320. [DOI] [PubMed] [Google Scholar]
- 361.Sun Y, Lu CJ, Chien KL, Chen ST, Chen RC. Efficacy of multivitamin supplementation containing vitamins B6 and B12 and folic acid as adjunctive treatment with a cholinesterase inhibitor in Alzheimer’s disease: a 26-week, randomized, double-blind, placebo-controlled study in Taiwanese patients. Clin Ther. 2007;29(10):2204–2214. doi: 10.1016/j.clinthera.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 362.van Dyck CH, Lyness JM, Rohrbaugh RM, Siegal AP. Cognitive and psychiatric effects of vitamin B12 replacement in dementia with low serum B12 levels: a nursing home study. Int Psychogeriatr. 2008:1–10. doi: 10.1017/S1041610208007904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.van Uffelen JG, Chin APMJ, Hopman-Rock M, van Mechelen W. The effect of walking and vitamin B supplementation on quality of life in community-dwelling adults with mild cognitive impairment: a randomized, controlled trial. Qual Life Res. 2007;16(7):1137–1146. doi: 10.1007/s11136-007-9219-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.van Uffelen JG, Chinapaw MJ, van Mechelen W, Hopman-Rock M. Walking or vitamin B for cognition in older adults with mild cognitive impairment? A randomised controlled trial. Br J Sports Med. 2008;42(5):344–351. doi: 10.1136/bjsm.2007.044735. [DOI] [PubMed] [Google Scholar]
- 365.Ruscin JM, Page RL, 2nd, Valuck RJ. Vitamin B(12) deficiency associated with histamine(2)-receptor antagonists and a proton-pump inhibitor. Ann Pharmacother. 2002;36(5):812–816. doi: 10.1345/aph.10325. [DOI] [PubMed] [Google Scholar]
- 366.Valuck RJ, Ruscin JM. A case-control study on adverse effects: H2 blocker or proton pump inhibitor use and risk of vitamin B12 deficiency in older adults. J Clin Epidemiol. 2004;57(4):422–428. doi: 10.1016/j.jclinepi.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 367.Mitchell SL, Rockwood K. The association between antiulcer medication and initiation of cobalamin replacement in older persons. J Clin Epidemiol. 2001;54(5):531–534. doi: 10.1016/s0895-4356(00)00340-1. [DOI] [PubMed] [Google Scholar]
- 368.Oh R, Brown DL. Vitamin B12 deficiency. Am Fam Physician. 2003;67(5):979–986. [PubMed] [Google Scholar]
- 369.Metz J. The deoxyuridine suppression test. Crit Rev Clin Lab Sci. 1984;20(3):205–241. doi: 10.3109/10408368409165775. [DOI] [PubMed] [Google Scholar]
- 370.Allen RH, Stabler SP, Savage DG, Lindenbaum J. Diagnosis of cobalamin deficiency I: usefulness of serum methylmalonic acid and total homocysteine concentrations. Am J Hematol. 1990;34(2):90–98. doi: 10.1002/ajh.2830340204. [DOI] [PubMed] [Google Scholar]
- 371.Clarke R, Sherliker P, Hin H, et al. Detection of vitamin B12 deficiency in older people by measuring vitamin B12 or the active fraction of vitamin B12, holotranscobalamin. Clin Chem. 2007;53(5):963–970. doi: 10.1373/clinchem.2006.080382. [DOI] [PubMed] [Google Scholar]
- 372.Herrmann W, Obeid R, Schorr H, Geisel J. Functional vitamin B12 deficiency and determination of holotranscobalamin in populations at risk. Clin Chem Lab Med. 2003;41(11):1478–1488. doi: 10.1515/CCLM.2003.227. [DOI] [PubMed] [Google Scholar]
- 373.Lindenbaum J, Savage DG, Stabler SP, Allen RH. Diagnosis of cobalamin deficiency: II. Relative sensitivities of serum cobalamin, methylmalonic acid, and total homocysteine concentrations. Am J Hematol. 1990;34(2):99–107. doi: 10.1002/ajh.2830340205. [DOI] [PubMed] [Google Scholar]
- 374.Miller JW, Garrod MG, Rockwood AL, et al. Measurement of total vitamin B12 and holotranscobalamin, singly and in combination, in screening for metabolic vitamin B12 deficiency. Clin Chem. 2006;52(2):278–285. doi: 10.1373/clinchem.2005.061382. [DOI] [PubMed] [Google Scholar]
- 375.Pennypacker LC, Allen RH, Kelly JP, et al. High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc. 1992;40(12):1197–1204. [PubMed] [Google Scholar]
- 376.Smith DL. Anemia in the elderly. Am Fam Physician. 2000;62(7):1565–1572. [PubMed] [Google Scholar]
- 377.Obeid R, Morkbak AL, Munz W, Nexo E, Herrmann W. The cobalamin-binding proteins transcobalamin and haptocorrin in maternal and cord blood sera at birth. Clin Chem. 2006;52(2):263–269. doi: 10.1373/clinchem.2005.057810. [DOI] [PubMed] [Google Scholar]
- 378.Krasinski SD, Russell RM, Samloff IM, et al. Fundic atrophic gastritis in an elderly population. Effect on hemoglobin and several serum nutritional indicators. J Am Geriatr Soc. 1986;34(11):800–806. doi: 10.1111/j.1532-5415.1986.tb03985.x. [DOI] [PubMed] [Google Scholar]
- 379.Carmel R. Mild transcobalamin I (haptocorrin) deficiency and low serum cobalamin concentrations. Clin Chem. 2003;49(8):1367–1374. doi: 10.1373/49.8.1367. [DOI] [PubMed] [Google Scholar]
- 380.Metz J, Bell AH, Flicker L, et al. The significance of subnormal serum vitamin B12 concentration in older people: a case control study. J Am Geriatr Soc. 1996;44(11):1355–1361. doi: 10.1111/j.1532-5415.1996.tb01407.x. [DOI] [PubMed] [Google Scholar]
- 381.Bor MV, Cetin M, Aytac S, Altay C, Nexo E. Nonradioactive vitamin B12 absorption test evaluated in controls and in patients with inherited malabsorption of vitamin B12. Clin Chem. 2005;51(11):2151–2155. doi: 10.1373/clinchem.2005.055509. [DOI] [PubMed] [Google Scholar]
- 382.McCaddon A, Hudson P, Abrahamsson L, Olofsson H, Regland B. Analogues, ageing and aberrant assimilation of vitamin B12 in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2001;12(2):133–137. doi: 10.1159/000051247. [DOI] [PubMed] [Google Scholar]
- 383.Refsum H, Smith AD. Low vitamin B12 status in confirmed Alzheimer’s disease as revealed by serum holotranscobalamin. J Neurol Neurosurg Psychiatry. 2003;74(7):959–961. doi: 10.1136/jnnp.74.7.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.McCracken C, Hudson P, Ellis R, McCaddon A. Methylmalonic acid and cognitive function in the Medical Research Council Cognitive Function and Ageing Study. Am J Clin Nutr. 2006;84(6):1406–1411. doi: 10.1093/ajcn/84.6.1406. [DOI] [PubMed] [Google Scholar]
- 385.Stabler SP, Allen RH, Savage DG, Lindenbaum J. Clinical spectrum and diagnosis of cobalamin deficiency. Blood. 1990;76(5):871–881. [PubMed] [Google Scholar]
- 386.Zeitlin A, Frishman WH, Chang CJ. The association of vitamin b 12 and folate blood levels with mortality and cardiovascular morbidity incidence in the old old: the Bronx aging study. Am J Ther. 1997;4:7–8. 275–281. doi: 10.1097/00045391-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 387.Dharmarajan TS, Norkus EP. Approaches to vitamin B12 deficiency. Early treatment may prevent devastating complications. Postgrad Med. 2001;110(1):99–105. doi: 10.3810/pgm.2001.07.977. quiz 106. [DOI] [PubMed] [Google Scholar]
- 388.Hultberg B, Isaksson A, Nilsson K, Gustafson L. Homocysteine and methylmalonic acid as markers of cobalamin/folate status. The association to neuropsychiatric symptoms in the elderly is explored. Lakartidningen. 2000;97(38):4131–4134. 4136. [PubMed] [Google Scholar]
- 389.Nilsson K, Gustafson L, Hultberg B. Optimal use of markers for cobalamin and folate status in a psychogeriatric population. Int J Geriatr Psychiatry. 2002;17(10):919–925. doi: 10.1002/gps.726. [DOI] [PubMed] [Google Scholar]
- 390.Savage DG, Lindenbaum J, Stabler SP, Allen RH. Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med. 1994;96(3):239–246. doi: 10.1016/0002-9343(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 391.Galimberti G, Conti E, Zini M, et al. Post-methionine load test: A more sensitive tool to reveal hyperhomocysteinemia in Alzheimer patients. Clin Biochem. 2008;41:10–11. 914–916. doi: 10.1016/j.clinbiochem.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 392.Kuzminski AM, Del Giacco EJ, Allen RH, Stabler SP, Lindenbaum J. Effective treatment of cobalamin deficiency with oral cobalamin. Blood. 1998;92(4):1191–1198. [PubMed] [Google Scholar]
- 393.Little DR. Ambulatory management of common forms of anemia. Am Fam Physician. 1999;59(6):1598–1604. [PubMed] [Google Scholar]
- 394.Hill DM, Johnson LJ, Burns PJ, Neale AM, Harmening DM, Kenney AC. Effects of temperature on stability of blood homocysteine in collection tubes containing 3-deazaadenosine. Clin Chem. 2002;48(11):2017–2022. [PubMed] [Google Scholar]
- 395.Hansrani M, Stansby G. Extended storage of whole blood with 3-deazaadenosine for homocysteine assay. Ann Clin Biochem. 2007;44(Pt 4):388–390. doi: 10.1258/000456307780945732. [DOI] [PubMed] [Google Scholar]
- 396.Joosten E, Pelemans W, Devos P, et al. Cobalamin absorption and serum homocysteine and methylmalonic acid in elderly subjects with low serum cobalamin. Eur J Haematol. 1993;51(1):25–30. doi: 10.1111/j.1600-0609.1993.tb00600.x. [DOI] [PubMed] [Google Scholar]
- 397.van Asselt DZ, Pasman JW, van Lier HJ, et al. Cobalamin supplementation improves cognitive and cerebral function in older, cobalamin-deficient persons. J Gerontol A Biol Sci Med Sci. 2001;56(12):M775–779. doi: 10.1093/gerona/56.12.m775. [DOI] [PubMed] [Google Scholar]
- 398.Annanmaki T, Pessala-Driver A, Hokkanen L, Murros K. Uric acid associates with cognition in Parkinson’s disease. Parkinsonism Relat Disord. 2008;14(7):576–578. doi: 10.1016/j.parkreldis.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 399.Moretti R, Torre P, Antonello RM, Cazzato G, Cattaruzza T, Scapicchio PL. Vitamin B12 and folate depletion: clinical evidence in a neurological population. Neurologist. 2004;10(6):338–343. doi: 10.1097/01.nrl.0000145597.28008.2e. [DOI] [PubMed] [Google Scholar]
- 400.Selhub J, Morris MS, Jacques PF. In vitamin B12 deficiency, higher serum folate is associated with increased total homocysteine and methylmalonic acid concentrations. Proc Natl Acad Sci U S A. 2007;104(50):19995–20000. doi: 10.1073/pnas.0709487104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401.Clarke R, Sherliker P, Hin H, et al. Folate and vitamin B12 status in relation to cognitive impairment and anaemia in the setting of voluntary fortification in the UK. Br J Nutr. 2008;100(5):1054–1059. doi: 10.1017/S0007114508958001. [DOI] [PubMed] [Google Scholar]
- 402.Santiesteban-Freixas R, Serrano-Verdura C, Gutierrez-Gil J, et al. The neuropathy epidemic in Cuba: eight years of investigation and follow-up. Rev Neurol. 2000;31(6):549–566. [PubMed] [Google Scholar]
- 403.The Cuba Neuropathy Field Investigation Team. Epidemic optic neuropathy in Cuba – clinical characterization and risk factors. N Engl J Med. 1995;333(18):1176–1182. doi: 10.1056/NEJM199511023331803. [DOI] [PubMed] [Google Scholar]
- 404.Dominguez YL, Hernandez M, Matos CM, Zhou D. Is B vitamins deficiency associated with prevalence of Alzheimer’s disease in Cuban elderly? Nutr Health. 2006;18(2):103–118. doi: 10.1177/026010600601800202. [DOI] [PubMed] [Google Scholar]
- 405.Hedges TR, 3rd, Hirano M, Tucker K, Caballero B. Epidemic optic and peripheral neuropathy in Cuba: a unique geopolitical public health problem. Surv Ophthalmol. 1997;41(4):341–353. doi: 10.1016/s0039-6257(96)00008-2. [DOI] [PubMed] [Google Scholar]
- 406.Roman GC. An epidemic in Cuba of optic neuropathy, sensorineural deafness, peripheral sensory neuropathy and dorsolateral myeloneuropathy. J Neurol Sci. 1994;127(1):11–28. doi: 10.1016/0022-510x(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 407.Roman GC. On politics and health: an epidemic of neurologic disease in Cuba. Ann Intern Med. 1995;122(7):530–533. doi: 10.7326/0003-4819-122-7-199504010-00009. [DOI] [PubMed] [Google Scholar]
- 408.Roman GC. Epidemic neuropathy in Cuba: a public health problem related to the Cuban Democracy Act of the United States. Neuroepidemiology. 1998;17(3):111–115. doi: 10.1159/000026161. [DOI] [PubMed] [Google Scholar]
- 409.Tucker K, Hedges TR. Food shortages and an epidemic of optic and peripheral neuropathy in Cuba. Nutr Rev. 1993;51(12):349–357. doi: 10.1111/j.1753-4887.1993.tb03766.x. [DOI] [PubMed] [Google Scholar]
- 410.The Flour Fortification Initiative. Countries with Mandatory Fortification of Flour with Iron and/or Folic Acid (Total = 57) [Accessed on August 11, 2009]. Available from: http://www.sph.emory.edu/wheatflour/COUNTRYDATA/Mandatory_countries.pdf.
- 411.Smithells RW, Sheppard S, Schorah CJ. Vitamin dificiencies and neural tube defects. Arch Dis Child. 1976;51(12):944–950. doi: 10.1136/adc.51.12.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Mills JL, McPartlin JM, Kirke PN, et al. Homocysteine metabolism in pregnancies complicated by neural-tube defects. Lancet. 1995;345(8943):149–151. doi: 10.1016/s0140-6736(95)90165-5. [DOI] [PubMed] [Google Scholar]
- 413.Bjorke-Monsen AL, Ueland PM, Schneede J, Vollset SE, Refsum H. Elevated plasma total homocysteine and C677T mutation of the methylenetetrahydrofolate reductase gene in patients with spina bifida. QJM. 1997;90(9):593–596. doi: 10.1093/qjmed/90.9.593. [DOI] [PubMed] [Google Scholar]
- 414.Vollset SE, Refsum H, Irgens LM, et al. Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland Homocysteine study. Am J Clin Nutr. 2000;71(4):962–968. doi: 10.1093/ajcn/71.4.962. [DOI] [PubMed] [Google Scholar]
- 415.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327(26):1832–1835. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 416.MRC Vitamin Study Research Group . Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338(8760):131–137. [PubMed] [Google Scholar]
- 417.Smithells RW, Sheppard S, Schorah CJ, et al. Possible prevention of neural-tube defects by periconceptional vitamin supplementation. Lancet. 1980;1(8164):339–340. doi: 10.1016/s0140-6736(80)90886-7. [DOI] [PubMed] [Google Scholar]
- 418.Laurence KM, James N, Miller MH, Tennant GB, Campbell H. Double-blind randomised controlled trial of folate treatment before conception to prevent recurrence of neural-tube defects. Br Med J (Clin Res Ed) 1981;282(6275):1509–1511. doi: 10.1136/bmj.282.6275.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Czeizel AE. Prevention of congenital abnormalities by periconceptional multivitamin supplementation. BMJ. 1993;306(6893):1645–1648. doi: 10.1136/bmj.306.6893.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Trends in wheat-flour fortification with folic acid and iron – worldwide, 2004 and 2007. MMWR Morb Mortal Wkly Rep. 2008;57(1):8–10. [PubMed] [Google Scholar]
- 421.Jacobsen DW. Homocysteine and vitamins in cardiovascular disease. Clin Chem. 1998;44(8 Pt 2):1833–1843. [PubMed] [Google Scholar]
- 422.Pitkin RM. Folate and neural tube defects. Am J Clin Nutr. 2007;85(1):285S–288S. doi: 10.1093/ajcn/85.1.285S. [DOI] [PubMed] [Google Scholar]
- 423.Liu S, West R, Randell E, et al. A comprehensive evaluation of food fortification with folic acid for the primary prevention of neural tube defects. BMC Pregnancy Childbirth. 2004;4(1):20. doi: 10.1186/1471-2393-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Lewis DP, Van Dyke DC, Stumbo PJ, Berg MJ. Drug and environmental factors associated with adverse pregnancy outcomes. Part III: Folic acid: pharmacology, therapeutic recommendations, and economics. Ann Pharmacother. 1998;32(10):1087–1095. doi: 10.1345/aph.17427. [DOI] [PubMed] [Google Scholar]
- 425.Leyva M. Folic acid intake and its effects on the prevention of neural tube defects, the masking of vitamin B12 deficiency and the reduction of homocysteine. J Okla State Med Assoc. 2002;95(5):339–342. [PubMed] [Google Scholar]
- 426.Malinow MR, Duell PB, Hess DL, et al. Reduction of plasma homocyst(e)ine levels by breakfast cereal fortified with folic acid in patients with coronary heart disease. N Engl J Med. 1998;338(15):1009–1015. doi: 10.1056/NEJM199804093381501. [DOI] [PubMed] [Google Scholar]
- 427.Kristensen MO, Gulmann NC, Christensen JE, Ostergaard K, Rasmussen K. Serum cobalamin and methylmalonic acid in Alzheimer dementia. Acta Neurol Scand. 1993;87(6):475–481. doi: 10.1111/j.1600-0404.1993.tb04140.x. [DOI] [PubMed] [Google Scholar]
- 428.Carmel R. Prevalence of undiagnosed pernicious anemia in the elderly. Arch Intern Med. 1996;156(10):1097–1100. [PubMed] [Google Scholar]
- 429.Kuroda S, Morita S. A case of pernicious anemia with type A gastritis in an extremely elderly patient with dementia and heart failure. Nippon Ronen Igakkai Zasshi. 2008;45(3):335–337. doi: 10.3143/geriatrics.45.335. [DOI] [PubMed] [Google Scholar]
- 430.Argyriadou S, Vlachonikolis I, Melisopoulou H, Katachanakis K, Lionis C. In what extent anemia coexists with cognitive impairment in elderly: a cross-sectional study in Greece. BMC Fam Pract. 2001;2:5. doi: 10.1186/1471-2296-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.McCaddon A, Kelly CL. Familial Alzheimer’s disease and vitamin B12 deficiency. Age Ageing. 1994;23(4):334–337. doi: 10.1093/ageing/23.4.334. [DOI] [PubMed] [Google Scholar]
- 432.Nilsson K, Gustafson L, Faldt R, Andersson A, Hultberg B. Plasma homocysteine in relation to serum cobalamin and blood folate in a psychogeriatric population. Eur J Clin Invest. 1994;24(9):600–606. doi: 10.1111/j.1365-2362.1994.tb01111.x. [DOI] [PubMed] [Google Scholar]
- 433.McCaddon A, Tandy S, Hudson P, et al. Absence of macrocytic anaemia in Alzheimer’s disease. Clin Lab Haematol. 2004;26(4):259–263. doi: 10.1111/j.1365-2257.2004.00618.x. [DOI] [PubMed] [Google Scholar]
- 434.Gross JS, Weintraub NT, Neufeld RR, Libow LS. Pernicious anemia in the demented patient without anemia or macrocytosis. A case for early recognition. J Am Geriatr Soc. 1986;34(8):612–614. doi: 10.1111/j.1532-5415.1986.tb05768.x. [DOI] [PubMed] [Google Scholar]
- 435.Fox JH, Topel JL, Huckman MS. Dementia in the elderly – a search for treatable illnesses. J Gerontol. 1975;30(5):557–564. doi: 10.1093/geronj/30.5.557. [DOI] [PubMed] [Google Scholar]
- 436.Ikeda T, Furukawa Y, Mashimoto S, Takahashi K, Yamada M. Vitamin B12 levels in serum and cerebrospinal fluid of people with Alzheimer’s disease. Acta Psychiatr Scand. 1990;82(4):327–329. doi: 10.1111/j.1600-0447.1990.tb01395.x. [DOI] [PubMed] [Google Scholar]
- 437.Kountouras J, Tsolaki M, Boziki M, et al. Association between Helicobacter pylori infection and mild cognitive impairment. Eur J Neurol. 2007;14(9):976–982. doi: 10.1111/j.1468-1331.2007.01827.x. [DOI] [PubMed] [Google Scholar]
- 438.Kountouras J, Boziki M, Gavalas E, et al. Eradication of Helicobacter pylori may be beneficial in the management of Alzheimer’s disease. J Neurol. 2009;256(5):758–767. doi: 10.1007/s00415-009-5011-z. [DOI] [PubMed] [Google Scholar]
- 439.Malaguarnera M, Bella R, Alagona G, Ferri R, Carnemolla A, Pennisi G. Helicobacter pylori and Alzheimer’s disease: a possible link. Eur J Intern Med. 2004;15(6):381–386. doi: 10.1016/j.ejim.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 440.Kountouras J, Tsolaki M, Gavalas E, et al. Relationship between Helicobacter pylori infection and Alzheimer disease. Neurology. 2006;66(6):938–940. doi: 10.1212/01.wnl.0000203644.68059.5f. [DOI] [PubMed] [Google Scholar]
- 441.Vasconcellos LF, Correa RB, Chimelli L, et al. Myelopathy due to vitamin B12 deficiency presenting as transverse myelitis. Arq Neuropsiquiatr. 2002;60(1):150–154. doi: 10.1590/s0004-282x2002000100028. [DOI] [PubMed] [Google Scholar]
- 442.Gottfries J, Blennow K, Lehmann MW, Regland B, Gottfries CG. One-carbon metabolism and other biochemical correlates of cognitive impairment as visualized by principal component analysis. J Geriatr Psychiatry Neurol. 2001;14(3):109–114. doi: 10.1177/089198870101400302. [DOI] [PubMed] [Google Scholar]
- 443.Faxen-Irving G, Basun H, Cederholm T. Nutritional and cognitive relationships and long-term mortality in patients with various dementia disorders. Age Ageing. 2005;34(2):136–141. doi: 10.1093/ageing/afi023. [DOI] [PubMed] [Google Scholar]
- 444.Jones S, Small BJ, Fratiglioni L, Backman L. Predictors of cognitive change from preclinical to clinical Alzheimer’s disease. Brain Cogn. 2002;49(2):210–213. [PubMed] [Google Scholar]
- 445.McCaddon A, Kelly CL. Alzheimer’s disease: a ‘cobalaminergic’ hypothesis. Med Hypotheses. 1992;37(3):161–165. doi: 10.1016/0306-9877(92)90074-m. [DOI] [PubMed] [Google Scholar]
- 446.Opala G, Jasinska-Myga B, Ochudlo S, Janiec K. Diagnostic problems in the case of dementia with coexisting hematological disorders. Wiad Lek. 2001;54:7–8. 456–461. [PubMed] [Google Scholar]
- 447.Carmel R, Ellenbogen L, Herbert VD. Dietary Supplement Fact Sheet Vitamin B12. Bethesda, MD: Office of Dietary Supplements, National Institutes of Health; 2006. [Accessed on January 18, 2009]. Available from: From http://ods.od.nih.gov/factsheets/VitaminB12_pf.asp. [Google Scholar]
- 448.American Regent I. Cyanocobalamin Injection, USP. Package Insert. Shirley, NY: American Regent; 2003. [Google Scholar]
- 449.Braun-Falco O, Lincke H. The problem of vitamin B6/B12 acne. A contribution on acne medicamentosa (author’s transl) MMW Munch Med Wochenschr. 1976;118(6):155–160. [PubMed] [Google Scholar]
- 450.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291(5):565–575. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 451.Schnyder G, Roffi M, Pin R, et al. Decreased rate of coronary restenosis after lowering of plasma homocysteine levels. N Engl J Med. 2001;345(22):1593–1600. doi: 10.1056/NEJMoa011364. [DOI] [PubMed] [Google Scholar]
- 452.Schnyder G, Roffi M, Flammer Y, Pin R, Hess OM. Effect of homocysteine-lowering therapy with folic acid, vitamin B12, and vitamin B6 on clinical outcome after percutaneous coronary intervention: the Swiss Heart study: a randomized controlled trial. JAMA. 2002;288(8):973–979. doi: 10.1001/jama.288.8.973. [DOI] [PubMed] [Google Scholar]
- 453.Albert CM, Cook NR, Gaziano JM, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: a randomized trial. JAMA. 2008;299(17):2027–2036. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Bonaa KH, Njolstad I, Ueland PM, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354(15):1578–1588. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 455.Ebbing M, Bleie O, Ueland PM, et al. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: a randomized controlled trial. JAMA. 2008;300(7):795–804. doi: 10.1001/jama.300.7.795. [DOI] [PubMed] [Google Scholar]
- 456.Lange H, Suryapranata H, De Luca G, et al. Folate therapy and in-stent restenosis after coronary stenting. N Engl J Med. 2004;350(26):2673–2681. doi: 10.1056/NEJMoa032845. [DOI] [PubMed] [Google Scholar]
- 457.Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354(15):1567–1577. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 458.Mann JF, Sheridan P, McQueen MJ, et al. Homocysteine lowering with folic acid and B vitamins in people with chronic kidney disease – results of the renal Hope-2 study. Nephrol Dial Transplant. 2008;23(2):645–653. doi: 10.1093/ndt/gfm485. [DOI] [PubMed] [Google Scholar]
- 459.Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a metaanalysis of randomized controlled trials. JAMA. 2006;296(22):2720–2726. doi: 10.1001/jama.296.22.2720. [DOI] [PubMed] [Google Scholar]
- 460.Wang X, Qin X, Demirtas H, et al. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007;369(9576):1876–1882. doi: 10.1016/S0140-6736(07)60854-X. [DOI] [PubMed] [Google Scholar]
- 461.Brattstrom L, Wilcken DE, Ohrvik J, Brudin L. Common methylenetetrahydrofolate reductase gene mutation leads to hyperhomocysteinemia but not to vascular disease: the result of a meta-analysis. Circulation. 1998;98(23):2520–2526. doi: 10.1161/01.cir.98.23.2520. [DOI] [PubMed] [Google Scholar]
- 462.Dangour AD, Breeze E, Clarke R, Shetty PS, Uauy R, Fletcher AE. Plasma homocysteine, but not folate or vitamin B-12, predicts mortality in older people in the United Kingdom. J Nutr. 2008;138(6):1121–1128. doi: 10.1093/jn/138.6.1121. [DOI] [PubMed] [Google Scholar]
- 463.den Heijer M, Rosendaal FR, Blom HJ, Gerrits WB, Bos GM. Hyperhomocysteinemia and venous thrombosis: a meta-analysis. Thromb Haemost. 1998;80(6):874–877. [PubMed] [Google Scholar]
- 464.Den Heijer M, Lewington S, Clarke R. Homocysteine, MTHFR and risk of venous thrombosis: a meta-analysis of published epidemiological studies. J Thromb Haemost. 2005;3(2):292–299. doi: 10.1111/j.1538-7836.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 465.Dhonukshe-Rutten RA, Pluijm SM, de Groot LC, Lips P, Smit JH, van Staveren WA. Homocysteine and vitamin B12 status relate to bone turnover markers, broadband ultrasound attenuation, and fractures in healthy elderly people. J Bone Miner Res. 2005;20(6):921–929. doi: 10.1359/JBMR.050202. [DOI] [PubMed] [Google Scholar]
- 466.Kuo HK, Sorond FA, Chen JH, Hashmi A, Milberg WP, Lipsitz LA. The role of homocysteine in multisystem age-related problems: a systematic review. J Gerontol A Biol Sci Med Sci. 2005;60(9):1190–1201. doi: 10.1093/gerona/60.9.1190. [DOI] [PubMed] [Google Scholar]
- 467.Sato Y, Honda Y, Iwamoto J, Kanoko T, Satoh K. Homocysteine as a predictive factor for hip fracture in stroke patients. Bone. 2005;36(4):721–726. doi: 10.1016/j.bone.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 468.Sato Y, Iwamoto J, Kanoko T, Satoh K. Homocysteine as a predictive factor for hip fracture in elderly women with Parkinson’s disease. Am J Med. 2005;118(11):1250–1255. doi: 10.1016/j.amjmed.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 469.Lehmann M, Regland B, Blennow K, Gottfries CG. Vitamin B12-B6- folate treatment improves blood-brain barrier function in patients with hyperhomocysteinaemia and mild cognitive impairment. Dement Geriatr Cogn Disord. 2003;16(3):145–150. doi: 10.1159/000071002. [DOI] [PubMed] [Google Scholar]
- 470.Flicker L, Martins RN, Thomas J, et al. B-vitamins reduce plasma levels of beta amyloid. Neurobiol Aging. 2008;29(2):303–305. doi: 10.1016/j.neurobiolaging.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 471.Stuerenburg HJ, Mueller-Thomsen T, Methner A. Vitamin B12 plasma concentrations in Alzheimer disease. Neuro Endocrinol Lett. 2004;25(3):176–177. [PubMed] [Google Scholar]
- 472.La Rue A, Koehler KM, Wayne SJ, Chiulli SJ, Haaland KY, Garry PJ. Nutritional status and cognitive functioning in a normally aging sample: a 6-y reassessment. Am J Clin Nutr. 1997;65(1):20–29. doi: 10.1093/ajcn/65.1.20. [DOI] [PubMed] [Google Scholar]
- 473.Bryan J, Calvaresi E, Hughes D. Short-term folate, vitamin B-12 or vitamin B-6 supplementation slightly affects memory performance but not mood in women of various ages. J Nutr. 2002;132(6):1345–1356. doi: 10.1093/jn/132.6.1345. [DOI] [PubMed] [Google Scholar]
- 474.Remington R, Chan A, Paskavitz J, Shea TB. Efficacy of a vitamin/nutriceutical formulation for moderate-stage to later-stage Alzheimer’s disease: a placebo-controlled pilot study. Am J Alzheimers Dis Other Demen. 2009;24(1):27–33. doi: 10.1177/1533317508325094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Ikeda T, Yamamoto K, Takahashi K, et al. Treatment of Alzheimer-type dementia with intravenous mecobalamin. Clin Ther. 1992;14(3):426–437. [PubMed] [Google Scholar]
- 476.Alfthan G, Tapani K, Nissinen K, Saarela J, Aro A. The effect of low doses of betaine on plasma homocysteine in healthy volunteers. Br J Nutr. 2004;92(4):665–669. doi: 10.1079/bjn20041253. [DOI] [PubMed] [Google Scholar]
- 477.Bell KM, Plon L, Bunney WE, Jr, Potkin SG. S-adenosylmethionine treatment of depression: a controlled clinical trial. Am J Psychiatry. 1988;145(9):1110–1114. doi: 10.1176/ajp.145.9.1110. [DOI] [PubMed] [Google Scholar]
- 478.Bottiglieri T. Ademetionine (S-adenosylmethionine) neuropharmacology: implications for drug therapies in psychiatric and neurological disorders. Expert Opin Investig Drugs. 1997;6(4):417–426. doi: 10.1517/13543784.6.4.417. [DOI] [PubMed] [Google Scholar]
- 479.Bressa GM. S-adenosyl-l-methionine (SAMe) as antidepressant: meta-analysis of clinical studies. Acta Neurol Scand Suppl. 1994;154:7–14. doi: 10.1111/j.1600-0404.1994.tb05403.x. [DOI] [PubMed] [Google Scholar]
- 480.D’Angelo A, Selhub J. Homocysteine and thrombotic disease. Blood. 1997;90(1):1–11. [PubMed] [Google Scholar]
- 481.Di Rocco A, Rogers JD, Brown R, Werner P, Bottiglieri T. S-Adenosyl- Methionine improves depression in patients with Parkinson’s disease in an open-label clinical trial. Mov Disord. 2000;15(6):1225–1229. doi: 10.1002/1531-8257(200011)15:6<1225::aid-mds1025>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 482.Dudman NP, Guo XW, Gordon RB, Dawson PA, Wilcken DE. Human homocysteine catabolism: three major pathways and their relevance to development of arterial occlusive disease. J Nutr. 1996;126(4 Suppl):1295S–1300S. doi: 10.1093/jn/126.suppl_4.1295S. [DOI] [PubMed] [Google Scholar]
- 483.Ho PI, Ashline D, Dhitavat S, et al. Folate deprivation induces neurodegeneration: roles of oxidative stress and increased homocysteine. Neurobiol Dis. 2003;14(1):32–42. doi: 10.1016/s0969-9961(03)00070-6. [DOI] [PubMed] [Google Scholar]
- 484.Jacob RA, Jenden DJ, Allman-Farinelli MA, Swendseid ME. Folate nutriture alters choline status of women and men fed low choline diets. J Nutr. 1999;129(3):712–717. doi: 10.1093/jn/129.3.712. [DOI] [PubMed] [Google Scholar]
- 485.Kagan BL, Sultzer DL, Rosenlicht N, Gerner RH. Oral S-adenosylmethionine in depression: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry. 1990;147(5):591–595. doi: 10.1176/ajp.147.5.591. [DOI] [PubMed] [Google Scholar]
- 486.Knopman D, Patterson M. An open-label, 24-week pilot study of the methyl donor betaine in Alzheimer disease patients. Alzheimer Dis Assoc Disord. 2001;15(3):162–165. doi: 10.1097/00002093-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 487.Lipinski JF, Cohen BM, Frankenburg F, et al. Open trial of S-adenosylmethionine for treatment of depression. Am J Psychiatry. 1984;141(3):448–450. doi: 10.1176/ajp.141.3.448. [DOI] [PubMed] [Google Scholar]
- 488.McCaddon A. Homocysteine and cognitive impairment; a case series in a General Practice setting. Nutr J. 2006;5:6. doi: 10.1186/1475-2891-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Melse-Boonstra A, Holm PI, Ueland PM, Olthof M, Clarke R, Verhoef P. Betaine concentration as a determinant of fasting total homocysteine concentrations and the effect of folic acid supplementation on betaine concentrations. Am J Clin Nutr. 2005;81(6):1378–1382. doi: 10.1093/ajcn/81.6.1378. [DOI] [PubMed] [Google Scholar]
- 490.Mitsuyama Y, Kogoh H. Serum and cerebrospinal fluid vitamin B12 levels in demented patients with CH3-B12 treatment – preliminary study. Jpn J Psychiatry Neurol. 1988;42(1):65–71. [PubMed] [Google Scholar]
- 491.Montero Brens C, Dalmau Serra J, Cabello Tomas ML, Garcia Gomez AM, Rodes Monegal M, Vilaseca Busca A. Homocystinuria: effectiveness of the treatment with pyridoxine, folic acid, and betaine. An Esp Pediatr. 1993;39(1):37–41. [PubMed] [Google Scholar]
- 492.Schwab U, Torronen A, Toppinen L, et al. Betaine supplementation decreases plasma homocysteine concentrations but does not affect body weight, body composition, or resting energy expenditure in human subjects. Am J Clin Nutr. 2002;76(5):961–967. doi: 10.1093/ajcn/76.5.961. [DOI] [PubMed] [Google Scholar]
- 493.Selhub J, Bagley LC, Miller J, Rosenberg IH. B vitamins, homocysteine, and neurocognitive function in the elderly. Am J Clin Nutr. 2000;71(2):614S–620S. doi: 10.1093/ajcn/71.2.614s. [DOI] [PubMed] [Google Scholar]
- 494.Shippy RA, Mendez D, Jones K, Cergnul I, Karpiak SE. S-adenosylmethionine (SAM-e) for the treatment of depression in people living with HIV/AIDS. BMC Psychiatry. 2004;4:38. doi: 10.1186/1471-244X-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Tavoni A, Jeracitano G, Cirigliano G. Evaluation of S-adenosylmethionine in secondary fibromyalgia: a double-blind study. Clin Exp Rheumatol. 1998;16(1):106–107. [PubMed] [Google Scholar]
- 496.Connelly PJ, Prentice NP, Cousland G, Bonham J. A randomised double-blind placebo-controlled trial of folic acid supplementation of cholinesterase inhibitors in Alzheimer’s disease. Int J Geriatr Psychiatry. 2008;23(2):155–160. doi: 10.1002/gps.1856. [DOI] [PubMed] [Google Scholar]
- 497.Ito T, Yamadera H, Ito R, Suzuki H, Asayama K, Endo S. Effects of vitamin B12 on bright light on cognitive and sleep-wake rhythm in Alzheimer-type dementia. Psychiatry Clin Neurosci. 2001;55(3):281–282. doi: 10.1046/j.1440-1819.2001.00860.x. [DOI] [PubMed] [Google Scholar]
- 498.Haidemenos A, Kontis D, Gazi A, Kallai E, Allin M, Lucia B. Plasma homocysteine, folate and B12 in chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(6):1289–1296. doi: 10.1016/j.pnpbp.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 499.King JC. Dietary Guidelines for Americans 2005. US Department of Health and Human Services and US Department of Agriculture; 2005. [Accessed on January 18, 2009]. Available from: http://www.cnpp.usda.gov/Publications/DietaryGuidelines/2005/2005DGPolicyDocument.pdf. [Google Scholar]
- 500.Dierkes J, Domrose U, Ambrosch A, et al. Supplementation with vitamin B12 decreases homocysteine and methylmalonic acid but also serum folate in patients with end-stage renal disease. Metabolism. 1999;48(5):631–635. doi: 10.1016/s0026-0495(99)90062-8. [DOI] [PubMed] [Google Scholar]
- 501.Hoffer LJ, Saboohi F, Golden M, Barre PE. Cobalamin dose regimen for maximum homocysteine reduction in end-stage renal disease. Metabolism. 2005;54(6):835–840. doi: 10.1016/j.metabol.2005.01.034. [DOI] [PubMed] [Google Scholar]
- 502.Eussen SJ, de Groot LC, Clarke R, et al. Oral cyanocobalamin supplementation in older people with vitamin B12 deficiency: a dose-finding trial. Arch Intern Med. 2005;165(10):1167–1172. doi: 10.1001/archinte.165.10.1167. [DOI] [PubMed] [Google Scholar]
- 503.Nyholm E, Turpin P, Swain D, et al. Oral vitamin B12 can change our practice. Postgrad Med J. 2003;79(930):218–220. doi: 10.1136/pmj.79.930.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Lederle FA. Oral cobalamin for pernicious anemia: back from the verge of extinction. J Am Geriatr Soc. 1998;46(9):1125–1127. doi: 10.1111/j.1532-5415.1998.tb06651.x. [DOI] [PubMed] [Google Scholar]
- 505.Kwong JC, Carr D, Dhalla IA, Tom-Kun D, Upshur RE. Oral vitamin B12 therapy in the primary care setting: a qualitative and quantitative study of patient perspectives. BMC Fam Pract. 2005;6(1):8. doi: 10.1186/1471-2296-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Hengstermann S, Hanemann A, Nieczaj R, et al. Does an association between increased homocystein levels and cognitive dysfunction also exist in multimorbid geriatric patients? Z Gerontol Geriatr. 2009;42(2):131–136. doi: 10.1007/s00391-008-0551-x. [DOI] [PubMed] [Google Scholar]
- 507.Hengstermann S, Laemmler G, Hanemann A, et al. Total serum homocysteine levels do not identify cognitive dysfunction in multimorbid elderly patients. J Nutr Health Aging. 2008;12(6):411–416. doi: 10.1007/BF02982675. [DOI] [PubMed] [Google Scholar]
- 508.Huang YC, Kuo YW, Lee TH, et al. Hypoalbuminemia and not hyperhomocysteinemia as a risk factor for dementia in hemodialysis patients. J Ren Nutr. 2008;18(4):347–354. doi: 10.1053/j.jrn.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 509.Stipanuk MH, Dominy JE, Jr, Lee JI, Coloso RM. Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J Nutr. 2006;136(6 Suppl):1652S–1659S. doi: 10.1093/jn/136.6.1652S. [DOI] [PubMed] [Google Scholar]
- 510.Fernandez HH, Wu CK, Ott BR. Pharmacotherapy of dementia with Lewy bodies. Expert Opin Pharmacother. 2003;4(11):2027–2037. doi: 10.1517/14656566.4.11.2027. [DOI] [PubMed] [Google Scholar]
- 511.McKeith I, Del Ser T, Spano P, et al. Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet. 2000;356(9247):2031–2036. doi: 10.1016/S0140-6736(00)03399-7. [DOI] [PubMed] [Google Scholar]