Abstract
Females are more susceptible to development of asthma than are males. In a mouse model of ovalbumin-induced airway inflammation, with aggravated disease in females compared with males, we studied interactions between immune and resident lung cells during asthma development to elucidate which processes are affected by sex. We studied numbers of regulatory T cells (Tregs), effector T cells, myeloid dendritic cells (mDCs), and alternatively activated macrophages (AAMΦ), and their functional capabilities. Male and female mice had comparable Treg numbers in lung tissue and comparable Treg function, but effector T cells had expanded to a greater extent in lungs of females after ovalbumin exposure. This difference in T cell expansion was therefore not the result of lack of Treg control, but appeared to be driven by a greater number of inflammatory mDCs migrating from the lungs to lymph nodes in females. Resident lung cells can influence mDC migration, and AAMΦ in lung tissue were found to be involved. Artificially elevating the number of AAMΦ in lung tissue increased the migration of mDCs and airway inflammation. We found greater numbers of AAMΦ in female lungs than in males; we therefore postulate that AAMΦ are involved in increased airway inflammation found in female mice.
Keywords: myeloid dendritic cells, allergy, regulatory T cells, lung, sex
CLINICAL RELEVANCE.
This research expands our knowledge of how immunological processes in asthma pathogenesis are affected by sex. This knowledge is necessary to develop proper therapeutic strategies for both men and women.
Asthma is a widely prevalent inflammatory disease of the airways with an exaggerated response of the airways to normally innocuous antigens. Adult women display greater immune responsiveness, which results in a higher incidence of asthma and autoimmune diseases as compared with men (1–4). Moreover, severe asthma is also more predominant in women (5–7), and females with asthma were found to benefit less from corticosteroid therapy than males with asthma (8).
Surprisingly little is known about intrinsic differences between males and females in the pathogenesis of asthma. We and others recently showed that female mice develop more severe ovalbumin (OVA)-induced allergic airway inflammation than male mice (9–11), and we subsequently used this model to further investigate sex differences in asthma development.
The allergic immune response starts with inhaled antigens taken up by dendritic cells (DCs) in the lung in the context of danger signals. DCs then migrate to organized lymphoid tissue while maturing on the way to initiate T cell responses in lymphoid tissue (12). There are at least two subsets of pulmonary DCs: myeloid DCs (mDCs) and plasmacytoid DCs (pDCs), with the mDCs being responsible for the induction of peripheral immune responses (13, 14). pDCs were found to promote tolerance, but how is still unknown.
T helper (Th) 2 lymphocytes that are generated in the lymph nodes subsequently infiltrate into the lungs in response to antigens (15). These peripheral T cell responses are being regulated by regulatory T cells (Tregs) that actively control or suppress effector T cells, and can abrogate features of allergic airway disease, including reduced airway hyperresponsiveness, IgE levels, and eosinophil numbers (16–18).
Other resident lung cells, like macrophages, can provide the danger signals required for mDC maturation. Alveolar macrophages, for instance, were shown to have a role in the steady-state regulation of DC migration and antigen-presenting cell (APC) functions of DC (19, 20). Macrophages can develop into different subsets and switch from one state to another, depending on the incoming threats they encounter (21). The subset that is characterized by expression of YM1, mannose receptors, chitinases, and arginase is implicated in the pathogenesis of asthma (22, 23). These macrophages are called alternatively activated macrophages (AAMΦ), and have been shown to promote Th2 responses (24, 25). How AAMΦ facilitate allergic inflammation remains elusive, but this may involve directly or indirectly modulating DC maturation.
The effects of sex on these immune processes in asthma pathogenesis have not yet been studied. From our own previous studies, we postulated that a reduced number of pulmonary Tregs and/or reduced function may explain the aggravated asthma phenotype found in females (9). Consequently, in a further set of experiments described in this article, we concentrated on the role of Tregs in OVA-induced allergic airway inflammation in female mice as compared with males. However, we found no differences in either number or function. We therefore continued our quest for processes that determine the difference between males and females in asthma development along the lines of DCs and lung macrophages.
MATERIALS AND METHODS
Location
Experiments for the present study were performed either at the Division of Pulmonology, Allergy, and Critical Care Medicine (Department of Medicine, University of Pittsburgh, Pittsburgh, PA) or at the Department of Pathology and Laboratory Medicine (University Medical Center Groningen, University of Groningen, Groningen, The Netherlands). The model of OVA-induced airway inflammation was found to yield similar results in both locations.
Animals
Male and female BALB/cByJ mice (8–10 wk old) were either obtained from The Jackson Laboratory (Bar Harbor, ME) or from Harlan (Zeist, The Netherlands), and were held under specific pathogen–free conditions. DO11.10 T-cell receptor–transgenic mice were bred and maintained in the Department of Laboratory Animal Resources at the University of Pittsburgh. The institutional animal care and use committees of the University of Pittsburgh and the University of Groningen approved the studies that used mice.
Airway Inflammation Induced by OVA and Alum
Male and female mice were immunized against OVA by a similar protocol in both locations (9, 26).
Pittsburgh.
OVA (10 μg; Sigma-Aldrich, St. Louis, MO) and 1 mg of alum (Resorptar; Intergen Co., New York, NY) were injected intraperitoneally on Days 1 and 7, followed by seven consecutive, daily, 20-minute aerosol challenges with 1% wt/vol OVA in sterile PBS with an ultrasonic nebulizer (Omron Healthcare, Bannockburn, IL) beginning 7 days after the intraperitoneal injection.
Groningen.
OVA (10 μg; Sigma-Aldrich) and 1.5 mg of alum (Aluminject; Pierce Chemical, Etten-Leur, The Netherlands) were injected intraperitoneally on Days 1 and 7, followed on Day 14 by 7 consecutive, daily, 20-minute aerosol challenges with 1% wt/vol OVA in sterile PBS with a Pari LC Sprint Star nebulizer driven by a PARI Boy SX compressor (both kind gifts from Pari GmbH, Starnberg, Germany).
Mice were killed for further analyses 24 hours after the last OVA challenge.
Confirmation of OVA-Induced Airway Inflammation
To confirm findings already published for the Groningen protocol (9), we subjected six male and six female mice to the Pittsburgh protocol of OVA-induced airway inflammation, and assessed the number of eosinophils in bronchoalveolar lavage fluid (BALF) and OVA-specific IgE in serum.
BAL.
BAL was performed 24 hours after the last aerosol challenge, and cell differentials and cytokines in BALF were assessed as described previously (26).
IgE.
OVA-specific serum IgE was measured by ELISA, as described previously (27).
Characterization of T Cell Subsets in Lung Tissue
Because of the robustness of the model, two experiments were pooled for characterization of T cell subsets: allergic airway inflammation was induced in seven male and eight female mice in Pittsburgh, and six male and six female mice in Groningen. The Pittsburgh experiment was further used to assess cell proliferation of lung, lung-draining lymph nodes (LDLNs), and spleen cells, and the Groningen experiment was further used to assess DC populations in LDLNs (see below).
Leukocytes enriched for lymphocytes were isolated from lung tissue 24 hours after the last OVA challenge, as described previously (9). The resulting single-cell suspensions were used for flow cytometric analysis of T cell subsets. Frequencies of effector T cells (CD4+CD25+Foxp3−), regulatory T cells (CD4+CD25+Foxp3+), and membrane-bound transforming growth factor (TGF)–β1–positive regulatory T cells were examined by four-color flow cytometry with antibodies directed against CD4, CD25, TGF-β1, and Foxp3. The membrane-bound TGF-β1–positive regulatory T cells were only assessed in the Pittsburgh experiment.
Before staining, cells were blocked for 15 minutes on ice with Fc-block (10% normal mouse serum). For cell surface staining, 1 × 106 CD4+ leukocytes were incubated with PerCP-labeled anti-mouse CD4 (Becton & Dickinson Biosciences, San Jose, CA), PE-labeled anti-mouse CD25 (Becton & Dickinson Biosciences), and biotin-labeled anti–TGF-β1 (R&D Systems, Minneapolis, MN) or the appropriate isotype control. As a second step, reagent for anti–TGF-β1, APCs (allophycocyanin) conjugated to streptavidin (Becton & Dickinson Biosciences) was used. For intracellular staining, the cells were subsequently fixed, permeabilized, and stained for Foxp3 with a kit from eBioscience (San Diego, CA) with FITC-labeled anti-Foxp3 or its isotype. A representative two-color fluorescence plot in Figure E1A in the online supplement depicts Treg and effector T cell populations.
Flow Cytometry
Cell populations were assessed with a FACScalibur flow cytometer (Becton & Dickinson, Franklin Lakes, NJ) with CellQuest software or an LSR II flow cytometer with FACSdiva software (Becton & Dickinson). Analysis was performed using FlowJo (TreeStar, San Carlos, CA). The frequency of positive cells was calculated by subtracting the value obtained with the respective isotype controls.
Ex Vivo Stimulation of Spleen, Lymph, and Lung Leukocytes
From the animals used for characterization of T cell subsets in Pittsburgh, we also harvested spleens and LDLNs to assess proliferation rate after ex vivo stimulation with OVA.
Spleens were disrupted by pressing through a 70-μm nylon mesh, and erythrocytes were eliminated from the suspensions by centrifugation on a Lympholyte-M (Cedarlane, Hornby, ON, Canada) gradient.
The LDLNs collected included the anterior mediastinal, posterior mediastinal, and parathymic. Cells obtained from these nodes were routinely examined by flow cytometry for absence of CD4/CD8 double-positive cells as a control for accidental inclusion of thymic tissue (data not shown). The lymph nodes were physically disrupted by pressing through a 70-μm nylon mesh.
The leukocyte suspensions harvested from spleens, LDLNs, and lungs from 8 mice were pooled per two mice to yield four suspensions per sex per organ. These cells were cultured with or without 20 μg/ml OVA in 96-well plates in RPMI 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated FBS, 2 mM l-glutamine, and 50 mM 2-mercaptoethanol penicillin/streptomycin (complete RPMI). The cells were incubated for 3 days with 1 μCi/well [3H]-thymidine (PerkinElmer, Waltham, MA) added during the final 18 hours of culture, at which time the cells were harvested onto filters (PerkinElmer) for scintillation counting to assess the rate of proliferation. Culture medium was stored at −80°C for subsequent analysis of cytokines.
Suppression Assay
Tregs were isolated from spleens of male and female mice (n = 4) tolerized against OVA by exposure to 1% OVA aerosols on 10 subsequent days (26) and harvesting the spleens on Day 21 with a magnetic bead isolation kit (Miltenyi Biotec, Auburn, CA). Effector T cells and γ-irradiated (2,000 rad) APCs isolated from spleens of naive male mice were used as target cells for Treg suppression and costimulation, respectively. Magnetic bead isolation was used to isolate CD4+ effector T cells, and the remaining T cell–depleted fraction was used as APCs. Cells were cultured in complete Bruff's medium with soluble anti-CD3ɛ (2 μg/ml; Becton & Dickinson Biosciences) in 96-well, round-bottom plates with 5 × 104 effector T cells and 5 × 104 APCs per well. Tregs were added in ratios of 2:1, 1:1, 0.5:1, and 0:1 Tregs:effector T cells. Cells were cultured for 3 days, with [3H]thymidine (1 μCi/well) included for the final 18 hours of culture, and [3H]thymidine incorporation was assessed as described previously here. All conditions were done in triplicate, and proliferation without Tregs present was set at 100%.
Number and Function of DCs Isolated from LDLNs
To study sex differences in the number and function of DCs after an initial sensitizing event in the lung, male and female mice (pool of n = 8 per sex, experiment done twice) were intranasally exposed to OVA and cholera toxin (CT). Under light anesthesia with isoflurane, 100 μg OVA was administered with 1 μg CT (List Biological Laboratories, Campbell, CA). The intranasal treatments were performed daily for 3 consecutive days. DCs were isolated 24 hours after the final intranasal administration.
For comparison, DC numbers were also assessed in the standard OVA plus alum model. LDLNs from the mice used to characterize T cell subsets in Groningen were harvested as described previously here.
Isolation of DCs from LDLNs.
LDLNs were harvested from mice as described above. DCs were isolated by first removing macrophages and part of the lymphocytes by centrifugation on a nycodenz gradient (1-Step Monocyte; Accurate Chemical and Scientific, Westbury, NY). Subsequent staining with anti–major histocompatibility complex (MHC) II–FITC (Southern Biotech, Birmingham, AL) and anti–CD11c–APC (Becton & Dickinson Biosciences) identified mDCs (CD11chi MHCIIhi) and pDCs (CD11chi MHCIIint) by flow cytometry. A representative two-color fluorescence plot in Figure E1B (OVA + alum model) and Figure E1C (OVA + CT model) depicts the mDC and pDC populations. mDCs from the OVA plus CT model were sorted for functional studies with a FACSAria cell sorter with FACSdiva software (Becton & Dickinson).
Assay of mDC function.
To test the capacity of mDCs to induce T cell proliferation, male and female sorted mDCs were coincubated in different ratios (10–1 × 104 cells/well) in complete RPMI in 96-well plates, with 2 × 105 purified splenic CD4+ T cells from DO11.10 T-cell receptor–transgenic mice, and 20 μg/ml specific antigenic OVA-peptide (OVA aa 323–339). These T cells were prepared by negative selection with a CD4 isolation kit (Miltenyi Biotec) and multiple rounds of magnetic sorting using AutoMACS (Miltenyi Biotec). Anti–MHC II magnetic beads (Miltenyi Biotec) were included in the purification procedure to guarantee that all APCs were removed from the T cell preparations. Purified DO11.10 T cells were routinely cultured with specific peptide alone to ensure that all APC activity had been removed (data not shown). The cells were incubated for 5 days, with 1 μCi/well [3H]-thymidine included for the final 18 hours of culture, and [3H]thymidine incorporation was assessed as described previously here. All conditions were done in triplicate.
Cytokine Assays
Thymic stromal lymphopoietin (TSLP) was measured by ELISA (R&D Systems, Abingdon, UK), and all other cytokines by multiplex assay (Bio-Rad) and a Luminex analyzer, according to each manufacturer's recommendations. For cytokine detection in total lung, the tissue was homogenized in 50 mM Tris-HCl (pH 7.5) containing 150 mM NaCl, 0.002% Tween-20, and protease inhibitor (Complete Mini, EDTA-free; Roche Applied Science, Almere, The Netherlands). After centrifugation, supernatants were collected and stored at −80°C until assayed. BALF and culture medium samples were similarly stored until assayed.
Flow Cytometric Analysis of Macrophages
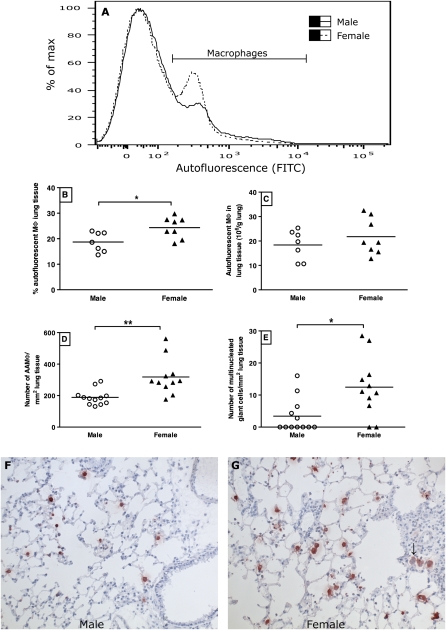
Male (n = 7) and female (n = 8) mice exposed to the OVA plus alum model were killed 24 hours after the last challenge. Lungs were removed, minced, and incubated for 45 minutes at 37°C in a shaking water bath in RPMI medium (Lonza, Verviers, Belgium) supplemented with 10% FBS (Lonza), DNase I (10 μg/ml; Roche Applied Science), and collagenase I (0.66 mg/ml; Sigma-Aldrich). Purified vital lung cells were obtained by first passing the digested lung tissue first through a 70-μm and then through a 35-μm nylon strainer. These cells were subsequently used for flow cytometric analysis of macrophages, as described by Vermaelen and Pauwels (28). Macrophages were defined as cells with autofluorescence in the FITC channel. Their identity was confirmed by expression of MHC II, CD11c, and F4/80 by staining for these markers with biotin-labeled anti–MHC II (Becton & Dickinson Biosciences) followed by PerCP-labeled streptavidin (Becton & Dickinson Biosciences), PE-labeled anti-CD11c (Biolegend, Uden, the Netherlands), and Pacific blue–labeled anti-F4/80 (Biolegend).
Immunohistochemical Staining of Alternatively Activated Macrophages
Male (n = 12) and female (n = 11) mice exposed to the OVA plus alum model were killed 24 hours after the last challenge. The lungs were carefully inflated with 50% Tissue-Tek O.C.T. compound (Sakura, Finetek Europe B.V., Zoeterwoude, The Netherlands) in PBS, the right lung was snap frozen for histological analysis, and the left lung was fixed in formalin. AAMΦ were identified in sections of frozen lung tissue with biotin-labeled antibodies against YM1 (R&D Systems, Abingdon, UK) and CD206 (macrophage mannose receptor; Biolegend) by standard immunohistochemical procedures. Both were visualized with 3-amino-9-ethylcarbazole (Sigma-Aldrich), and YM1-positive cells were quantified in lung tissue by manual counting of the number of positively stained cells in 25 microscopic fields (magnification, ×200) by a blinded observer. The tissue area in these microscopic fields was subsequently quantified by morphometric analysis, and the numbers of cells were expressed per square millimeter.
The Effect of AAMΦ on Airway Inflammation
To test whether AAMΦ contribute to airway inflammation, eight male Balb/c mice were sensitized with OVA and alum on Days 1 and 7. On Day 13, 4 mice received 50 μl PBS and 4 mice received 0.15 × 106 male AAMΦ in 50 μl of PBS intratracheally. All mice were subsequently challenged with 1% OVA on Days 14–20, as described previously here. Mice were killed on Day 21, and eosinophils in BALF were quantified, as were T cell subsets in lung tissue and DC subsets in LDLNs.
Male macrophages were cultured from bone marrow with 10 ng/ml macrophage colony-stimulating factor (M-CSF; Peprotech, Hamburg, Germany), as described in detail in the protocol of Fortier and Falk (29). AAMΦ were generated by stimulating mature macrophages for 24 hours with 10 ng/ml IL-4 and 10 ng/ml IL-13 (both from Peprotech), and their phenotype was checked by flow cytometry (30). Stimulated cells were found to have up-regulated expression of mannose receptors.
Statistical Analysis
Comparisons between groups were performed with the nonparametric Mann-Whitney U test. In the case of the suppression assays, one-way ANOVA with Dunnet's post-test was performed. Differences were considered significant when P was less than 0.05. All results are expressed as the mean ± standard error of the mean (SEM).
RESULTS
Female Mice Have More Pulmonary Effector T Cells than Male Mice
Induction of acute airway inflammation with intraperitoneal OVA and alum and seven subsequent OVA challenges induced a more severe type of airway inflammation in female mice as compared with male mice, as we have reported previously (9). Female mice had higher levels of OVA-specific IgE and a higher percentage of eosinophils among those leukocytes (Table 1).
TABLE 1.
CONFIRMATION OF PREVIOUSLY DESCRIBED DIFFERENCES BETWEEN MALE AND FEMALE MICE WITH OVALBUMIN-INDUCED AIRWAY INFLAMMATION
| Male | Female | |
|---|---|---|
| (n = 6) |
(n = 6) |
|
| Eosinophils in BALF (106 cells/ml) | 0.4 ± 0.1 | 1.6 ± 0.8* |
| OVA-IgE, kU/ml | 3.5 ± 0.5 | 6.8 ± 1* |
Definition of abbreviations: BALF, bronchoalveolar lavage fluid; OVA, ovalbumin.
Values shown are means (±SEM).
P < 0.05 females versus males; tested with Mann-Whitney U test.
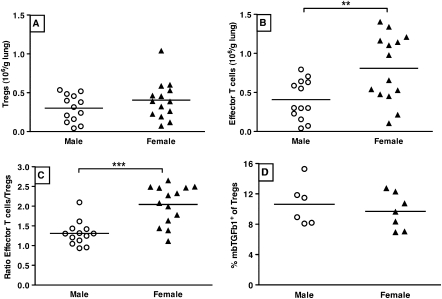
When analyzing T cell subsets in the lung by isolation of leukocytes from lung tissue, we found that females had slightly elevated numbers of total CD4+ T cells compared with males, as we have reported previously (Figure E2) (9). Within this population, male and female mice had the same number of Tregs (defined as CD4+CD25+Foxp3+ lymphocytes), but differed significantly in the number of effector T cells present (defined as CD4+CD25+Foxp3− lymphocytes; Figures 1A and 1B). Female mice had more than twice as many effector T cells in lung tissue as compared with males, which resulted in a mean effector T cell to Treg ratio of 2.1 (±0.1) for females versus 1.3 (±0.1) for males (Figure 1C).
Figure 1.
(A) Male (n = 13) and female (n = 14) mice with ovalbumin (OVA)-induced airway inflammation have the same number of regulatory T cells (Tregs) present in lung tissue. Tregs were defined by the phenotype CD4+CD25+Foxp3+ lymphocytes. Crossbars depict the median. Differences between males and females were tested with Mann Whitney U. (B) Female mice (n = 14) with OVA-induced airway inflammation have more than twice as many effector T cells in lung tissue than their male counterparts (n = 13). Effector T cells were defined by the phenotype CD4+CD25+Foxp3− lymphocytes. **P < 0.01, Mann-Whitney U test. (C) The ratio of effector T cells to Tregs clearly shows a preponderance of effector T cells in lung tissue of female mice (n = 14) with OVA-induced airway inflammation as compared with similar male mice (n = 13). ***P < 0.001, Mann-Whitney U test. (D) No difference in the percentage of TGF-β1+ Tregs is observed between Tregs isolated from lung tissue of male (n = 7) and female (n = 8) mice with OVA-induced airway inflammation.
Because membrane-bound TGF-β1 on Tregs is an important functional characteristic of Tregs (26), we studied the percentage of Tregs in lung tissue positive for TGF-β1, and found no differences between males and females (Figure 1D).
Longer lasting effector T cells and memory T cells down-regulate CD25, and we therefore also compared the CD4+CD25−Foxp3− subset, but found no differences between males and females (males, 2.5 ± 0.3 × 106 cells/g lung tissue, versus females, 2.4 ± 0.4 × 106 cells/g lung tissue).
Male and Female Tregs Are Equally Effective in Suppressing Inflammation
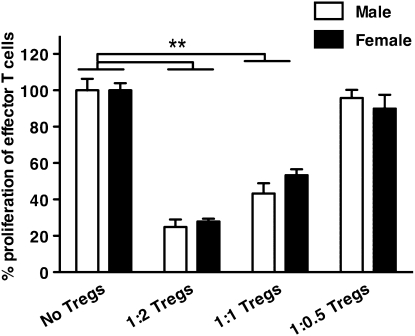
To study whether the greater number of effector T cells in females is due to a difference in suppressive capacities between males and females, we isolated Tregs from spleens of male and female mice tolerized against OVA. In this model, mice are exposed to OVA for 10 consecutive days, rested for another 10 days, and then killed. In this way, we could isolate both naturally occurring Tregs and antigen-experienced Tregs. The only way to isolate Tregs to date is to select for CD4 and CD25, which, unfortunately, are also expressed on effector T cells. However, effector T cells usually do not live beyond a 10-day rest period (31), and our FACS analysis showed that 90% of the CD4+CD25+ T cell fraction was indeed Foxp3+ (i.e., Tregs), and that only a small percentage (∼6%) was Foxp3− (i.e., effector T cells). Both male and female Tregs were subsequently cultured with T cells, and irradiated APCs were isolated from male naive mice with anti-CD3ɛ stimulation. No differences in suppressing capabilities were found between the male and female Tregs (Figure 2).
Figure 2.
Male and female Tregs (n = 3) isolated from mice tolerized against OVA are equally effective in suppressing α-CD3ɛ-stimulated proliferation of naive male effector T cells in the presence of naive male irradiated antigen-presenting cells. Cells were isolated from 4 spleens per group. **P < 0.01, one-way ANOVA with Dunnet's post-test. Error bars indicate SEM.
More Proliferation of Lung and LDLN Cells in Females Compared with Males
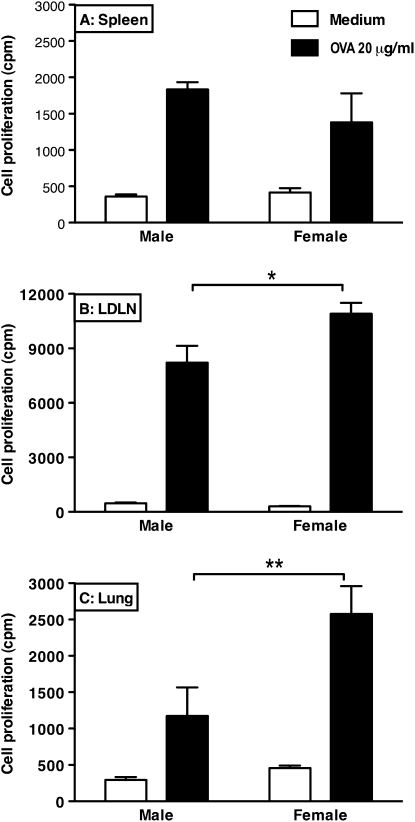
To study whether the effector T cells of females also proliferated more actively than male cells after OVA stimulation, ex vivo proliferation assays were performed. Cell suspensions generated from spleens of male and female mice with OVA-induced airway inflammation and stimulated with 20 μg/ml OVA did not show a difference in proliferation between male and female cells (Figure 3A). However, stimulation of suspensions generated from LDLNs and lungs did show a clear sex difference (Figures 3B and 3C). Female cells responded to OVA with more proliferation, and also with higher levels of Th2 cytokines in the culture medium, than male cells (data not shown).
Figure 3.
Cell suspensions generated from spleens of male and female mice with OVA-induced airway inflammation respond with the same amount of proliferation after ex vivo stimulation with OVA (A), whereas cells isolated from female lung-draining lymph nodes (LDLNs) (B) and lungs (C) respond with significantly more proliferation as compared with male cells. The suspensions were cultured with and without 20 μg/ml OVA for 3 days to study OVA-induced cell proliferation of the cells in spleens (n = 4 per group), LDLNs (n = 4 per group), or lungs (n = 4 per group). *P < 0.05 and **P < 0.01, Mann-Whitney U test. Error bars indicate SEM.
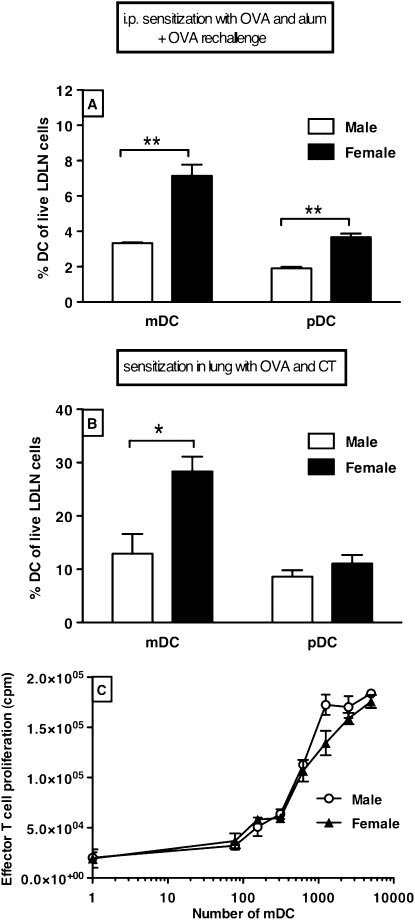
Females Have Higher Numbers of mDCs in LDLNs than Males
After being exposed to intraperitoneal injections of OVA and alum and OVA aerosols, female mice had twice as many mDCs and pDCs traveling to LDLNs as did male mice (Figure 4A). In this model, however, the initial sensitizing event does not occur in the lungs. To test whether sensitization to an antigen in the lungs also leads to more DCs traveling to LDLNs in females compared with males, OVA-specific lung DCs were generated by exposing mice intranasally to a combination of OVA and CT for 3 days. On Day 4, females again had twice as many mDCs as males in LDLNs, but the percentage of pDCs was now similar between the sexes (Figure 4B). The reason we isolated a smaller percentage of DCs from LDLNs in the OVA plus alum model is that the LDLNs had been stimulated for longer in this model, and therefore contained more proliferated T and B cells.
Figure 4.
(A) After intraperitoneal sensitization with OVA and alum and OVA aerosol challenges (total protocol length 21 d), female LDLNs contain twice as many myeloid dendritic cells (mDCs) and plasmacytoid DCs (pDCs) as male LDLNs (n = 6). **P < 0.01, Mann-Whitney U test. (B) After lung sensitization and challenge with OVA and cholera toxin (CT) (total protocol length, 3 d), female LDLNs contain twice as many mDCs as male LDLNs (n = 2 of 8 pooled mice). There is no difference in the percentage of pDC between cells isolated from male and female LDLNs. Error bars indicate SEM; *P < 0.05, Mann-Whitney U test. (C) mDCs isolated from pooled male and female LDLNs (n = 2 of 8 pooled mice per group) after exposure to OVA and CT are equally effective in inducing proliferation of male DO11.10 T cells after coculturing with 20 μg/ml specific antigenic OVA-peptide (aa 323–339). Cells were plated in triplicate for each condition. Error bars indicate SEM.
There was no appreciable difference between the male and female mDCs in stimulating T cell proliferation (Figure 4C).
Female Mice Have Higher Numbers of AAMΦ in Lung Tissue
We assessed the levels of several cytokines mainly produced by macrophages in lungs of male and female mice with OVA-induced airway inflammation. Female mice had significantly higher levels of TNF-α, IL-1β, IL-10, and macrophage inflammatory protein–1α than males in lung tissue, and similar levels of IL-12 (p70) and IL-6 (Table 2). Subsequently, we assessed the percentage and absolute number of macrophages in lung tissue by flow cytometric analysis (Figures 5B and 5C). Although females had a significantly higher percentage of macrophages in isolated lung cells, the absolute numbers per gram of lung tissue were the same between males and females, making it hard to explain the higher cytokine levels in females. Another explanation could be a different shift in macrophage subtypes between males and females.
TABLE 2.
LEVELS OF CYTOKINES MAINLY PRODUCED BY MACROPHAGES IN LUNG TISSUE OF MICE WITH OVALBUMIN-INDUCED AIRWAY INFLAMMATION
| Male | Female | |
|---|---|---|
| Cytokine, pg/ml |
(n = 6) |
(n = 6) |
| TNF-α | 9 ± 3 | 24 ± 2* |
| IL-1β | 64 ± 18 | 168 ± 19* |
| IL-6 | 273 ± 65 | 198 ± 23 |
| IL-10 | 26 ± 4 | 64 ± 10* |
| IL-12 (p70) | 57 ± 12 | 105 ± 28 |
| MIP-1α | 1,001 ± 121 | 1,504 ± 137† |
Definition of abbreviation: MIP, macrophage inflammatory protein.
Values shown are means (±SEM).
P < 0.01 females versus males; tested with Mann-Whitney U test.
P < 0.05 females versus males; tested with Mann-Whitney U test.
Figure 5.
(A) Representative plots of cells isolated from digested lung tissue of male and female mice with OVA-induced airway inflammation (male, solid line; female, dashed line). FITC-autofluorescent cells are macrophages within this cell suspension. (B) Female mice (n = 8) with OVA-induced airway inflammation have significantly higher percentages of autofluorescent macrophages than male OVA mice (n = 7). *P < 0.05, Mann-Whitney U test. (C) The absolute number of autofluorescent macrophages in lung tissue of male (n = 7) and female mice (n = 8) with OVA-induced airway inflammation is similar. (D) Female mice (n = 11) with OVA-induced airway inflammation have significantly more alternatively activated macrophages (AAMΦ) than their male counterparts (n = 12), as judged by counting YM1-positive cells in lung tissue sections. **P < 0.01, Mann-Whitney U test. Quantification of the staining shown in F and G. Induction of airway inflammation increased the number AAMΦ in both sexes (data for control mice shown in Figure E3). (E) Female mice (n = 11) with OVA-induced airway inflammation have significantly more multinucleated giant cells in lung tissue than their male counterparts (n = 12). *P < 0.05, Mann-Whitney U test. Induction of airway inflammation increased the number of giant cells in both sexes (data not shown). (F and G) Representative photographs of sections of lung tissue from mice with OVA-induced allergic airway inflammation stained for YM1 (red staining), an important marker of AAMΦ (magnification, ×200). The arrow in G points at a multinucleated giant cell, which are more frequently found in female lung tissue with airway inflammation than in male lung tissue.
We then studied the macrophage subtype, AAMΦ, which has been implicated in the pathogenesis of asthma (25), by staining for YM1 and macrophage mannose receptors, both expressed by AAMΦ. Lungs of female mice with airway inflammation had significantly more AAMΦ (YM1-positive cells) than lungs of their male counterparts (Figures 5D, 5F, and 5G), and identical results were found for the mannose receptor (data not shown). Incidentally, induction of airway inflammation increased the number of AAMΦ in both males and females as compared with control mice, but we found no differences between male and female control mice (Figure E3).
When counting YM1-positive cells, we also noted that females had significantly higher numbers of multinucleated giant cells as compared with males (Figure 5E). These giant cells stained positive for both YM1 and the mannose receptor. An example can be seen in Figure 5G.
AAMΦ Contribute to Allergic Airway Inflammation
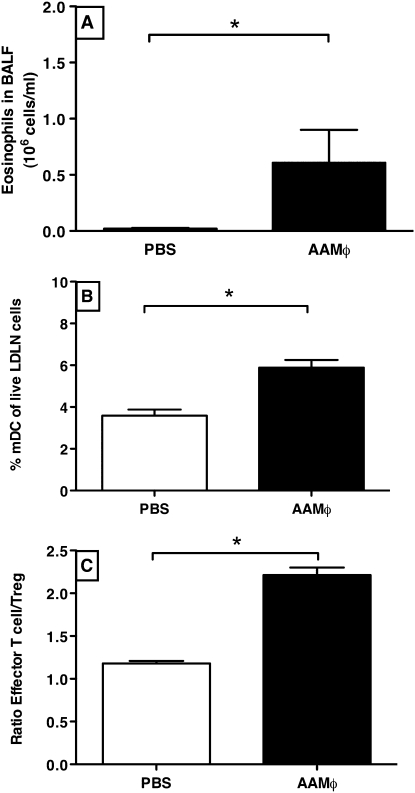
The observation of increased AAMΦ in females did not tell whether these cells were actually contributing to increased inflammation or were just the result of it. We therefore treated male mice intratracheally with male AAMΦ before OVA challenges, and found that this dramatically increased allergic airway inflammation as compared with mice treated with PBS. We used male mice and male AAMΦ because we expected an increase in inflammation, and it would be easier to detect it in the males because they have less inflammation than females. The AAMΦ-treated OVA-mice had approximately 30 times more eosinophils in BALF than did PBS-treated OVA mice (Figure 6A). They also had significantly more mDCs migrating to LDLNs (Figure 6B). In addition, AAMΦ-treated mice had a significantly higher effector T cell:Treg ratio than control animals, which was caused by a small increase in effector T cells and a small but significant decrease in Tregs (Figure 6C).
Figure 6.
(A) Intratracheal instillation of AAMΦ (n = 4) as compared with PBS (n = 4) before OVA challenges significantly increases the number of eosinophils found in bronchoalveolar lavage fluid. *P < 0.05, Mann-Whitney test. (B) After AAMΦ instillation (n = 4), LDLNs contain twice as many mDCs as compared with instillation with PBS (n = 4). *P < 0.05, Mann-Whitney test. (C) The ratio of effector T cells to Tregs shows a preponderance of effector T cells in lung tissue of AAMΦ-treated mice (n = 4) as compared with mice treated with PBS (n = 4). *P < 0.05, Mann-Whitney test.
DISCUSSION
In this article, we report that, although male and female Balb/c mice have comparable Treg numbers and function, effector T cells have expanded to a greater extent in lungs of female mice after being exposed to OVA than in lungs of male mice. This difference in T cell expansion appears to be driven by a greater number of mDCs migrating from the lungs to the lymph nodes in females as compared with males. We also found that female mice have higher numbers of AAMΦ in lung tissue than males, and that these cells increase allergic airway inflammation, which is accompanied by increased mDC migration. An increased number of AAMΦ in lung tissue of female mice may therefore be (partly) responsible for the aggravated airway inflammation that these mice develop as compared with males.
The experiments described in this article were performed at two different laboratories. Due to the robustness of the model, however, we were able to pool data from experiments from both laboratories, further emphasizing the significance of the greater number of effector T cells found in lung tissue of female mice as compared with males. This pointed at a possible difference in Treg control of effector T cells between male and female mice. However, neither Treg numbers in lung tissue nor the basal suppressive capacities of Tregs were found to be different between the sexes. However, it cannot be excluded that the local inflammatory environment in the lung might have influenced Treg function (32). In our study, it was impossible to isolate male and female Tregs from lung tissue of mice with airway inflammation for suppression assays, because these lungs contain large numbers of effector T cells and Tregs. Both are CD4 and CD25 positive, and the only definitive marker is Foxp3, which cannot be used to isolate live cells. However, recent studies from our laboratory have shown that the suppressive function of Tregs isolated from lung tissue of Foxp3EGFP-knockin mice with airway inflammation is still present, but is inhibited when IL-4 is added to the suppression assay (33). Female mice with airway inflammation have higher levels of IL-4 in lung tissue than male mice (9), and Treg function could therefore have been reduced in females.
Because ex vivo stimulation of spleen cells with OVA from males and females with allergic airway inflammation did not show any differences in proliferation, it seemed unlikely that intrinsic differences between male and female effector T cells are causing the differences in the lung. The fact that we found increased OVA-induced ex vivo proliferation of cells isolated from LDLNs and lungs in females (and Th2 cytokines produced by those cells) alerted us to the possibility of involvement of local cells that do not circulate through the spleen, but do migrate to the LDLNs. Likely candidates for this role are DCs. The lung has a network of DCs that, upon recognition of antigen in the context of a danger signal, migrate to the LDLNs, maturing into mDCs on their way. In the lymph nodes, mDCs interact with T cells to induce and polarize the T cell responses (12). A difference in the number of mDCs traveling to the lymph nodes, or a difference in T cell stimulating capabilities, between males and females may be an alternative explanation for the increased number of effector T cells found in females. We found the first option, a difference in the number of migrating mDCs, to be the most likely explanation. Male and female mDCs stimulated T cell proliferation equally well, but there was a substantial difference between the number of migrating mDCs between males and females. We and others recently showed that the severity of airway inflammation was proportional to the number of mDCs (13, 34), indicating that females could indeed have increased airway inflammation due to increased numbers of mDCs.
This increased number of mDCs in female LDLNs did not seem to be a result of a higher number of total DCs in female lung tissue, either under normal, healthy conditions or under inflammatory conditions. It is therefore more likely that other resident lung cells and/or their products are involved in stimulating DC maturation to mDCs and migration to LDLNs (35). One such product is TSLP. It is produced by epithelial cells, and is instrumental in activating DCs and inducing Th2 responses (36). The increase in mDCs in females could therefore also be the result of increased TSLP production by female epithelial cells. However, we did not find significant differences in BALF levels of TSLP between male and female mice after OVA challenges (male, 16 ± 6 pg/ml, versus female, 9 ± 1 pg/ml). This could be due to the fact that we only sampled at one time point, at the end of the asthma protocol, and we may therefore have missed an earlier peak of TSLP.
Differences in male and female pulmonary cytokines produced by macrophages, among others, alerted us to a possible role of macrophages in modulating DC maturation and migration (19, 20). Besides higher levels of Th2 cytokines (9), female lung tissue contained significantly higher levels of the macrophage products, TNF-α, IL-1β, IL-10, and macrophage inflammatory protein–1α, as compared with males. They nevertheless had the same absolute number of macrophages in lung tissue. We therefore hypothesized that females might have increased numbers of a different subset of macrophages. We chose to focus on the AAMΦ subset, because the cytokines mentioned here can all be produced by AAMΦ (30), and we did not find significant differences in IL-12 levels, typically produced by the classically activated subset. Furthermore, AAMΦ are implicated in the pathogenesis of asthma (22, 23), and have been shown to promote Th2 responses (25), whereas classically activated macrophages have been found to inhibit allergic airway inflammation (19, 37). Using the AAMΦ-specific marker, YM1 (38), we subsequently found higher numbers of AAMΦ in lung tissue of females as compared with males. These results were confirmed by using the mannose receptor as another marker of AAMΦ (data not shown) (39). The increase in AAMΦ numbers corresponded with increased numbers of multinucleated giant cells in females. Giant cells originate from fusion of AAMΦ, and are characteristic of chronic, granulomatous inflammation (40, 41), again emphasizing the increased inflammation found in females as compared with males.
The elevated number of AAMΦ in females may be involved in causing the aggravated airway inflammation when compared with males, but it may also be the result of the higher levels of IL-4 and IL-13 in lung tissue of females (30). Our further study with adoptive transfer of male AAMΦ into the airways of male mice showed that an increased number of AAMΦ could indeed dramatically increase airway inflammation, thereby giving more credit to their possible role in the aggravated inflammation found in females. Interestingly, two recent publications investigating macrophage activation during wound healing and myocarditis showed that estrogen and progesterone contribute to alternative activation of macrophages, and that testosterone inhibits alternative activation (42, 43). Female sex hormones may therefore increase susceptibility for airway inflammation through alternative activation of macrophages in females, whereas testosterone does the opposite in males. As sex hormones affect a myriad of processes and cell types, further studies are needed to test these hypotheses.
Although the instillation of male AAMΦ in lungs of male mice mimicked important features of the female inflammation, such as greater numbers of pulmonary eosinophils and mDCs in LDLNs, we did not find significantly elevated numbers of effector T cells in lung tissue. This may be related to the time of sampling. We used a surrogate model of naturally occurring AAMΦ by culturing them artificially from bone marrow, and this may have affected the kinetics of the inflammatory response, causing us to miss the increase in effector T cells. It is also likely that we only reproduced part of the differences between males and females by introducing AAMΦ to the lung, and that other, possibly sex hormone–related, mechanisms have remained unaffected. Interesting further experiments in this respect would be the transfer of female AAMΦ into male lungs. An additional increase in inflammation and/or generation of the full female inflammatory phenotype would then suggest intrinsic differences between male and female AAMΦ. Transfer of male or female AAMΦ into female lungs as compared with transfer of these cells into male lungs will subsequently give valuable information as to whether the female lung microenvironment also affects the inflammatory response. These experiments, and the effects of sex hormones, will be included in future research.
How AAMΦ could facilitate allergic inflammation remains elusive. It may involve directly or indirectly modulating DC maturation, as we found more mDCs in LDLNs after intratracheally instilling AAMΦ as compared with PBS. As interactions between DC and AAMΦ have not been studied yet, further studies are required to elucidate the intriguing role that AAMΦ may have in regulating DC maturation after exposure to antigens.
In conclusion, the aggravated type of airway inflammation found in female mice as compared with males is not likely the result of a difference in Treg or mDC function. The greater numbers of mDCs in LDLNs and AAMΦ in lungs of females that we found suggest an interaction between these cells that needs further investigation. To the best of our knowledge, this is the first article to show that AAMΦ in the lung aggravate allergic airway inflammation in mice.
Supplementary Material
Acknowledgments
The authors thank Bea Rutgers for setting up the immunohistological staining for CD206, Yvette de Reus for setting up macrophage cultures, Petra van der Wouden for laboratory assistance, and Pieter Klok for biotechnical assistance.
This work was supported by grant 3.4.05.041 from the Netherlands Asthma Foundation (B.N.M.) and National Institutes of Health grants AI48927 and HL77430 (A.R.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2009-0016OC on July 2, 2009
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Melgert BN, Ray A, Hylkema MN, Timens W, Postma DS. Are there reasons why adult asthma is more common in females? Curr Allergy Asthma Rep 2007;7:143–150. [DOI] [PubMed] [Google Scholar]
- 2.Verthelyi D. Sex hormones as immunomodulators in health and disease. Int Immunopharmacol 2001;1:983–993. [DOI] [PubMed] [Google Scholar]
- 3.Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum Reprod Update 2005;11:411–423. [DOI] [PubMed] [Google Scholar]
- 4.Beeson PB. Age and sex associations of 40 autoimmune diseases. Am J Med 1994;96:457–462. [DOI] [PubMed] [Google Scholar]
- 5.Schatz M, Camargo CA Jr. The relationship of sex to asthma prevalence, health care utilization, and medications in a large managed care organization. Ann Allergy Asthma Immunol 2003;91:553–558. [DOI] [PubMed] [Google Scholar]
- 6.Lee JH, Haselkorn T, Chipps BE, Miller DP, Wenzel SE, Tenor Study Group. Gender differences in IgE-mediated allergic asthma in the epidemiology and natural history of asthma: outcomes and treatment regimens (TENOR) study. J Asthma 2006;43:179–184. [DOI] [PubMed] [Google Scholar]
- 7.ENFUMOSA. The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. European Network for Understanding Mechanisms of Severe Asthma. Eur Respir J 2003;22:470–477. [DOI] [PubMed] [Google Scholar]
- 8.Dijkstra A, Vonk JM, Jongepier H, Koppelman GH, Schouten JP, ten Hacken NH, Timens W, Postma DS. Lung function decline in asthma: association with inhaled corticosteroids, smoking and sex. Thorax 2006;61:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melgert BN, Postma DS, Kuipers I, Geerlings M, Luinge MA, van der Strate BW, Kerstjens HA, Timens W, Hylkema MN. Female mice are more susceptible to the development of allergic airway inflammation than male mice. Clin Exp Allergy 2005;35:1496–1503. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi T, Adachi Y, Hasegawa K, Morimoto M. Less sensitivity for late airway inflammation in males than females in Balb/c mice. Scand J Immunol 2003;57:562–567. [DOI] [PubMed] [Google Scholar]
- 11.Corteling R, Trifilieff A. Gender comparison in a murine model of allergen-driven airway inflammation and the response to budesonide treatment. BMC Pharmacol 2004;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammad H, Lambrecht BN. Recent progress in the biology of airway dendritic cells and implications for understanding the regulation of asthmatic inflammation. J Allergy Clin Immunol 2006;118:331–336. [DOI] [PubMed] [Google Scholar]
- 13.Oriss TB, Ostroukhova M, Seguin-Devaux C, Dixon-McCarthy B, Stolz DB, Watkins SC, Pillemer B, Ray P, Ray A. Dynamics of dendritic cell phenotype and interactions with CD4+ T cells in airway inflammation and tolerance. J Immunol 2005;174:854–863. [DOI] [PubMed] [Google Scholar]
- 14.de Heer HJ, Hammad H, Soullie T, Hijdra D, Vos N, Willart MA, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med 2004;200:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umetsu DT, DeKruyff RH. The regulation of allergy and asthma. Immunol Rev 2006;212:238–255. [DOI] [PubMed] [Google Scholar]
- 16.Kearley J, Robinson DS, Lloyd CM. CD4+CD25+ regulatory T cells reverse established allergic airway inflammation and prevent airway remodeling. J Allergy Clin Immunol 2008;122:617–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinson DS, Larche M, Durham SR. Tregs and allergic disease. J Clin Invest 2004;114:1389–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strickland DH, Stumbles PA, Zosky GR, Subrata LS, Thomas JA, Turner DJ, Sly PD, Holt PG. Reversal of airway hyperresponsiveness by induction of airway mucosal CD4+CD25+ regulatory T cells. J Exp Med 2006;203:2649–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holt PG, Oliver J, Bilyk N, McMenamin C, McMenamin PG, Kraal G, Thepen T. Downregulation of the antigen presenting cell function(s) of pulmonary dendritic cells in vivo by resident alveolar macrophages. J Exp Med 1993;177:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakubzick C, Tacke F, Llodra J, van Rooijen N, Randolph GJ. Modulation of dendritic cell trafficking to and from the airways. J Immunol 2006;176:3578–3584. [DOI] [PubMed] [Google Scholar]
- 21.Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol 2005;142:481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Z, Zheng T, Homer RJ, Kim YK, Chen NY, Cohn L, Hamid Q, Elias JA. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 2004;304:1678–1682. [DOI] [PubMed] [Google Scholar]
- 23.Vercelli D. Arginase: marker, effector, or candidate gene for asthma? J Clin Invest 2003;111:1815–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 2009;27:451–483. [DOI] [PubMed] [Google Scholar]
- 25.Ramalingam TR, Reiman RM, Wynn TA. Exploiting worm and allergy models to understand Th2 cytokine biology. Curr Opin Allergy Clin Immunol 2005;5:392–398. [DOI] [PubMed] [Google Scholar]
- 26.Ostroukhova M, Seguin-Devaux C, Oriss TB, Dixon-McCarthy B, Yang L, Ameredes BT, Corcoran TE, Ray A. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and Foxp3. J Clin Invest 2004;114:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melgert BN, Postma DS, Geerlings M, Luinge MA, Klok PA, van der Strate BW, Kerstjens HA, Timens W, Hylkema MN. Short-term smoke exposure attenuates ovalbumin-induced airway inflammation in allergic mice. Am J Respir Cell Mol Biol 2004;30:880–885. [DOI] [PubMed] [Google Scholar]
- 28.Vermaelen K, Pauwels R. Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: methodology and new insights. Cytometry A 2004;61:170–177. [DOI] [PubMed] [Google Scholar]
- 29.Fortier AH, Falk LA. Isolation of murine macrophages. Hoboken, NJ: John Wiley & Sons, Inc.; 2003.
- 30.Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol 2006;80:1298–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merica R, Khoruts A, Pape KA, Reinhardt RL, Jenkins MK. Antigen-experienced CD4 T cells display a reduced capacity for clonal expansion in vivo that is imposed by factors present in the immune host. J Immunol 2000;164:4551–4557. [DOI] [PubMed] [Google Scholar]
- 32.Pillemer BB, Xu H, Oriss TB, Qi Z, Ray A. Deficient socs3 expression in CD4+CD25+Foxp3+ regulatory T cells and SOCS3-mediated suppression of Treg function. Eur J Immunol 2007;37:2082–2089. [DOI] [PubMed] [Google Scholar]
- 33.Pillemer BL, Qi ZB, Melgert BN, Oriss TB, Ray P, Ray A. Stat6 activation confers upon T helper cells resistance to suppression by regulatory T cells. J Immunol 2009;183:155–163. [DOI] [PMC free article] [PubMed]
- 34.Koya T, Kodama T, Takeda K, Miyahara N, Yang ES, Taube C, Joetham A, Park JW, Dakhama A, Gelfand EW. Importance of myeloid dendritic cells in persistent airway disease after repeated allergen exposure. Am J Respir Crit Care Med 2006;173:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: Linking innate and adaptive immunity in asthma. Nat Rev Immunol 2008;8:193–204. [DOI] [PubMed] [Google Scholar]
- 36.Liu YJ. Thymic stromal lymphopoietin and OX40 ligand pathway in the initiation of dendritic cell–mediated allergic inflammation. J Allergy Clin Immunol 2007;120:238–244; quiz 245–236. [DOI] [PubMed] [Google Scholar]
- 37.Korf JE, Pynaert G, Tournoy K, Boonefaes T, Van Oosterhout A, Ginneberge D, Haegeman A, Verschoor JA, De Baetselier P, Grooten J. Macrophage reprogramming by mycolic acid promotes a tolerogenic response in experimental asthma. Am J Respir Crit Care Med 2006;174:152–160. [DOI] [PubMed] [Google Scholar]
- 38.Raes G, De Baetselier P, Noel W, Beschin A, Brombacher F, Hassanzadeh Gh G. Differential expression of FIZZ1 and YM1 in alternatively versus classically activated macrophages. J Leukoc Biol 2002;71:597–602. [PubMed] [Google Scholar]
- 39.Van Ginderachter JA, Movahedi K, Hassanzadeh Ghassabeh G, Meerschaut S, Beschin A, Raes G, De Baetselier P. Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion. Immunobiology 2006;211:487–501. [DOI] [PubMed] [Google Scholar]
- 40.Helming L, Gordon S. Macrophage fusion induced by IL-4 alternative activation is a multistage process involving multiple target molecules. Eur J Immunol 2007;37:33–42. [DOI] [PubMed] [Google Scholar]
- 41.Brodbeck WG, Anderson JM. Giant cell formation and function. Curr Opin Hematol 2009;16:53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Routley CE, Ashcroft GS. Effect of estrogen and progesterone on macrophage activation during wound healing. Wound Repair Regen 2009;17:42–50. [DOI] [PubMed] [Google Scholar]
- 43.Frisancho-Kiss S, Coronado MJ, Frisancho JA, Lau VM, Rose NR, Klein SL, Fairweather D. Gonadectomy of male Balb/c mice increases TIM-3(+) alternatively activated M2 macrophages, TIM-3(+) T cells, Th2 cells and Treg in the heart during acute coxsackievirus-induced myocarditis. Brain Behav Immun 2009;23:649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.