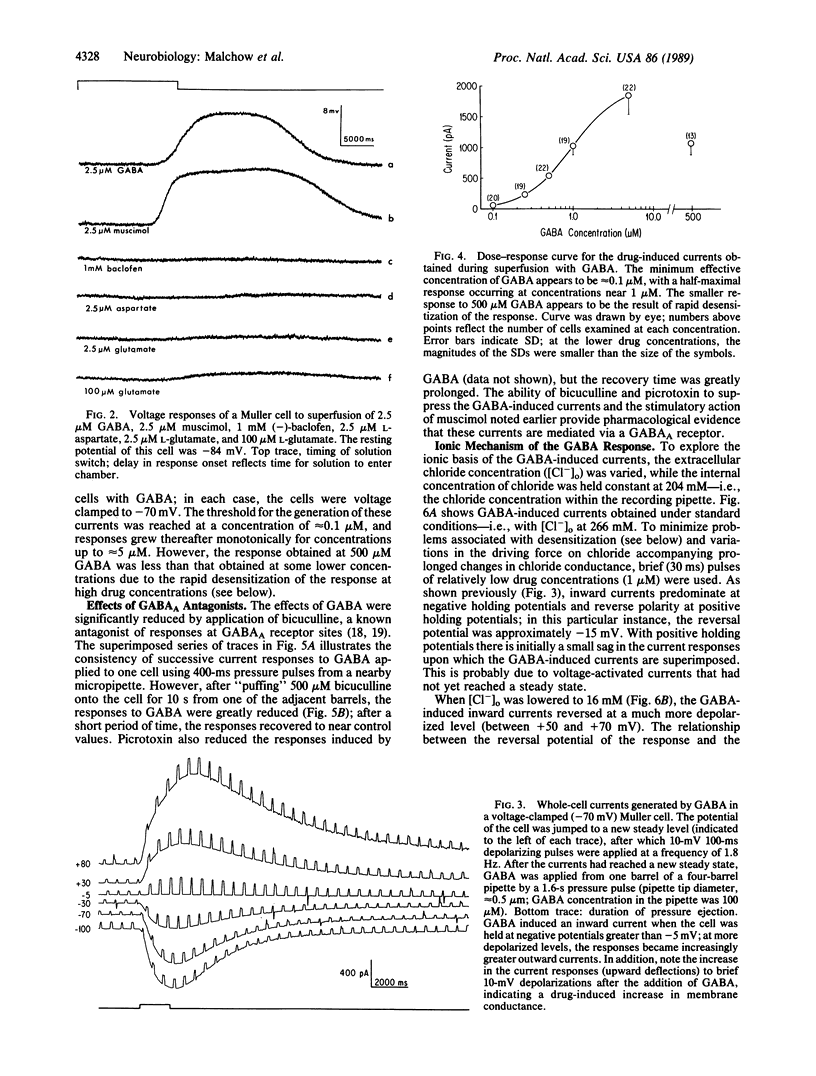
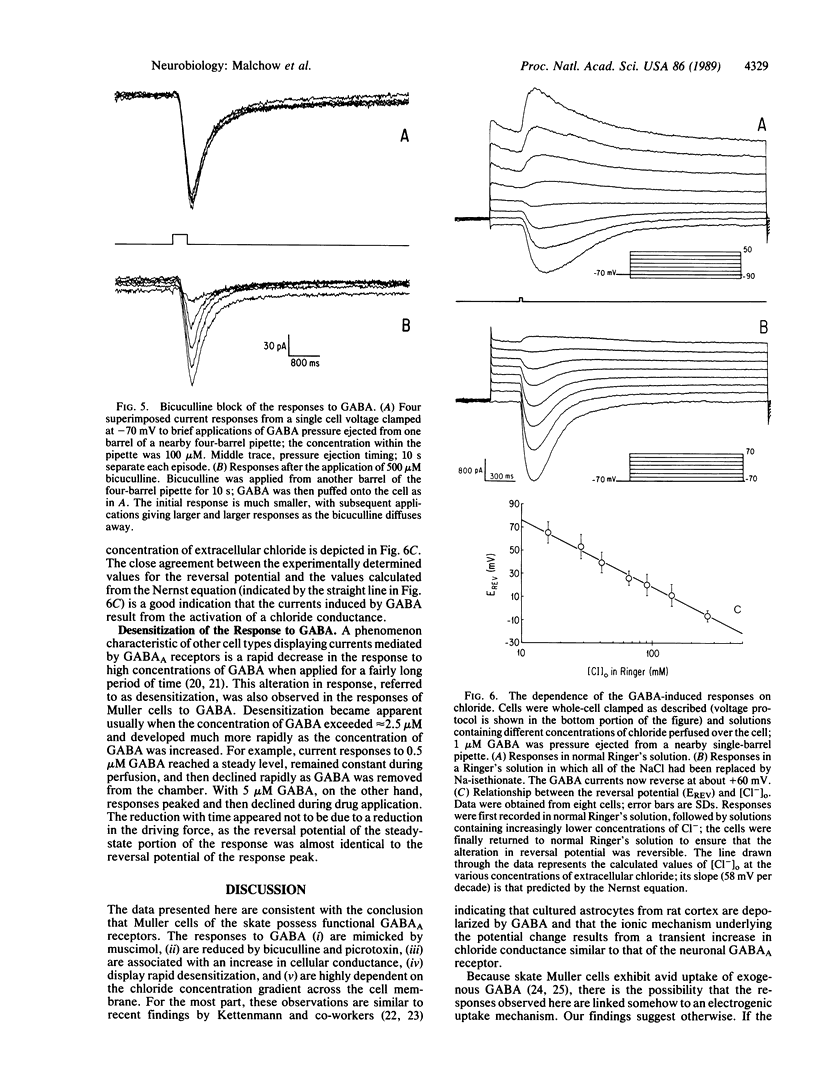
Abstract
Radial glia (Muller cells) of the vertebrate retina appear to be intimately involved in regulating the actions of amino acid neurotransmitters. One of the amino acids thought to be important in mediating retinal information flow is gamma-aminobutyric acid (GABA). The findings of this study indicate that enzymatically isolated skate Muller cells are depolarized by GABA and the GABAA agonist muscimol and that the actions of these agents are reduced by bicuculline and picrotoxin. Membrane currents induced by GABA under voltage clamp were dose dependent, were associated with an increase in membrane conductance, and showed marked desensitization when the concentration of GABA exceeded 2.5 microM. The responses had a reversal potential close to that calculated for chloride, indicating that the currents were generated by ions passing through channels. These data support the view that skate Muller cells possess functional GABAA receptors. The presence of such receptors on retinal glia may have important implications for the role of Muller cells in maintaining the constancy of the extracellular milieu, for neuron-glia interactions within the retina, and for theories concerning the generation of the electroretinogram.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A. Actions of gamma-aminobutyric acid on sympathetic ganglion cells. J Physiol. 1975 Aug;250(1):85–120. doi: 10.1113/jphysiol.1975.sp011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Inomata N., Tokutomi N. Contribution of chloride shifts to the fade of gamma-aminobutyric acid-gated currents in frog dorsal root ganglion cells. J Physiol. 1987 Oct;391:219–234. doi: 10.1113/jphysiol.1987.sp016735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour B., Brew H., Attwell D. Electrogenic glutamate uptake in glial cells is activated by intracellular potassium. Nature. 1988 Sep 29;335(6189):433–435. doi: 10.1038/335433a0. [DOI] [PubMed] [Google Scholar]
- Barker J. L., Mathers D. A. GABA analogues activate channels of different duration on cultured mouse spinal neurons. Science. 1981 Apr 17;212(4492):358–361. doi: 10.1126/science.6259733. [DOI] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N., Mathers D. A. Convulsant-induced depression of amino acid responses in cultured mouse spinal neurones studied under voltage clamp. Br J Pharmacol. 1983 Dec;80(4):619–629. doi: 10.1111/j.1476-5381.1983.tb10051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres B. A., Chun L. L., Corey D. P. Ion channel expression by white matter glia: I. Type 2 astrocytes and oligodendrocytes. Glia. 1988;1(1):10–30. doi: 10.1002/glia.440010104. [DOI] [PubMed] [Google Scholar]
- Bevan S., Raff M. Voltage-dependent potassium currents in cultured astrocytes. Nature. 1985 May 16;315(6016):229–232. doi: 10.1038/315229a0. [DOI] [PubMed] [Google Scholar]
- Bonaventure N., Wioland N., Mandel P. Antagonists of the putative inhibitory transmitter effects of taurine and GABA in the retina. Brain Res. 1974 Nov 15;80(2):281–289. doi: 10.1016/0006-8993(74)90691-x. [DOI] [PubMed] [Google Scholar]
- Bowman C. L., Kimelberg H. K. Excitatory amino acids directly depolarize rat brain astrocytes in primary culture. Nature. 1984 Oct 18;311(5987):656–659. doi: 10.1038/311656a0. [DOI] [PubMed] [Google Scholar]
- Boyle P. J., Conway E. J. Potassium accumulation in muscle and associated changes. J Physiol. 1941 Aug 11;100(1):1–63. doi: 10.1113/jphysiol.1941.sp003922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew H., Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. 1987 Jun 25-Jul 1Nature. 327(6124):707–709. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- Brunken W. J., Witkovsky P., Karten H. J. Retinal neurochemistry of three elasmobranch species: an immunohistochemical approach. J Comp Neurol. 1986 Jan 1;243(1):1–12. doi: 10.1002/cne.902430102. [DOI] [PubMed] [Google Scholar]
- Bruun A., Ehinger B., Sytsma V. M. Neurotransmitter localization in the skate retina. Brain Res. 1984 Mar 19;295(2):233–248. doi: 10.1016/0006-8993(84)90972-7. [DOI] [PubMed] [Google Scholar]
- Bruun A., Ehinger B. Uptake of certain possible neurotransmitters into retinal neurons of some mammals. Exp Eye Res. 1974 Nov;19(5):435–447. doi: 10.1016/0014-4835(74)90052-9. [DOI] [PubMed] [Google Scholar]
- Cohen J. L. The action of gamma-aminobutyric acid on the horizontal cells of the skate retina. Brain Res. 1988 Jul 12;455(2):366–369. doi: 10.1016/0006-8993(88)90096-0. [DOI] [PubMed] [Google Scholar]
- Conner J. D., Detwiler P. B., Sarthy P. V. Ionic and electrophysiological properties of retinal Müller (glial) cells of the turtle. J Physiol. 1985 May;362:79–92. doi: 10.1113/jphysiol.1985.sp015664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res. 1971 Sep 10;32(1):69–96. doi: 10.1016/0006-8993(71)90156-9. [DOI] [PubMed] [Google Scholar]
- De Vries G. W., Friedman A. H. GABA, picrotoxin and retinal sensitivity. Brain Res. 1978 Jun 16;148(2):530–535. doi: 10.1016/0006-8993(78)90743-6. [DOI] [PubMed] [Google Scholar]
- Dunlap K. Two types of gamma-aminobutyric acid receptor on embryonic sensory neurones. Br J Pharmacol. 1981 Nov;74(3):579–585. doi: 10.1111/j.1476-5381.1981.tb10467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erecińska M. The neurotransmitter amino acid transport systems. A fresh outlook on an old problem. Biochem Pharmacol. 1987 Nov 1;36(21):3547–3555. doi: 10.1016/0006-2952(87)90001-3. [DOI] [PubMed] [Google Scholar]
- Gilbert P., Kettenmann H., Schachner M. gamma-Aminobutyric acid directly depolarizes cultured oligodendrocytes. J Neurosci. 1984 Feb;4(2):561–569. doi: 10.1523/JNEUROSCI.04-02-00561.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlob I., Wündsch L., Tuppy F. K. The rabbit electroretinogram: effect of GABA and its antagonists. Vision Res. 1988;28(2):203–210. doi: 10.1016/0042-6989(88)90147-2. [DOI] [PubMed] [Google Scholar]
- Grafe P., Ballanyi K. Cellular mechanisms of potassium homeostasis in the mammalian nervous system. Can J Physiol Pharmacol. 1987 May;65(5):1038–1042. doi: 10.1139/y87-164. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HOROWICZ P. The influence of potassium and chloride ions on the membrane potential of single muscle fibres. J Physiol. 1959 Oct;148:127–160. doi: 10.1113/jphysiol.1959.sp006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Kaila K., Voipio J. Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature. 1987 Nov 12;330(6144):163–165. doi: 10.1038/330163a0. [DOI] [PubMed] [Google Scholar]
- Kettenmann H., Backus K. H., Schachner M. gamma-Aminobutyric acid opens Cl-channels in cultured astrocytes. Brain Res. 1987 Feb 24;404(1-2):1–9. doi: 10.1016/0006-8993(87)91349-7. [DOI] [PubMed] [Google Scholar]
- Kettenmann H., Schachner M. Pharmacological properties of gamma-aminobutyric acid-, glutamate-, and aspartate-induced depolarizations in cultured astrocytes. J Neurosci. 1985 Dec;5(12):3295–3301. doi: 10.1523/JNEUROSCI.05-12-03295.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline R. P., Ripps H., Dowling J. E. Generation of b-wave currents in the skate retina. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5727–5731. doi: 10.1073/pnas.75.11.5727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasater E. M., Dowling J. E., Ripps H. Pharmacological properties of isolated horizontal and bipolar cells from the skate retina. J Neurosci. 1984 Aug;4(8):1966–1975. doi: 10.1523/JNEUROSCI.04-08-01966.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linser P. J., Smith K., Angelides K. A comparative analysis of glial and neuronal markers in the retina of fish: variable character of horizontal cells. J Comp Neurol. 1985 Jul 8;237(2):264–272. doi: 10.1002/cne.902370210. [DOI] [PubMed] [Google Scholar]
- Marshall J., Voaden M. Autoradiographic identification of the cells accumulating 3H gamma-aminobutyric acid in mammalian retinae: a species comparison. Vision Res. 1975 Mar;15(3):459–461. doi: 10.1016/0042-6989(75)90102-9. [DOI] [PubMed] [Google Scholar]
- Miller R. F. Role of K + in generation of b-wave of electroretinogram. J Neurophysiol. 1973 Jan;36(1):28–38. doi: 10.1152/jn.1973.36.1.28. [DOI] [PubMed] [Google Scholar]
- Minchin M. C., Iversen L. L. Release of (3H)gamma-aminobutyric acid from glial cells in rat dorsal root ganglia. J Neurochem. 1974 Sep;23(3):533–540. doi: 10.1111/j.1471-4159.1974.tb06056.x. [DOI] [PubMed] [Google Scholar]
- Murphy S., Jeremy J., Pearce B., Dandona P. Eicosanoid synthesis and release from primary cultures of rat central nervous system astrocytes and meningeal cells. Neurosci Lett. 1985 Oct 24;61(1-2):61–65. doi: 10.1016/0304-3940(85)90401-x. [DOI] [PubMed] [Google Scholar]
- Murphy S., Pearce B. Functional receptors for neurotransmitters on astroglial cells. Neuroscience. 1987 Aug;22(2):381–394. doi: 10.1016/0306-4522(87)90342-3. [DOI] [PubMed] [Google Scholar]
- Neal M. J., Iversen L. L. Autoradiographic localization of 3 H-GABA in rat retina. Nat New Biol. 1972 Feb 16;235(59):217–218. doi: 10.1038/newbio235217a0. [DOI] [PubMed] [Google Scholar]
- Newman E. A., Frambach D. A., Odette L. L. Control of extracellular potassium levels by retinal glial cell K+ siphoning. Science. 1984 Sep 14;225(4667):1174–1175. doi: 10.1126/science.6474173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E. A., Odette L. L. Model of electroretinogram b-wave generation: a test of the K+ hypothesis. J Neurophysiol. 1984 Jan;51(1):164–182. doi: 10.1152/jn.1984.51.1.164. [DOI] [PubMed] [Google Scholar]
- Newman E. A. Voltage-dependent calcium and potassium channels in retinal glial cells. 1985 Oct 31-Nov 6Nature. 317(6040):809–811. doi: 10.1038/317809a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Reichenbach A. Efficient K+ buffering by mammalian retinal glial cells is due to cooperation of specialized ion channels. Pflugers Arch. 1988 Jun;411(6):654–660. doi: 10.1007/BF00580862. [DOI] [PubMed] [Google Scholar]
- Pearce B., Cambray-Deakin M., Morrow C., Grimble J., Murphy S. Activation of muscarinic and of alpha 1-adrenergic receptors on astrocytes results in the accumulation of inositol phosphates. J Neurochem. 1985 Nov;45(5):1534–1540. doi: 10.1111/j.1471-4159.1985.tb07224.x. [DOI] [PubMed] [Google Scholar]
- Ripps H. Night blindness revisited: from man to molecules. Proctor lecture. Invest Ophthalmol Vis Sci. 1982 Nov;23(5):588–609. [PubMed] [Google Scholar]
- Sarthy P. V. Release of [3H]gamma-aminobutyric acid from glial (Müller) cells of the rat retina: effects of K+, veratridine, and ethylenediamine. J Neurosci. 1983 Dec;3(12):2494–2503. doi: 10.1523/JNEUROSCI.03-12-02494.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtmeister L. Further studies of the chemical sensitivity of the oscillatory potentials of the electroretinogram (ERG) I. GABA- and glycine antagonists. Acta Ophthalmol (Copenh) 1980 Oct;58(5):712–725. doi: 10.1111/j.1755-3768.1980.tb06684.x. [DOI] [PubMed] [Google Scholar]
- Walz W., Schlue W. R. Ionic mechanism of a hyperpolarizing 5-hydroxytryptamine effect on leech neuropile glial cells. Brain Res. 1982 Oct 28;250(1):111–121. doi: 10.1016/0006-8993(82)90957-x. [DOI] [PubMed] [Google Scholar]



