Abstract
The insulin-like growth factor (IGF) system plays an important role in regulating ovarian follicular development and steroidogenesis. IGF binding proteins (IGFBP) mostly inhibit IGF actions, and IGFBP proteolysis is a major mechanism for regulating IGF bioavailability. Pregnancy-associated plasma protein-A (PAPPA) is a secreted metalloprotease responsible for cleavage of IGFBP4 in the ovary. The aim of this study was to investigate whether PAPPA plays a role in regulating ovarian functions and female fertility by comparing the reproductive phenotype of wild-type (WT) mice with mice heterozygous or homozygous for a targeted Pappa gene deletion (heterozygous and PAPP-A knockout [KO] mice, respectively). When mated with WT males, PAPP-A KO females demonstrated an overall reduction in average litter size. PAPP-A KO mice had a reduced number of ovulated oocytes, lower serum estradiol levels following equine chorionic gonadotropin administration, lower serum progesterone levels after human chorionic gonadotropin injection, and reduced expression of ovarian steroidogenic enzyme genes, compared to WT controls. In PAPP-A KO mice, inhibitory IGFBP2, IGFBP3, and IGFBP4 ovarian gene expression was reduced postgonadotropin stimulation, suggesting some compensation within the ovarian IGF system. Expression levels of follicle-stimulating hormone receptor, luteinizing hormone receptor, and genes required for cumulus expansion were not affected. Analysis of preovulatory follicular fluid showed complete loss of IGFBP4 proteolytic activity in PAPP-A KO mice, demonstrating no compensation for loss of PAPPA proteolytic activity by other IGFBP proteases in vivo in the mouse ovary. Taken together, these data demonstrate an important role of PAPPA in modulating ovarian function and female fertility by control of the bioavailability of ovarian IGF.
Keywords: follicle, growth factors, insulin-like growth factor binding protein proteolysis, mechanisms of hormone action, ovary
A functional pregnancy-associated plasma protein-A (Pappa) gene, and thus insulin-like growth factor (IGF) binding protein-4 (IGFBP4) proteolysis, is required for optimal female fertility and ovarian steroidogenesis in the mouse.
INTRODUCTION
Pituitary gonadotropins and the intraovarian insulin-like growth factor (IGF) system are key modulators of ovarian follicle growth, selection, atresia, cellular differentiation, steroidogenesis, oocyte maturation, cumulus expansion, and follicle dynamics [1]. The IGF family is comprised of IGF peptides (IGF-I, IGF-II), IGF receptors, IGF binding proteins (IGFBPs), and IGFBP proteases. IGF peptides are mitogenic, differentiating survival factors that act primarily through the Type I IGF membrane tyrosine kinase receptor [2]. The type II receptor regulates IGF-II turnover [3] and has little signaling capacity [4]. IGFBPs have higher affinity for the IGFs than the receptors, and they mostly inhibit IGF action by peptide sequestration [5–7]. IGFBP proteases cleave IGFBPs, decreasing their affinities for the IGFs and increasing IGF peptide bioavailability [5]. In human ovary at the time of dominant follicle selection, IGF-II is increased in granulosa cells, IGFBPs are decreased, and the IGFBP4 protease is increased in the follicular antrum [8–11]. The net result of this repertoire of IGF family expressions is to increase IGF bioavailability in order to act as a co-gonadotropin in stimulating granulosa steroidogenesis [1, 12]. In contrast, in atretic follicles, IGF2 levels are low, IGFBPs are increased, and the IGFBP4 protease is nearly undetectable [8, 9, 11], resulting in limited IGF bioavailability and compromised follicle survival [1, 12].
IGFBP4 is the main inhibitory IGFBP in the ovary, and the ovarian IGFBP4 protease has been identified as pregnancy-associated plasma protein-A (PAPPA) in humans [10, 13] and domestic animals [14, 15]. PAPPA expression in granulosa is increased by gonadotropins, and ovarian PAPPA is a marker of follicle selection and the corpus luteum [10, 13, 16, 17]. Recently, the development of Pappa knockout (KO) mice revealed a phenotype of viable, proportional dwarfs 60% the size of wild-type (WT) littermates [18]. However, the reproductive phenotype has not been characterized. The purpose of the current study was to examine the hypothesized reduced reproductive potential of the Pappa KO mouse (based on known functions of IGFs, IGFBPs, and IGFBP proteases) and to delineate a potential mechanism for any observed effects by analyzing ovarian expression of genes encoding steroidogenic enzymes after equine chorionic gonadotropin (eCG) priming and human chorionic gonadotropin (hCG) stimulation. Thus, herein, we report on the reproductive capability of the Pappa KO mouse and the steroidogeneic capacity of the Pappa KO mouse ovary. The data support a key role of the IGF system in folliculogenesis and steroidogenesis and underscore the importance of PAPPA in these processes and in female fertility.
MATERIALS AND METHODS
Targeted Disruption of the Pappa Gene in Mice
Generation of Pappa KO (Pappatm1Cac/Pappatm1Cac, hereafter PAPP-A KO) mice in a C57BL/6x129 background has been described [18]. The mice were housed and bred at Stanford University Research Animal Facility, and new breeding pairs were established in the Laboratory Animal Resources Center at the University of California, San Francisco, for further validation of some experiments. All animal protocols were approved by the Administrative Panel on Laboratory Animal Care at Stanford University and by the IACUC Panel at the University of California, San Francisco. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Genotyping
Mouse genomic DNA was isolated from tail tips using the DNeasy Tissue Kit (Qiagen, Valencia, CA). Genotyping was performed using a multiplex PCR reaction containing 1× HotStarTaq Master Mix (Qiagen), 1 μl DNA template, 0.4 μM common forward primer (5′-TAAGCAGGGGTGGGTCCTTT-3′) annealing to the Pappa gene in immediate proximity to the Neo-gene insertion site, 0.3 μM Pappa reverse primer (5′-CACTCCTCAGCTTCGGCTTTCA-3′), and 0.4 μM Neo reverse primer (5′-TCGCCTTCTATCGCCTTCTTG-3′) in a final volume of 25 μl. PCR was carried out with an initial step of 94°C at 15 min followed by 38 cycles of 94°C for 30 sec, 60°C for 30 sec, and 72°C for 1 min. The PCR fragments were separated on a 2% agarose gel, and genotypes were determined according to band sizes, with a WT allele giving rise to a 306-bp band, and a Neo (i.e., a PAPP-A KO) allele giving rise to a 481-bp band.
Superovulation
Immature (20- to 22-day-old) female mice were primed with eCG (National Hormone and Peptide Program, NIDDK, Torrance, CA) for 47 h by i.p. injection (5 IU per mouse). Some mice also received i.p. injection with hCG (5 IU per mouse; National Hormone and Peptide Program, NIDDK, Torrance, CA). At various time points after gonadotropin treatments, mice were euthanized using cervical dislocation and weighed, and the ovaries were removed, weighed, flash frozen in liquid nitrogen, and stored at −80°C. The ovaries were analyzed for gene expression and follicular fluid IGFBP-4 protease activity. To determine ovulatory capacity, cumulus oocyte complexes (COCs) were isolated from the oviducts 16 h after hCG injection in 22- and 42-day-old female mice and counted. Ovulation is expected to occur 12 h after hCG injection in this model.
Histology
Ovaries from 22-day-old unprimed mice, 22-day-old eCG-primed mice, and 22-day-old eCG-primed mice 8 h after hCG injection were fixed in Bouin solution overnight, paraffin embedded, sectioned at 5 μm, and stained with hemotoxylin and eosin (H&E). Photomicrographs were taken under a standard light microscope with a 4× objective lens. Histological analysis of folliculogenesis was carried out on ovaries from 22-day-old unprimed mice as described previously [19]. The presence of follicles at preantral, early antral, and mid- to large antral stages was surveyed in every third section, and only follicles with visible germinal vesicle were counted. Ovaries of three WT, four heterozygous (HET), and four PAPP-A KO mice were used for this analysis, and a 49, 54, and 75 sections of each WT, HET, and PAPP-A KO ovary, respectively, were surveyed. Two mice of each genotype were used for the assessment of ovarian responses to eCG and hCG stimulation. The dominances of large antral follicles in the ovarian cortex and follicles with expanded cumulus cells were considered to be normal responses to eCG and hCG,respectively.
Determination of Serum Steroid Hormone Levels
Using cardiac puncture, whole blood was collected from female mice at indicated times after hormone priming. The blood samples were allowed to clot for 1.5 h at room temperature and spun for 15 min at 1600 × g, after which the serum was aspirated and stored at −20°C. Levels of estradiol (E2) and progesterone (P4) in mouse serum were measured using the 3rd generation Estradiol DA RIA (DSL-39100) and Progesterone EIA (DSL-3400), respectively, obtained from Diagnostic Systems Laboratories, Inc. (Webster, TX). All serum samples were diluted 1:8 in PBS (for E2) or 1:5 in Std A from the manufacturer (for P4) and assayed in duplicate. Identical dilutions were performed for all samples analyzed to ensure the best possible comparability.
RNA Isolation
Frozen ovaries were mechanically homogenized in 600 μl RLT lysis buffer (RNeasy mini kit; Qiagen) with 1% β-mercaptoethanol using a hand-held Polytron homogenizer (Kinematica, Bohemia, NY). The resulting homogenate was further homogenized by spinning through a QIAshredder spin column (Qiagen). An equal volume of 70% EtOH was added to the lysate, and total RNA was isolated using the RNeasy mini kit. The RNA was eluted in a volume of 50 ul water and stored at −80°C. RNA purity and concentration were assessed by optical density (OD)260 and OD280 measurements.
Reverse Transcription and Real-Time Quantitative PCR
Total RNA (2 μg) was reverse transcribed using the Omniscript RT Kit (Qiagen), 10 U of Ribonuclease Inhibitor (Invitrogen), a final concentration of 5 μM Oligo(dT)12–18 (Invitrogen, Carlsbad, CA), and Random Primers (Invitrogen) in a 40-μl reaction. The reaction was performed at 37°C for 1 h then 65°C for 5 min, and subsequently diluted 1:10 with RNase-free water. The levels of mRNA encoding (a) the transporter and enzymes in the steroidogenic pathway (Star, Cyp11a1, Cyp19a1, Cyp17a1, Hsd17b1, Hsd17b3, Hsd3b1, and Hsd3b6), (b) the IGF system (Pappa, Igf1, Igf2, Igf1r, Igfbp1, Igfbp2, Igfbp3, Igfbp4, Igfbp5, and Igfbp6), (c) genes required for the cumulus expansion process (Has2, Ptgs2, and Tnfaip6), and (d) the luteinizing hormone (LH)/choriogonadotropin (CG) receptor (Lhcgr) and the follicle stimulating hormone (FSH) receptor (Fshr) were then assessed (primer sequences are shown in Table 1) by real-time quantitative PCR (QPCR) using the above cDNA as template. QPCR was performed on an Mx4000 Multiplex Quantitative PCR System (Stratagene, La Jolla, CA) by determining the threshold cycle values (CT). Each QPCR reaction contained 1× SYBR Green PCR Master Mix (Qiagen), 0.2 μM forward primer, 0.2 μM reverse primer, and 5 μl cDNA (diluted 10×) in a total volume of 25 μl. The cycle conditions were an initial step of 94°C for 15 min, 40 cycles of 94°C for 15 sec, 60°C for 30 sec, and 72°C for 30 sec. Each sample was amplified in duplicate, and water blanks were included as controls. Data were collected and analyzed using Mx4000 v4.00 software as previously described [20]. For each gene, the amplification efficiency (Egoi) of the primer set was determined by performing QPCR using a dilution series of the cDNA as template.
TABLE 1.
Primers for QPCR.
The Rpl19 gene was used as a normalizer gene to correct for the total amount of RNA in each sample [21–23]. In addition, β-actin (Actb) and β2-microglobulin (B2m) were analyzed in a subset of samples to verify minimal variation between these normalizer genes and Rpl19 (data not shown). The ratio between the expression level of each gene of interest (goi) and Rpl19 (Rgoi/norm) at the indicated time points for each genotype was determined by the equation:
The expression levels were determined relative to the average expression level for 22-day-old WT mice at the indicated time points for each gene, except for the cumulus expansion genes (Has2, Ptgs2, and Tnfaip6), where the WT level at the time of hCG injection was used as the reference. To avoid potential amplification of contaminating genomic DNA in the QPCR, all primer pairs were designed to anneal to different exons interspersed with at least one intron at the genomic level. In addition, the PCR products were analyzed on a 2% agarose gel to ensure that only one band of the expected size was present following the reaction.
Measurement of IGFBP4 Proteolytic Activity in Ovarian Follicular Fluid
Follicular fluid (FF) was collected 47 h after eCG injection. For each mouse, the ovaries were placed directly in 4-cm Petri dishes containing 50 μl 1× PBS. All follicles were punctured with a pair of 27-gauge needles, and the released FF was spun for 15 min at 350 × g to remove remaining cellular debris. The diluted FF was stored at −20°C. IGFBP4 proteolytic activity in FF was essentially performed as described [24]. In brief, 5 μl FF was incubated at room temperature for 24 h with purified [125I]-IGFBP4 in a final concentration of 20 mM Tris-HCl (pH 7.5) and 1 mM CaCl2 in a 10-μl reaction. Incubations were carried out in the presence or absence of added IGF-II (5 nM final concentration; Bachem, Torrence, CA). Samples were separated by nonreducing SDS-PAGE (12%), and the degree of cleavage was assessed by autoradiography using a STORM PhosphorImager (Molecular Dynamics, Sunnyvale, CA). Purified recombinant human IGFBP4 was generously provided by Prof. Claus Oxvig (University of Aarhus, Aarhus, Denmark) and labeled with 125I as described [25]. Generation of recombinant human PAPPA has previously been described [26].
Statistical Analysis
All data are presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism version 4 for windows (GraphPad Software, Inc., La Jolla, CA). Litter sizes and ovary:body weight ratios were compared with one-way ANOVA, followed by Dunnett multiple-comparison post hoc test. For the number of ovulated COCs, the data from WT and HET mice were pooled and compared to PAPP-A KOs for 22- and 42-day-old mice using the Student unpaired t-test after log transformation. All gene expression comparisons between genotypes were made with one-way ANOVA using log-transformed fold-change data at individual time points, followed by Dunnett multiple-comparison post hoc test. Comparisons of gene expression levels in gonadotropin-stimulated versus unstimulated mice were done with one-way ANOVA followed by Dunnett multiple-comparisons post hoc test.
RESULTS
PAPP-A KO Female Mice Have Reduced Litter Size and Reduced Ovulatory Capacity
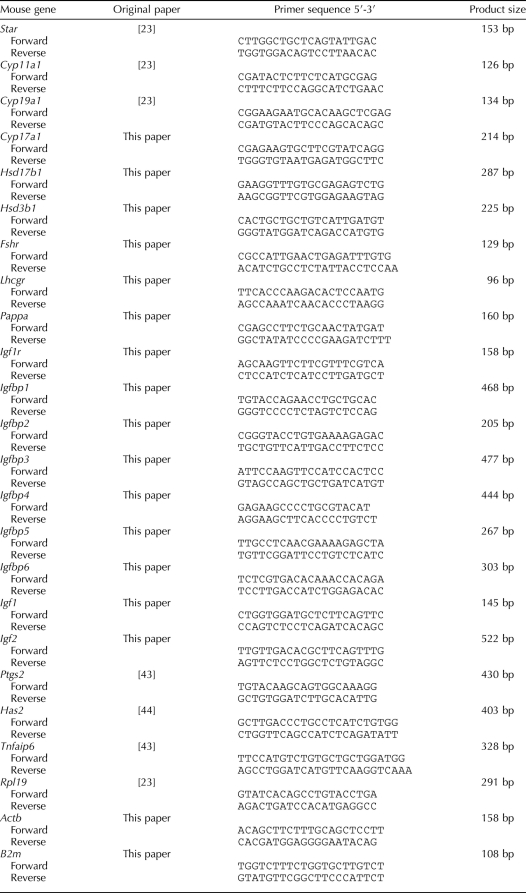
When mated with WT males, the average size of litters produced by PAPP-A KO females (5.1 ± 0.7) was markedly and significantly (P < 0.01) lower than that produced by WT (8.7 ± 0.9) or HET (8.1 ± 0.4) female mice (Fig. 1A). Furthermore, when female mice underwent superovulation cycles at 22 and 42 days of age, PAPP-A KO mice ovulated a significantly (P < 0.05) reduced number of COCs, compared to WT or HET mice (50.0 ± 4.0 vs. 71.1 ± 7.3 and 10.7 ± 3.3 vs. 25.5 ± 3.0, respectively; Fig. 1B). As previously published [18], the PAPP-A KO mice were smaller than WT or HET littermates (8.8 ± 0.3g vs. 14.4 ± 0.3g and 13.1 ± 0.3g, respectively, at 24 days of age), and they demonstrated proportionally smaller ovary size in unstimulated and eCG-primed mice. Upon hCG injection, an apparent lack of ovarian weight gain in the PAPP-A KO mice demonstrated a diminished response to hCG (Fig. 1C), resulting in a significantly lower ovary:body weight ratio in the PAPP-A KO compared to HET or WT mice at 8 h after hCG (Fig. 1D). The ovary:body weight ratio is a measure of the specific ovary response to the hormonal stimulation, which is directly comparable between genotypes of different sizes. Collectively, these data suggest that mice lacking a functional Pappa gene have reduced fertility potential compared to HET or WT mice. Although smaller ovaries were observed in the PAPP-A KO mice, morphologically, all follicle stages were present, and no gross difference, except the proportionally smaller size, was found between PAPP-A KO and WT or HET ovaries in 22-day-old mice before and after eCG priming (not shown). Cumulus of mutant mice underwent normal expansion, as determined by the formation of a normal matrix surrounding the oocyte 8 h after hCG administration (Fig. 1E).
FIG. 1.
Characterization of PAPP-A KO reproductive potential. A) Average litter size (+ SEM) from WT (black bar), HET (gray bar) and PAPP-A KO (white bar) female mice mated with WT males, determined from 10–20 litters per genotype. The average litter size in PAPP-A KO females is significantly reduced vs. WT female mice. **P < 0.01 vs. WT. B) Number of ovulated COCs (+ SEM) after induction of superovulation in 3- and 6-wk-old mice. PAPP-A KO (white bars) ovulate a significantly lower number of COCs compared to WT or HET mice (gray bars). Each bar represents between three and seven mice. *P < 0.05 vs. WT. C) Ovary weight (mg) (+ SEM) in 22-day-old mice determined 47 h after eCG priming and 4 h and 8 h after hCG injection. *P < 0.05 and **P < 0.01 compared to same genotype at 47 h eCG (0 h hCG). D) Ovary (in mg) to body weight (in g) ratio (+ SEM) in 22-day-old mice determined 47 h after eCG priming and 4 and 8 h after hCG injection. *P < 0.05 vs. WT or HET mice. E) Representative micrograph of ovarian histology in WT (left panel), HET (middle panel), and PAPP-A KO (right panel) mice. Ovaries from 22-day-old eCG-primed mice 8 h after hCG injection were fixed overnight in Bouin solution, paraffin embedded, sectioned at 5 μm, and stained with H&E. One intact ovary cross section is seen in each micrograph.
Serum Steroid Hormone Levels
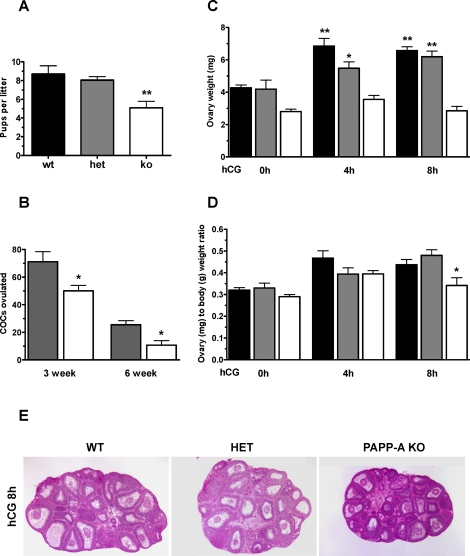
As an initial approach to determine the possible causes of the reduced fertility of PAPP-A KO mice, steroid hormone concentrations were measured in serum from 22-day-old female mice 47 h after eCG priming (0 h hCG), or 4, 8, and 24 h after hCG injection (given 47 h after the initial eCG priming). As evident from Figure 2A, serum E2 levels of all genotypes were decreased at 4 h after hCG injection and were decreased further over time, reaching an almost undetectable level at 24 h for WT and HET mice. Levels of P4 (Fig. 2B) were dramatically increased at 4 h after hCG administration, then leveled off at 8 and 24 h, yet at a significantly higher level than at the time of hCG injection (P < 0.01 for all time points). The patterns of serum steroid concentrations were compatible with the shift from E2 to P4 production of the luteinizing ovarian granulosa cells following the LH peak in an unstimulated cycle and were similar to our previously published studies [23]. The E2 levels in PAPP-A KO mice were significantly lower (P < 0.05) than that in WT mice after eCG stimulation (0 h hCG; WT = 132.5 ± 14.9, PAPP-A KO = 83.5 ± 23.0 pg/ml; Fig. 2A). In contrast, serum E2 levels in PAPP-A KO mice were significantly higher than in WT mice at 24 h after hCG injection (WT = 7.1 ± 0.3, PAPP-A KO = 10.0 ± 1.2 pg/ml; P < 0.05) and showed a tendency to be higher at 8 h without reaching statistical significance (WT = 16.6 ± 4.1, PAPP-A KO = 21.9 ± 13.4 pg/ml). It proved essential to use an assay of high sensitivity (see Materials and Methods) to determine the serum E2 levels. Significant reductions in the P4 levels in PAPP-A KO vs. WT mice were found (Fig. 2B) at 8 h (P < 0.01) and 24 h (P < 0.05) after hCG injection (8 h: WT = 44.4 ± 3.9, PAPP-A KO = 20.5 ± 3.1 ng/ml; 24 h: WT = 34.7 ± 3.1, PAPP-A KO = 19.2 ± 5.4 ng/ml). At the time of hCG injection, the mean P4 level in the PAPP-A KO mice was also lower than in the WT mice without reaching statistical significance (WT = 11.3 ± 1.9, PAPP-A KO = 7.6 ± 2.2 ng/ml).
FIG. 2.
Serum steroid levels. Serum steroid E2 (A) and P4 (B) levels in WT (black bars), HET (gray bars), and PAPP-A KO (white bars) mice determined at indicated times after hCG injection 47 h following eCG priming. Each data point represents the average of between four and fourteen mice (+ SEM). *P < 0.05 and **P < 0.01 vs. WT.
Ovarian mRNA Levels of Genes Encoding Proteins Involved in the Steroidogenic Pathway
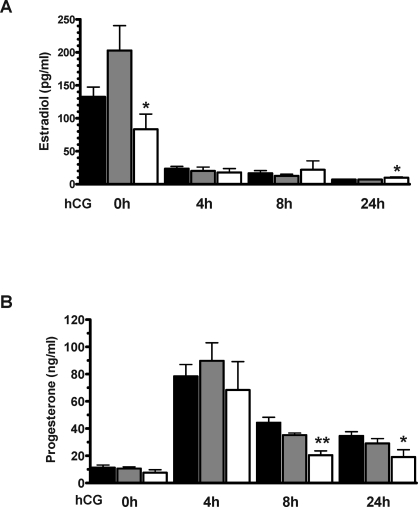
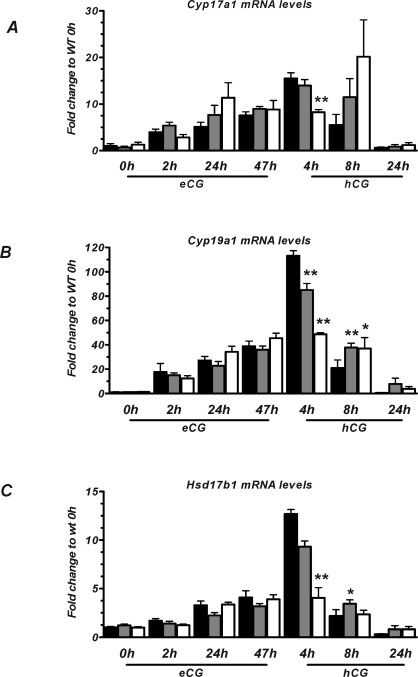
To delineate the potential cause of the altered levels of steroid hormones, we investigated ovarian levels of transcripts encoding enzymes in the steroidogenic pathway using real-time QPCR. Figure 3 shows the expression level of genes encoding enzymes in the pathway of P4 synthesis: steroidogenic acute regulatory protein (Star; Fig. 3A), cytochrome P450 side chain cleavage (Cyp11a1; Fig. 3B), and 3β-hydroxysteroid dehydrogenase type 1(Hsd3b1; Fig. 3C). Figure 4 shows the expression level of genes encoding enzymes converting P4 to E2: P45017α-hydroxylase/C17–20 lyase (Cyp17a1; Fig. 4A), 17β-hydroxysteroid dehydrogenase type 1 (Hsd17b1; Fig. 4B), and cytochrome P450 aromatase (Cyp19a1; Fig. 4C). When comparing the observed expression levels in WT mice with the publicly available microarray data in the Rat Ovarian Gene Database (rOGED), which contains rat ovarian gene expression data from the time points of 0, 12, and 48 h after eCG priming and 6 and 12 h following hCG stimulation [27], highly similar expression profiles were observed for all genes in the steroidogenic pathway.
FIG. 3.
Ovarian expression levels of genes involved in P4 synthesis, Star (A), Cyp11a1 (B), and Hsd3b1 (C), following gonadotropin stimulation. The indicated time points are after eCG priming or after hCG injection (given 47 h after eCG). Data are from WT (black bars), HET (gray bars), and PAPP-A KO (white bars) mice. Expression levels are shown as fold change compared to WT level at 0 h (+ SEM). Each data point represents the average of between four and fourteen samples. *P < 0.05 and **P < 0.01 vs. WT.
FIG. 4.
Ovarian expression levels of E2 synthesizing genes, Cyp17a1 (A), Cyp19a1 (B), and Hsd17b1 (C), following gonadotropin stimulation. The indicated time points are after eCG priming or after hCG injection (given 47 h after eCG). Data are from WT (black bars), HET (gray bars), and PAPP-A KO (white bars) mice. Expression levels are shown as fold change compared to WT level at 0 h (+ SEM). Each data point represents the average of between four and fourteen samples. *P < 0.05 and **P < 0.01 vs. WT.
Overall, in comparing the PAPP-A KO and WT expression levels, these data suggest a delayed shutdown of E2-synthesizing genes after hCG administration. Furthermore, since the PAPP-A KO mice appear to have a blunted response to hCG stimulation, we determined the time-dependent expression levels of the FSH and LH/CG receptors following eCG and hCG administration. No significant differences in the levels of Fshr or Lhcgr mRNA were observed between the PAPP-A KO and the WT mice at any of the time points investigated (data not shown).
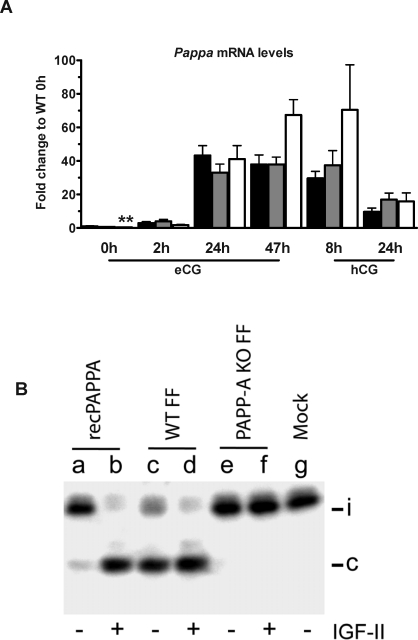
Ovarian Pappa mRNA Levels
The mRNA level for Pappa is significantly increased in WT ovary after eCG stimulation in a time-dependent manner (P < 0.05 at 2 h, P < 0.01 for all other time points; Fig. 5A), confirming previous findings [16]. No further stimulation was observed after hCG administration, and, in fact, levels of WT Pappa mRNA decreased after hCG (Fig. 5A). No significant differences were observed between WT and PAPP-A KO or HET mice, except for a lower Pappa mRNA level in the untreated PAPP-A KO mouse. The targeted deletion of the Pappa gene in the PAPP-A KO mouse did not remove the promotor or most of the mRNA, but transcription resulted in a truncated, nonfunctional mRNA that can be detected by the primers used in the QPCR assay for Pappa.
FIG. 5.
Ovarian Pappa mRNA levels and IGFBP4 protease activity. A) Pappa mRNA levels following gonadotropin stimulation in WT (black bars), HET (gray bars), and PAPP-A KO (white bars) mice. The indicated time points are after eCG priming or after hCG injection (given 47 h after eCG). Each data point represents the average of between four and fourteen samples. *P < 0.05 and **P < 0.01 vs. unstimulated mice. B) IGFBP4 proteolytic activity in follicular fluid from WT and PAPP-A KO mice. SDS-PAGE separation of 125I-labeled IGFBP4 incubated with medium from HEK293 cells transfected with an expression plasmid encoding recombinant human PAPPA (lanes a, b) or an empty plasmid (lane g), or follicular fluid from eCG-stimulated ovaries from WT (lanes c, d) or PAPP-A KO (lanes e, f) mice, in the presence or absence of IGF-II (indicated by - or +, respectively). The top bands represent intact (i) IGFBP4, bottom bands the cleaved (c) form of IGFBP4.
PAPPA Is the Only IGFBP4 Protease in Murine Ovaries
To determine whether IGFBP4 proteolytic activity was indeed absent in the ovarian follicular compartment in PAPP-A KO mice, ovaries were harvested from WT and PAPP-A KO mice stimulated 47 h prior with eCG. Follicular fluid was obtained from large antral follicles and analyzed for IGFBP4 proteolytic activity, with or without added IGF-II (Fig. 5B). As seen in lanes a and b, the recombinant PAPPA control shows an IGF-dependent IGFBP4 proteolytic activity, consistent with the known IGF2 (IGF-II)-dependence of this enzyme [28]. No cleaved IGFBP4 fragment was detected in the mock group treated only with control medium (negative control, lane g). Lanes c and d show that the WT ovarian follicular fluid contained high levels of IGFBP4 proteolytic activity that is enhanced by IGF addition. In contrast, lanes e and f reveal a total absence of IGFBP4 proteolytic activity in follicular fluid from PAKO mice, with or without addition of IGF2. Interestingly, WT follicular fluid likely (and expectedly) contained some bio-available IGF, since the observed proteolytic activity was not totally dependent on addition of exogenous IGF. These results demonstrate unequivocally that no other protease can substitute for PAPPA IGFBP4 proteolytic activity in the murine ovarian compartment. Furthermore, the total absence of IGFBP4 proteolysis in PAPP-A KO FF strongly suggests that the observed reduction of reproductive capacity of the PAPP-A KO female mouse is a consequence of reduced IGF availability in the follicular compartment.
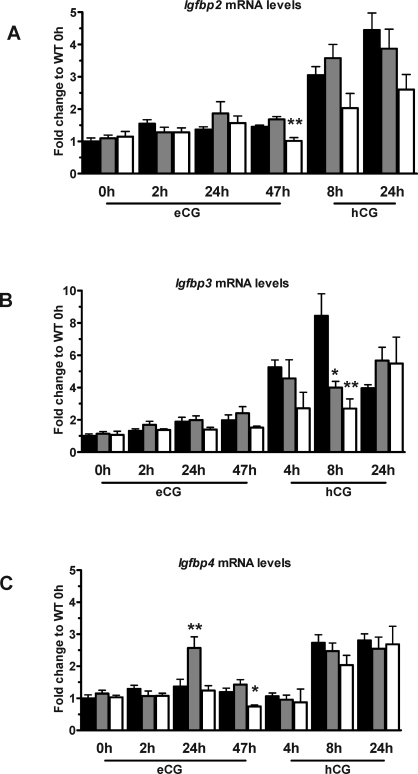
Ovarian mRNA Levels of Genes in the IGF-System
No increase in the levels of Igf1 or Igfr1 mRNA was detected in WT mouse ovaries in response to gonadotropin stimulation, which is consistent with the expression data in rOGED [27], and no significant differences in the Igf1 or Igfr1 mRNA levels between WT and HET or PAPP-A KO mouse ovaries were found for any of the time points investigated (data not shown). Lower expression levels of Igfbp2, Igfbp3, and Igfbp4 at certain time points after eCG and hCG stimulation were observed in PAPP-A KO vs. WT (Fig. 6, A–C), and no significant differences in the expression levels for Igfbp5 and Igfbp6 were observed at any of the studied time points during the stimulated cycle (data not shown). Igfbp1 and Igf2 expression levels were too low to be detected (all genotypes, data not shown).
FIG. 6.
Ovarian expression levels of IGF system genes, Igfbp2 (A), Igfbp3 (B), and Igfbp4 (C). The indicated time points are after eCG priming or after hCG injection (given 47 h after eCG). Data are from WT (black bars), HET (gray bars), and PAPP-A KO (white bars) mice. Expression levels are shown as fold change compared to WT level at 0 h (+ SEM). Each data point represents the average of between four and fourteen samples. *P < 0.05 and **P < 0.01 vs. WT.
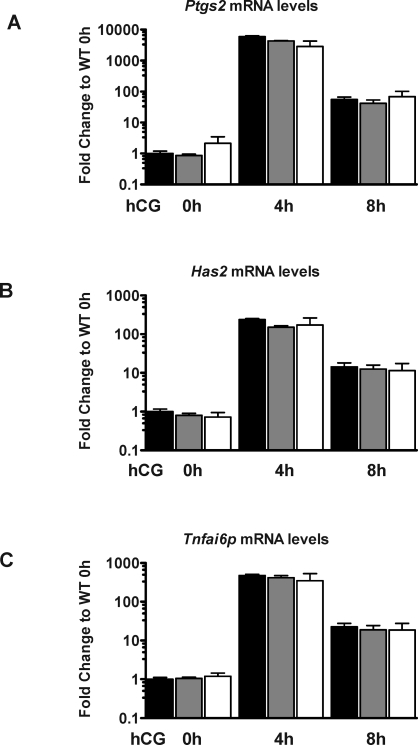
Ovarian mRNA Levels of Genes Required for Cumulus Expansion
Because there is a significant reduction in the number of ovulated COCs in PAPP-A KO mice, we investigated the expression levels, following hCG injection, of genes previously shown to be required for the cumulus expansion process (Has2, Ptgs2, and Tnfaip6 [29]). As seen in Figure 7, all three genes show a dramatic and transient increase in transcript levels at 4 h, with a rapid decline by 8 h in PAPP-A KO, HET, and WT ovaries without significant differences among the genotypes. These data and the observation of formation of normal extracellular matrix surrounding COCs indicate that timing of the cumulus expansion process is normal in PAPP-A KO ovaries and is not the cause for reduced ovulation rate and subfertility in these mice.
FIG. 7.
Ovarian expression of genes required for normal cumulus expansion following hCG injection, Ptgs2 (A), Has2 (B), and Tnfai6p (C). Data are from WT (black bars), HET (gray bars), and PAPP-A KO (white bars) mice. Expression levels are shown as fold change compared to WT level at 0 h hCG (47 h eCG) (+ SEM).
DISCUSSION
The intraovarian IGF system plays an important role in augmenting gonadotropin actions on granulosa and theca and also during dominant follicle selection and oocyte maturation [1]. Furthermore, the IGFBP-4 protease in the ovary, PAPP-A, is a marker of follicle selection and the corpus luteum [10, 13]. Data in the current study demonstrate that PAPP-A is essential for normal fertility, based on observations in the PAPP-A KO mouse of lower litter size, lower numbers of oocytes ovulated upon gonadotropin stimulation, and lower E2 and P4 levels during superovulation cycles. Of interest, and unexpectedly, the phenotype is not one of sterility but rather reduced fecundity (lower litter size), likely due to decreased numbers of ovulated oocytes in natural cycles, with genes governing cumulus expansion appropriately expressed in the PAPP-A KO mouse as in WT and HET. With decreased intrafollicular IGFBP-4 proteolysis, IGF peptide bioavailability is presumed to be decreased within the PAPP-A KO ovary, and this phenotype is consistent with this process. However, an implantation defect cannot be ruled out, especially in view of significantly lower levels of E2 after eCG treatment and of P4 after hCG treatment in PAPP-A KO vs. WT mice, although gestational age of term pregnancy was not reduced. It is noteworthy, however, that in contrast to the very high primate placental PAPPA expression level, the murine placenta demonstrates a remarkably low level of PAPPA expression [30, 31]. The PAPP-A KO mouse has a remarkably different phenotype compared to the Igf1- and Igf1r-null mice, which have a more extreme phenotype with the absence of IGFI and IGF type I receptor signaling. Igf1-null females are infertile due to several reasons [32], including (a) diminished sex drive in both sexes in adulthood, (b) diminished lordosis in adult females, (c) E2 synthesis is decreased about 50%, (d) ovarian weight is significantly diminished, (e) formation of small Graafian follicles is rare, (f) some antral follicle granulosa exhibit features of early atresia, (g) the thecal layers are not well delineated, (h) there is no evidence of ovulation, and (i) animals display hypoplastic myometrium and dramatically reduced uterine size. These are in marked contrast to the PAPP-A KO mouse whose follicles have normal histology and where uterine size is not appreciably diminished (data not shown). Furthermore, FSH receptor expression is decreased in Igf1-null mice, and Igf1- and Fshr-null animals similarly have arrested follicular development [33]. Fshr-null animals also exhibit female infertility due to a block in folliculogenesis that occurs prior to antral follicle formation [34], lack of estrous cyclicity, decreased ovarian and uterine mass, and no evidence of corpora lutea. In addition, serum levels of FSH and LH are significantly elevated, and inhibin A and B levels are undetectable [35]. Of interest, in the PAPP-A KO mouse ovary, Fshr mRNA level was not different compared to WT ovary. This is not unexpected, since the impact of the Pappa-null mutation on ovarian function is observed herein at a much later stage than in the Igf1- or Fshr-null mutations. As discussed below, PAPPA appears to be a moderator or an accelerator of IGF bioavailability, and the phenotype is not expected to be as dramatic as a total removal of IGFI or the FSHR, and indeed is not, as described herein.
With regard to the PAPP-A KO mouse, lower circulating levels of E2 were observed after eCG stimulation. However, E2 levels were significantly higher after 24 h of hCG administration and showed a tendency to be elevated at 8 h, although this did not reach significance. This could be the result of a delayed response to hCG of E2 synthesizing genes (Cyp19a1 and Cyp17a1), such that the switch from E2 production to P4 of granulosa cells may be slightly delayed due to decreased bioavailability of IGF in the PAPP-A KO ovary that may be critical for “correct” activation of the appropriate pathways essential for the switch of steroidogenic enzyme gene expression [23]. We have previously shown that activation of the MAPK-pathway (by LH) triggers a shift in granulosa steroidogenic output from E2 to P4 (Cyp19a1 down-regulated, Star and Cyp11a1 up-regulated [23]), and this has also recently been elegantly demonstrated in vivo by Fan et al. [36]. EGF-like growth factors are important ovarian components upstream of the MAPK pathway [37], and whether the EGF network is affected in the PAPP-A KO will require further experiments. We do not find this likely, however, since the expression level of genes involved in cumulus expansion (Has2, Ptgs2, and Tnfai6p) are not affected. Instead, we speculate that co-stimulation of the MAPK pathway by IGF may be needed for an optimal steroidogenic shift, based on the apparent delay in luteinization in the PAPP-A KO mouse, which was indicated by delayed shutdown of aromatase (evident at 8 h hCG) and higher E2 levels at 24 h hCG. Experimental evidence to support this conjecture is not yet available and is worthy of further evaluation.
The PAPP-A KO mouse demonstrates a disruption of the steroidogenic pathway that becomes manifest after hCG stimulation. Diminished expression of enzymes in the rate-limiting step of steroid hormone biosynthesis and enzymes important in P4 synthesis and subsequent throughput for E2 biosynthesis, including Star and Cyp11a1, likely results in lower E2 and P4 circulating levels, although we cannot exclude the possibility of a decrease in antral follicle numbers or changes in posttranscriptional regulation. Furthermore, interestingly, during eCG treatment, expression of enzymes important for E2 biosynthesis (Cyp17a1, Cyp19a1, and Hsd17b1) were equivalent among PAPP-A KO, HET, and WT mice; however, all three enzymes were decreased in the PAPP-A KO ovary within 4 h after hCG administration, suggesting compromised LH responsiveness. Our previous study in human ovary [10] and studies in human and rodent ovary by Adashi and colleagues [16, 17] revealed high expression of PAPPA in the corpus luteum. The current study suggests that PAPPA is an important regulator of triggering luteinization and steroidogenesis in the corpus luteum in the mouse, and that a reduction in LH/CG receptor is not a mechanism for the observed diminution of steroid hormone biosynthesis. Alterations in receptor responsiveness and postreceptor signaling, however, cannot be ruled out.
The fact that we observe fewer ovulated oocytes in the superstimulated PAPP-A KO animals does not necessarily explain the changes seen in serum steroid levels or the altered expression levels of steroidogenic enzymes, since these measurements are inherently normalized to the smaller size of the PAPP-A KO animals (steroid levels per volume serum and expression levels to the total ovary mRNA population). Also, an effect of fewer mature follicles could not explain the simultaneous higher level of E2-synthesizing genes and lower level of P4-synthesizing genes in PAPP-A KO compared to WT mice, observed at 8 h hCG. Hence, we conclude that deletion of functional PAPPA has a direct impact on steroidogenesis. However, the mechanism underlying why fewer ovulated oocytes in stimulated PAPP-A KO animals occurs is currently not well understood and is worthy of further investigation.
The current study also unequivocally demonstrates that PAPPA is the IGFBP4 protease in mouse ovarian follicular fluid, without compensation from other proteases. Furthermore, we demonstrate that PAPPA expression in ovary is dramatically regulated (increased) by eCG. With regard to IGF, IGFBP, and IGFR expression, we found our results to be consistent with the rOGED array data [27].
Furthermore, interestingly, the rOGED database [27] reveals a very tight correlation between total ovary mRNA levels and that of the isolated compartment (theca or granulosa) with the predominant expression for each particular gene of interest, following gonadotropin stimulation. Also, Pappa mRNA is primarily expressed in the granulosa compartment in the murine ovary [16], and Matsui et al. [38] demonstrated that the increase in rat Pappa mRNA levels following eCG stimulation was the same for whole ovary and isolated granulosa cells, suggesting that the whole ovary may reflect compartmental expression of PAPPA, as in the studies herein. While PAPPA is exclusively a granulosa product in rodent and human ovary, interestingly, in isolated small and large bovine granulosa and theca cell cultures, Aad et al. [39] found that insulin with or without LH decreased (P < 0.05) Pappa mRNA. Furthermore, they found that E2 alone decreased Pappa mRNA levels and amplified insulin-induced inhibition of Pappa mRNA abundance in large theca cells. In the bovine model, it has been concluded that regulation of Pappa mRNA expression differs in these cell types [39]. Further evaluation of Pappa regulation in ovarian cell cultures from murine and human ovaries would be of value in comparing species differences and control of this important regulator of IGF action in the ovary.
Taken together, the data presented herein suggest that the phenotype of lowered fertility in the PAPP-A KO mouse is due to lower (local) IGF bioavailability in the ovary. When extending the analysis to include ovarian expression of other IGFBPs, we observed a possible compensation in the PAPP-A KO mouse, in that the expression of IGFBP2, 3, and 4 was reduced vs. WT at certain time points during an induced superovulation cycle. This strongly suggests that IGF bioavailability is crucial for optimal gonadotropin-induced steroidogenesis, and further suggests that fertility is rescued by compensatory expression of IGF-system components (e.g., lower IGFBP2/3/4). Another potential mechanism of compensation could be an increase in other IGFBP proteolytic enzymes. The data presented herein demonstrate, however, that this is not the case for IGFBP4 proteolysis.
Finally, IGFBP protease activity is a key regulatory process for IGF action [5, 40] in a variety of tissues and fluids, and as PAPPA is investigated in more and more systems, it is increasingly apparent that it is a key modulator of IGF activity. In the PAPP-A KO mouse, in addition to the delay in the switch from E2 to P4 production of granulosa after hCG treatment observed herein, delayed development of the fetus, delayed wound healing, and delayed healing of bone fractures have been observed [18, 41, 42], underscoring a common delay or reduction in IGF-assisted physiological processes in this model. An important pursuit in human ovarian physiology is to determine the function of PAPPA in the corpus luteum and whether there is a role for compromised PAPPA activity in follicles resistant to gonadotropin stimulation and in the setting of compromised corpus luteum production of P4 in some women with infertility and luteal insufficiency. This is worthy of further investigation, as IGFs and regulation of their actions are key to ovarian follicle development and function in women.
Footnotes
Supported by National Institutes of Health HD 31579-10 (to L.C.G.), an unrestricted grant from Diagnostic Systems Laboratories, Inc. (to M.N. and M.T.O.), and a fellowship (to M.T.O.) from the Alfred Benzon Foundation.
These authors contributed equally to this work
REFERENCES
- Kwintkiewicz J, Giudice LC.The interplay of insulin-like growth factors, gonadotropins, and endocrine disruptors in ovarian follicular development and function. Semin Reprod Med 2009; 27: 43–51. [DOI] [PubMed] [Google Scholar]
- Dupont J, Dunn SE, Barrett JC, LeRoith D.Microarray analysis and identification of novel molecules involved in insulin-like growth factor-1 receptor signaling and gene expression. Recent Prog Horm Res 2003; 58: 325–342. [DOI] [PubMed] [Google Scholar]
- Samani AA, Yakar S, LeRoith D, Brodt P.The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev 2007; 28: 20–47. [DOI] [PubMed] [Google Scholar]
- Rose PP, Bogyo M, Moses AV, Fruh K.Insulin-like growth factor II receptor-mediated intracellular retention of cathepsin B is essential for transformation of endothelial cells by Kaposi's sarcoma-associated herpesvirus. J Virol 2007; 81: 8050–8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boldt HB, Conover CA.Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm IGF Res 2007; 17: 10–18. [DOI] [PubMed] [Google Scholar]
- Clemmons DR.Role of insulin-like growth factor binding proteins in controlling IGF actions. Mol Cell Endocrinol 1998; 140: 19–24. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR.Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev 1995; 16: 3–34. [DOI] [PubMed] [Google Scholar]
- Cataldo NA, Giudice LC.Insulin-like growth factor binding protein profiles in human ovarian follicular fluid correlate with follicular functional status. J Clin Endocrinol Metab 1992; 74: 821–829. [DOI] [PubMed] [Google Scholar]
- Chandrasekher YA, Van Dessel HJ, Fauser BC, Giudice LC.Estrogen- but not androgen-dominant human ovarian follicular fluid contains an insulin-like growth factor binding protein-4 protease. J Clin Endocrinol Metab 1995; 80: 2734–2739. [DOI] [PubMed] [Google Scholar]
- Conover CA, Faessen GF, Ilg KE, Chandrasekher YA, Christiansen M, Overgaard MT, Oxvig C, Giudice LC.Pregnancy-associated plasma protein-a is the insulin-like growth factor binding protein-4 protease secreted by human ovarian granulosa cells and is a marker of dominant follicle selection and the corpus luteum. Endocrinology 2001; 142: 2155 [DOI] [PubMed] [Google Scholar]
- Thierry van Dessel HJ, Chandrasekher Y, Yap OW, Lee PD, Hintz RL, Faessen GH, Braat DD, Fauser BC, Giudice LC.Serum and follicular fluid levels of insulin-like growth factor I (IGF-I), IGF-II, and IGF-binding protein-1 and -3 during the normal menstrual cycle. J Clin Endocrinol Metab 1996; 81: 1224–1231. [DOI] [PubMed] [Google Scholar]
- Giudice LC.Insulin-like growth factor family in Graafian follicle development and function. J Soc Gynecol Investig 2001; 8: S26–S29. [DOI] [PubMed] [Google Scholar]
- Conover CA, Oxvig C, Overgaard MT, Christiansen M, Giudice LC.Evidence that the insulin-like growth factor binding protein-4 protease in human ovarian follicular fluid is pregnancy associated plasma protein-A. J Clin Endocrinol Metab 1999; 84: 4742–4745. [DOI] [PubMed] [Google Scholar]
- Mazerbourg S, Overgaard MT, Oxvig C, Christiansen M, Conover CA, Laurendeau I, Vidaud M, Tosser-Klopp G, Zapf J, Monget P.Pregnancy-associated plasma protein-A (PAPP-A) in ovine, bovine, porcine, and equine ovarian follicles: involvement in IGF binding protein-4 proteolytic degradation and mRNA expression during follicular development. Endocrinology 2001; 142: 5243–5253. [DOI] [PubMed] [Google Scholar]
- Monget P, Mazerbourg S, Delpuech T, Maurel MC, Maniere S, Zapf J, Lalmanach G, Oxvig C, Overgaard MT.Pregnancy-associated plasma protein-A is involved in insulin-like growth factor binding protein-2 (IGFBP-2) proteolytic degradation in bovine and porcine preovulatory follicles: identification of cleavage site and characterization of IGFBP-2 degradation. Biol Reprod 2003; 68: 77–86. [DOI] [PubMed] [Google Scholar]
- Hourvitz A, Kuwahara A, Hennebold JD, Tavares AB, Negishi H, Lee TH, Erickson GF, Adashi EY.The regulated expression of the pregnancy-associated plasma protein-A in the rodent ovary: a proposed role in the development of dominant follicles and of corpora lutea. Endocrinology 2002; 143: 1833–1844. [DOI] [PubMed] [Google Scholar]
- Hourvitz A, Widger AE, Filho FL, Chang RJ, Adashi EY, Erickson GF.Pregnancy-associated plasma protein-A gene expression in human ovaries is restricted to healthy follicles and corpora lutea. J Clin Endocrinol Metab 2000; 85: 4916–4920. [DOI] [PubMed] [Google Scholar]
- Conover CA, Bale LK, Overgaard MT, Johnstone EW, Laursen UH, Fuchtbauer EM, Oxvig C, van Deursen J.Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development 2004; 131: 1187–1194. [DOI] [PubMed] [Google Scholar]
- Rankin TL, O'Brien M, Lee E, Wigglesworth K, Eppig J, Dean J.Defective zonae pellucidae in Zp2-null mice disrupt folliculogenesis, fertility and development. Development 2001; 128: 1119–1126. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC.Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 2006; 147: 1097–1121. [DOI] [PubMed] [Google Scholar]
- Jo Y, King SR, Khan SA, Stocco DM.Involvement of protein kinase C and cyclic adenosine 3′,5′-monophosphate-dependent kinase in steroidogenic acute regulatory protein expression and steroid biosynthesis in Leydig cells. Biol Reprod 2005; 73: 244–255. [DOI] [PubMed] [Google Scholar]
- Mizuyachi K, Son DS, Rozman KK, Terranova PF.Alteration in ovarian gene expression in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin: reduction of cyclooxygenase-2 in the blockage of ovulation. Reprod Toxicol 2002; 16: 299–307. [DOI] [PubMed] [Google Scholar]
- Su YQ, Nyegaard M, Overgaard MT, Qiao J, Giudice LC.Participation of mitogen-activated protein kinase in luteinizing hormone-induced differential regulation of steroidogenesis and steroidogenic gene expression in mural and cumulus granulosa cells of mouse preovulatory follicles. Biol Reprod 2006; 75: 859–867. [DOI] [PubMed] [Google Scholar]
- Laursen LS, Overgaard MT, Soe R, Boldt HB, Sottrup-Jensen L, Giudice LC, Conover CA, Oxvig C.Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett 2001; 504: 36–40. [DOI] [PubMed] [Google Scholar]
- Boldt HB, Overgaard MT, Laursen LS, Weyer K, Sottrup-Jensen L, Oxvig C.Mutational analysis of the proteolytic domain of pregnancy-associated plasma protein-A (PAPP-A): classification as a metzincin. Biochem J 2001; 358: 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overgaard MT, Haaning J, Boldt HB, Olsen IM, Laursen LS, Christiansen M, Gleich GJ, Sottrup-Jensen L, Conover CA, Oxvig C.Expression of recombinant human pregnancy-associated plasma protein-A and identification of the proform of eosinophil major basic protein as its physiological inhibitor. J Biol Chem 2000; 275: 31128–31133. [DOI] [PubMed] [Google Scholar]
- Jo M, Gieske MC, Payne CE, Wheeler-Price SE, Gieske JB, Ignatius IV, Curry TE, Jr, Ko C.Development and application of a rat ovarian gene expression database. Endocrinology 2004; 145: 5384–5396. [DOI] [PubMed] [Google Scholar]
- Laursen LS, Overgaard MT, Nielsen CG, Boldt HB, Hopmann KH, Conover CA, Sottrup-Jensen L, Giudice LC, Oxvig C.Substrate specificity of the metalloproteinase pregnancy-associated plasma protein-A (PAPP-A) assessed by mutagenesis and analysis of synthetic peptides: substrate residues distant from the scissile bond are critical for proteolysis. Biochem J 2002; 367: 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JS.Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol 2005; 234: 75–79. [DOI] [PubMed] [Google Scholar]
- Overgaard MT, Oxvig C, Christiansen M, Lawrence JB, Conover CA, Gleich GJ, Sottrup-Jensen L, Haaning J.Messenger ribonucleic acid levels of pregnancy-associated plasma protein-A and the proform of eosinophil major basic protein: expression in human reproductive and nonreproductive tissues. Biol Reprod 1999; 61: 1083–1089. [DOI] [PubMed] [Google Scholar]
- Soe R, Overgaard MT, Thomsen AR, Laursen LS, Olsen IM, Sottrup-Jensen L, Haaning J, Giudice LC, Conover CA, Oxvig C.Expression of recombinant murine pregnancy-associated plasma protein-A (PAPP-A) and a novel variant (PAPP-Ai) with differential proteolytic activity. Eur J Biochem 2002; 269: 2247–2256. [DOI] [PubMed] [Google Scholar]
- Baker J, Hardy MP, Zhou J, Bondy C, Lupu F, Bellve AR, Efstratiadis A.Effects of an Igf1 gene null mutation on mouse reproduction. Mol Endocrinol 1996; 10: 903–918. [DOI] [PubMed] [Google Scholar]
- Liu JL, Yakar S, LeRoith D.Conditional knockout of mouse insulin-like growth factor-1 gene using the Cre/loxP system. Proc Soc Exp Biol Med 2000; 223: 344–351. [DOI] [PubMed] [Google Scholar]
- Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P.Impairing follicle-stimulating hormone (FSH) signaling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci U S A 1998; 95: 13612–13617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM.The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology 2000; 141: 1795–1803. [DOI] [PubMed] [Google Scholar]
- Fan HY, Liu Z, Shimada M, Sterneck E, Johnson PF, Hedrick SM, Richards JS.MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science 2009; 324: 938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M.EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 2004; 303: 682–684. [DOI] [PubMed] [Google Scholar]
- Matsui M, Sonntag B, Hwang SS, Byerly T, Hourvitz A, Adashi EY, Shimasaki S, Erickson GF.Pregnancy-associated plasma protein—a production in rat granulosa cells: stimulation by follicle-stimulating hormone and inhibition by the oocyte-derived bone morphogenetic protein-15. Endocrinology 2004; 145: 3686–3695. [DOI] [PubMed] [Google Scholar]
- Aad PY, Voge JL, Santiago CA, Malayer JR, Spicer LJ.Real-time RT-PCR quantification of pregnancy-associated plasma protein-A mRNA abundance in bovine granulosa and theca cells: effects of hormones in vitro. Domest Anim Endocrinol 2006; 31: 357–372. [DOI] [PubMed] [Google Scholar]
- Bunn RC, Fowlkes JL.Insulin-like growth factor binding protein proteolysis. Trends Endocrinol Metab 2003; 14: 176–181. [DOI] [PubMed] [Google Scholar]
- Miller BS, Bronk JT, Nishiyama T, Yamagiwa H, Srivastava A, Bolander ME, Conover CA.Pregnancy associated plasma protein-A is necessary for expeditious fracture healing in mice. J Endocrinol 2007; 192: 505–513. [DOI] [PubMed] [Google Scholar]
- Resch ZT, Simari RD, Conover CA.Targeted disruption of the pregnancy-associated plasma protein-A gene is associated with diminished smooth muscle cell response to insulin-like growth factor-I and resistance to neointimal hyperplasia after vascular injury. Endocrinology 2006; 147: 5634–5640. [DOI] [PubMed] [Google Scholar]
- Ochsner SA, Russell DL, Day AJ, Breyer RM, Richards JS.Decreased expression of tumor necrosis factor-alpha-stimulated gene 6 in cumulus cells of the cyclooxygenase-2 and EP2 null mice. Endocrinology 2003; 144: 1008–1019. [DOI] [PubMed] [Google Scholar]
- Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM.Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol 1999; 13: 1035–1048. [DOI] [PubMed] [Google Scholar]