Abstract
Background
Transglutaminase 2 (TG2), a cross-linking enzyme that confers supra-molecular structures with extra rigidity and resistance against proteolytic degradation, is expressed in the shoulder regions of human atherosclerotic plaques. It has been proposed that TG2 prevents tearing and promotes plaque repair at these potential weak points, and also promotes ectopic calcification of arteries. TG2 is also expressed within plaques that develop within the brachiocephalic arteries of apolipoprotein E (apoE) deficient mice.
Objectives
To determine the role that TG2 plays in plaque development and calcification, mice were bred that were doubly deficient in apoE and TG2, and were maintained on a high-fat diet for 6 months.
Results
Lesion size and composition were not significantly altered in the apoE/TG2 double-knockout mice, with the exception of a 9.7% decrease in the proportion of the plaque occupied by lipid (p = 0.032). The frequency of buried fibrous caps within brachiocephalic plaques was significantly higher in male than in female mice, but TG2 deficiency had no effect on either gender. The extent of lesion calcification varied markedly between individual mice, but it was not decreased in the apoE/TG2 double-knockout mice.
Conclusion
These data indicate that, in the apoE knockout mouse model of atherosclerosis, TG2 does not influence plaque composition or calcification. The data further suggest that TG2 does not influence plaque stability or repair in these mice.
Keywords: Transglutaminase 2, Apolipoprotein E, Plaque stability, Calcification, Mouse
1. Introduction
Plaque rupture exposes the internal constituents of the atherosclerotic plaque to the blood-stream. This promotes platelet adhesion and blood coagulation, leading to thrombosis within the affected artery. In humans, approximately 90% of these events are repaired asymptomatically [1]. Repeated cycles of rupture and repair can occur, but a strong fibrous cap covering the lesion and decreased plaque lipid content are associated with increased plaque stability. Atherosclerosis is also associated with arterial calcification, the extent of which strongly predicts the incidence of cardiovascular events [2].
Tissue repair and stabilization involve the incorporation of structural proteins into the extracellular matrix and their subsequent remodelling by proteinases and cross-linking enzymes. Amongst the various cross-linking enzymes which protect proteins from proteolysis and mechanical disruption is transglutaminase 2 (TG2) (reviewed in [3,4]). Despite lacking a conventional signal sequence, TG2 is exported from cells in response to stress, enabling it to associate with and to cross-link extracellular matrix proteins [3,4]. TG2 on the surface of macrophages also facilitates apoptotic cell engulfment [5,6]. Intracellular TG2 activity is suppressed within healthy cells, but becomes activated following injury and cross-links cellular components to minimize pro-inflammatory leakage [4]. TG2 may therefore play multiple roles in the body's response to tissue damage, and it is induced in human atherosclerotic plaques where it may stabilize against rupture [7,8]. Recently it has been suggested that TG2 enables smooth muscle cells to transform into chondrocyte-like cells [9], thus promoting ectopic calcification of atherosclerotic arteries and exerting deleterious as well as beneficial effects on lesion development [10].
Apolipoprotein E (apoE) deficient mice, maintained on a high-fat diet, develop advanced and complex plaques in the proximal brachiocephalic artery [11–13], although such plaques are rare at other anatomical sites [14]. It has been proposed that these plaques undergo cycles of rupture and repair [14]. The use of mice doubly deficient for apoE and for individual proteinases has identified some that promote lesion development, while indicating others that may be protective [15,16]. We are using an analogous approach to define the roles of the transglutaminases, examining, in the first instance, TG2. TG2 is a widely expressed and abundant protein, and unlike most other abundant transglutaminases, which have very restricted tissue distributions and functions, TG2 has been implicated in arterial repair. Mice with a targeted disruption of the TG2 gene develop normally for the first year of life [17,18] but subsequently develop mild abnormalities including glucose intolerance [19]. In the present study, apoE/TG2 double-knockout mice were bred and were compared (at less than 12 months of age), with matched apoE single knockout controls to determine the role of TG2 in plaque rupture and repair and in arterial calcification.
2. Materials and methods
2.1. Animals
The maintenance of the animals, and the procedures used in these studies, were performed in accordance with the guidelines and regulations of the University of Bristol and the United Kingdom Home Office. ApoE [20] and TG2 [17] single knockout mice, both on a mixed C57BL/6, 129 strain background, were crossed and the doubly heterozygous F1 mice were interbred. Tail-tip DNA from the F2 mice and subsequent litters was genotyped by PCR. In the F2 generation, only 1 double homozygote was obtained among 98 mice (p < 0.02 for 1 or 0). Relative frequencies of the other genotypes were as expected. Intercrossing of apoE−/−TG2+/− F2 mice yielded only 10 double homozygotes among 98 mice (p < 0.01 for 10 or fewer). However, the resulting double homozygotes bred successfully, generating sufficient apoE−/−TG2−/− mice for study. ApoE single knockout littermates of the double-knockout mice were used to generate apoE−/−TG2+/+ controls.
2.2. Experimental design
Fuller experimental details are included in the online supplement. Briefly, starting at 6–8 weeks of age, 51 apoE single knockout and 66 apoE/TG2 double-knockout mice were maintained for 6 months on a high-fat rodent diet. Paraffin sections of mouse brachiocephalic arteries were used to measure the parameters listed in Table 1 [13], for immunohistochemistry and to detect the presence of Ca3(PO4)2 deposits. Aortic sinuses from 24 randomly selected mice (six males and six females per genotype) were also processed to assess atherosclerosis. In all cases, plaque morphometry was analysed in a blinded fashion.
Table 1.
Proximal brachiocephalic artery morphometry.
| Gender | Genotype | Vessel area (×103 μm2) | Media area (×103 μm2) | Plaque area (×103 μm2) | Lumen area (×103 μm2) | Cap thickness (μm) | Plaque lipid (%) | Buried fibrous caps |
|---|---|---|---|---|---|---|---|---|
| Male (n = 20) | ApoE−/−TG2+/+ | 331.7 ± 20.3 | 83.5 ± 4.7 | 156.9 ± 19.0 | 141.4 ± 12.4 | 2.80 ± 0.26a | 35.7 ± 1.7 | 2.35 ± 0.33 |
| Female (n = 31) | ApoE−/−TG2+/+ | 317.8 ± 15.9 | 77.3 ± 4.4 | 165.1 ± 15.2 | 122.9 ± 7.5 | 3.62 ± 0.29 | 39.8 ± 1.7 | 1.74 ± 0.23 |
| Both (n = 51) | ApoE−/−TG2+/+ | 323.3 ± 12.4 | 79.7 ± 3.3 | 161.9 ± 11.8 | 130.2 ± 6.7 | 3.30 ± 0.21 | 38.1 ± 1.3b | 1.98 ± 0.20 |
| Male (n = 36) | ApoE−/−TG2−/− | 335.7 ± 8.8 | 82.5 ± 3.1 | 154.9 ± 7.6 | 143.7 ± 5.2 | 4.12 ± 0.45a | 34.3 ± 1.5 | 2.14 ± 0.22 |
| Female (n = 30) | ApoE−/−TG2−/− | 298.9 ± 12.6 | 82.0 ± 3.7 | 140.8 ± 11.6 | 122.3 ± 7.7 | 3.90 ± 0.33 | 34.3 ± 1.7 | 1.51 ± 0.25 |
| Both (n = 66) | ApoE−/−TG2−/− | 318.7 ± 7.8 | 81.9 ± 2.4 | 148.9 ± 6.7 | 132.7 ± 4.6 | 4.02 ± 0.29 | 34.4 ± 1.1b | 1.88 ± 0.17 |
Groups with the same superscript letter are significantly different from each other (p < 0.05).
2.3. Immunohistochemical analysis
Sections (3 μm) of human carotid artery atheroma or mouse artery were incubated with rabbit anti-guinea pig TG2 anti-serum, or with a mouse monoclonal antibody to α-smooth muscle actin. Bound antibodies were complexed with peroxidase-linked secondary antibodies and detected with diaminobenzidine. Sections were counterstained with haematoxylin.
2.4. Determination of calcium deposits
De-waxed sections were stained with 2% Alizarin Red S in water pH 6.8, washed extensively and then counterstained with 0.005% Fast Green.
2.5. Statistical analysis
Double-knockout group values were compared with apoE single knockout controls using the computer programs InStat and Prism (both GraphPad Software, San Diego, CA, USA). Means were compared using an unpaired two-tailed Student's t-test, applying Welch's correction in instances where variances were significantly different. Data that were discontinuous (incidence of buried fibrous layers) or not normally distributed (lesion calcification) were analysed using the Mann–Whitney test. The correlation between plaque rupture and calcification was assessed by Spearman's test. Results are shown as the mean ± SEM. Statistical significance was concluded where the probability was less than 0.05.
3. Results
3.1. Plasma analysis
Blood samples obtained at termination from the fat-fed mice were used either for lipid measurements or for determining insulin and glucose concentrations. There were no significant differences in final mean lipid values between 26 apoE single knockout and 20 TG2 apoE double-knockout mice: total cholesterol, 19.7 ± 1.6 mmol/L and 18.8 ± 1.4 mmol/L respectively; HDL cholesterol, 1.1 ± 0.1 mmol/L and 1.1 ± 0.1 mmol/L; LDL cholesterol, 18.3 ± 1.6 mmol/L and 17.4 ± 1.5 mmol/L; triacyglycerols, 1.11 ± 0.1 mmol/L and 1.1 ± 0.2 mmol/L. Blood obtained from 18 apoE single knockout and 12 TG2 apoE double-knockout mice showed no significant differences in mean plasma insulin levels (0.70 ± 0.18 ng/mL and 0.48 ± 0.13 ng/mL respectively, p = 0.37), or plasma glucose levels (8.20 ± 0.87 mmol/L and 7.56 ± 0.77 mmol/L respectively, p = 0.616).
3.2. Immunohistochemistry
The commercial TG2 anti-serum detected a single band in total mouse liver proteins that co-migrated with guinea pig liver TG2 (not shown). The specificity of immunohistochemical staining of the TG2 anti-serum was confirmed by showing that staining was minimal in spleen from TG2 knockout mice but abundant in spleen from wild-type mice, and that the antibody strongly stained capillary endothelial cells in intestinal villi (Supplementary Fig. 1). In agreement with previous reports, the antibody intensely stained formations of cells in the shoulder regions of human carotid atherosclerotic plaques including the intima of capillaries within the arterial wall (Supplementary Fig. 2). The TG2 antibody strongly stained intact endothelium in brachiocephalic arteries from apoE deficient mice TG2 but also detected small clusters of cells within the shoulder regions of mouse plaques and within the core of the plaque (Fig. 1).
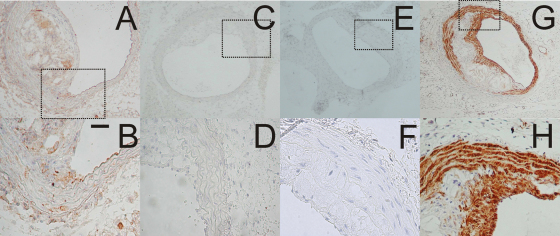
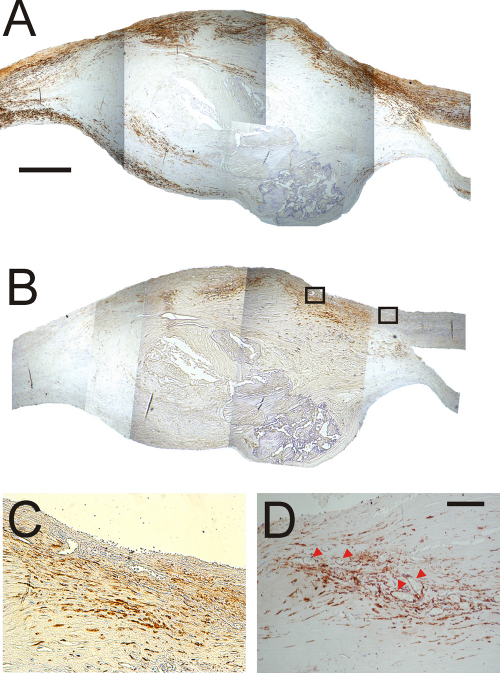
Fig. 1.
Mouse proximal brachiocephalic artery plaques. Scale bar represents 25 μm in Panels B, D, F and H. Panel (A): TG2 wild-type mouse stained for TG2. TG2 antigen is present on the apparently normal endothelium and on various cells within the shoulder region and in the necrotic core, but is not present on the cap surface. Panel (B): Higher power detail of view of part of Panel A. Panel (C): Negative control, TG2 wild-type mouse processed without the primary antibody. Panel (D): Higher power detail of view of part of Panel C. Panel (E): TG2 knockout mouse stained for TG2 antigen. Panel (F): Higher power detail of view of part of Panel E. Panel (G): TG2 wild-type mouse stained for α-smooth muscle actin. Panel (H): Higher power detail of view of part of Panel G.
3.3. Vessel morphometry
The overall extent of atherosclerosis and other morphometric characteristics examined in fat-fed apoE knockout mice was generally unaffected by the presence or absence of TG2 (Table 1). However, the plaque lipid content was slightly decreased from 38.1 ± 1.3% to 34.4 ± 1.1% in apoE/TG2 double-knockout animals (p = 0.032). Also, fibrous cap thickness was increased significantly from 2.8 ± 0.3 μm to 4.1 ± 0.5 μm in male double-knockout mice (p = 0.028). This effect lost statistical significance when the genders were combined (p = 0.135). Additionally, aortic sinus plaques were measured in a sample of 12 single and 12 double-knockout mice. Vessel cross-sectional area was essentially identical between the two groups, and there were no significant differences in aortic sinus morphological characteristics (Supplementary Table 1 and Supplementary Fig. 3).
3.4. Buried and ruptured fibrous caps
The frequency of buried caps per mouse was significantly greater in males than females (2.21 ± 0.18 vs. 1.66 ± 0.17 respectively, p = 0.021), but there was no effect of TG2 deficiency in either sex. Up to six buried caps were detected in individual double-knockout mice (Fig. 2A). Rare instances of current unhealed ruptures were also observed (Fig. 2B) and occurred at similar frequencies in both groups (apoE/TG2 double-knockouts: 1 observed in 66 mice; apoE single knockouts: 2 observed in 51 mice).
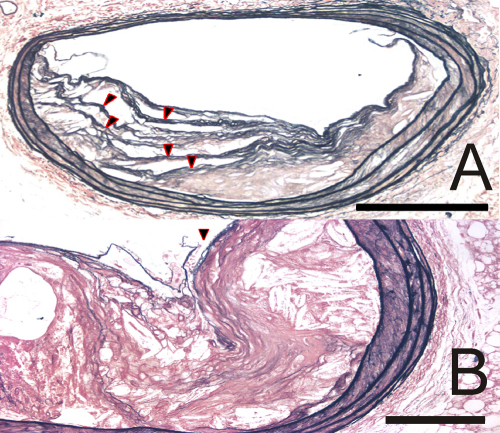
Fig. 2.
Sections of mouse brachiocephalic plaques stained for elastin. Scale bars represent 200 μm in Panel A and 100 μm in Panel B. Panel (A): Multiple buried caps (arrowheads) present in a plaque from an apoE/TG2 double-knockout mouse. Panel (B): A current rupture in a plaque from an apoE/TG2 double-knockout mouse (arrowhead). Red blood cells have permeated the plaque at the point of rupture.
3.5. Calcification
Complexing Ca3(PO4)2 deposits with Alizarin Red S [21] in sections from 28 randomly selected mice showed that the extent of calcification varied widely within both the single and double-knockout groups. Human carotid plaques were processed alongside as a positive control (Fig. 3). When expressed either as whole tissue section calcification (calcified area/total tissue area) or as interlamellar calcification (calcified media area/total media area), there were no significant differences between groups (apoE/TG2 double-knockouts: tissue section 0.60 ± 0.22%, media 1.41 ± 0.49%; apoE single knockouts: tissue section 0.25 ± 0.08% (p = 0.181), media 0.61 ± 0.20% (p = 0.165). It thus appears that TG2 is not required for arterial calcification. In human subjects, calcification is associated with advanced atherosclerosis [2]. However, no correlation was apparent between the incidence of buried plaques and plaque calcification amongst the mice sampled (p = 0.974).
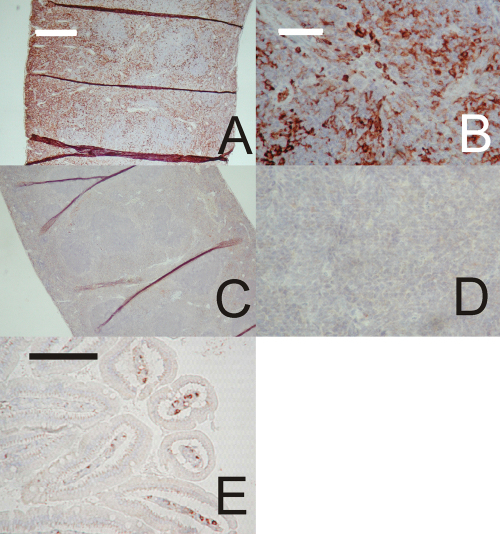
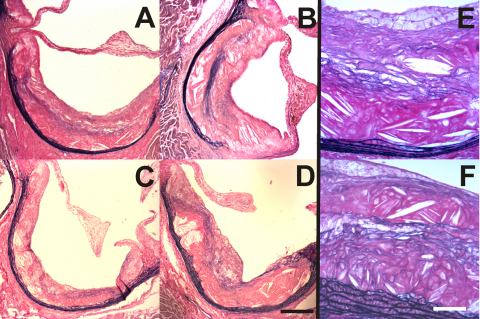
Fig. 3.
Sections of human carotid and mouse brachiocephalic arteries stained for calcium. Scale bars represent 500 μm in Panel A, 100 μm in Panels B and C, 50 μm in Panels D and E. Panel (A): Crystalline calcium deposits in a longitudinal section of a human carotid arterial plaque. Panel (B): Higher power view of part of Panel A. Panel (C): Extensive calcification of both the plaque and arterial wall in a lesion from the brachiocephalic artery of an apoE/TG2 double-knockout mouse. Panel (D): Higher power view of a region of the lesion shown in Panel C. Panel (E): Low to moderate calcification in the brachiocephalic artery of an apoE single knockout mouse.
4. Discussion
Transglutaminase 2 (TG2) is induced at sites of tissue injury including atherosclerotic plaques [7,8] and is strongly expressed in endothelial cells where it establishes a protective barrier against permeation and invasion of foreign cells into the vessel wall [4].
We could readily detect TG2 within human arterial plaques (Supplementary Fig. 1), and observed staining of small islands of TG2-positive cells in the shoulder regions of mouse brachiocephalic plaques. As expected, TG2 was detected on mouse endothelial cells. Despite this, we did not observe any increase in plaque volume or gross changes in plaque composition in brachiocephalic arteries or aortic sinuses in apoE/TG2 double-knockout mice. While we observed a slight and unexpected reduction in brachiocephalic plaque lipid accumulation in the double-knockout animals (Table 1), it is unclear whether this is biologically significant. Similarly, a statistically significant increase in cap thickness was observed in male mice, but this was due to large changes in a few animals rather than a consistent increase across the group. Of greater importance, we did not see the major changes that would be anticipated if TG2 limited lipoprotein permeation into the arterial wall or if TG2 promoted macrophage-mediated clearance of plaque material. Some caution must be exercised in extrapolating to the human situation, since the accelerated atherosclerosis induced in fat-fed apoE knockout mice could have overwhelmed the protection that TG2 affords in slowly developing human plaques. In contrast to our findings, Boisvert et al. [22] observed increases in total, and necrotic core size of aortic sinus lesions size using LDL-receptor knockout mice that had been transplanted with TG2 knockout as opposed to TG2-wild-type bone marrow. They also reported qualitative differences in plaque appearance, although we observed very similar plaque morphology irrespective of the presence of TG2 (Supplementary Fig. 2). Boisvert et al. also reported differences in macrophage distribution within the plaques which might account for differences in plaque composition, but assuming that TG2 is necessary for optimal macrophage function [5,6], this requirement can only manifest in vivo under particular sets of conditions otherwise TG2 knockout mice would exhibit severe developmental abnormalities. Since we observed very similar plasma lipid profiles in TG2+/+ and TG2−/− mice, it is unlikely that a compensatory change in lipid levels was induced by the breeding strategy which offset the deleterious effect of TG2 deficiency. Although there was some variability in random glucose and insulin levels at termination, the differences between genotypes were not statistically significant difference and could not have masked a protective effect of TG2. Older TG2−/− mice become insulin resistant [19], but if this occurred in the current study, TG2 deficiency would be expected to promote atherosclerosis and hence bias towards a protective effect for TG2, rather than no effect.
Lesions within the brachiocephalic artery display a layered morphology, interpreted as arising from the burying of fibrous caps following successive rounds of plaque rupture [14]. Our data therefore suggest that TG2 is essential neither for the repair of previous ruptures in mice (since up to six buried caps were detected in individual TG2 deficient mice), nor for stabilizing plaques (since frequencies of buried caps were similar in both groups). We have also assessed current acute ruptures in TG2-deficient mice, where erythrocyte intrusion into the plaque is apparent. These events, although rare, occur with similar frequency in both groups, reinforcing the suggestion that lesions in TG2-deficient mice are not rendered unstable.
Johnson et al. suggested that TG2 is essential for the ectopic calcification of arteries, since cultured TG2-deficient arterial smooth muscle cells were unable to differentiate to chondrocytes and calcify [9]. This failure was partially reversed with exogenous FXIIIA [9], a situation which resembles the model proposed for bone calcification where TG2 and FXIIIA have overlapping functions [23]. Here, we observed that calcification was unimpaired in apoE/TG2 double-knockout lesions, even within the intact arterial wall where permeation by blood-borne soluble FXIIIA would be prevented. Matlung et al. recently reported that FXIIIA-positive macrophages co-localise with areas of calcification within human lesions [24], and similarly macrophages could release FXIIIA into the mouse arterial wall. However, our recent unpublished observations indicate that TG2−/−FXIIIA−/− double-knockout mice are viable and apparently normal and healthy at 3 months of age. This precludes the possibility that either one of FXIIIA and TG2 is essential for bone calcification and possibly therefore for arterial calcification. It also questions the proposed overlap in roles of these enzymes in other processes relevant to atherosclerosis, including physiological arterial remodelling [25].
In summary, we were unable to detect any protective role for TG2 against lesion development at either of two anatomical sites in a large cohort of mice. We were unable to show any requirement for TG2 in arterial calcification. Further studies are planned to examine these events in FXIIIA knockout and FXIIIA/TG2 double-knockout mice. These studies will help to establish whether plaque composition and stability are primarily maintained by FXIIIA, which itself has tissue repair activities [26,27], and whether one or the other enzyme must be present to enable arterial calcification or whether there are unexpected roles for other members of the transglutaminase family. Defining the role of these transglutaminases in arterial calcification versus tissue repair is important for evaluating their potential as therapeutic targets in atherosclerosis.
Acknowledgements
The authors wish to thank Mr. Peter Jackson and Mrs. Sally Gray (Histopathology Laboratory, Department of Pathology, Leeds General Infirmary, Leeds, UK) for providing samples of human carotid endarterectomy specimens and for expert guidance on the immunohistochemistry; Dr. Ray Bush (Bristol Heart Institute) for help and advice with the mouse breeding programme; and Miss Katrina Redding (Bristol Heart Institute) for excellent technical assistance. This work was supported by a grant from the British Heart Foundation.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.atherosclerosis.2009.11.014.
Appendix A. Supplementary data
References
- 1.Burke A.P., Kolodgie F.D., Farb A. Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation. 2001;103(7):934–940. doi: 10.1161/01.cir.103.7.934. [DOI] [PubMed] [Google Scholar]
- 2.Budoff M.J., Gul K.M. Expert review on coronary calcium. Vasc Health Risk Manage. 2008;4(2):315–324. doi: 10.2147/vhrm.s1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lorand L., Graham R.M. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4(2):140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 4.Verderio E.A., Johnson T., Griffin M. Tissue transglutaminase in normal and abnormal wound healing: review article. Amino Acids. 2004;26(4):387–404. doi: 10.1007/s00726-004-0094-4. [DOI] [PubMed] [Google Scholar]
- 5.Rose D.M., Sydlaske A.D., Agha-Babakhani A., Johnson K., Terkeltaub R. Transglutaminase 2 limits murine peritoneal acute gout-like inflammation by regulating macrophage clearance of apoptotic neutrophils. Arthritis Rheum. 2006;54(10):3363–3371. doi: 10.1002/art.22137. [DOI] [PubMed] [Google Scholar]
- 6.Falasca L., Iadevaia V., Ciccosanti F. Transglutaminase type II is a key element in the regulation of the anti-inflammatory response elicited by apoptotic cell engulfment. J Immunol. 2005;174(11):7330–7340. doi: 10.4049/jimmunol.174.11.7330. [DOI] [PubMed] [Google Scholar]
- 7.Haroon Z.A., Wannenburg T., Gupta M. Localization of tissue transglutaminase in human carotid and coronary artery atherosclerosis: implications for plaque stability and progression. Lab Invest. 2001;81(1):83–93. doi: 10.1038/labinvest.3780214. [DOI] [PubMed] [Google Scholar]
- 8.Auld G.C., Ritchie H., Robbie L.A., Booth N.A. Thrombin upregulates tissue transglutaminase in endothelial cells: a potential role for tissue transglutaminase in stability of atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 2001;21(10):1689–1694. doi: 10.1161/hq1001.097063. [DOI] [PubMed] [Google Scholar]
- 9.Johnson K.A., Polewski M., Terkeltaub R.A. Transglutaminase 2 is central to induction of the arterial calcification program by smooth muscle cells. Circ Res. 2008;102(5):529–537. doi: 10.1161/CIRCRESAHA.107.154260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.VanBavel E., Bakker E.N. A vascular bone collector: arterial calcification requires tissue-type transglutaminase. Circ Res. 2008;102(5):507–509. doi: 10.1161/CIRCRESAHA.108.173013. [DOI] [PubMed] [Google Scholar]
- 11.Rosenfeld M.E., Polinsky P., Virmani R. Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arterioscler Thromb Vasc Biol. 2000;20(12):2587–2592. doi: 10.1161/01.atv.20.12.2587. [DOI] [PubMed] [Google Scholar]
- 12.Calara F., Silvestre M., Casanada F. Spontaneous plaque rupture and secondary thrombosis in apolipoprotein E-deficient and LDL receptor-deficient mice. J Pathol. 2001;195(2):257–263. doi: 10.1002/path.915. [DOI] [PubMed] [Google Scholar]
- 13.Williams H., Johnson J.L., Carson K.G., Jackson C.L. Characteristics of intact and ruptured atherosclerotic plaques in brachiocephalic arteries of apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol. 2002;22(5):788–792. doi: 10.1161/01.atv.0000014587.66321.b4. [DOI] [PubMed] [Google Scholar]
- 14.Jackson C.L., Bennett M.R., Biessen E.A., Johnson J.L., Krams R. Assessment of unstable atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2007;27(4):714–720. doi: 10.1161/01.ATV.0000261873.86623.e1. [DOI] [PubMed] [Google Scholar]
- 15.Johnson J.L., George S.J., Newby A.C., Jackson C.L. Divergent effects of matrix metalloproteinases 3, 7, 9, and 12 on atherosclerotic plaque stability in mouse brachiocephalic arteries. Proc Natl Acad Sci USA. 2005;102(43):15575–15580. doi: 10.1073/pnas.0506201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodgers K.J., Watkins D.J., Miller A.L. Destabilizing role of cathepsin S in murine atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2006;26(4):851–856. doi: 10.1161/01.ATV.0000203526.75772.4b. [DOI] [PubMed] [Google Scholar]
- 17.Nanda N., Iismaa S.E., Owens W.A. Targeted inactivation of Gh/tissue transglutaminase II. J Biol Chem. 2001;276(23):20673–20678. doi: 10.1074/jbc.M010846200. [DOI] [PubMed] [Google Scholar]
- 18.De Laurenzi V., Melino G. Gene disruption of tissue transglutaminase. Mol Cell Biol. 2001;21(1):148–155. doi: 10.1128/MCB.21.1.148-155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernassola F., Federici M., Corazzari M. Role of transglutaminase 2 in glucose tolerance: knockout mice studies and a putative mutation in a MODY patient. FASEB J. 2002;16(11):1371–1378. doi: 10.1096/fj.01-0689com. [DOI] [PubMed] [Google Scholar]
- 20.Plump A.S., Smith J.D., Hayek T. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71(2):343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 21.Dahl L.K. A simple and sensitive histochemical method for calcium. Proc Soc Exp Biol Med. 1952;80(3):474–479. doi: 10.3181/00379727-80-19661. [DOI] [PubMed] [Google Scholar]
- 22.Boisvert W.A., Rose D.M., Boullier A. Leukocyte transglutaminase 2 expression limits atherosclerotic lesion size. Arterioscler Thromb Vasc Biol. 2006;26(3):563–569. doi: 10.1161/01.ATV.0000203503.82693.c1. [DOI] [PubMed] [Google Scholar]
- 23.Nakano Y., Al Jallad H.F., Mousa A., Kaartinen M.T. Expression and localization of plasma transglutaminase factor XIIIA in bone. J Histochem Cytochem. 2007;55(7):675–685. doi: 10.1369/jhc.6A7091.2007. [DOI] [PubMed] [Google Scholar]
- 24.Matlung H.L., Groen H.C., de Vos J. Calcification locates to transglutaminases in advanced human atherosclerotic lesions. Am J Pathol. 2009;174(5.):1374–1379. doi: 10.2353/ajpath.2009.090012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bakker E.N., Pistea A., Spaan J.A. Flow-dependent remodeling of small arteries in mice deficient for tissue-type transglutaminase: possible compensation by macrophage-derived factor XIII. Circ Res. 2006;99(1):86–92. doi: 10.1161/01.RES.0000229657.83816.a7. [DOI] [PubMed] [Google Scholar]
- 26.Inbal A., Lubetsky A., Krapp T. Impaired wound healing in factor XIII deficient mice. Thromb Haemost. 2005;94(2):432–437. doi: 10.1160/TH05-04-0291. [DOI] [PubMed] [Google Scholar]
- 27.Dardik R., Loscalzo J., Eskaraev R., Inbal A. Molecular mechanisms underlying the proangiogenic effect of factor XIII. Arterioscler Thromb Vasc Biol. 2005;25(3):526–532. doi: 10.1161/01.ATV.0000154137.21230.80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.