Abstract
The mossy fiber (MF) system targets the apical dendrites of CA3 pyramidal cells in the stratum lucidum (SL). In mice overexpressing the growth-associated protein GAP-43 there is an apparent ectopic growth of these MFs into the stratum oriens (SO) targeting the basal dendrites of these same pyramidal cells (Aigner et al. (1995) Cell 83:269–278). This is the first evidence to our knowledge that links increased GAP-43 expression with growth of central axons. Here we studied the Aigner et al. transgenic mice but were unable to confirm such growth into SO. However, using quantitative methods we did observe enhanced growth within the regions normally targeted by MFs, for example, the SL in the CA3a region. These contrasting results led us to study MFs with double-immunostaining using an immunohistochemical marker for MFs, the zinc transporter, ZnT3, to visualize the colocalization of transgenic GAP-43 within MFs. Unexpectedly, using both fluorescence and confocal microscopy, we were unable to detect colocalization of GAP-43-positive axons with ZnT3-positive MF axons within the MF pathways, either in the region of the MF axons or in the SL, where MF terminals are abundant. In contrast, the plasma membrane-associated presynaptic marker SNAP-25 did colocalize with transgenic GAP-43-positive terminals in the SL. Synaptophysin, the vesicle-associated presynaptic terminal marker, colocalized with ZnT3 but did not appear to colocalize with GAP-43. The present findings raise important questions about the properties of granule cells and the MF mechanisms that differentially regulate axonal remodeling in the adult hippocampus: (1) Because there appears to be at least two populations of granule cells defined by their differential protein expression, this points to the existence of an intrinsic heterogeneity of granule cell expression beyond that contributed by adult neurogenesis; (2) Given the present evidence that growth is induced in mice overexpressing GAP-43 in adjacent non-GAP-43 containing MFs, the potential exists for a heretofore unexplored interaxonal communication mechanism.
Keywords: mossy fibers, hippocampus, GAP-43, ZnT3, granule cell
INTRODUCTION
Null mutation of GAP-43, the growth-associated protein, has provided convincing evidence that this protein kinase C (PKC) substrate is critical for axonal pathfinding during development (Strittmatter et al., 1995; Meiri et al., 1998; Maier et al., 1999). Aigner et al. (1995) reported that transgenic mice overexpressing chicken GAP-43 (termed G-Phos here) induce ectopic mossy fibers (MFs) located beyond the normal terminal zone of the stratum lucidum (SL) extending into the stratum oriens (SO) of CA3 up to CA3a, at the border with CA2. Such innervation, which is not normally observed in wild-type C57BL6 mice (e.g., Cantallops and Routtenberg, 2000; Rekart et al., 2007b), may be due to expansion into SO of the major suprapyramidal mossy fiber pathway that is typically restricted in its termination target zone to SL.
There are several reasons why the Aigner et al. report is of significance, warranting further study. First, it demonstrated that GAP-43 actually induced growth. This finding was consistent with subsequent work examining the role of transcriptional regulation of the development of mouse hippocampal mossy fibers detected with a lacZ reporter driven by the GAP-43 promoter (Cantallops and Routtenberg, 1999). Second, the critical role of GAP-43 in mossy fiber remodeling suggested by Aigner et al. provides a molecular substrate for such growth seen after experimental induction of status epilepticus in which gene expression of this protein is upregulated after seizures and before the onset of supragranular mossy fiber sprouting (Meberg et al., 1993; McNamara and Routtenberg, 1995; Cantallops and Routtenberg, 1996; Bendotti et al., 1997). Third, the proposed role for GAP-43 in synaptic remodeling underlying learning and memory in adult animals, (Routtenberg, 1985), is supported by prior studies of the hippocampus (Routtenberg et al., 2000; Young et al., 2002) and of the anterior cingulate cortex, where GAP-43 has been linked to consolidation of long-term memory (Maviel et al., 2004).
The potential role of GAP-43 in MFs is more complicated, because a distinctive feature of hippocampal granule cells first noted by Rosenthal et al. (1987) is that GAP-43 mRNA expression is undetectable in adult granule cells. As would be expected the GAP-43 protein is also not detected in the adult MF system. It is, however, present in developing postnatal and postepileptic granule cells, (Meberg and Routtenberg, 1991; Cantallops and Routtenberg, 1996, 1999). Surprisingly, though GAP-43 mRNA is not detected in hippocampal granule cells, they do express the full length heterogeneous nuclear RNA (hnRNA; Namgung and Routtenberg, 2000). In the G-Phos mouse studied here, transgenic chick GAP-43 mRNA is expressed in adult granule cells and GAP-43 protein is observed in MFs and their terminal fields (MFTFs). As there is no quantitative information on the extent of growth and the characteristics of the GAP-43-positive MF system in transgenic mice, we measured the distribution of the MFTFs with Timm’s staining in G-Phos mice and their wild-type controls. Additionally, key axonal and presynaptic markers were studied using double-labeling immunohistochemistry visualized with fluorescence and confocal microscopy to examine their distribution and potential colocalization within MFs and their terminal fields.
MATERIALS AND METHODS
Animals
G-Phos animals originally described by Aigner et al. (1995) have been previously behaviorally and electrophysiologically phenotyped (Routtenberg et al., 2000). They were genotyped using novel PCR primers specifically designed to the 3′ region of the transgenic GAP-43 cDNA insert (forward: 5′ GACAC GGGCTCAGAGCAG 3′; reverse: 5′ TTCAGGCATTTTCTT GGTCC 3′). The primers did not have significant homology to any known mouse genes (BLASTn) and yielded a 379 nt amplicon. Training took place during the light cycle (between 10 AM and 5 PM CST). Only hemizygous animals were used for the purposes of this study as these are the “maze bright” strain of G-Phos mouse that exhibit superior learning and memory relative to wild-type mice (Holahan et al., 2007). Animal use and care were in accordance with Northwestern University Center for Comparative Medicine guidelines and approved by the IACUC.
Histology
Animals were sacrificed by cardiac perfusion following an overdose of sodium pentobarbital. Brains were then processed for Timm’s heavy metal staining (as described in Cantallops and Routtenberg, 2000) or for in situ hybridization/immunofluorescence (fixation in 4% paraformaldehyde, cryoprotected in 30% sucrose, stored in 0.1 M phosphate buffer, pH 7.4 with 0.1% sodium azide). Fixed tissue was sectioned in the coronal plane (Timm’s: 40 µm; in situ hybridization/immunofluorescence: 20 µm).
Timm’s Stain: Quantitative Analysis
Timm’s-stained tissue from the septal hippocampus (every fifth section, starting from a systematically randomly chosen point from the septal pole of the hippocampus) was analyzed in hemizygous (n = 6) and wild-type (n = 9) adult male mice. Using an Olympus BX61 microscope with a DP70 camera (12.5 megapixels), digital images of the entire regio inferior were obtained. All analyses were carried out with experimenters blind to specimen genotype using the methodology outlined in Rekart et al. (2007a,b). Briefly, a point counting grid (squares = 25 µm/side) was randomly placed over the entire regio inferior. Every grid intersection overlying dark Timm’s silver precipitate was then counted as either belonging to the stratum lucidum (SL), or the stratum oriens (SO) or stratum pyramidale (SP). Because the SP labeling appears to arise from SL, the SO + SP measure from this and prior studies is taken as a measure of the sprouting into SO from SL. Additionally, SO and SP were combined due to their close proximity and, in places, an unclear border between the two (Claiborne et al., 1986). An area was identified as part of the SL if it was superior to the pyramidal cell layer. The area of both the SL and the SO + SP were then estimated by multiplying the number of points on the grid overlaying a Timm’s-positive granule in each region by the area per point (625 µm2). Comparisons between groups were made using the ratio of the estimated area in SO + SP to that of SL to account for size differences between individual animals.
Immunofluorescence
Animals were sacrificed using methods modified from the descriptions of Namgung and Routtenberg (2000). After brief rinses in tris-buffered saline, pH 7.4 (TBS), free-floating coronal sections (20 µm) were blocked in TBS with 0.1% triton (T-TBS) with 1% normal goat serum for 1 h. Sections were incubated overnight at room temperature in primary antibodies. Primary antibodies were mouse anti-GAP43 (1:2,000; Sigma), mouse antisynaptophysin (1:1,000; Sigma), rabbit anti-ZnT3 (1:400; gift of Dr. R. Palmiter), rabbit anti-GAP43 (1:400; Chemicon), rabbit antisynaptophysin (1:400; Pierce), mouse anti-SNAP-25 (1:2,000, Chemicon), rabbit anti-GFAP (1:1,000; gift of Dr. O. Steward), rabbit anticalretinin (1:2,000; gift of Dr. J. Rogers). To rule-out cross reactivity of antibodies, double-labeling experiments with sections incubated in the respective antibodies in successive fashion (e.g., ZnT3 first, GAP-43 second, and vice versa) and single-labeling experiments (e.g., ZnT3-only, GAP-43-only) were first performed. We did not find any qualitative differences in the intensity or pattern of immunoreactivity of adjacent sections from the same brain stained using the coincubation procedure outlined here. The next day, sections were rinsed in TBS and incubated in secondary Alexa594- or Alexa546-conjugated goat antirabbit, highly cross-adsorbed secondary antibody and/or Alexa 488-conjugated goat antimouse, highly cross-adsorbed secondary antibody in T-TBS for 2 h at room temperature. Sections were then rinsed 2 h in TBS followed by a final series of rinses in 0.1 M tris, pH 7.4. Sections were mounted on Superfrost Plus slides (Fisher Scientific), and covered with VectorShield Hardset mounting medium either with or without DAPI nuclear counterstain (Vector Labs) and coverslipped. The specificity of immunolabeling was verified in all experiments by examining controls in which the primary antibody was omitted. Images were obtained with either an Olympus BX61 microscope, Plan-Apo objectives, and a DP70 camera (12.5 megapixels, Olympus) or a Leica DM RXE-7 laser-scanning confocal microscope. When using the confocal microscope, we collected optical sections every 0.5 µm through the entire tissue sample. Qualitative analysis of separate channels resulted in positive identification of colocalized signals for structures that (1) were pseudo-colored “yellow” to “yellowish-orange” (given relatively equivalent single-channel intensities) and (2) sizes and distribution of structures were similar.
In Situ Hybridization
The in situ hybridization procedure follows that as first described by Meberg and Routtenberg (1991) and detailed in Wisden and Morris (2002). Antisense (AS) and sense (S) 45mer oligonucleotides specific to the exogenous GAP-43 insert were designed for the present study to be complementary to nucleotides 281–325 of the chicken cDNA per Baizer et al. (1990). The sequences were: S:5′GATGCCCCCGCATCCGAGTCT GAGGCCGCCGACAAGAAGGACGAA 3′ and AS: 5′TTCGT CCTTCTTGTCGGCGGCCTCAGACTCGGATGCGGGGG CATC 3′. In addition, AS and S 50mer probes specific to endogenous mouse GAP-43 (nucleotides 154–203 per Cimler et al., 1987) were also designed. The sequences were: S:5′TGTGCTG TATGAGAAGAACCAAACAGGTTGAAAAGAATGATGAGG ACCAA 3′ & AS: 5′ TTGGTCCTCATCATTCTTTTCAAC CTGTTTGGTTCTTCTCATACAGCACA 3′.
BLASTn searches on all oligos confirmed the (1) specificity of probes to transgenic (and not endogenous) GAP-43 and that (2) probe sequences were not homologous to any known rodent genes. Desalted AS and S oligonucleotides were synthesized commercially (Invitrogen), diluted to a concentration of 3 pmol/µl in diethylpyrocarbonate (DEPC)-treated water, radioactively labeled using terminal deoxynucleotidyl transferase (Promega) per the manufacturer’s instructions with 35S-ATP (Perkin Elmer) and activity was determined using a liquid scintillation counter (Beckman). Before hybridization, slides with 20-µm thick coronal brains sections were placed at −20° for 1 h and then rapidly thawed at 55° for 5 min. Slides were rinsed in 0.1 M triethanolamine/0.25% acetic anhydride for 10 min, rinsed in 0.3M sodium chloride/0.03 M sodium citrate (2× SSC), dehydrated through an ethanol series (70%, 95%, 100%) and allowed to dry. Radiolabeled probes were diluted to a concentration of 200,000 cpm/100 µl in 50% formamide/4× SSC/10% dextran sulfate and then 100 µl/slide was applied and slides were covered with a parafilm coverslip. Sections were incubated 18 (GAP-43) h at 42° in a chamber humidified with 4× SSC and 50% formamide. After hybridization, coverslips were gently removed and slides were incubated in 1× SSC at room temperature for 10 min and then in 1× SSC at 55° for 30 min (GAP-43). Sections were then rinsed in 0.1× SSC and dehydrated through an ethanol series (50%, 70%, 95%) and allowed to dry. Hybridized slides were apposed to Biomax MR film (Kodak) and stored at room temperature for 5 days. Films were developed under safelight for 5 min in GBX developer (Kodak), rinsed in deionized water for 5 min, fixed for 10 min (Kodak), and then rinsed for 15 min in deionized water before drying.
RESULTS
Transgenic GAP-43 mRNA is Highly Expressed in Adult Hippocampal Granule Cells
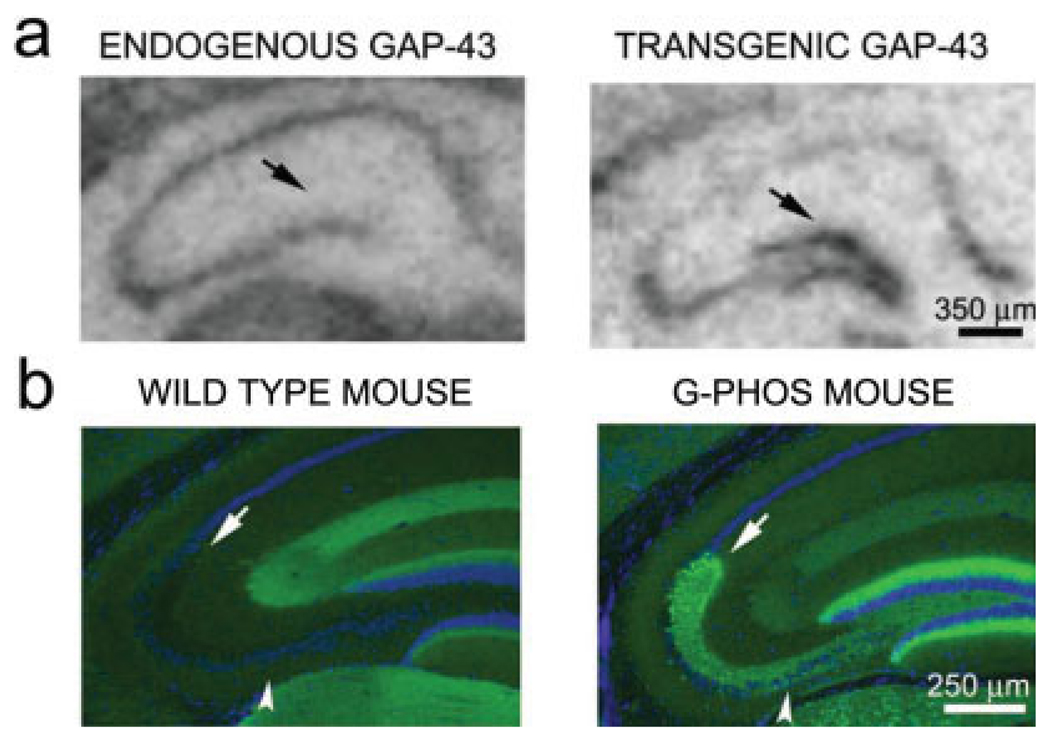
In wild type mice, endogenous GAP-43 mRNA is not observed in granule cells of the dentate gyrus (DG), duplicating prior findings in the rat (Meberg and Routtenberg, 1991). But in transgenic mice, chick GAP-43 mRNA was highly expressed in granule cells (Fig. 1a). As expected from this expression of transgenic mRNA, in G-Phos mice but not wild-type controls, GAP-43 protein was abundant in MFs and in the SL, the mossy fiber terminal field (MFTF; Figs. 1c,d). No detectable endogenous mouse GAP-43 mRNA was observed in the hippocampal granule cells of transgenic animals. Transgenic GAP-43 protein was not detected in the granule cell dendrites indicating axon-selective transport of transgenic protein, similar to that observed for wild-type GAP-43 in other systems (Benowitz and Routtenberg, 1997). Because of the location and distribution of transgenic GAP-43 at low magnification, it appears, as it did to Aigner et al., that expression of transgenic GAP-43 protein is within hippocampal mossy fibers (but see what follows).
FIGURE 1.
In situ hybridization of mouse GAP-43 mRNA in wild type and chick GAP-43 mRNA in transgenic G-Phos mice. Transgenic GAP-43 mRNA was driven by the Thy1 promoter and is present in hippocampal granule cells of G-Phos mice; wild-type mouse GAP-43 mRNA was driven by the GAP-43 promoter. As WT mice show no expression of transgenic GAP-43 mRNA the probe for chick mRNA does not hybridize to mouse mRNA. (a) Though endogenous GAP-43 mRNA is not expressed by hippocampal granule cells, the transgene is present at high levels (black arrow, right panel). (b) The expression of chick mRNA in granule cell soma is paralleled by chick GAP-43 protein immunoreactivity in mossy fiber axons and its termination zones in the suprapyramidal (arrow) and the infra- and intrapyramidal mossy fiber (IIPMF) pathways(arrowhead). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Overexpression of GAP-43 is not Associated With Increases in the Size of Mossy Fiber Terminal Fields in Stratum Oriens
We did not observe the pronounced SO innervation throughout the hippocampal CA3 subregion that was reported by Aigner et al. (1995). It did appear, however, that there were localized differences in the length of the expansion of the normally minor infra/intrapyramidal mossy fiber pathway (IIPMF) which terminates both on CA3c pyramidal neurons proximal to the dentate gyrus (CA3c), as well as in the pyramidal cell layer (SP; Gaarskjaer, 1978) in the more anterior hippocampal sections from G-Phos mice.
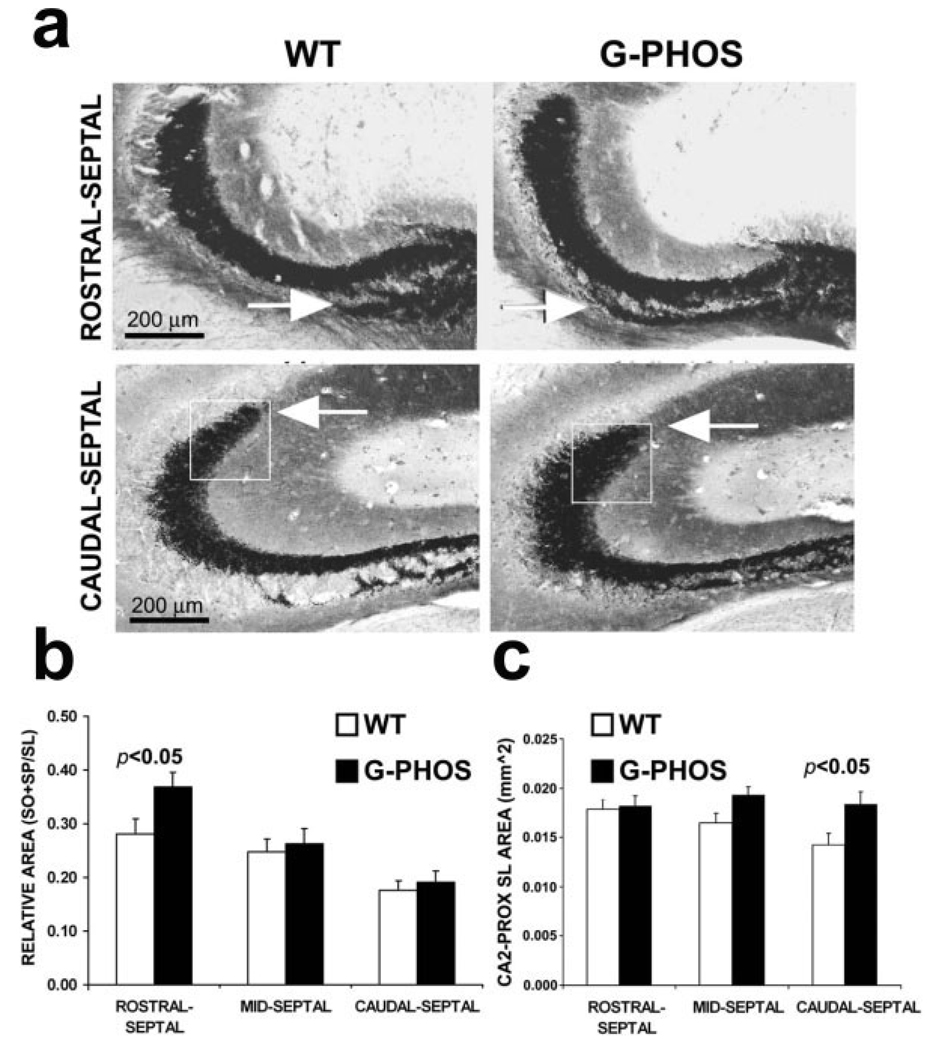
In order to evaluate quantitatively the size of mossy fibers in GAP-43 transgenic mice the same method described in Rekart et al. (2007a) was used to characterize the area of MFTF. A significant increase in the Timm’s-stained MFTF area of transgenic mice was evident in rostral-septal sections after quantifying SO + SP (see Methods) of G-Phos and wild-type mice. G-Phos had a significantly larger SO + SP area than wild type mice (Student’s t-test t(13) = 2.70; P < 0.025) and this difference remained as well even after correcting for individual differences in hippocampal size by normalizing the SO + SP using the size of the SL, ([SO + SP]/SL; Fig. 2b; Student’s t-test t(13) = 2.23; P < 0.05). Note that this increase was principally due to IIPMF in CA3b/CA3c, which was distinct from that reported by Aigner et al. (1995).
FIGURE 2.
Overexpression of GAP-43 increases the size of mossy fiber terminal fields. (a) In G-Phos mice we observed an increment in the size of the IIPMF (here designated by its termination zone in CA3b, SO + SP) that was restricted to the rostral levels of the hippocampus (arrow) and a shift in the distribution of Timm’s reactivity along the CA3 pyramidal cells near the border of CA2 (boxed area in a). Quantitatively, the increment in Timm’s staining in G-Phos mice appeared to be due to a lengthening of the IIPMF, primarily within the rostral-septal CA3b (in b) and an outgrowth of the dorsolateral SL in the caudal-septal CA3a (in c). WT, wild type; G-Phos, transgenic GAP-43 overexpression mouse; SL, stratum lucidum.
Growth was also observed as an expansion within the SL. Comparison of Timm’s-reactivity in the SL of G-Phos and wild-type mice revealed that the distribution of Timm’s reactivity within the SL most proximal to CA2 was more bulbous in G-Phos relative to wild-type mice, especially in more caudal sections (see Fig. 2a). Though we did not detect any effect of genotype on the overall area of the SL in the caudal-septal hippocampus (Student’s t-test t(13) = −0.86; P = n.s), a more circumscribed analysis of the SL in this bulbous region (Figs. 2a,c) was carried out. To quantify the potential difference in innervation of the CA2-proximal SL in G-Phos mice, we positioned a virtual square 200 µm/side such that the upper right hand corner was situated at the proximal-most crest of the Timm’s-positive SL and then calculated the area within the square using a modified version of our standard areal technique (see Methods). Indeed, quantitation of Timm’s staining within the 0.04 mm2 subsection of the SL proximal to CA2 (i.e., CA3a) revealed that this portion of SL of G-Phos mice was significantly larger than wild type mice at more caudal levels of the septal hippocampus (Fig. 2c; Student’s t-test t(13) = 2.28; P < 0.05).
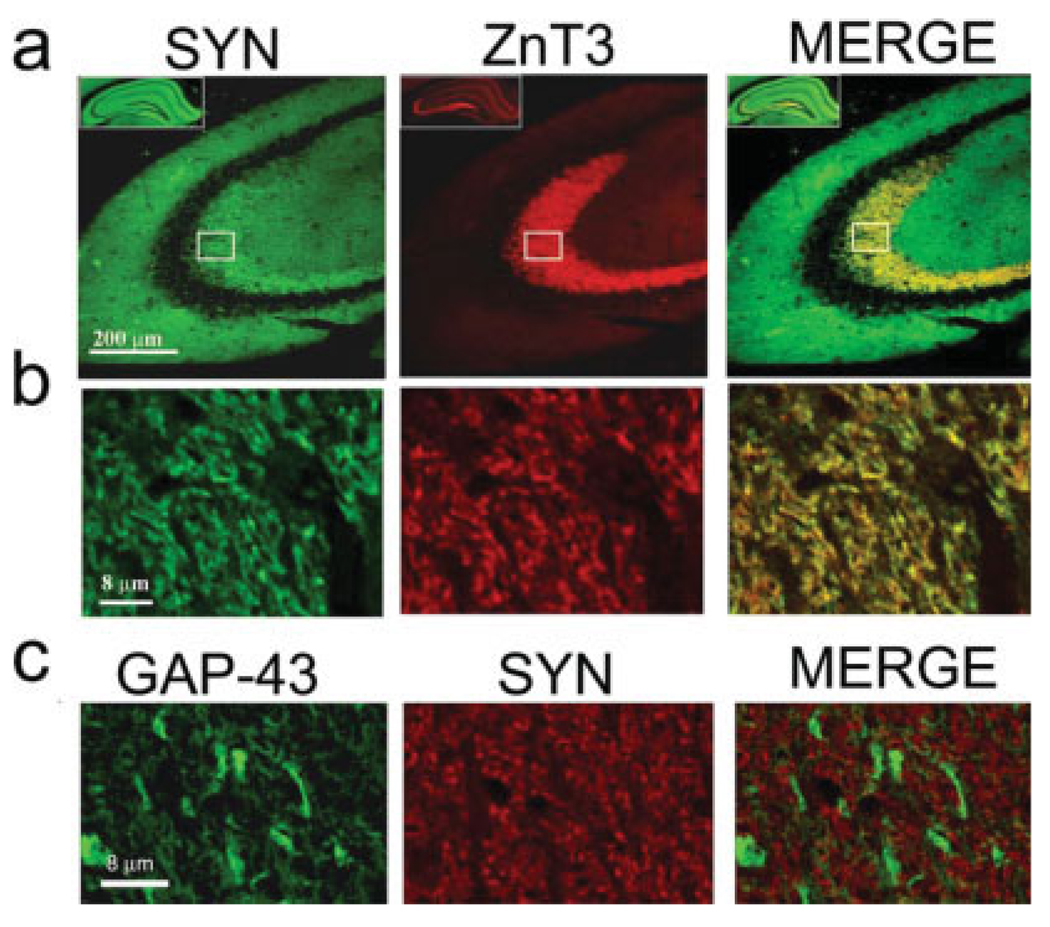
While Zinc Transporter 3 (ZnT3) and GAP-43 Were “Copresent” Within MFs in the SL, Unexpectedly, There was no Evidence at High Magnification That They Were Colocalized
Aigner et al. (1995) assumed that the growth of the mossy fiber terminal fields into SO from SL of GAP-43 transgenic mice was due to the presence of transgenic GAP-43 within the mossy fibers. This predicts that with double-immunolabeling methods that GAP-43 should colocalize with a marker for mossy fibers, the zinc transporter 3 (ZnT3; Palmiter et al., 1996), which is found in high abundance in zinc-containing mossy fibers.
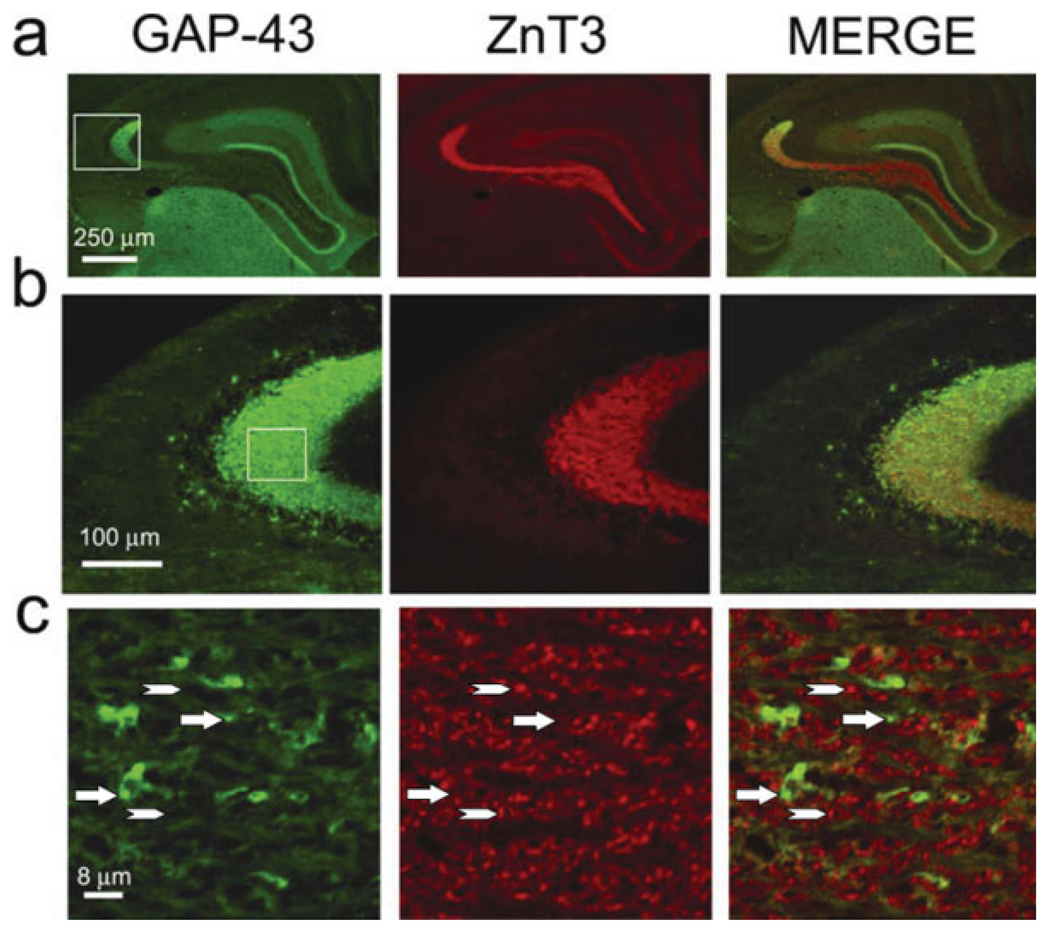
Hippocampal sections from wild-type and transgenic mice were labeled with an antibody that recognized transgenic GAP-43 (as well as endogenous, which was not present in MFs) and with another antibody that recognized the MF marker ZnT3. To our surprise there was a paucity of transgenic GAP-43 colocalization with ZnT3 in mossy fibers and their terminal fields. (Figs. 3 and 4). Indeed, as seen in both fluorescence and confocal imaging, there were few detectable yellow puncta in the SL that would indicate colocalization. This contrasts sharply with the images of the clear colocalization of GAP-43 and SNAP-25 within MFs and their terminal fields (Fig. 8c). Thus, it is reasonable to consider the possibility that there exists at least two populations of MFs (Fig. 3c), one of which is denoted as GAP-43-positive and ZnT3-negative (G+/Z−) and the other GAP-43-negative and ZnT3-positive (G−/Z+; Fig. 4).
FIGURE 3.
Zinc transporter-positive staining in the mossy fiber terminal field of the stratum lucidum does not appear to colocalize with GAP-43-positive staining in this same MF field. (a) Low-power imaging of a double-labeled hippocampal section from a G-Phos mouse shows that though immunoreactivity for GAP-43 and the zinc transporter (ZnT3) overlap, there does not appear to be any detectable colocalization (b). (c) Confocal image from the boxed area in (b) identifies several GAP-43-positive, ZnT3-negative (arrows), and GAP-43-negative, ZnT3-positive puncta (arrowhead). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
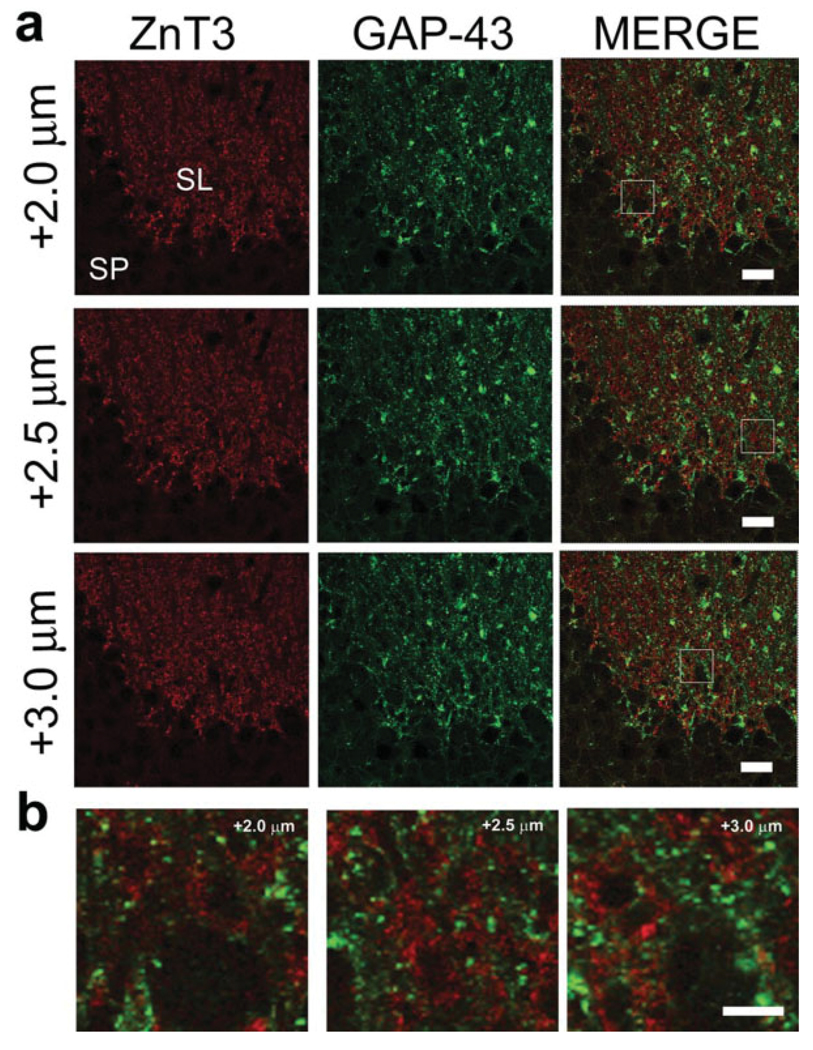
FIGURE 4.
Confocal z-stack series through a GAP-43/ZnT3 double-immunostained hippocampal section demonstrates that GAP-43+ structures are distinct from ZnT3+ puncta. Three separate 40X optical sections, separated by 0.5 µm, of double-immunostained tissue from the dorsal hippocampal stratum lucidum of a G-Phos mouse are presented in (a). Note the lack of colocalized puncta, highlighted by the zoomed in areas in (b). Scale bar in (a) = 20 µm; scale bar in (b) = 5 µm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
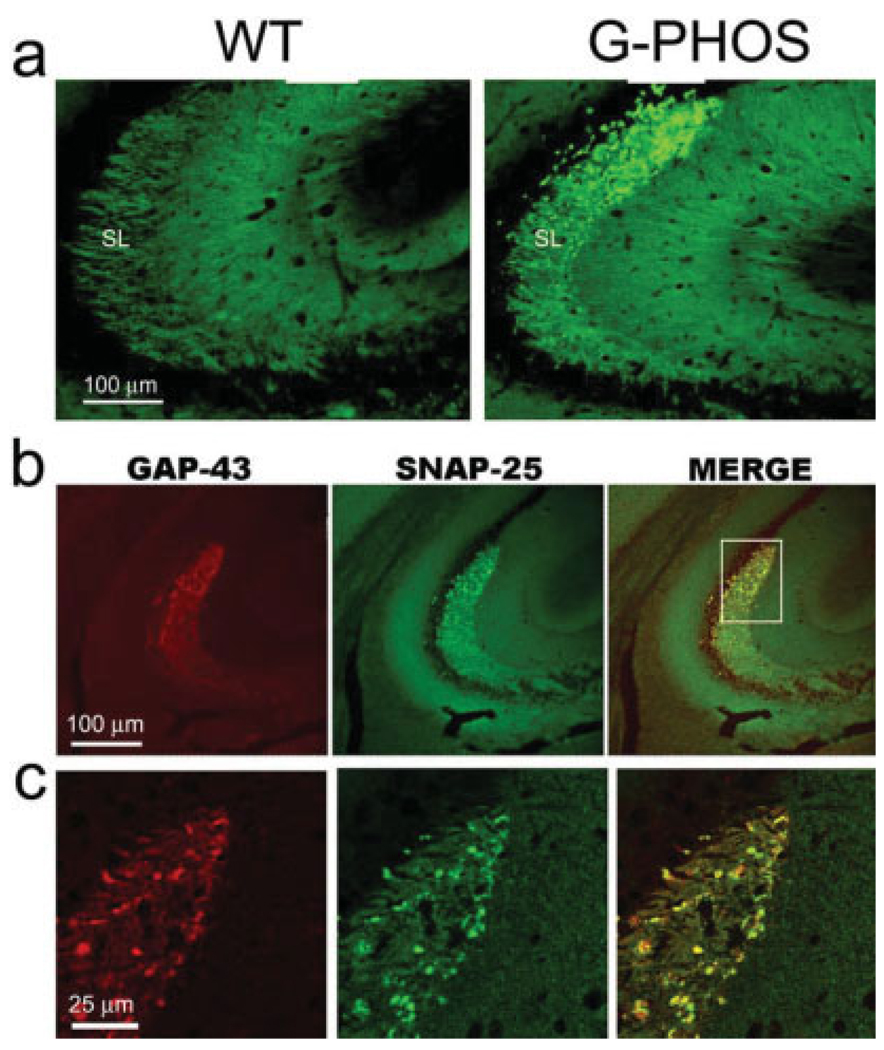
FIGURE 8.
The presynaptic terminal and axonal marker SNAP-25 colocalizes with GAP-43 in G-Phos varicosities. (a) Though SNAP-25 staining in the wild-type (WT) mouse is uniform throughout the stratum lucidum, in the same region of transgenic mice overexpressing GAP-43 (G-Phos), it becomes more intense particularly at distal-most portions of CA3a. (b) Low-power images of hippocampal tissue from a G-Phos mouse double-labeled for GAP-43 and SNAP-25 suggest that the two axonal proteins are colocalized in the presynaptic varicosities in G-Phos mice. (c) Confocal 0.5 µm optical section of the boxed area from (b) confirms the colocalization of the two proteins in the irregular-shaped, varicosities of G-Phos mice. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
There are some areas in Figure 3c that could be interpreted as evidence for the presence of both proteins in some MFs in the SL given the high density of mossy fiber terminals and axons in the area examined and the overlapping of GAP-43 (green) and ZnT3 (red) fibers. However, closer examination of a confocal microsopic z-series (rather than an individual optical section) through the SL of the hippocampus of a G-Phos mouse (Fig. 4) clearly shows the distinct separation of GAP-43+ (green) and ZnT3+ terminals in the SL. Moreover, the lack of specific colocalization of the signals at high magnification stands in contrast to that seen with other antigen pairings (see Figs. 8b,c and 9a,b).
FIGURE 9.
(a) Both synaptophysin (SYN), a synaptic vesicle-associated protein, and zinc transporter (ZnT3) protein, that transports zinc into vesicles, are located in the mossy fiber terminals situated in the stratum lucidum of CA3. (b) Confocal imaging (0.5 micron optical section) shows the similar punctate pattern and the colocalization of these two markers. (c) However, confocal imaging from an adjacent section stained for GAP-43 and synaptophysin (SYN) demonstrates no apparent colocalization of these two proteins. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
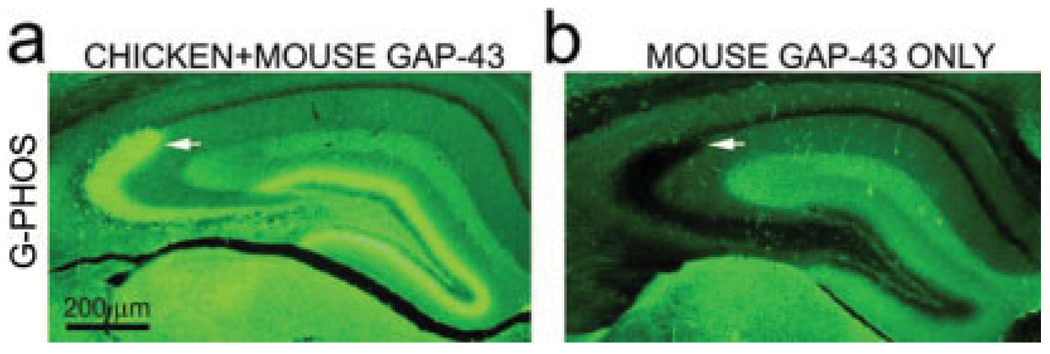
To determine whether G+/Z− varicosities contained transgenic and/or endogenous GAP-43, a comparison was made of the staining of adjacent sections from a G-Phos mouse using two different antibodies, one which only recognizes endogenous mouse GAP-43, and the other which recognizes both the endogenous mouse GAP-43 and transgenic chicken GAP-43 protein (Figs. 5a,b). As can be seen, no staining of varicosities was observed in the sections stained with the antibody that only recognizes endogenous, mouse GAP-43 indicating that these varicosities only contain the transgenic chick GAP-43 protein.
FIGURE 5.
GAP-43 immunoreactivity in the stratum lucidum is due to the presence of transgenic not wild type protein. (a) Low-power photomicrographs of hippocampal sections from a G-Phos mouse show that a section stained with an antibody that recognizes both chicken and mouse GAP-43 (Sigma) clearly has GAP-43 immunoreactivity in MF termination zones(arrow); (b) However, in an adjacent section from the same mouse stained with an antibody that only recognizes mouse GAP-43 (Chemicon) there is no detectable GAP-43 immunoreactivity in the MF region. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
GAP-43-Positive Staining Originates From Granule Cells, and not From Mossy Cells or Astrocytes
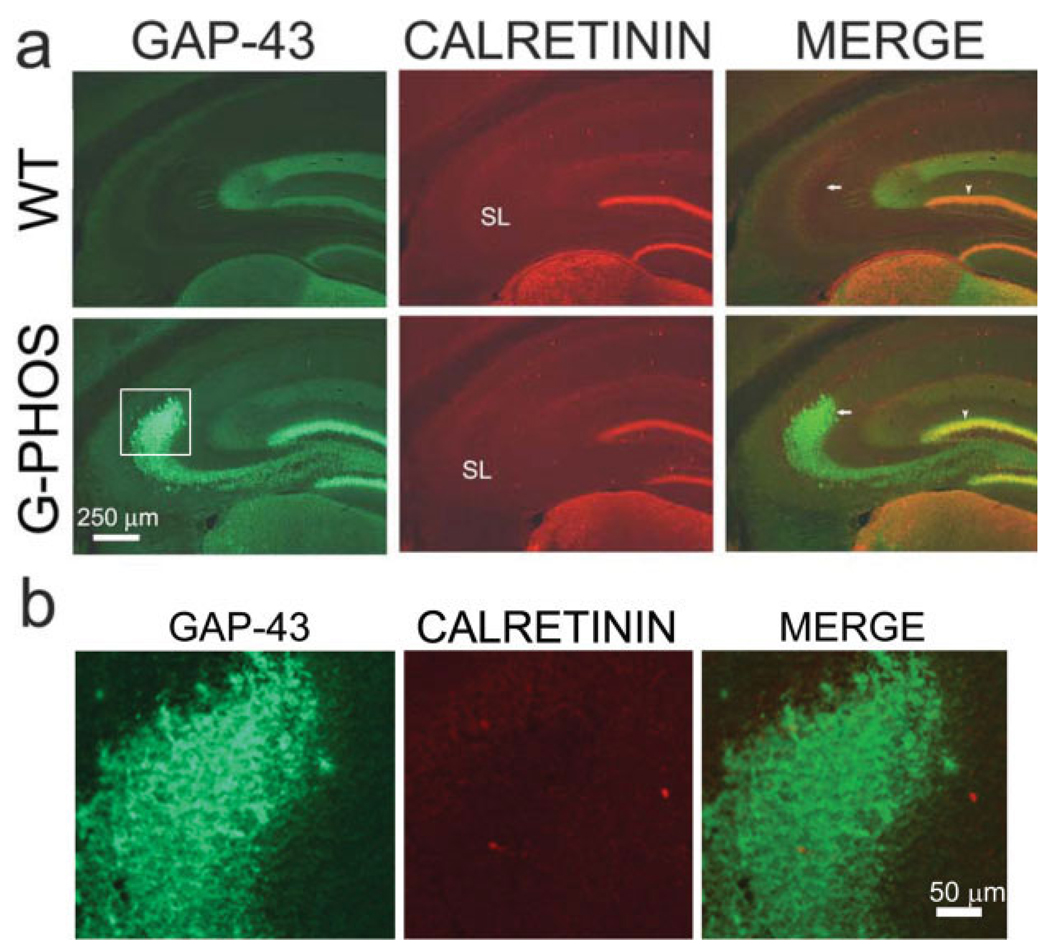
Because we were unable to detect colocalization of transgenic GAP-43 and ZnT3, we explored the possibility that the transgenic GAP-43-positive staining seen in SL was derived from neurons other than granule cells. We therefore assessed the contribution of nearby hilar mossy cells, which express high levels of endogenous GAP-43 (Namgung et al., 1997). As these hilar cells, but not GCs, express high levels of calretinin, a calcium-binding protein (Gulyas et al., 1992; Liu et al., 1996) we double-immunostained for calretinin and transgenic GAP-43. Mossy cells project to the inner molecular layer of both the ipsi- and the contralateral dentate gyrus (Amaral, 1978; Ribak et al., 1985; Namgung et al., 1997), are positive for calretinin, a calcium-binding protein, and indeed, in mouse they are the only cells in the hilus that stain for this protein (Liu et al., 1996). In the inner molecular layer of both wild-type and transgenic mice dentate gyrus, there was colocalization (yellow) of calretinin (red) and GAP-43 (green; Figs. 6a,b) from the GAP-43 in mossy cell axon terminals. However, in the SL, there was no apparent colocalization of calretinin with the GAP-43 in the transgenic granule cell axon (mossy fiber) varicosities. This experiment clearly illustrates the difference in the same section between colocalization and its absence, the former in the inner molecular layer, the latter within SL. The absence of colocalization in SL would appear to rule out a contribution from mossy cells, leaving the more likely alternative that GAP-43+ varicosities originate from dentate gyrus granule cells.
FIGURE 6.
GAP-43 immunoreactivity in mossy fiber termination zones of G-Phos mice is not from axons of mossy cells which are marked by their expression of calretinin. Sections from wild type (WT) and transgenic GAP-43 overexpression mice (G-Phos) were double-labeled for GAP-43 and calretinin. As would be expected, these two proteins appear to colocalize (yellow) in the inner molecular layer of the dentate gyrus (arrowhead in a), the termination zone for mossy cells in both G-Phos and WT sections. However, no such colocalization is observed in the stratum lucidum of the G-Phos section (SL; arrow in a; area shown in b), the termination zone of granule cells. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
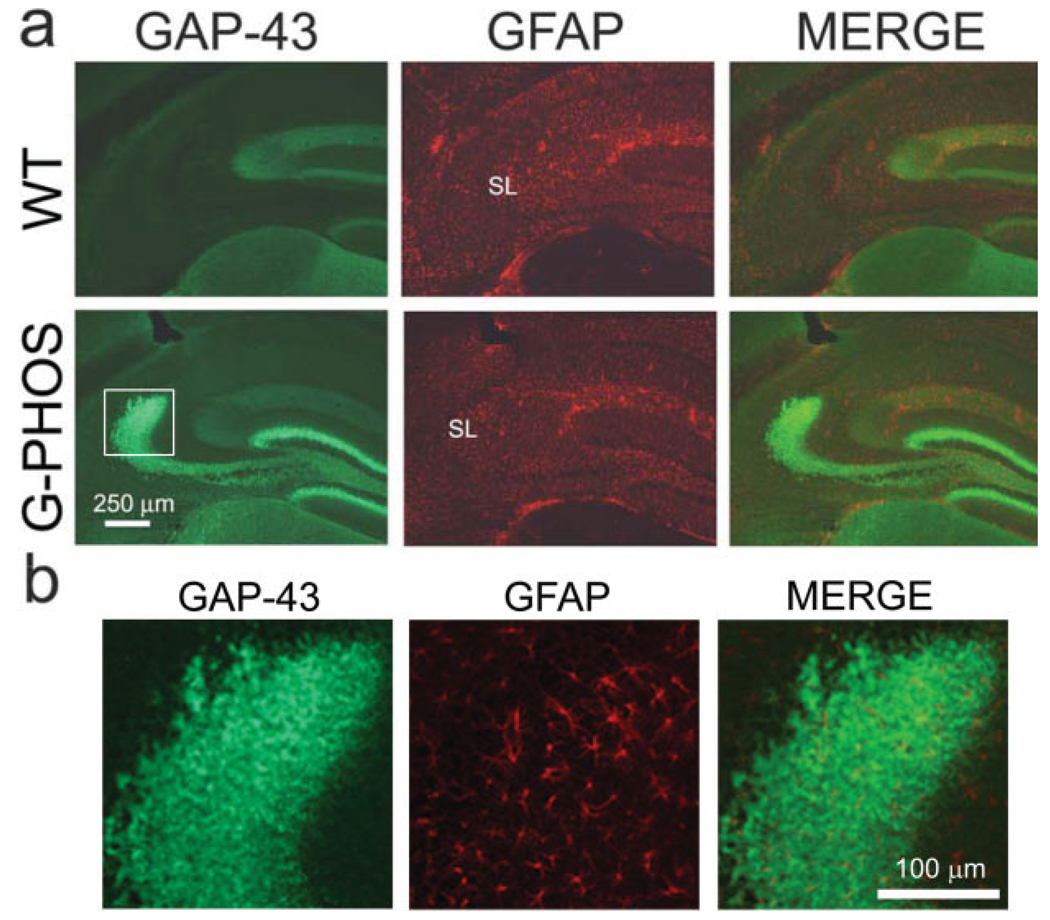
Though the expression of transgenic GAP-43 appears localized to MFs this does not rule out the potential contribution of glial cells as there are instances in cell culture of astrocytes expressing GAP-43 mRNA (Benowitz and Routtenberg, 1997). In wild-type and transgenic mice, astrocytic soma and processes were identified by staining with an antibody specific for glial fibrillary acidic protein (GFAP). GFAP immunoreactivity revealed that astrocytes were visible in the SL of both wild-type and transgenic mice (Figs. 7a,b). However, there were no detectable similarities in the patterns of GFAP and GAP-43+ staining nor was there specific overlap of GAP-43 with that of the GFAP-positive astrocytes making it unlikely that the GAP-43 in SL is of astroglial origin.
FIGURE 7.
There is no evidence that transgenic GAP-43 is located within astroglial cells. The astrocytic marker glial fibrillary acidic protein (GFAP) reveals widespread labeling throughout the hippocampus of both G-Phos and WT mice (a) but does not appear to be colocalized with the GAP-43 transgenic protein (b). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
GAP-43+ Varicosities Colocalize With the Presynaptic Protein SNAP-25
In an attempt to determine whether GAP-43 in SL was present within axonal terminals, albeit those that do not contain ZnT3, we studied SNAP-25, a membrane-bound (Gerst, 1999) soluble N-ethylamide response element (SNARE) protein that is associated with calcium-evoked neurotransmission at excitatory synapses (Sorensen et al., 2002), axonal outgrowth during development (Igarashi et al., 1997), and regenerating axons (Patanow et al., 1997).
Indeed, at low magnification SNAP-25-positive varicosities were “colocated” within MFs: the GAP-43-positive varicosities in hippocampal tissue from G-Phos mice double-stained for SNAP-25 and GAP-43. At high magnification confocal images of SNAP-25-positive structures demonstrated colocalization (yellow) with structures expressing transgenic GAP-43 protein (Fig. 8c), contrasting with the results with GAP-43 and ZnT3 (Fig. 3c). The presence of SNAP-25 in transgenic GAP-43-containing varicosities in G-Phos mice indicates that these enlargements are likely presynaptic terminals.
It may also be noted that there is an apparent increase in SNAP-25 staining in G-Phos mice. The mechanism for this remains unclear, though it is consistent with the idea that compensatory gene expression mechanisms in transgenic mice are not unexpected (Routtenberg, 1996).
GAP-43+ Varicosities Lack Immunoreactivity for Synaptophysin
Given the colocalization of transgenic GAP-43 with SNAP-25, a presynaptic terminal marker, we wished to determine whether transgenic GAP-43 would also colocalize with synaptophysin, a presynaptic vesicle marker. Because the expression of both SNAP-25 and GAP-43 proteins is presynaptic (Duc and Catsicas, 1995; Benowitz and Routtenberg, 1997), it seemed likely that synaptophysin, another presynaptic marker (Sudhof and Jahn, 1991), would also localize to these structures. We were surprised to find, however, no such detectable colocalization (Fig. 9c). In contrast, synaptophysin did colocalize with ZnT3 suggesting colocalization of these two proteins within synaptic terminals of the SL of transgenic mice MFTFs (Figs. 9a,b). The apparent absence of localization of synaptophysin with GAP-43 indicates that either these two antigens are in different terminals, or, less likely but possible, in different compartments of the same terminal.
DISCUSSION
When Aigner et al. (1995) reported on the hypertrophic and ectopic growth of mossy fibers into the SO, it was the first in vivo demonstration that increased levels of GAP-43 protein induced increased axonal outgrowth. Although such ectopic expansion was not observed in the present report, nonetheless growth was indeed observed in G-Phos mice within the SL, the known target region of the mossy fibers, consistent with the growth-promoting function of GAP-43.
After the unexpected differences in Timm’s staining between WT and G-Phos mice were seen, the mossy fiber system in these transgenic mice was characterized in some detail. In low power photomicrographs the ZnT3 marker for MFs and GAP-43 appeared to be within MFs and MFTFs, but, unexpectedly, confocal images taken at higher power revealed little evidence for colocalization. The overall pattern of immunohistochemical results suggested the possibility of at least two populations of granule cells in the G-Phos mouse: one containing elevated transgenic GAP-43 but no apparent zinc transporter and the other elevated zinc transporter but no apparent transgenic GAP-43, designated G+/Z− and G−/Z+ respectively.
Because the above claim of absence of colocalization is limited by and therefore a function of the sensitivity of the method of detection, the present studies nonetheless strongly encourage the view that GAP-43 is not detected in zincergic terminals marked by ZnT3. The apparent absence in confocal images of yellow colocalization indices in the z-stack of Figure 4, provides persuasive evidence for the absence of colocalization of GAP-43 and ZnT3 staining. This stands in stark contrast to the pattern of dual channel immunofluorescence and multiple yellow containing terminal-like structures seen using confocal microscopy for tissue stained with antibodies specific for SNAP-25 and GAP-43 (Figs. 8b,c). Given this pattern of results, the provisional conclusion seems warranted that GAP-43 and ZnT3 are in different mossy fiber terminals in the G-Phos mouse. The distribution and size of G+/Z− terminals and the lack of any colocalization with either calretinin or GFAP strongly support our assertion that these structures extend from hippocampal granule cells. Furthermore, the pattern of transgenic GAP-43 immunoreactivity throughout the stratum lucidum and, in particular, along the IIPMF (see Fig. 1b) follows the stereotypic pattern observed for zinc-positive mossy fibers. Although it cannot be definitively ruled out that some of the terminals in SL originate from mossy fiber-associated interneurons (Vida and Frotscher, 2000), we find it highly likely, given the high density of GAP-43 staining in MFs, that the vast majority of GAP-43 structures in SL are indeed in nonzinc containing MFs. Dual channel immunoelectron microscopy using two different diameters of gold particles is one converging approach that would be useful in addressing this issue.
The apparently dichotomous (G+/Z− and G−/Z+) nature of the MF pathway seen in transgenic mice may arise because GAP-43 expression is driven by the Thy-1 promoter which is not activated in all granule cells (Feng et al., 2000; Vuksic et al., 2008). While DNA integration can contribute to some of this variation, Feng et al. (2000) were quite explicit in their assertion that GFP expression driven by the Thy-1 promoter is primarily due to endogenous Thy-1 expression. Indeed, in Thy-1/GFP mice there is a high correlation of GFP expression with expression of endogenous Thy-1 protein (Feng et al., 2000). Such differentiation of Thy-1 promoter regulation suggests the possibility of a differential array of transcription factors in the individual granule cells. Indeed, there are other examples of such gene expression heterogeneity as specific molecules are expressed by some but not all MF terminals. For example, the neuropeptide dynorphin I is expressed by a seemingly random subset of MF terminals in the mouse SL (Gall, 1988).
Aigner et al. (1995) reported that the three different isoforms of GAP-43, G-Phos, G-Perm, and G-NonP (as denoted in Routtenberg et al., 2000) demonstrated ectopic growth of MFs with the G-Phos mice studied here showing more prominent growth than the other two isoforms. The surprising results of the present report prompted a follow-up study (Holahan et al., 2009) of the G-NonP and G-Perm mice, the former overexpresses a nonphosphorylatable GAP-43, the latter a pseudophosphorylated GAP-43. Unlike the G-Phos mice of the present study, to our surprise G-NonP animals demonstrated ectopic growth of MFs into the SO of CA3. By what mechanisms unphosphorylatable GAP-43, but not phosphorylatable GAP-43, promotes ectopic MF growth is yet to be determined (Routtenberg, 2009).
The present findings would appear to require an alternative interpretation to that offered by Aigner et al. (1995) concerning the growth promotion by GAP-43 in MFs. An important assumption made in the present report is that the ZnT3 antibody is in fact marking MFs. The evidence appears persuasive. First, gross expression of the MF marker, ZnT3, is identical to the pattern of results observed with the Timm’s staining method (Palmiter et al., 1996). Second, there is a dose-dependence between the amount of Timm’s staining that is present and the gene dosage of ZnT3 as hemizygous ZnT3 mutants display less Timm’s staining than wild-type mice and null mutants exhibit no detectable Timm’s staining at all (Cole et al., 1999). Third, it has been shown that the presence of the ZnT3 gene is critical for the transport into MF boutons of elemental zinc, which is stained by the Timm’s method (Linkous et al., 2008). Thus, the same MFs detected using the ZnT3 method are also detected using Timm’s staining. Finally, several recent reports from our laboratory (e.g., Rekart et al., 2007a) indicate that when learning-induced MF growth into SO is observed with Timm’s stain it is paralleled by an increase in ZnT3 staining.
Given the strong linkage between Timm’s and ZnT3 staining it appears that MF growth occurs in axons that do and do not overexpress transgenic GAP-43. Thus, growth may occur as a “trans” effect because increased growth occurred in mossy fiber axons that did not contain GAP-43 (G−/Z+). While several potential explanations exists, one of interest is that of transaxonal communication occurring between the two hypothesized populations of mossy fibers within the hippocampus which may be mediated by cell adhesion molecule interactions (e.g., Zhang et al., 2005).
The idea that there is more than one population of granule cells may be related to the different ages of the granule cells as a consequence of adult neurogenesis. Could the unique neurogenic properties of adult granule cells thus contribute to the present results? One possibility is that the G+/Z− axons arise from more recently born granule cells in which the Thy-1 transcription factor “package” is in place to activate the GAP-43 transgene. This transcription array is then not present in older granule cells with the G−/Z+ expression profile. Thus, the “zinc only” (i.e., G−/Z+) axons would be derived from axons originating from granule cells expressing zinc in the mossy fiber termination zones of the mouse as early as the third postnatal day (P3; Slomianka and Geneser, 1997). Note that this is several days before the Thy1.2 promoter driving the GAP-43 transgene is active (Aigner et al., 1995; Caroni, 1997). Indeed, such an arrangement may explain why G+/Z− axons were SNAP-25-positive but failed to express detectable levels of synaptophysin. In the developing rat forebrain axon terminals express SNAP-25 several days before synaptophysin (Igarashi et al., 1997). This suggests that GAP-43-positive MFs (G+/Zn−) may be derived from “younger” granule cells than those MFs expressing synaptophysin and zinc (G−/Zn+). These developmental mechanisms and the earlier proposed different transcription factor arrays may both contribute to the granule cell heterogeneity observed here.
Acknowledgments
The authors thank Dr. Pico Caroni for providing the GAP-43 transgenic mice, Dr. R. Palmiter for providing the ZnT3 antibody, Dr. O. Steward for the GFAP antibody, and Dr. J. Rogers for providing the antibody against calretinin. The authors also thank Ms. Sarah Hatch Berth for her careful technical assistance.
Grant sponsor: NIMH traineeship; Grant number: MH TG 067564 (to J.L.R.); Grant sponsor: NSF; Grant number: 980090723; Grant sponsor: NIMH; Grant number: MH65436–06 (to A.R.).
REFERENCES
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner HR, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. doi: 10.1016/0092-8674(95)90168-x. [DOI] [PubMed] [Google Scholar]
- Amaral DG. A Golgi study of cell types in the hilar region of the hippocampus in the rat. J Comp Neurol. 1978;182(4 Pt 2):851–914. doi: 10.1002/cne.901820508. [DOI] [PubMed] [Google Scholar]
- Baizer L, Alkan S, Stocker K, Ciment G. Chicken growth-associated protein (GAP)-43: Primary structure and regulated expression of mRNA during embryogenesis. Brain Res Mol Brain Res. 1990;7:61–68. doi: 10.1016/0169-328x(90)90074-n. [DOI] [PubMed] [Google Scholar]
- Bendotti C, Baldessari S, Pende M, Southgate T, Guglielmetti F, Samanin R. Relationship between GAP-43 expression in the dentate gyrus and synaptic reorganization of hippocampal mossy fibres in rats treated with kainic acid. Eur J Neurosci. 1997;9:93–101. doi: 10.1111/j.1460-9568.1997.tb01357.x. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Routtenberg A. GAP-43: An intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/s0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- Cantallops I, Routtenberg A. Rapid induction by kainic acid of both axonal growth and F1/GAP-43 protein in the adult rat hippocampal granule cells. J Comp Neurol. 1996;366:303–319. doi: 10.1002/(SICI)1096-9861(19960304)366:2<303::AID-CNE9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Cantallops I, Routtenberg A. Activity-dependent regulation of axonal growth: Posttranscriptional control of the GAP-43 gene by the NMDA receptor in developing hippocampus. J Neurobiol. 1999;41:208–220. [PubMed] [Google Scholar]
- Cantallops I, Routtenberg A. Kainic acid induction of mossy fiber sprouting: Dependence on mouse strain. Hippocampus. 2000;10:269–273. doi: 10.1002/1098-1063(2000)10:3<269::AID-HIPO7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Caroni P. Overexpression of growth-associated proteins in the neurons of adult transgenic mice. J Neurosci Methods. 1997;71:3–9. doi: 10.1016/s0165-0270(96)00121-5. [DOI] [PubMed] [Google Scholar]
- Cimler BM, Giebelhaus DH, Wakim BT, Storm DR, Moon RT. Characterization of murine cDNAs encoding P-57, a neural-specific calmodulin-binding protein. J Biol Chem. 1987;262:12158–12163. [PubMed] [Google Scholar]
- Claiborne BJ, Amaral DG, Cowan WM. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J Comp Neurol. 1986;246:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- Cole TB, Wenzel HJ, Kafer KE, Schwartzkroin PA, Palmiter RD. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc Natl Acad Sci USA. 1999;96:1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duc C, Catsicas S. Ultrastructural localization of SNAP-25 within the rat spinal cord and peripheral nervous system. J Comp Neurol. 1995;356:152–163. doi: 10.1002/cne.903560111. [DOI] [PubMed] [Google Scholar]
- Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- Gaarskjaer FB. Organization of the mossy fiber system of the rat studied in extended hippocampi. II. Experimental analysis of fiber distribution with silver impregnation methods. J Comp Neurol. 1978;178:73–88. doi: 10.1002/cne.901780105. [DOI] [PubMed] [Google Scholar]
- Gall C. Seizures induce dramatic and distinctly different changes in enkephalin, dynorphin, and CCK immunoreactivities in mouse hippocampal mossy fibers. J Neurosci. 1988;8:1852–1862. doi: 10.1523/JNEUROSCI.08-06-01852.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerst JE. SNAREs and SNARE regulators in membrane fusion and exocytosis. Cell Mol Life Sci. 1999;55:707–734. doi: 10.1007/s000180050328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Miettinen R, Jacobowitz DM, Freund TF. Calretinin is present in non-pyramidal cells of the rat hippocampus. I. A new type of neuron specifically associated with the mossy fibre system. Neuroscience. 1992;48:1–27. doi: 10.1016/0306-4522(92)90334-x. [DOI] [PubMed] [Google Scholar]
- Holahan MR, Honegger KS, Routtenberg A. Ectopic growth of hippocampal mossy fibers in a mutated GAP-43 transgenic mouse with impaired spatial memory retention. Hippocampus. 2009 doi: 10.1002/hipo.20635. 10.1002/hipo.20635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holahan MR, Honegger KS, Tabatadze N, Routtenberg A. GAP-43 gene expression regulates information storage. Learn Mem. 2007;14:407–415. doi: 10.1101/lm.581907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi M, Tagaya M, Komiya Y. The soluble N-ethylmaleimide-sensitive factor attached protein receptor complex in growth cones: Molecular aspects of the axon terminal development. J Neurosci. 1997;17:1460–1470. doi: 10.1523/JNEUROSCI.17-04-01460.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkous DH, Flinn JM, Koh JY, Lanzirotti A, Bertsch PM, Jones BF, Giblin LJ, Frederickson CJ. Evidence that the ZNT3 protein controls the total amount of elemental zinc in synaptic vesicles. J Histochem Cytochem. 2008;56:3–6. doi: 10.1369/jhc.6A7035.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fujise N, Kosaka T. Distribution of calretinin immunoreactivity in the mouse dentate gyrus. I. General description. Exp Brain Res. 1996;108:389–403. doi: 10.1007/BF00227262. [DOI] [PubMed] [Google Scholar]
- Maier DL, Mani S, Donovan SL, Soppet D, Tessarollo L, McCasland JS, Meiri KF. Disrupted cortical map and absence of cortical barrels in growth- associated protein (GAP)-43 knockout mice. Proc Natl Acad Sci USA. 1999;96:9397–9402. doi: 10.1073/pnas.96.16.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science. 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- McNamara RK, Routtenberg A. NMDA receptor blockade prevents kainate induction of protein F1/GAP-43 mRNA in hippocampal granule cells and subsequent mossy fiber sprouting in the rat. Brain Res Mol Brain Res. 1995;33:22–28. doi: 10.1016/0169-328x(95)00083-5. [DOI] [PubMed] [Google Scholar]
- Meberg PJ, Gall CM, Routtenberg A. Induction of F1/GAP-43 gene expression in hippocampal granule cells after seizures [corrected] Brain Res Mol Brain Res. 1993;17:295–299. doi: 10.1016/0169-328x(93)90014-g. [DOI] [PubMed] [Google Scholar]
- Meberg PJ, Routtenberg A. Selective expression of protein F1/(GAP-43) mRNA in pyramidal but not granule cells of the hippocampus. Neuroscience. 1991;45:721–733. doi: 10.1016/0306-4522(91)90284-u. [DOI] [PubMed] [Google Scholar]
- Meiri KF, Saffell JL, Walsh FS, Doherty P. Neurite outgrowth stimulated by neural cell adhesion molecules requires growth-associated protein-43 (GAP-43) function and is associated with GAP-43 phosphorylation in growth cones. J Neurosci. 1998;18:10429–10437. doi: 10.1523/JNEUROSCI.18-24-10429.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namgung U, Matsuyama S, Routtenberg A. Long-term potentiation activates the GAP-43 promoter: Selective participation of hippocampal mossy cells. Proc Natl Acad Sci USA. 1997;94:11675–11680. doi: 10.1073/pnas.94.21.11675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namgung U, Routtenberg A. Transcriptional and post-transcriptional regulation of a brain growth protein: Regional differentiation and regeneration induction of GAP-43. Eur J Neurosci. 2000;12:3124–3136. doi: 10.1046/j.1460-9568.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- Palmiter RD, Cole TB, Quaife CJ, Findley SD. ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc Natl Acad Sci USA. 1996;93:14934–14939. doi: 10.1073/pnas.93.25.14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patanow CM, Day JR, Billingsley ML. Alterations in hippocampal expression of SNAP-25, GAP-43, stannin and glial fibrillary acidic protein following mechanical and trimethyltin-induced injury in the rat. Neuroscience. 1997;76:187–202. doi: 10.1016/s0306-4522(96)00335-1. [DOI] [PubMed] [Google Scholar]
- Rekart JL, Sandoval CJ, Bermudez-Rattoni F, Routtenberg A. Remodeling of hippocampal mossy fibers is selectively induced seven days after the acquisition of a spatial but not a cued reference memory task. Learn Mem. 2007a;14:416–421. doi: 10.1101/lm.516507. [DOI] [PubMed] [Google Scholar]
- Rekart JL, Sandoval CJ, Routtenberg A. Learning-induced axonal remodeling: Evolutionary divergence and conservation of two components of the mossy fiber system within Rodentia. Neurobiol Learn Mem. 2007b;87:225–235. doi: 10.1016/j.nlm.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Seress L, Amaral DG. The development, ultrastructure and synaptic connections of the mossy cells of the dentate gyrus. J Neurocytol. 1985;14:835–857. doi: 10.1007/BF01170832. [DOI] [PubMed] [Google Scholar]
- Rosenthal A, Chan SY, Henzel W, Haskell C, Kuang WJ, Chen E, Wilcox JN, Ullrich A, Goeddel DV, Routtenberg A. Primary structure and mRNA localization of protein F1 (GAP-43), a growth-related protein kinase C substrate associated with synaptic plasticity. Embo J. 1987;6:3641–3646. doi: 10.1002/j.1460-2075.1987.tb02696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routtenberg A. Protein kinase C activation leading to protein F1 (GAP-43) phosphorylation may regulate synaptic plasticity by presynaptic terminal growth. Behav Neural Biol. 1985;44:186–200. doi: 10.1016/s0163-1047(85)90184-0. [DOI] [PubMed] [Google Scholar]
- Routtenberg A. Reverse piedpiperase: Is the knockout mouse leading neuroscientists to a watery end? Trends Neurosci. 1996;19:47–48. doi: 10.1016/S0166-2236(96)20051-7. [DOI] [PubMed] [Google Scholar]
- Routtenberg A, Cantallops I, Zaffuto S, Serrano P, Namgung U. Enhanced learning after genetic overexpression of a brain growth protein. Proc Natl Acad Sci USA. 2000;97:7657–7662. doi: 10.1073/pnas.97.13.7657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routtenberg A. Adult learning and remodeling of hippocampal mossy fibers: unheralded participant in circuitry for long-lasting spatial memory. Hippocampus. 2009 doi: 10.1002/hipo.20664. 10.1002/hipo.20664. [DOI] [PubMed] [Google Scholar]
- Slomianka L, Geneser FA. Postnatal development of zinc-containing cells and neuropil in the hippocampal region of the mouse. Hippocampus. 1997;7:321–340. doi: 10.1002/(SICI)1098-1063(1997)7:3<321::AID-HIPO7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Sorensen JB, Matti U, Wei SH, Nehring RB, Voets T, Ashery U, Binz T, Neher E, Rettig J. The SNARE protein SNAP-25 is linked to fast calcium triggering of exocytosis. Proc Natl Acad Sci USA. 2002;99:1627–1632. doi: 10.1073/pnas.251673298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter SM, Fankhauser C, Huang PL, Mashimo H, Fishman MC. Neuronal pathfinding is abnormal in mice lacking the neuronal growth cone protein GAP-43. Cell. 1995;80:445–452. doi: 10.1016/0092-8674(95)90495-6. [DOI] [PubMed] [Google Scholar]
- Sudhof TC, Jahn R. Proteins of synaptic vesicles involved in exocytosis and membrane recycling. Neuron. 1991;6:665–677. doi: 10.1016/0896-6273(91)90165-v. [DOI] [PubMed] [Google Scholar]
- Vida I, Frotscher M. A hippocampal interneuron associated with the mossy fiber system. Proc Natl Acad Sci USA. 2000;97:1275–1280. doi: 10.1073/pnas.97.3.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuksic M, Del Turco D, Bas Orth C, Burbach GJ, Feng G, Muller CM, Schwarzacher SW, Deller T. 3D-reconstruction and functional properties of GFP-positive and GFP-negative granule cells in the fascia dentata of the Thy1-GFP mouse. Hippocampus. 2008;18:364–375. doi: 10.1002/hipo.20398. [DOI] [PubMed] [Google Scholar]
- Wisden W, Morris BJ. In situ hybridization with radiolabelled probes. In: Wisden W, Morris BJ, editors. In Situ Hybridization Protocols for the Brain. 2 ed. London: Academic Press; 2002. pp. 3–59. [Google Scholar]
- Young E, Cesena T, Meiri KF, Perrone-Bizzozero NI. Changes in protein kinase C (PKC) activity, isozyme translocation, and GAP-43 phosphorylation in the rat hippocampal formation after a single-trial contextual fear conditioning paradigm. Hippocampus. 2002;12:457–464. doi: 10.1002/hipo.10015. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bo X, Schoepfer R, Holtmaat AJ, Verhaagen J, Emson PC, Lieberman AR, Anderson PN. Growth-associated protein GAP-43 and L1 act synergistically to promote regenerative growth of Purkinje cell axons in vivo. Proc Natl Acad Sci USA. 2005;102:14883–14888. doi: 10.1073/pnas.0505164102. [DOI] [PMC free article] [PubMed] [Google Scholar]