Abstract
This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org. Immunoglobulin E (IgE) plays a pivotal role in allergic reactions and asthma through its ability to bind to the mast cell Fc receptor for IgE (FcεRI). Current therapies to suppress such reactions include passive treatment with neutralizing antibodies to IgE that block its binding to FcεRI. In theory, induction of immune tolerance in the B lymphocytes that carry IgE antigen receptors and give rise to IgE secreting cells should provide longer term efficacy. However, recent data have suggested that such memory cells may lack cell surface IgE. Using a gene therapy approach, we show that a recombinant single-chain neutralizing anti-IgE could not only neutralize circulating IgE, but also reduce IgE+ B cell numbers and H-chain transcripts. Therapeutic anti-IgE stimulated a calcium response in primary B cells or in a B cell line expressing membrane IgE and suppressed IgE secretion in vitro suggesting that active signaling through membrane IgE likely promoted tolerance. Interestingly, upon subsequent challenge of anti-IgE treated mice with an IgE crosslinking reagent capable of inducing activation of IgE-decorated mast cells, an anaphylaxis reaction was induced, apparently via a FcγRIII pathway involving recognition of anti-IgE antibody itself. These studies have important implications for the optimal design of safe and effective anti-IgE therapies and suggest that the IgE memory B cells may be targeted by such genetic antibody therapies.
Keywords: Tolerance, B Cells, Mast Cells, Allergy, Gene Therapy
Introduction
IgE plays critical roles in allergic diseases including asthma, atopic dermatitis and anaphylactic reactions (1). IgE binds to FcεRI α-chain expressed on mast cells and basophils (2). In people with antigen-specific IgE, contact with allergen induces IgE aggregation on mast cells, triggering their activation through the FcεRI signaling machinery. Activated mast cells release chemical mediators such as histamine that can induce life-threatening anaphylaxis. Two approaches can be considered to protect patients from allergic reactions. One is simply to remove IgE from the body. Alternatively, one could attempt to block the binding of IgE to mast cells, an approach that is currently used clinically with the anti-human IgE antibody Omalizumab (3,4).
In this study we wished to investigate whether it was possible to not only block the interaction of IgE with Fc receptors, but also to suppress new IgE responses by providing a tolerogenic stimulus to developing or preexisting IgE memory B cells. In mammalian immunoglobulin E heavy chain (IgHε) loci, there exist exons encoding not only secretory but also membrane forms of ε H-chain. Although it has been suggested that, in the mouse, the memory B cells that give rise to IgE responses express surface IgG1 rather than membrane IgE (mIgE)2 (5), other studies point to a biologically active role of the mIgE as an antigen receptor (6-8). For example, germline truncations of the ε-chain membrane form result in reduced IgE titers, particularly in the secondary response (7). Moreover, exons encoding the membrane form of IgE are conserved over evolution. Previous studies have indicated it should be possible to suppress new IgE production by targeting membrane IgE on B cells (6,8). We show here additional functional evidence that mIgE is expressed on the surface of some activated B cells and can transmit biologically relevant signals. Importantly, we show that mIgE can transmit tolerogenic signals to B cells upon binding of a single chain anti-IgE delivered by a gene therapy approach.
Materials and methods
Mice
Eight to 12-week-old BALB/c mice were used in most experiments. C.129S2-Fcer1atm1Knt/J (FcεRI-/-) mice were purchased from the Jackson Laboratory. Mice were bred and maintained in the TSRI Animal Resources facility according to Institutional Animal Care and Use guidelines.
Cells
293F cells were purchased from Invitrogen. Anti-IgE hybridoma cells R1E4 (9) and EM95 (10) were kindly provided from Drs. D. Conrad and F. Finkelman. Anti-FcR hybridoma cells 2.4G2 (11) were purchased from ATCC.
Development R1E4 scFv
R1E4 variable region sequence was obtained using a 5’-RACE kit (Ambion) following the manufacturer's protocol. The following oligos were used for H chain and L chain 5’-RACE, respectively 5’-AATAGCCCTTGACCAGGCATCC-3’, 5’-CCAGTTGCTAACTGTTCCGTGGA-3’. H chain and L chain genes were PCR amplified respectively (T34, T31 for L chain and T35 and T33 for H chain) and R1E4 scFv (pUb-R1E4) was generated with nested PCR. To generate a membrane tethered single chain antibody, we subcloned this fragment into Spe1 and Xma1 digested pUb-187.1 plasmid (12). This strategy fuses the single chain Fv with rat-IgG1 hinge, CH2 and CH3 domains, followed by a C terminal MHC class I transmembrane/ intracellular domain.
T34: 5'-ctattaattattacaggtgcctgtgcaGACATTGTCTTGACCCAGTCT-3'; T31: 5'-ACCGCCAGAGCCACCTCCGCCTGAACCGCCTCCACCCCGTTTCAATTCCAGCTTGGTGC-3'; T35: 5'-GCGGTTCAGGCGGAGGTGGCTCTGGCGGTGGCGGATCGCAGGTGACTCTGAAAGAGTCT-3'; T33: 5'-cctcccgggtttctgggggctgttgtttcagcTGAGGAGACTGTGACCATGACTC-3' To decrease the antigenicity of R1E4, rat-IgG1 was replaced with mouse IgG1 and several amino acid reversion mutations were introduced to L chain framework regions (Supplementary Fig 1). To generate a secreted form in the final construct (pR1E4) the MHC transmembrane and cytoplasmic domains were excised. The specificity of recombinant single chain membrane R1E4 to IgE was confirmed by the transient transfection to 293F cells and flow cytometry analysis of IgE binding (see Figure 1B). All the plasmids used in in vivo injection were purified with EndoFree Plasmid Maxi kit (QIAGEN). For recombinant R1E4 (rR1E4), pR1E4 antibody coding sequences were inserted into pIRES-Zeocin hrGFP plasmid (pIRES-pR1E4). pIRES-pR1E4 (3 μg) was transfected into 293F cells with Lipofectamine2000 (Invitrogen) and stable cells were established. From this supernatant rR1E4 was purified with rProtein A column (GE).
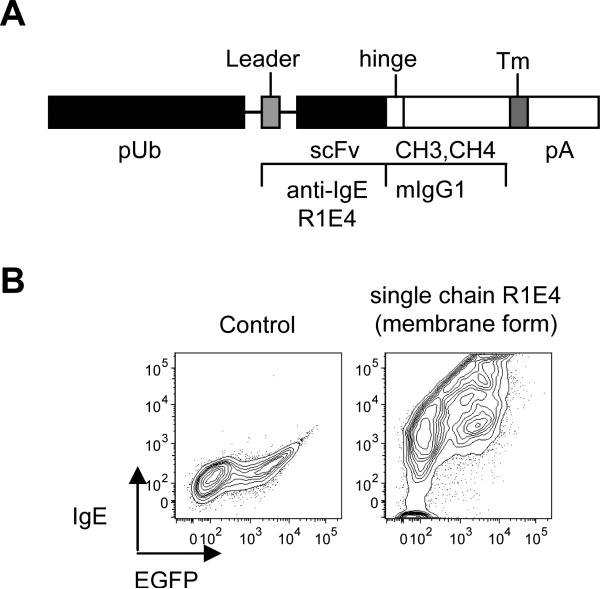
Figure 1.
Generation and characterization of recombinant neutralizing anti-IgE single chain antibody construct.
(A) The gene construct encoding recombinant R1E4 (anti-IgE) showing intron/exon structure and selected features. Introns are depicted as thin lines. R1E4 scFv express under the control of human ubiquitin promoter. The R1E4 scFv was fused to hinge and Fc encoding exons of mouse IgG1 H-chain. For membrane bound anti-IgE expression H-2Kb major histocompatibility complex class I transmembrane coding sequences were included downstream.
(B) Control or recombinant plasmid expressing the membrane form of single chain R1E4 were transiently co-transfected with EGFP plasmid into 293 cells and the ability of the cells to bind to IgE was analyzed by flow cytometry.
qPCR
Total RNA was purified from 2-3 million spleen cells of control or pR1E4-treated mice using RNEazy Plus kit (QIAGEN). Reverse transcription was performed with QuanteTect Rev. Transcription Kit (QIAGEN) following the manufacture's protocol. IgE mRNA was quantitated using SYBR GreenER qPCR Supermix (Invitrogen) with 7900HT (ABI) and normalized with CD19 mRNA. Oligonucleotide primers used for IgE detection were 5’-acactcggagatgcccagatc-3’ and 5’-ggagcaccgttttgatacaggtc-3’; for CD19 detection, 5’-aggtcattgcaaggtcagcagtgtg-3’ and 5’-ggcgtcactttgaagaatctcctg-3’.
Flow cytometry analysis
Erythrocyte-depleted cells were suspended in ice cold staining buffer (HANKS buffer including 0.5 mM EDTA, 0.05 mM Sodium Azide, 0.5% BSA) with appropriately titrated antibodies. The following antibodies were used: CD45R/B220 (RA3-6B2, BD)(Pacific Blue), FcεRIα (MAR-1, eBio)(PE), IgE (23G3, eBio or EM95)(FITC, PE, Alexa-647), CD49b (HMa2, BD)(PE, APC, Bio), c-kit (2B8, eBio)(APC, Bio), CD4 (GK1.5, BD)(PerCP-Cy5.5), CD8 (53-6.7, BD) ( PerCP-Cy5.5), SA-PE-Cy7 (eBio). For intracellular IgE staining, cells incubated during surface staining with unlabeled anti-IgE (EM95); after fixation and permeabilization using a kit (Cytofix/Cytoperm, BD) cells were stained with labeled EM95 conjugate. These antibodies were purchased from eBiosciences, or BD Biosciences as indicated. Propidium iodide (Invitrogen) was included in some experiments to exclude dead cells. To calculate total FcεRI expression level on basophils based on IgE binding capacity, Fcγ receptors were pre-blocked with 2.4G2 for 10 minutes, the cells further incubated with purified IgE (IgELa) at 10μg/ml for 30 minutes. Cells were then washed twice with FACS buffer and bound IgE quantitated with anti-IgE conjugate. Data collection was done on LSRII flow cytometer (BD) and was analyzed using FlowJo software (TriStar).
Hydrodynamic injection
Thirty μg purified plasmid (pR1E4 or pUb control plasmid) was dissolved in 1.8 ml TransIT®-EE Delivery Solution (Mirus Bio Corporation) and injected via tail-vein. In the experiments depicted in Figure 2, 10 μg of a second plasmid driving human placental secreted alkaline phosphatase (pLIVE-SEAP, Mirus Bio Corporation) was coinjected, allowing one to monitor the efficiency of transfection by enzyme activity appearing in blood. All of the injected mice (5/5 control and 8/8 pR1E4-treated) analyzed on d13 post plasmid injection were alkaline phosphatase positive (data not shown).
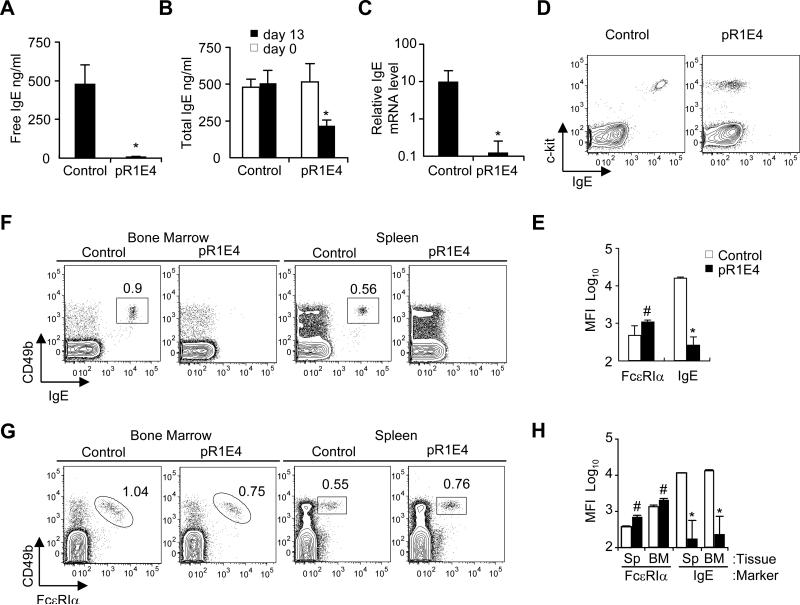
Figure 2.
Effect of in vivo expression of secreted form of chimeric single chain anti-IgE on markers of IgE expression. Two month old BALB/c mice were given pR1E4 or control plasmid i.v. and analyzed 13 days later.
(A) Free serum IgE level measured on d13 after plasmid treatment.
(B) Total serum IgE concentration before treatment (open bars) and 13 days after treatment (filled bars).
(C) qPCR comparison of relative IgE mRNA levels in the spleen of control or pR1E4-treated mice at d13 of treatment.
(D,E) Flow cytometry analysis of IgE bound to peritoneal mast cells in control and pR1E4-treated mice. Peritoneal mast cells were defined as c-kit+FcεRI+CD4-CD8-B220- cells. Mean fluorescence intensity (MFI) of binding by FcεRIα and anti-IgE antibodies was monitored.
(F,G) Analysis of IgE bound to basophils in the spleen and bone marrow of control or treated individual mice. Basophils were identified as CD49b+FcεRI+CD4-CD8-B220-.
(H) Levels of surface IgE and FcεRI on basophils in the spleen and bone marrow on day 13 post plasmid treatment. Results are means ± s.d. of 8 mice receiving pR1E4 compared to 5 mice receiving empty vector. Statistical significance of differences between pR1E4-treated and control-treated mice was calculated using Student's T test: *P < .001; #P < 0.2.
IgE-eliciting immunizations
Ovalbumin (Sigma) was prepared with alum (Imject, Pierce) at a ratio of 10 μg protein/100 μg alum/mouse and was given intraperitoneally. Goat anti-mouse IgD (0.2 ml, eBio) was injected intraperitoneally.
IgE ELISA
IgE ELISA quantitation kit was purchased from Bethyl and used following the kit instructions. In experiments involving pR1E4, purified EM95 (anti-IgE) was coated on Nunc Maxisorp plates and bound serum IgE detected with HRP conjugated goat anti-mouse IgE (Bethyl). The presence of rR1E4 did not interfere with the total IgE concentration measurement because R1E4 and EM95 see non-overlapping epitopes; R1E4 blocks IgE binding to FcεR1 whereas EM95 cannot. For quantitation of free IgE, rR1E4 was coated on the plate and bound IgE detected with HRP conjugated goat anti-mouse IgE. Color was developed with Ultra-TMB (Pierce).
Cell culture
Splenocytes (2×105/ml) were cultured at 37°C, 5% CO2 in a final volume of 2.5 ml with 25 ng/ml IL-4, 1 μg/ml anti-CD40 (1C10, eBio) with or without 20 μg/ml rR1E4 in Advanced-RPMI1640 medium supplemented with 5% FCS, 1x Penicillin-Streptomycin-Glutamine (Gibco), 2 mM GlutaMAX™-I (Gibco), 55 μM 2-Mercaptoethanol for 4 days. For Ca++ mobilization analysis, splenocytes were cultured with 25 μg/ml IL-4, 10 μg/ml anti-CD40. Culture supernatants were then tested for IgE concentration by ELISA; recovered cells were washed 2 times with FACS buffer prior to flow cytometry or Ca++ mobilization analysis.
Calcium response
Cultured splenocytes were loaded with Fluo-4 (Invitrogen) per manufacturer's instructions. Calcium mobilization was induced by addition of 20 μg/ ml mAbs at a cell concentration of 106/ml in volume of 0.5 ml. In some experiments, cells were preincubated with 10 μg/ml anti-mouse IgG1 antibody for 30 min prior to challenge and fluorescence analysis. Analysis was carried out using the FL1 channel of LSR-II flow cytometer and analyzed with FlowJo software.
Anaphylaxis
100 μg EM95 was given per mouse retroorbitally after anesthesia; rectal temperature was monitored with RET-3 probe (Physitemp Instruments) for 60 minutes. In some experiments 0.5 mg 2.4G2 (anti-FcγRII/III) antibody was given to each mouse 24 hours before challenge. In case of rR1E4 injection, 10μg TNP-BSA and 100μg IgE anti-TNP (IgELa) was given per mouse and 50μg rR1E4 was given retroorbitally; rectal temperature was monitored for 60 minutes.
Results
Development of recombinant single chain anti-IgE
In contrast to passive anti-IgE administration, anti-IgE delivery via gene therapy has the potential to provide permanent therapy of IgE-mediated disease. To study the effects of anti-IgE gene therapy in the mouse (an intensively studied model for human allergy, asthma and anaphylaxis (13-15)) we generated a chimeric single chain anti-IgE gene based on neutralizing rat anti-mouse IgE monoclonal antibody (mAb) R1E4 (9). R1E4, like Omalizumab in the human system, is unable to activate mast cells via IgE bound to FcεRI, but binds free IgE. R1E4 immunoglobulin heavy and light chain variable region codons were cloned, sequenced, and joined together with a short linker sequence, yielding a single chain variable fragment (scFv) gene (Figure 1A). The scFv gene was placed upstream of mouse IgG1 hinge and membrane proximal codons. Plasmids driving plasma membrane expression under the control of the human ubiquitin promoter (16) were prepared and validated (Figure 1B). A modified plasmid, called pR1E4, encoding a secreted version of the recombinant R1E4 chimeric protein (rR1E4) was generated. The biological effects of in vivo expression of plasmids encoding membrane or secreted proteins was tested by the hydrodynamic (naked DNA) injection method, which leads to transient expression in the liver. Because tolerance of cognate B cells to protein antigens can be induced either by membrane expression on the liver (17) or by soluble protein (18-20), we also compared in vivo efficacy of plasmids encoding membrane and secreted forms of recombinant anti-IgE.
Neutralizing serum IgE in vivo
pR1E4 plasmid given to BALB/c mice led to single chain anti-IgE secretion lasting at least 30 days that diminished at later times (not shown). At day 13 post pR1E4 injection, levels of free IgE were reduced to ~1% of control mice (Figure 2A). There was also a marked decline in levels of “total IgE”, which includes both free IgE and IgE:rR1E4 complexes (Figure 2B). Plasmid encoding membrane-bound single chain anti-IgE was also effective in reducing IgE levels, though the effect was less long-lasting (Supplementary Figure 2). For unknown reasons, plasmids encoding membrane-bound single chain constructs were apparently somewhat toxic to expressing liver cells, even if they lacked specificity for IgE (data not shown). Therefore, further experiments focused on the effects of the soluble form. Importantly, RNA analysis of the spleens of mice treated with pR1E4 revealed a >99% reduction in levels of IgE H-chain mRNA (Figure 2C). Consistent with the reduced IgE levels in pR1E4-treated mice, c-kit+ peritoneal mast cells and CD49b+ basophils in the bone marrow and spleens of anti-IgE treated mice lacked detectable surface IgE, indicating that their FcεRs had lost bound IgE (Figure 2D-H). Basophils were still present in these tissues as indicated by FcεRIα/CD49b double staining (Figure 2G). In treated mice, FcεRI expression levels in basophils appeared higher than in control plasmid treated mice, however, total IgE binding capacity of basophils was somewhat reduced (Supplementary Figure 3A,B). This discrepancy was probably because anti-FcεRI mAb MAR-1 binds more tightly to FcεRI lacking bound IgE than to FcεRI carrying IgE (Supplementary Figure 3C). Reduced levels of bioactive IgE in pR1E4-treated mice did not lower the percentages or absolute numbers of mast cells and basophils (Table I). Overall, these data support the conclusions that a) gene therapy with recombinant single chain anti-IgE is feasible and can effectively neutralize circulating IgE, b) reduced levels of bioactive IgE have surprisingly minimal negative effects on mast cell and basophil survival, at least at the time point tested, and c) recombinant single chain anti-IgE treatment could actively suppress new IgE production by B cells.
Recombinant anti-IgE treatment of pre-immunized mice
We next determined if pR1E4 could be used to treat and suppress ongoing, established IgE responses. Basal serum IgE levels in mice are quite low (about 500 ng/ml in BALB/c mice and under 100 ng/ml in C57BL/6 mice). To evaluate the anti-IgE treatment, we used two different model responses: ovalbumin immunization with alum adjuvant (ova/alum), which is commonly used to elicit an antigen-specific IgE response, and anti-IgD treatment, a non-specific B cell activator which elicits a stronger, but polyclonal IgE response (21,22).
Ten days after receiving IgE-eliciting stimulus, mice were treated with pR1E4 or control plasmids, then analyzed 12 days later, i.e., 22 days post immunization (Figure 3). Ova/alum immunization increased total serum IgE levels many-fold above preimmune levels, a response that was essentially fully suppressed by treatment with pR1E4 (Figure 3A). IgE mRNA qPCR analysis showed that pR1E4 treatment reduced IgE H-chain mRNA levels in the spleen by 99% (Figure 3B). pR1E4 treatment also reduced the levels of the low affinity IgE receptor CD23 (23) on follicular B cells by ~50% (Supplementary Figure 4A,B). We conclude that in mice with ongoing IgE responses, treatment with the soluble recombinant anti-IgE-producing plasmid pR1E4 could significantly (p=0.0023) suppress levels of serum IgE, new IgE synthesis, and B cell-expressed CD23.
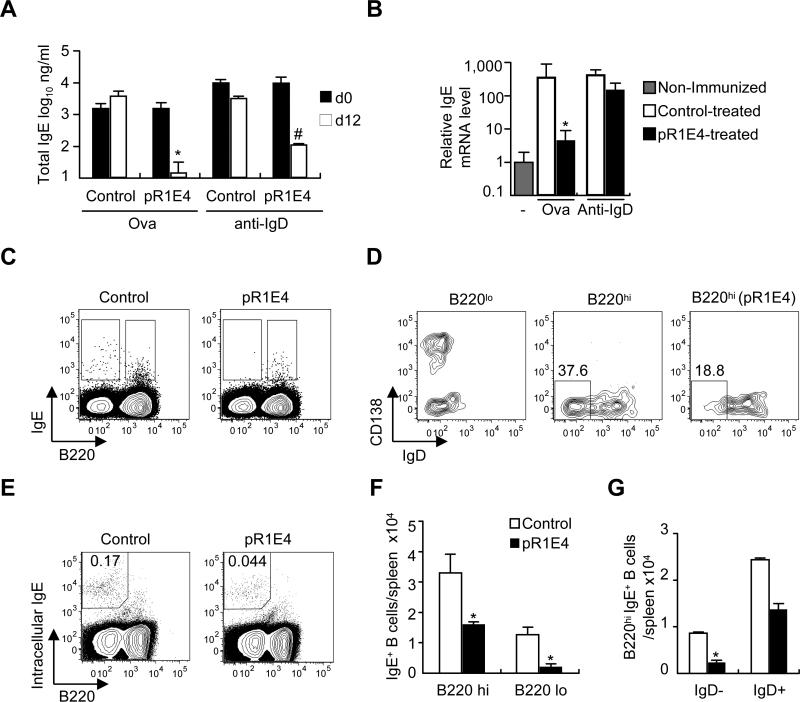
Figure 3.
Effects of pR1E4 plasmid treatment on ongoing IgE responses induced by ovalbumin/alum immunization or goat-anti mouse IgD treatment. Two-month old BALB/c mice injected i.p. ten days previously (day -10) with either 10μg ova/alum or 200μl goat anti-mouse IgD serum were treated with control or pR1E4 plasmids i.v. on d0 and analyzed on d0 and d12 for suppression of the IgE response. (A) Total serum IgE levels on d0 (filled bars) and d12 (open bars). (B) Splenic IgE H-chain mRNA levels assessed by qPCR. (C-G) Flow cytometry analysis of IgE+ B cells in spleens of mice injected with anti-IgD and subsequently treated with control or pR1E4 plasmid. (C) Analysis of the frequencies of B220hi and B220loIgE+ B cells among CD4-CD8-c-kit-CD49b- gated viable spleen cells. (D) Comparison of CD138 and IgD expression of B220lo and B220hi populations in control plasmid and pR1E4-treated mice, as gated in C. Left, B220lo cells from control plasmid-treated mouse; center panel, B220hi cells from control plasmid-treated mouse; right, B220hi cells of pR1E4-treated mouse. (E) Analysis of treated and control anti-IgD-injected mice for the presence of cells that expressed high levels of intracellular IgE. (F) Summary of pR1E4-induced reductions in IgE+B220hi and IgE+B220lo subsets using the analysis in C. (G) Quantitation of pR1E4-induced reduction in surface B220hiIgE+IgD- B cells, as in D. Significance calculated using Student's T test. *P < .01, #P < .05. n= 4-6 mice/group.
Treatment of mice after anti-IgD injection served as a more stringent test of the ability to tolerize ongoing responses because IgE responses were over 10-fold further enhanced over the ova/alum induced response. Anti-IgD injection induced about 10 μg/ml IgE in sera in control mice, which could be markedly (99%) suppressed by subsequent in vivo expression of pR1E4 (Figure 3A). However, IgE mRNA qPCR analysis revealed that mice given pR1E4 after anti-IgD treatment still had high levels 12d later, suggesting that IgE mRNA-producing cells remained in the spleen (Figure 3B).
To identify the source of the IgE mRNA, we analyzed splenocytes from anti-IgD-treated mice for the presence of mIgE+ B cells (Figure 3C-G). In control plasmid-treated mice, B220hi and B220low IgE+ B cells were detected (Figure 3C). B220hi B cells were mostly IgD+CD138- cells, and possibly represented background staining of naive B cells (Figure 3D, center). B220lo B cells were mostly IgD-CD138+ or CD138- cells indicating that they were IgE preplasma cells or B220lo memory B cells (Figure 3D, left). In pR1E4-treated mice, B220hiIgE+ B cells were detected, but most B220loIgE+ B cells were lost. The remaining B220hiIgE+ B cells in pR1E4-treated mice were IgD+CD138- (Figure 3D), suggesting that pR1E4 blocked development of IgE memory B cells and preplasma cells. The frequency of the B220loIgE+ fraction declined in pR1E4-treated mice (p=0.0024, Figure 3F). Intracellular staining for IgE (preblocked for surface staining) was carried out, revealing that plasma cells remained in the spleens of pR1E4-treated mice (Figure 3E). Total numbers of B220lo;intracellular IgE+ cells were on average lower in pR1E4-treated mice compared to control, but not to a statistically significant extent. We conclude that in anti-IgD stimulated mice pR1E4 could neutralize circulating IgE and suppress the numbers of mIgE+ B cells, but could not suppress fully developed IgE plasma cells.
Anti-IgE can block IgE secretion in vitro
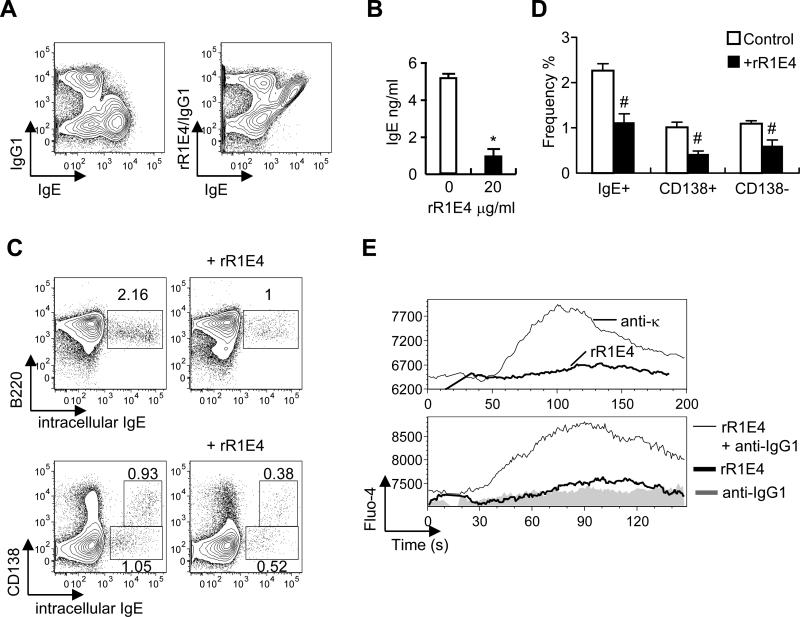
To determine mechanistically how soluble recombinant R1E4 affects IgE B cells, we assessed the effects of R1E4 mAb on cultured naive B cells induced to switch to IgE by the addition of IL-4 and anti-CD40. Although the binding of R1E4 has been mapped to the ε CH3 domain (9), it was not clear if this epitope would be accessible on mIgE+ B cells because on B cells mIg is oriented differently than in solution and is associated with Igα/β signal transducers. We found that mIgE+ B cells could be clearly identified with both R1E4 IgG and recombinant R1E4 antibody (Figure 4A). Moreover, inclusion of recombinant R1E4 both inhibited the secretion of IgE in the supernatant and also reduced the frequency of CD138+ and CD138- IgE+ B cells emerging in the cultures (Figure 4B-D). Ca++ signaling analysis indicated that rR1E4 antibody could induce Ca++ mobilization in mIgE+ B cells upon super-crosslinking by a second antibody (rat anti-mouse IgG1) (Figure 4E). These data indicate that anti-IgE can block the development of IgE plasma cells in vitro and may in part explain the in vivo effects of this treatment.
Figure 4.
Ability of recombinant R1E4 (rR1E4) anti-IgE protein to affect the biological responses of IgE+ B cells generated in tissue culture.
(A) BALB/c spleen cells were induced to undergo IgE class switch by culture at 2×105 cells/ml for 4 days with IL-4 (25 ng/ml) and anti-CD40 (10μg/ml); binding by rR1E4 was examined. Right panel shows staining with rR1E4 (10μg/ml) followed by FITC anti-mouse IgG1 and Alexa647 anti-IgE (EM95). (B,C,D) Spleen cells (2×105 cells/ml) were stimulated for 4 days with IL-4 (25 ng/ml) and anti-CD40 (1μg/ml) with or without 20μg rR1E4. (B) IgE levels in culture supernatants on d4 as measured by ELISA. (C) Analysis of IgE+ B cell and IgE+ plasma cell frequencies at d4 of culture, as determined by staining for intracellular IgE, B220 and CD138. (D) Summary of rR1E4-induced changes in IgE subpopulations, as shown in C. (E) rR1E4-induced calcium flux in fluo-4 loaded B cells recovered at d4 of culture. Top panel, B cells were treated with either anti-Igκ or rR1E4 alone. Lower panel, cells were precultured with anti-IgG1 alone for 30 min (to obscure the response of IgG1 cells) then stimulated with either anti-IgG1 or rR1E4, which is super-crosslinked by free anti-IgG1. Results presented in B and D represent the means and standard deviations of 4 separate experiments. *P < 10-4, #P < .05.
Anaphylaxis reaction
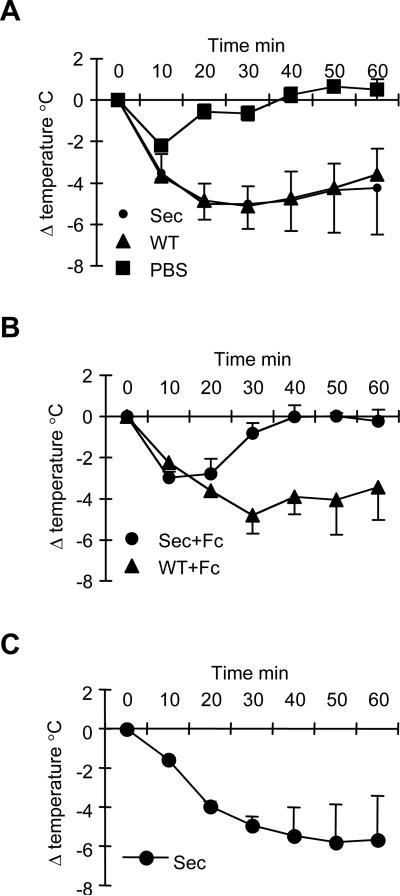
The ability of in vivo pR1E4 treatment to suppress allergic responses was tested by challenging mice on d13 post treatment with an activating anti-IgE, EM95, which has the ability to induce mast cell degranulation and anaphylactic manifestations. Surprisingly, and notwithstanding their suppressed IgE levels, pR1E4-treated mice challenged in this way showed a systemic anaphylaxis reaction as indicated by rapidly lowered body temperature (Figure 5A). Recently, an alternative pathway for anaphylaxis has been elucidated in which IgG1 immune complexes activate FcγRIII on basophils, releasing platelet activating factor (15,24-27). To test the possibility that this pathway was triggered by clustering of anti-IgE antibodies upon challenge, pR1E4-treated or control mice were given FcγRII/III blocking monoclonal antibody 2.4G2 (28) one day prior to challenge. Indeed, anaphylaxis was blocked in pR1E4-treated but not control mice receiving 2.4G2 (Figure 5B). To test if mouse IgG1 R1E4 could induce anaphylaxis directly in an FcεRI independent manner, we pre-injected TNP-BSA and IgE anti-TNP into FcεRIα-/- mice and challenged one hour later with rR1E4. As shown in Figure 5C, mice that received rR1E4 manifested an anaphylaxis reaction, indicating that rR1E4 itself triggered the reaction through Fcγ receptors. Collectively, these data indicate that pR1E4 treatment suppresses FcεRI-mediated anaphylaxis, but permits and in fact promotes FcγRIII-mediated anaphylaxis.
Figure 5.
Systemic anaphylaxis reactions in rR1E4-treated mice.
(A) Untreated BALB/c (WT) mice or pR1E4-treated (Sec) mice were challenged with 100μg activating anti-IgE mAb EM95 and rectal temperatures were measured over 60 minutes.
(B) Mice received 0.5 mg Fc blocking mAb 2.4G2 twenty-four hours before challenge with 100μg anti-IgE EM95. Untreated BALB/c (WT+Fc), pR1E4-treated (Sec+Fc).
(C) FcεRIα-/- mice received 10μg TNP-BSA and 100μg anti-TNP IgE (IgELa) one hr before challenge with 50μg rR1E4. Shown are means and standard deviations of results obtained with the following numbers of mice/group. All groups included 3 mice except pR1E4-treated mice in A which included 4 mice.
Discussion
Although it has been known that anti-IgE antibodies that block IgE:FcεRI interactions prevent acute allergic reactions, it has not been clear that such antibodies have strong effects on mIgE+ B cells nor that a gene therapy approach to their application would be possible. Our data support earlier work of Haba and Nisonoff who demonstrated that high doses of syngeneic anti-IgE could reduce IgE antibody forming responses when given just prior to immunization (6). We show here that expression of an anti-IgE single chain fusion construct can drive prolonged expression of a presumably dimeric anti-IgE protein in vivo, and that this recombinant anti-IgE can block IgE binding to mast cells and basophils, and suppress new IgE production. Because recombinant R1E4 antibody was able to bind to mIgE+ B cells in vitro and to trigger an altered in vitro response of these cells, we conclude that it can trigger signals in mIgE+ B cells. Omalizumab (Xolair) neutralizes circulating IgE in humans, providing relief from allergic symptoms, apparently without reducing the underlying IgE stimulus. In a human trial of Xolair, the serum concentration of free IgE dropped rapidly (to 13.9 ng/ml), whereas the total IgE concentration increased over time (to >1000 ng/ml for 120 days) (29). By contrast, in the present study pR1E4 treatment suppressed both free IgE and total IgE, and RNA analysis indicated that it also suppressed new IgE synthesis. These results may suggest that Xolair fails to suppress mIgE+ B cells. If this is the case, our data suggest the possibility that more effective anti-IgE mAbs may be found with the potential for longer-term benefit. Such antibodies would ideally share Xolair's ability to block IgE:FcεRI interactions, but also be able to suppress IgE+ B cells. Our preliminary studies indicate that Xolair is able to trigger Ca++ mobilization in a B cell line carrying human mIgE. However, it may be that Xolair promotes rather than inhibits IgE production because of the quality of this signal or through its ability to generate high order immune complexes.
We presume that in vivo expression of rR1E4 mediates negative regulation of developing memory IgE B cells, probably by induction of apoptosis. However, the numbers of mIgE+ B cells were too low to directly demonstrate this. Indeed, there is some controversy over whether mIgE+ B cells stably exist in vivo (5). Several studies demonstrate that IgE class switching often occurs through an IgG1 intermediate (30-33). On the other hand, there are clear indications that B cells giving rise to IgE responses must carry mIgE and functionally signal through mIgE, at least for a short time. The membrane exons and protein sequences of mammalian IgEs are well conserved and similar to other membrane immunoglobulins (34). Moreover, mutations or truncations of the membrane exons of IgE severely inhibit IgE responses, particularly secondary responses (7). The membrane form of ε H-chain mRNA may be poorly expressed owing to inefficient polyadenylation signals (35), suggesting that IgE memory B cells may express relatively low mIgE+ levels. Nevertheless, antibodies directed to the membrane form of IgE can have toleragenic effects (8). In the present studies we detected mIgE+ cells and showed that their numbers increased in appropriately immunized mice. Moreover, mIgE+ cells were specifically reduced in pR1E4-treated mice (Figure 3C), indicating that negative regulation of developing IgE B cells by IgE-reactive ligands is possible.
The potency of IgE in allergic reactions is a result of the extraordinarily high affinity of FcεRI for monomeric IgE (Ka=1010 M-1) combined with the powerful biological consequences of FcεRI ligation on mast cells and basophils (2). But the levels of IgE in blood are very low compared to other types of immunoglobulins, facilitating the effectiveness of treatment with passively administered IgE neutralizing antibodies and, as we show here, gene therapy using recombinant anti-IgE plasmid. We expressed the anti-IgE neutralizing antibody as scFv fusion protein with a naked DNA injection method. The merit of this method is that gene expression is potentially long lasting and it also permits the expression of membrane bound proteins in vivo. Importantly, soluble recombinant R1E4 expression lasted more than 3 weeks after plasmid injection and could completely neutralize serum IgE, as measured by the level of free IgE and the levels of IgE bound to mast cells and basophils. Though convenient for proof of principle, plasmid injection provides only transient expression. Other modes of gene transfer, including retroviral transduction, should ultimately be more effective and practical in a clinical setting and may provide permanent IgE suppression. Long term anti-IgE gene expression is predicted to not only suppress new plasma cell formation but also to neutralize IgE secreted by long lived plasma cells that are no longer subject to regulation by surface Ig.
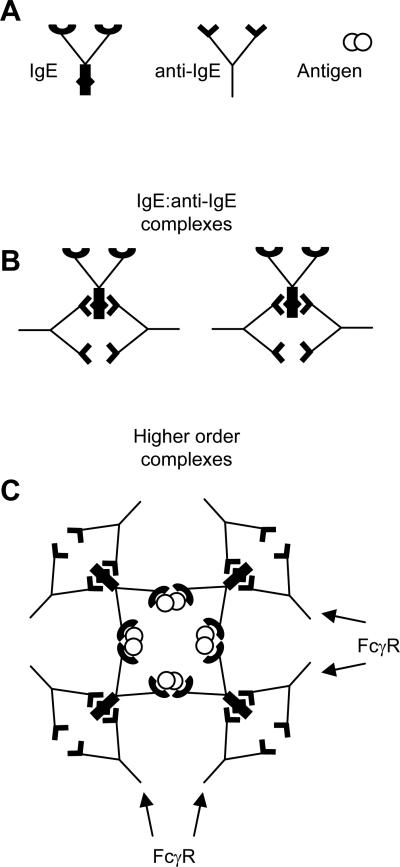
An unexpected finding was that even when IgE was neutralized mice still underwent anaphylaxis upon challenge with an activating anti-IgE stimulus, presumably through a FcγRIII-mediated pathway triggered by crosslinking IgE:rR1E4 complexes. We also showed that rR1E4 administration induced anaphylaxis in mice with IgE:antigen complexes. A comparable reaction may occur in a small fraction of anti-IgE-treated patients. According to the US Food and Drug Administration (http://www.fda.gov/Cder/drug/InfoSheets/HCP/omalizumabHCP.htm), the frequency of anaphylaxis attributed to Xolair use in patients was ≥0.2%. Of reported cases, 39% occurred after the first dose of Xolair, while only 19% occurred with the second dose, indicating the cause of anaphylaxis was not anti-idiotype (anti-Xolair) antibody. Xolair is a humanized IgG1 monoclonal antibody that makes complexes with IgE at various ratios, the dominant complex being a trimer (36). If patients have both IgE and IgG antibodies against the same antigen, or IgE-bound foreign antigen itself, Xolair infusion may form higher order immune complexes and induce anaphylaxis though clustering of FcγRIII (Fig 6). Conceivably, such reactions may be influenced by polymorphisms in FcγRIII and FcγRII (37,38). To prevent anaphylaxis, mutagenesis of therapeutic anti-IgE to prevent FcγRIII binding may be desirable. Alternatively, expression of soluble monovalent or membrane tethered anti-IgE may be used. Overall, our data suggest several ways that anti-IgE therapy can be improved to facilitate safety and longer term effectiveness. We also show the feasibility of providing the antibody by gene therapy, which may provide a strategy to permanently suppress IgE reactions.
Figure 6.
Schematic of how IgE:anti-IgE complexes may promote FcγR activation.
(A) Depicted are IgE, anti-IgE, and a bivalent antigen.
(B) IgG anti-IgE antibodies such as Xolair carry Fc portions able to interact with FcγRs. Upon interaction with IgE, anti-IgE may form small complexes that block binding of IgE to FcεRI and that are of too low valency to bind strongly to Fcγ receptors.
(C) Higher order complexes of IgE:anti-IgE formed by crosslinking with an additional ligand, such as the IgE's cognate antigen (allergen) shown here, permits multipoint binding to Fcγ receptors.
Supplementary Material
Acknowledgments
We thank Dr Fred D. Finkelman and Dr. Daniel Conrad providing R1E4 and EM95 hybridoma cells. We wish to thank Patrick Skog for technical assistance.
Footnotes
Supported by NIH grant R21AI069866 and by a Pfizer Postdoctoral Fellowship to T.O.
Abbreviations used in this paper: mIgE, membrane immunoglobulin E; scFv, single chain immunoglobulin variable fragment; qPCR, quantitative polymerase chain reaction.
References
- 1.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat.Rev.Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 2.Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu.Rev.Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 3.Avila PC. Does anti-IgE therapy help in asthma? Efficacy and controversies. Annu.Rev.Med. 2007;58:185–203. doi: 10.1146/annurev.med.58.061705.145252. [DOI] [PubMed] [Google Scholar]
- 4.Strunk RC, Bloomberg GR. Omalizumab for asthma. N.Engl.J.Med. 2006;354:2689–2695. doi: 10.1056/NEJMct055184. [DOI] [PubMed] [Google Scholar]
- 5.Erazo A, Kutchukhidze N, Leung M, Christ AP, Urban JF, Jr., Curotto de Lafaille MA, Lafaille JJ. Unique maturation program of the IgE response in vivo. Immunity. 2007;26:191–203. doi: 10.1016/j.immuni.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haba S, Nisonoff A. Effects of syngeneic anti-IgE antibodies on the development of IgE memory and on the secondary IgE response. J.Immunol. 1994;152:51–57. [PubMed] [Google Scholar]
- 7.Achatz G, Nitschke L, Lamers MC. Effect of transmembrane and cytoplasmic domains of IgE on the IgE response. Science. 1997;276:409–411. doi: 10.1126/science.276.5311.409. [DOI] [PubMed] [Google Scholar]
- 8.Feichtner S, Infuhr D, Achatz-Straussberger G, Schmid D, Karnowski A, Lamers M, Rhyner C, Crameri R, Achatz G. Targeting the Extracellular Membrane-Proximal Domain of Membrane-Bound IgE by Passive Immunization Blocks IgE Synthesis In Vivo. J.Immunol. 2008;180:5499–5505. doi: 10.4049/jimmunol.180.8.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keegan AD, Fratazzi C, Shopes B, Baird B, Conrad DH. Characterization of new rat anti-mouse IgE monoclonals and their use along with chimeric IgE to further define the site that interacts with Fc epsilon RII and Fc epsilon RI. Mol.Immunol. 1991;28:1149–1154. doi: 10.1016/0161-5890(91)90030-n. [DOI] [PubMed] [Google Scholar]
- 10.Baniyash M, Eshhar Z. Inhibition of IgE binding to mast cells and basophils by monoclonal antibodies to murine IgE. Eur.J.Immunol. 1984;14:799–807. doi: 10.1002/eji.1830140907. [DOI] [PubMed] [Google Scholar]
- 11.Benhamou M, Bonnerot C, Fridman WH, Daeron M. Molecular heterogeneity of murine mast cell Fc gamma receptors. J.Immunol. 1990;144:3071–3077. [PubMed] [Google Scholar]
- 12.Aït-Azzouzene D, Verkoczy L, Peters J, Gavin A, Skog P, Vela JL, Nemazee D. An immunoglobulin C{kappa}-reactive single chain antibody fusion protein induces tolerance through receptor editing in a normal polyclonal immune system. J.Exp.Med. 2005;201:817–828. doi: 10.1084/jem.20041854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kraft S, Kinet JP. New developments in FcepsilonRI regulation, function and inhibition. Nat.Rev.Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 14.Pichavant M, Goya S, Hamelmann E, Gelfand EW, Umetsu DT. Animal models of airway sensitization. Curr.Protoc.Immunol. 2007 doi: 10.1002/0471142735.im1518s79. Chapter 15. [DOI] [PubMed] [Google Scholar]
- 15.Finkelman FD. Anaphylaxis: lessons from mouse models. J.Allergy Clin.Immunol. 2007;120:506–515. doi: 10.1016/j.jaci.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 16.Schorpp M, Jager R, Schellander K, Schenkel J, Wagner EF, Weiher H, Angel P. The human ubiquitin C promoter directs high ubiquitous expression of transgenes in mice. Nucleic Acids Res. 1996;24:1787–1788. doi: 10.1093/nar/24.9.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell DM, Dembic Z, Morahan G, Miller JF, Burki K, Nemazee D. Peripheral deletion of self-reactive B cells. Nature. 1991;354:308–311. doi: 10.1038/354308a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiller JM, Habicht GS, Weigle WO. Kinetic differences in unresponsiveness of thymus and bone marrow cells. Science. 1971;171:813–815. doi: 10.1126/science.171.3973.813. [DOI] [PubMed] [Google Scholar]
- 19.Goodnow CC, Crosbie J, Adelstein S, Lavoie TB, Smith-Gill SJ, Brink RA, Pritchard-Briscoe H, Wotherspoon JS, Loblay RH, Raphael K. Altered immunoglobulin expression and functional silencing of self-reactive B lymphocytes in transgenic mice. Nature. 1988;334:676–682. doi: 10.1038/334676a0. a. et. [DOI] [PubMed] [Google Scholar]
- 20.Goodnow CC, Crosbie J, Jorgensen H, Brink RA, Basten A. Induction of self-tolerance in mature peripheral B lymphocytes [see comments]. Nature. 1989;342:385–91. doi: 10.1038/342385a0. [DOI] [PubMed] [Google Scholar]
- 21.Le Gros G, Schultze N, Walti S, Einsle K, Finkelman F, Kosco-Vilbois MH, Heusser C. The development of IgE+ memory B cells following primary IgE immune responses. Eur.J.Immunol. 1996;26:3042–3047. doi: 10.1002/eji.1830261233. [DOI] [PubMed] [Google Scholar]
- 22.Thyphronitis G, Katona IM, Gause WC, Finkelman FD. Germline and productive C epsilon gene expression during in vivo IgE responses. J.Immunol. 1993;151:4128–4136. [PubMed] [Google Scholar]
- 23.Conrad DH, Ford JW, Sturgill JL, Gibb DR. CD23: an overlooked regulator of allergic disease. Curr.Allergy Asthma Rep. 2007;7:331–337. doi: 10.1007/s11882-007-0050-y. [DOI] [PubMed] [Google Scholar]
- 24.Tsujimura Y, Obata K, Mukai K, Shindou H, Yoshida M, Nishikado H, Kawano Y, Minegishi Y, Shimizu T, Karasuyama H. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity. 2008;28:581–589. doi: 10.1016/j.immuni.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Strait RT, Morris SC, Finkelman FD. IgG-blocking antibodies inhibit IgE-mediated anaphylaxis in vivo through both antigen interception and Fc gamma RIIb cross-linking. J.Clin.Invest. 2006;116:833–841. doi: 10.1172/JCI25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyajima I, Dombrowicz D, Martin TR, Ravetch JV, Kinet JP, Galli SJ. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J.Clin.Invest. 1997;99:901–914. doi: 10.1172/JCI119255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ujike A, Ishikawa Y, Ono M, Yuasa T, Yoshino T, Fukumoto M, Ravetch JV, Takai T. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J.Exp.Med. 1999;189:1573–1579. doi: 10.1084/jem.189.10.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Unkeless JC. Characterization of a monoclonal antibody directed against mouse macrophage and lymphocyte Fc receptors. J.Exp.Med. 1979;150:580–596. doi: 10.1084/jem.150.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milgrom H, Fick RB, Jr., Su JQ, Reimann JD, Bush RK, Watrous ML, Metzger WJ. Treatment of allergic asthma with monoclonal anti-IgE antibody. rhuMAb-E25 Study Group. N.Engl.J.Med. 1999;341:1966–1973. doi: 10.1056/NEJM199912233412603. [DOI] [PubMed] [Google Scholar]
- 30.Takahama H, Ovary Z, Furusawa S. Murine IgG1 and IgE memory B cells. Cell Immunol. 1994;157:369–380. doi: 10.1006/cimm.1994.1234. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida K, Matsuoka M, Usuda S, Mori A, Ishizaka K, Sakano H. Immunoglobulin switch circular DNA in the mouse infected with Nippostrongylus brasiliensis: evidence for successive class switching from mu to epsilon via gamma 1. Proc.Natl.Acad.Sci.U.S.A. 1990;87:7829–7833. doi: 10.1073/pnas.87.20.7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siebenkotten G, Esser C, Wabl M, Radbruch A. The murine IgG1/IgE class switch program. Eur.J.Immunol. 1992;22:1827–1834. doi: 10.1002/eji.1830220723. [DOI] [PubMed] [Google Scholar]
- 33.Mandler R, Finkelman FD, Levine AD, Snapper CM. IL-4 induction of IgE class switching by lipopolysaccharide-activated murine B cells occurs predominantly through sequential switching. J.Immunol. 1993;150:407–418. [PubMed] [Google Scholar]
- 34.Reth M. Antigen receptors on B lymphocytes. Annu.Rev.Immunol. 1992;10:97–121. doi: 10.1146/annurev.iy.10.040192.000525. [DOI] [PubMed] [Google Scholar]
- 35.Karnowski A, Achatz-Straussberger G, Klockenbusch C, Achatz G, Lamers MC. Inefficient processing of mRNA for the membrane form of IgE is a genetic mechanism to limit recruitment of IgE-secreting cells. Eur.J.Immunol. 2006;36:1917–1925. doi: 10.1002/eji.200535495. [DOI] [PubMed] [Google Scholar]
- 36.Fox JA, Hotaling TE, Struble C, Ruppel J, Bates DJ, Schoenhoff MB. Tissue distribution and complex formation with IgE of an anti-IgE antibody after intravenous administration in cynomolgus monkeys. J.Pharmacol.Exp.Ther. 1996;279:1000–1008. [PubMed] [Google Scholar]
- 37.Huizinga TW, Kleijer M, Tetteroo PA, Roos D, dem Borne AE. Biallelic neutrophil Na-antigen system is associated with a polymorphism on the phospho-inositol-linked Fc gamma receptor III (CD16). Blood. 1990;75:213–217. [PubMed] [Google Scholar]
- 38.Leeuwenberg JF, Van de Winkel JG, Jeunhomme TM, Buurman WA. Functional polymorphism of IgG FcRII (CD32) on human neutrophils. Immunology. 1990;71:301–304. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.