Abstract
Spinal muscular atrophy (SMA) is an autosomal recessive neurodegenerative disorder and a leading genetic cause of infantile mortality. SMA is caused by mutation or deletion of Survival Motor Neuron-1 (SMN1). The clinical features of the disease are caused by specific degeneration of α-motor neurons in the spinal cord, leading to muscle weakness, atrophy and, in the majority of cases, premature death. A highly homologous copy gene (SMN2) is retained in almost all SMA patients but fails to generate adequate levels of SMN protein due to its defective splicing pattern. The severity of the SMA phenotype is inversely correlated with SMN2 copy number and the level of full-length SMN protein produced by SMN2 (∼10–15% compared with SMN1). The natural history of SMA has been altered over the past several decades, primarily through supportive care measures, but an effective treatment does not presently exist. However, the common genetic etiology and recent progress in pre-clinical models suggest that SMA is well-suited for the development of therapeutic regimens. We summarize recent advances in translational research that hold promise for the progression towards clinical trials.
INTRODUCTION
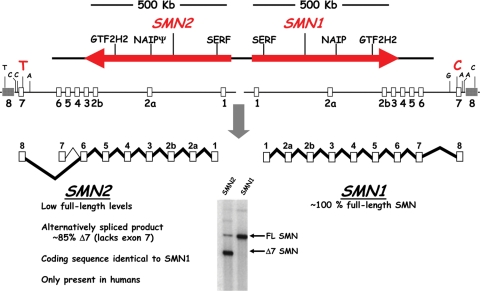
5q-Spinal muscular atrophy (SMA) is an inherited autosomal neurodegenerative disease caused by the homozygous deletion of Survival Motor Neuron-1 (SMN1) (1). The carrier frequency of SMA is ∼1:35 with an incidence of 1 in 6000 live births, making it a leading genetic cause of infantile mortality (2). Clinically, SMA severity spans a broad spectrum based upon the age of onset and the severity of symptoms, including a severe form (type I; Werdnig–Hoffmann disease), an intermediate form (type II) and a less severe disease or ‘juvenile’ form (type III; Kugelberg–Welander disease). All forms of SMA are caused by the loss of SMN1. Humans are the only species that also carry a nearly identical gene called SMN2 (3); however, the majority of SMN2-derived pre-mRNA transcripts are alternatively spliced and subsequently encode a truncated, dysfunctional protein, SMNΔ7 (Fig. 1) (1,4–7). Increasing SMN2 copy number, and more importantly, the small amount of full-length SMN produced by SMN2, is observed in milder forms of the disease (2). Therefore, SMN2 is the primary disease-modifying gene in humans and in transgenic models of disease (2,8). Although not conclusively demonstrated yet, Plastin-3 may prove to be an additional genetic modifier based upon expression studies that correlated with a decrease in severity in some female SMA patients (9). The precise SMN-associated function that is abrogated in SMA is currently controversial; however, two principal hypotheses have developed: (i) SMN performs an axonal-specific function potentially involving mRNA transport, such as β-actin; or (ii) SMA is caused by decreased SMN activity in snRNP biogenesis, and presumably, motor neurons are especially vulnerable to SMN-dependent snRNP perturbations (10).
Figure 1.
Schematic of the human SMN locus. The human SMN genes, SMN1 and SMN2, are located in close proximity on chromosome 5. The SMN2 locus is likely derived from a recent duplication event of a genomic region spanning ∼500 kb which contains additional genes and microsatellite markers. The SMN genes comprise nine exons and eight introns and encode an identical protein product. A silent C–T transition in exon 7 of SMN2 alters a critical exonic splice enhancer and results in a strong reduction of exon 7 inclusion during splicing. Consequently, ∼85% of the mature mRNA lacks exon 7 (Δ7), highlighted by the RT–PCR in the bottom panel. The truncated protein is defective in SMN self-association and is degraded rapidly.
SMN REGULATION
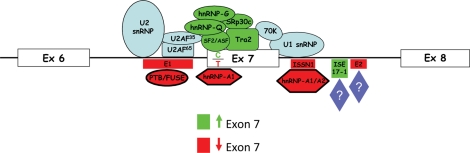
SMN1 and SMN2 maintain identical coding sequences; however, a silent cytosine-to-thymine (C–T) transition within exon 7 (+6) induces the alternative splicing event common to the majority of SMN2-derived transcripts (Fig. 1). Exon 7 is a highly regulated region comprised of 54 nucleotides and contains the translation termination signal for all full-length products, whereas the translational termination of the exon-skipped product is at the 5′ end of exon 8. The balance between full-length expression and exon-skipping is accomplished through a complex interplay between positively and negatively acting regulatory elements (Fig. 2). A critical AG-rich exonic splicing enhancer (ESE) within exon 7 is bound and regulated by Tra2-β1, an SR-like family member (11). Tra2-β1 likely serves as a nucleation point for several additional regulatory factors that indirectly associate with SMN exon 7, including SRp30c, hnRNP-Q, hnRNP-G and RBMY (12–14). Overlapping the C–T transition is an important ESE bound by SF2/ASF (15,16). This high-affinity SF2/ASF motif is disrupted in the SMN2 context. Concomitantly, the C–T transition appears to create a novel inhibitory region called Extinct that can also be bound by hnRNP-A1 (17–19). Flanking exon 7 are evolutionarily conserved positively acting regulatory sequences (20) as well as several potent negatively regulating elements, including intronic splice silencer (ISS) regions such as element 1 in intron 6 at the −75/−89 position (relative to exon 7) and a sequence immediately downstream of exon 7 called ISS-N1 (21,22). Although the precise mode of inhibition for the ISS elements is currently unknown, PTB, FUBP and hnRNP-A1/A2 have been identified in complexes with these ISS regions (23–25).
Figure 2.
Schematic of the exon 7 region and the factors involved in the inclusion or exclusion of exon 7 within SMN pre-mRNA. Components of the machinery are shown in blue. The positively acting sequences and splicing factors are shown in green. The negatively acting sequences and splicing factors are shown in red. The C–T transition at +6 is indicated.
The natural history of SMA has been altered over the past several decades, primarily through supportive care measures, many of which are summarized within the recently published consensus statement for standards of care (26,27). However, a treatment or cure has not been identified. Since all SMA patients retain varying copy numbers of SMN2, the SMN2 gene and gene products have become a focal point for SMA therapeutic development. In this review, the primary focus will be on recent translational strategies that are SMN-dependent and SMN-independent approaches to develop therapeutics for SMA.
THERAPEUTIC RNA MOLECULES: RNA-BASED MODULATION OF SMN2
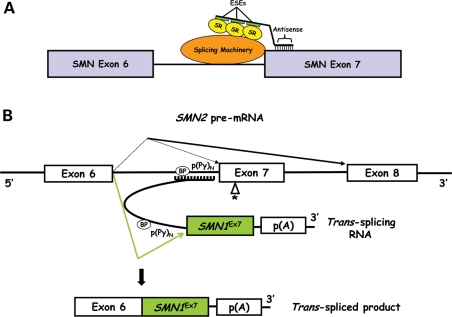
Utilizing small RNA molecules to reprogram the splicing of a faulty pre-mRNA is an expanding area of focus for a wide range of genetic diseases including SMA (28,29). Modulating SMN2 pre-mRNA splicing is a direct approach to restore proper expression of the normal SMN protein. Initial efforts involved antisense oligonucleotides (ASOs) to redirect splice decisions by blocking the 3′ splice site (ss) of exon 8 (30) and to inhibit the function of a negative splicing regulator (E1) within intron 6 (21). The ASO targeting the 3′ ss of exon 8 was incorporated into U7 snRNA for stable expression (31) and increased exon 7 inclusion and SMN protein levels following delivery into SMA type-I patient fibroblasts (3813 cells) using adeno-associated virus (AAV-5) (32). The antisense strategy was further extended by developing alternative chemistries and through the incorporation of an untethered binding platform for positively acting splicing factors to the SMN2 exon 7 region. This was accomplished by combining the antisense region with either a covalently bound synthetic peptide (16) or with a non-complementary ESE sequence acting as a binding platform for SR proteins (bifunctional RNAs) (Fig. 3A) (33,34). Conceptually similar to the synthetic RNAs, bifunctional RNAs were able to be expressed from AAV-2 vectors, leading to increased SMN protein levels in cell-based models (33). An alternative version of the bifunctional RNAs targeted the intron 7–exon 8 border and contained binding motifs for the negatively acting splicing factor, hnRNP-A1 (35). As opposed to the previous bifunctional RNAs that were designed to directly stimulate SMN2 exon 7 inclusion by recruiting positively acting splicing factors, this alternative class of RNAs recruited negatively acting factors to the 5′ end of exon 8 as a means of shifting the equilibrium towards full-length expression. Intravenous injection into the temporal vein of SMA mice with these synthetic 2′-O-methyl bifunctional RNAs enhanced SMN protein expression in the liver and kidney, whereas intracerebroventricular (ICV) injections increased SMN levels in brain tissue from treated SMA mice (35).
Figure 3.
(A) Schematic of the exon 7 region and the proposed function of a bifunctional RNA. The bifunctional RNA is illustrated with an antisense-targeting domain specific to 5′ end of exon 7, a short spacer region and a domain comprising three tandem repeats of ESEs shown in green. Positive splicing factors, interacting with the splicing machinery, are shown in yellow. (B) Schematic of trans-splicing in the context of SMN2 pre-mRNA splicing. The antisense domain of the trans-splicing RNA binds to endogenous SMN2 pre-mRNA at the intron 6 region by complementary base-pairing. The SMN1 exon 7 is contained within the trans-splicing RNA and precedes a polyadenylation signal. The product of an interaction between the 5′ ss of intron 6 and the 3′ ss of the trans-splicing RNA is a trans-spliced mRNA that contains SMN1 exon 7. BP, branch point; p(Py), polypyrimidine tract; p(A), polyadenylation signal.
The sequence targeted by the ASOs must be carefully selected to avoid masking any regulatory region that is crucial for exon 7 splicing. Ideally, a bifunctional RNA would have two modes of action: (i) the inhibition of a splicing silencer mediated by the antisense region, and (ii) the recruitment of SR proteins. Therefore, regions such as the hTra2β1 ESE represent poor molecular targets (11,36), whereas blocking its flanking regions (nucleotides 7–21 and 34–48; positions A and B, respectively) greatly improves the level of exon 7 splicing and SMN protein in cell-based models (36). Consistent with this refined targeting notion, a bifunctional RNA that blocked position B and contained SF2/ASF ESEs was delivered into 3813 cells, using a lentivirus vector (37). In cell-based assays, the outcome was 97% inclusion of exon 7 and a ∼2–3-fold increase in SMN protein (37). The viability of this approach was further illustrated through the development of a transgene expressing a similar RNA within the context of a very severe model of SMA (38). In all measured outcomes, bifunctional RNA expression resulted in levels of SMN protein that rescued the severe SMA phenotype (38).
Through detailed molecular studies, an intertwined series of enhancers and silencers have been identified. In particular, the identification of intronic splicing silencers (ISSs) has greatly impacted the design of exon 7-stimulating ASOs and bifunctional RNAs. In addition to the use of minigene systems, ASO arrays or tiling has proven to be a powerful and unbiased means to analyze ASOs in disease-specific cellular contexts (25,36,39,40). ISS-N1 ASOs systemically injected into unaffected SMN2-transgenic, heterozygous SMA mice gave rise to ∼90% inclusion of exon 7 in the liver and kidney, whereas the effect was modest in thigh muscles and not evident in the spinal cord (25). However, a recent report indicated that multiple ICV injections of ISS-N1 ASO increase SMN protein in the brain and spinal cord of the SMAΔ7 mouse model (41). Remarkably, uptake of uncoupled 2′-O-methyl ASO was significantly greater compared with ASO incorporated into previously described carriers. The weight and righting reflex, monitored until post-natal day 12, were also improved relative to a control group. As further confirmation of this regulatory sequence as a bona fide target, recently, an 8-mer ASO that binds to five nucleotides of ISS-N1 was reported to increase SMN and SMN-associated proteins in 3813 cells (39). It is still unclear whether such a short ASO can function specifically in vivo or whether the high number of cognate sites within the genome will result in unwanted off-targets effects.
In addition to ISS-N1, the detection of the inhibitory element E1 within intron 6 led to the synthesis of a bifunctional RNA that played a dual role by simultaneously blocking E1 and recruiting SR proteins (23). Originally, E1 was identified using an exon-trapping vector and its functionality was subsequently confirmed in a genomic minigene system and shown to form a complex with two RNA-binding factors, PTB and FUSE-BP (21,23). The antisense moiety consisted of two non-sequential sequences designed to inhibit E1 by hybridizing to the flanking regions of E1. E1-bifunctional RNAs contained the ESE for either ASF/SF2 or hTra2-β. Plasmid-derived and 2′-O-methyl RNAs increased SMN protein levels in 3813 cells. Furthermore, delivery of 2′-O-methyl bifunctional RNAs into the CNS of SMAΔ7 mice resulted in SMN levels comparable with that of carrier heterozygous mice in the brain and spinal cord. More importantly, E1-hTra2-β extended the lifespan and weight in a more severe mouse model of SMA (23). The ability of small therapeutic RNAs to reach and penetrate motor neurons, as well as their intracellular stability, will be key to the further development of these types of strategies in SMA as well as other disorders of the CNS.
TRANS-SPLICING RNAS
SMN trans-splicing is an alternative RNA therapy with promising outcomes. Trans-splicing requires a synthetic RNA (tsRNA) consisting of three domains: (i) a binding domain to interact with a specific target; (ii) a splicing domain to undergo a splicing reaction with the selected intron; and (iii) an intact exon or series of exons to replace the defective gene segment. The trans-spliced product is a chimeric mRNA that translates into a functional protein. The original SMN tsRNA targeted intron 6 and replaced SMN2 exon 7 with SMN1 exon 7 (Fig. 3B) (42). AAV-2 delivery of this construct in 3813 cells significantly increased SMN protein levels, which was validated by snRNP assembly assay for functionality (42). To improve the in vivo efficiency, the tsRNA vector was co-expressed with a short ASO expressed from a separate promoter (43). The ASO was designed to block the downstream splicing at exon 8 and, therefore, promote a trans-splicing event. The combination of the tsRNA and ASO was highly effective in vivo as demonstrated by increased levels of the trans-spliced RNA and SMN protein in the brain and spinal cord of injected SMAΔ7 mice (43). Consistent with this, a single ICV injection of the tsRNA/ASO vector in a severe model of SMA lessens disease severity by extending the lifespan nearly 70% (44).
SMA DRUG DEVELOPMENT
The mode-of-action for a potential SMA therapy using small molecules may include increasing exon 7 inclusion, activating the SMN2 promoter, extending the half-life of SMN mRNA or protein and lengthening the protein at the C-terminus—or a combination of these activities. Following extensive high-throughput screening of SMN promoter-activating compounds and medicinal chemistry optimization, novel quinazoline derivatives were recently developed, which not only increased SMN in vitro, but also altered the SMA phenotype in the SMNΔ7 mouse model (45–47). Several derivatives crossed the blood–brain barrier and increased SMN in the brain of neonatal mice. Using protein microarrays, the RNA-decapping protein DcpS was identified as a target of C5-quinazolines (48). The exact mechanisms by which DcpS increases SMN levels are not fully understood and need further investigation. Nevertheless, oral bioavailability and positive results in safety studies make quinazolines candidates for SMA clinical trials.
Another group of compounds, histone deacetylase (HDAC) inhibitors, has shown promise in several models of neurodegeneration (49). Several different HDAC inhibitors have been tested in SMA mouse models and in patients. Notably, administration of TSA increased expression of SMN and improved lifespan and motor performance in the SMAΔ7 model, especially when combined with nutritional support (50,51). Positive results were also obtained with sodium butyrate and valproic acid (52,53). To date, despite good safety profiles in clinical trials, valproic acid and phenylbutyrate have not resulted in dramatic clinical outcomes and efficacy has been incremental (54,55). However, novel HDAC inhibitor compounds may hold promise since it has been shown that LBH589 increased SMN levels in cells from patients unresponsive to valproic acid (56), and SAHA administration increased lifespan in an SMA mouse model (57).
Heterologous sequences can at least partially substitute for the reduced oligomerization and functionality of the SMNΔ7 protein (4–6,58). Based upon these molecular observations, it was reasoned that the use of compounds that induced a translational readthrough event of the SMNΔ7 protein would lengthen the C-terminus of SMNΔ7 and ultimately increase SMN levels. Aminoglycosides, a class of antibiotics, can suppress the recognition of termination codons and have been used in culture to increase SMN protein levels (4,59,60). The aminoglycoside G418, which was previously shown to confer improvement in a mouse model harboring a vasopressin receptor nonsense mutation (61), also increased SMN protein levels and improved motor function in the SMAΔ7 model despite an adverse toxicity profile (4). An aminoglycoside derivative obtained through a medicinal chemistry approach resulted in the elongation of lifespan and functional improvements in this model, suggesting that this class of compounds is amenable to optimization (62,63). Successful readthrough can also be achieved using different scaffolds with acceptable safety profiles as shown by PTC Therapeutics in a clinical trial with cystic fibrosis patients (64). Therefore, readthrough compounds with improved safety features and increased suppressive capacities and specificities may become candidates for SMA drug treatment regimens.
STEM CELLS
The possibility to replace lost neurons and to support the remaining neural cell population by neural stem cells is currently receiving considerable attention. Cell replacement may be achieved by transplantation of stem cell-derived cells which have undergone maturation in vitro, or by activation of endogenous stem cells in the CNS. However, bone marrow transplantation is the only stem cell therapy currently in use. In SMA animal studies, significant progress has recently been reported by Corti et al. (65,66), who injected primary neural stem cells from the spinal cord, as well as ES cell-derived neural cell precursors, into the spinal cord of the relatively severe SMAΔ7 mouse model. Approximately 15% of the injected cells engrafted into the spinal cord where they exhibited mostly astrocyte and, to a much lesser extent, motor neuron characteristics. This resulted in significant increases in lifespan, weight gain and improvements in muscle morphology. Intriguingly, the loss of ventral horn cells typically seen in this model was mitigated to an extent that could not be explained by the acquisition of new, stem cell-derived motor neurons alone, suggesting that the transplanted cells exerted a neuroprotective effect. Indeed, the stem cells secreted neurotrophic factors in culture and transplanted spinal cords had increased levels as well (66). Neuroprotective effects of transplanted stem cells were also described in several other models of neurodegeneration, including ALS (67), Purkinje neuron degeneration (68) and retinal disease (69). Although intrathecal administration of trophic factors generally had little clinical effect (70), genetically engineered stem cells may provide enhanced neuroprotection and trophic support in situ after differentiation into glial cells, in addition to replenishing the motor neuron pool.
The successful generation of induced pluripotent stem (iPS) cells from patient fibroblast was an important step towards the generation of genetically compatible neurons for stem cell therapy (71). iPS cells from an SMA patient can be differentiated in culture into motor neurons expressing specific transcription factors and markers such as choline acetyltransferase (72). This raises the possibility that the underlying genetic defect can be repaired in vitro and pre-differentiated cells then be returned to the patient without eliciting an adverse immune response. Additionally, valuable human motor neuron cultures can now be probed for biological answers or be utilized as disease-appropriate drug discovery platforms. Traditionally, iPS cells are generated using a cocktail of four factors delivered by lentivirus vectors, making them unsuitable for clinical use because of the potential for integrational mutagenesis and oncogenicity. To overcome this limitation, efforts are directed towards generating iPS cells without permanent genome modification, employing non-integrating or excisable vectors, or eventually activating the reprogramming process via protein factors or small molecules (73–80). Although these developments are very exciting, it remains to be demonstrated whether transplant motor neurons can form substantial numbers of functional neuromuscular junctions in an SMA model. Thus, at present, stem cells might be expected to provide primarily trophic support, and transplantation at the earliest possible time point should provide maximum benefit.
SMN-INDEPENDENT PATHWAYS
Muscle enhancement
Muscle has also been examined as a potential target for SMA therapeutics. Although recent work with novel transgenic animals expressing SMN in muscle under the control of the HSA promoter demonstrates that SMN restoration in skeletal muscle alone does not reverse the SMA phenotype (81), the possibility exists that enhanced muscle may contribute to the maintenance or stabilization of an intact motor unit. Two contrasting studies examined various inhibitors of the myostatin pathway, one demonstrating a modest extension in lifespan and gross motor function, following delivery of recombinant follistatin, the other detecting no phenotypic alteration in the SMNΔ7 mice treated with ActRIIB-Fc or transgenic overexpression of follistatin (82,83). The basis for this discrepancy is unclear; however, the possibility remains that motor neurons may require additional support to optimally respond to SMN-based therapeutics.
Actin dynamics
Using a novel milder SMA mouse model called Smn2B/−, a dramatic increase in survival was reported in animals treated with a pharmacological inhibitor of Rho-kinase. Neuromuscular junctions were also enhanced and appeared more mature following treatment. Remarkably, these alterations in the SMA phenotype occurred independently of SMN increases. These results not only provide new therapeutic targets, but also may offer insight into the SMN function.
CONCLUSIONS
A tremendous amount of translational work is progressing rapidly towards the pre-clinical stage in the SMA field. Clearly, obstacles will exist. Blood–brain barrier penetration is an impediment for all CNS disorders, especially for vector-based technology; however, with the analysis of new serotypes, exciting CNS penetration from an IV injection has been accomplished using AAV-9 (84,85). Important questions are still unanswered, which directly impact therapeutic development, such as: (i) what SMN-associated defect leads to SMA development; (ii) when can a therapeutic be delivered and still result in a beneficial effect; (iii) how do existing animal models correlate with clinical trial success; and (iv) will all therapeutic applications work similarly across SMA types? SMA has benefited tremendously by leveraging the basic scientific knowledge into translational research, and while questions remain, ongoing research is poised to address many of the current challenges within the SMA landscape.
FUNDING
This work was supported by grants from the National Institutes of Health (C.L.L., R01 NS41584, R01 HD054413) and SMA Europe (M.S.).
ACKNOWLEDGEMENTS
We would like to thank members of the Lorson Lab for their helpful discussions.
Conflict of Interest statement. None declared.
REFERENCES
- 1.Lefebvre S., Burglen L., Reboullet S., Clermont O., Burlet P., Viollet L., Benichou B., Cruaud C., Millasseau P., Zeviani M., et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. doi: 10.1016/0092-8674(95)90460-3. doi:10.1016/0092-8674(95)90460-3. [DOI] [PubMed] [Google Scholar]
- 2.Feldkotter M., Schwarzer V., Wirth R., Wienker T.F., Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am. J. Hum. Genet. 2002;70:358–368. doi: 10.1086/338627. doi:10.1086/338627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rochette C.F., Gilbert N., Simard L.R. SMN gene duplication and the emergence of the SMN2 gene occurred in distinct hominids: SMN2 is unique to Homo sapiens. Hum. Genet. 2001;108:255–266. doi: 10.1007/s004390100473. doi:10.1007/s004390100473. [DOI] [PubMed] [Google Scholar]
- 4.Heier C.R., DiDonato C.J. Translational readthrough by the aminoglycoside geneticin (G418) modulates SMN stability in vitro and improves motor function in SMA mice in vivo. Hum. Mol. Genet. 2009;18:1310–1322. doi: 10.1093/hmg/ddp030. doi:10.1093/hmg/ddp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lorson C.L., Androphy E.J. An exonic enhancer is required for inclusion of an essential exon in the SMA-determining gene SMN. Hum. Mol. Genet. 2000;9:259–265. doi: 10.1093/hmg/9.2.259. doi:10.1093/hmg/9.2.259. [DOI] [PubMed] [Google Scholar]
- 6.Mattis V.B., Bowerman M., Kothary R., Lorson C.L. A SMNDelta7 read-through product confers functionality to the SMNDelta7 protein. Neurosci. Lett. 2008;442:54–58. doi: 10.1016/j.neulet.2008.06.059. doi:10.1016/j.neulet.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novelli G., Calza L., Amicucci P., Giardino L., Pozza M., Silani V., Pizzuti A., Gennarelli M., Piombo G., Capon F., et al. Expression study of survival motor neuron gene in human fetal tissues. Biochem. Mol. Med. 1997;61:102–106. doi: 10.1006/bmme.1997.2590. doi:10.1006/bmme.1997.2590. [DOI] [PubMed] [Google Scholar]
- 8.Monani U.R., Coovert D.D., Burghes A.H. Animal models of spinal muscular atrophy. Hum. Mol. Genet. 2000;9:2451–2457. doi: 10.1093/hmg/9.16.2451. doi:10.1093/hmg/9.16.2451. [DOI] [PubMed] [Google Scholar]
- 9.Oprea G.E., Krober S., McWhorter M.L., Rossoll W., Muller S., Krawczak M., Bassell G.J., Beattie C.E., Wirth B. Plastin 3 is a protective modifier of autosomal recessive spinal muscular atrophy. Science. 2008;320:524–527. doi: 10.1126/science.1155085. doi:10.1126/science.1155085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burghes A.H., Beattie C.E. Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat. Rev. Neurosci. 2009;10:597–609. doi: 10.1038/nrn2670. doi:10.1038/nrn2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofmann Y., Lorson C.L., Stamm S., Androphy E.J., Wirth B. Htra2-beta 1 stimulates an exonic splicing enhancer and can restore full-length SMN expression to survival motor neuron 2 (SMN2) Proc. Natl Acad. Sci. USA. 2000;97:9618–9623. doi: 10.1073/pnas.160181697. doi:10.1073/pnas.160181697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H.H., Chang J.G., Lu R.M., Peng T.Y., Tarn W.Y. The RNA binding protein hnRNP Q modulates the utilization of exon 7 in the survival motor neuron 2 (SMN2) gene. Mol. Cell. Biol. 2008;28:6929–6938. doi: 10.1128/MCB.01332-08. doi:10.1128/MCB.01332-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmann Y., Wirth B. hnRNP-G promotes exon 7 inclusion of survival motor neuron (SMN) via direct interaction with Htra2-beta1. Hum. Mol. Genet. 2002;11:2037–2049. doi: 10.1093/hmg/11.17.2037. doi:10.1093/hmg/11.17.2037. [DOI] [PubMed] [Google Scholar]
- 14.Young P.J., DiDonato C.J., Hu D., Kothary R., Androphy E.J., Lorson C.L. SRp30c-dependent stimulation of survival motor neuron (SMN) exon 7 inclusion is facilitated by a direct interaction with hTra2beta1. Hum. Mol. Genet. 2002;11:577–587. doi: 10.1093/hmg/11.5.577. doi:10.1093/hmg/11.5.577. [DOI] [PubMed] [Google Scholar]
- 15.Cartegni L., Krainer A.R. Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat. Genet. 2002;30:377–384. doi: 10.1038/ng854. doi:10.1038/ng854. [DOI] [PubMed] [Google Scholar]
- 16.Cartegni L., Krainer A.R. Correction of disease-associated exon skipping by synthetic exon-specific activators. Nat. Struct. Biol. 2003;10:120–125. doi: 10.1038/nsb887. doi:10.1038/nsb887. [DOI] [PubMed] [Google Scholar]
- 17.Kashima T., Manley J.L. A negative element in SMN2 exon 7 inhibits splicing in spinal muscular atrophy. Nat. Genet. 2003;34:460–463. doi: 10.1038/ng1207. doi:10.1038/ng1207. [DOI] [PubMed] [Google Scholar]
- 18.Singh N.N., Androphy E.J., Singh R.N. An extended inhibitory context causes skipping of exon 7 of SMN2 in spinal muscular atrophy. Biochem. Biophys. Res. Commun. 2004;315:381–388. doi: 10.1016/j.bbrc.2004.01.067. doi:10.1016/j.bbrc.2004.01.067. [DOI] [PubMed] [Google Scholar]
- 19.Singh R.N. Unfolding the mystery of alternative splicing through a unique method of in vivo selection. Front. Biosci. 2007;12:3263–3272. doi: 10.2741/2310. doi:10.2741/2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gladman J.T., Chandler D.S. Intron 7 conserved sequence elements regulate the splicing of the SMN genes. Hum. Genet. 2009;126:833–841. doi: 10.1007/s00439-009-0733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyajima H., Miyaso H., Okumura M., Kurisu J., Imaizumi K. Identification of a cis-acting element for the regulation of SMN exon 7 splicing. J. Biol. Chem. 2002;277:23271–23277. doi: 10.1074/jbc.M200851200. doi:10.1074/jbc.M200851200. [DOI] [PubMed] [Google Scholar]
- 22.Singh N.K., Singh N.N., Androphy E.J., Singh R.N. Splicing of a critical exon of human survival motor neuron is regulated by a unique silencer element located in the last intron. Mol. Cell. Biol. 2006;26:1333–1346. doi: 10.1128/MCB.26.4.1333-1346.2006. doi:10.1128/MCB.26.4.1333-1346.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baughan T.D., Dickson A., Osman E.Y., Lorson C.L. Delivery of bifunctional RNAs that target an intronic repressor and increase SMN levels in an animal model of spinal muscular atrophy. Hum. Mol. Genet. 2009;18:1600–1611. doi: 10.1093/hmg/ddp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kashima T., Rao N., Manley J.L. An intronic element contributes to splicing repression in spinal muscular atrophy. Proc. Natl Acad. Sci. USA. 2007;104:3426–3431. doi: 10.1073/pnas.0700343104. doi:10.1073/pnas.0700343104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hua Y., Vickers T.A., Okunola H.L., Bennett C.F., Krainer A.R. Antisense masking of an hnRNP A1/A2 intronic splicing silencer corrects SMN2 splicing in transgenic mice. Am. J. Hum. Genet. 2008;82:834–848. doi: 10.1016/j.ajhg.2008.01.014. doi:10.1016/j.ajhg.2008.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oskoui M., Levy G., Garland C.J., Gray J.M., O'Hagen J., De Vivo D.C., Kaufmann P. The changing natural history of spinal muscular atrophy type 1. Neurology. 2007;69:1931–1936. doi: 10.1212/01.wnl.0000290830.40544.b9. doi:10.1212/01.wnl.0000290830.40544.b9. [DOI] [PubMed] [Google Scholar]
- 27.Wang C.H., Lunn M.R. Spinal muscular atrophy: advances in research and consensus on care of patients. Curr. Treat. Options Neurol. 2008;10:420–428. doi: 10.1007/s11940-008-0044-7. doi:10.1007/s11940-008-0044-7. [DOI] [PubMed] [Google Scholar]
- 28.Khoo B., Krainer A.R. Splicing therapeutics in SMN2 and APOB. Curr. Opin. Mol. Ther. 2009;11:108–115. [PMC free article] [PubMed] [Google Scholar]
- 29.Wood M., Yin H., McClorey G. Modulating the expression of disease genes with RNA-based therapy. PLoS Genet. 2007;3:e109. doi: 10.1371/journal.pgen.0030109. doi:10.1371/journal.pgen.0030109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim S.R., Hertel K.J. Modulation of survival motor neuron pre-mRNA splicing by inhibition of alternative 3′ splice site pairing. J. Biol. Chem. 2001;276:45476–45483. doi: 10.1074/jbc.M107632200. doi:10.1074/jbc.M107632200. [DOI] [PubMed] [Google Scholar]
- 31.Madocsai C., Lim S.R., Geib T., Lam B.J., Hertel K.J. Correction of SMN2 Pre-mRNA splicing by antisense U7 small nuclear RNAs. Mol. Ther. 2005;12:1013–1022. doi: 10.1016/j.ymthe.2005.08.022. doi:10.1016/j.ymthe.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 32.Geib T., Hertel K.J. Restoration of full-length SMN promoted by adenoviral vectors expressing RNA antisense oligonucleotides embedded in U7 snRNAs. PLoS ONE. 2009;4:e8204. doi: 10.1371/journal.pone.0008204. doi:10.1371/journal.pone.0008204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baughan T., Shababi M., Coady T.H., Dickson A.M., Tullis G.E., Lorson C.L. Stimulating full-length SMN2 expression by delivering bifunctional RNAs via a viral vector. Mol. Ther. 2006;14:54–62. doi: 10.1016/j.ymthe.2006.01.012. doi:10.1016/j.ymthe.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 34.Skordis L.A., Dunckley M.G., Yue B., Eperon I.C., Muntoni F. Bifunctional antisense oligonucleotides provide a trans-acting splicing enhancer that stimulates SMN2 gene expression in patient fibroblasts. Proc. Natl Acad. Sci. USA. 2003;100:4114–4119. doi: 10.1073/pnas.0633863100. doi:10.1073/pnas.0633863100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickson A., Osman E., Lorson C. A negatively-acting bifunctional RNA increases survival motor neuron in vitro and in vivo. Hum. Gene. Ther. 2008;19:1307–1315. doi: 10.1089/hum.2008.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hua Y., Vickers T.A., Baker B.F., Bennett C.F., Krainer A.R. Enhancement of SMN2 exon 7 inclusion by antisense oligonucleotides targeting the exon. PLoS Biol. 2007;5:e73. doi: 10.1371/journal.pbio.0050073. doi:10.1371/journal.pbio.0050073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marquis J., Meyer K., Angehrn L., Kampfer S.S., Rothen-Rutishauser B., Schumperli D. Spinal muscular atrophy: SMN2 pre-mRNA splicing corrected by a U7 snRNA derivative carrying a splicing enhancer sequence. Mol. Ther. 2007;15:1479–1486. doi: 10.1038/sj.mt.6300200. [DOI] [PubMed] [Google Scholar]
- 38.Meyer K., Marquis J., Trub J., Nlend Nlend R., Verp S., Ruepp M.D., Imboden H., Barde I., Trono D., Schumperli D. Rescue of a severe mouse model for spinal muscular atrophy by U7 snRNA-mediated splicing modulation. Hum. Mol. Genet. 2009;18:546–555. doi: 10.1093/hmg/ddn382. doi:10.1093/hmg/ddn382. [DOI] [PubMed] [Google Scholar]
- 39.Singh N.N., Shishimorova M., Cao L.C., Gangwani L., Singh R.N. A short antisense oligonucleotide masking a unique intronic motif prevents skipping of a critical exon in spinal muscular atrophy. RNA Biol. 2009;6:341–350. doi: 10.4161/rna.6.3.8723. doi:10.4161/rna.6.3.8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Singh N.N., Singh R.N., Androphy E.J. Modulating role of RNA structure in alternative splicing of a critical exon in the spinal muscular atrophy genes. Nucleic Acids Res. 2007;35:371–389. doi: 10.1093/nar/gkl1050. doi:10.1093/nar/gkl1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williams J.H., Schray R.C., Patterson C.A., Ayitey S.O., Tallent M.K., Lutz G.J. Oligonucleotide-mediated survival of motor neuron protein expression in CNS improves phenotype in a mouse model of spinal muscular atrophy. J. Neurosci. 2009;29:7633–7638. doi: 10.1523/JNEUROSCI.0950-09.2009. doi:10.1523/JNEUROSCI.0950-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coady T.H., Shababi M., Tullis G.E., Lorson C.L. Restoration of SMN function: delivery of a trans-splicing RNA re-directs SMN2 pre-mRNA splicing. Mol. Ther. 2007;15:1471–1478. doi: 10.1038/sj.mt.6300222. doi:10.1038/sj.mt.6300222. [DOI] [PubMed] [Google Scholar]
- 43.Coady T.H., Baughan T.D., Shababi M., Passini M.A., Lorson C.L. Development of a single vector system that enhances trans-splicing of SMN2 transcripts. PLoS ONE. 2008;3:e3468. doi: 10.1371/journal.pone.0003468. doi:10.1371/journal.pone.0003468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coady T.H., Lorson C.L. Trans-splicing-mediated improvement in a severe mouse model of spinal muscular atrophy. J. Neurosci. 2010;30:126–130. doi: 10.1523/JNEUROSCI.4489-09.2010. doi:10.1523/JNEUROSCI.4489-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butchbach M.E., Singh J., Thorsteinsdottir M., Saieva L., Slominski E., Thurmond J., Andresson T., Zhang J., Edwards J.D., Simard L.R., et al. Effects of 2,4-diaminoquinazoline derivatives on SMN expression and phenotype in a mouse model for spinal muscular atrophy. Hum. Mol. Genet. 2010;19:454–467. doi: 10.1093/hmg/ddp510. doi:10.1093/hmg/ddp510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jarecki J., Chen X., Bernardino A., Coovert D.D., Whitney M., Burghes A., Stack J., Pollok B.A. Diverse small-molecule modulators of SMN expression found by high-throughput compound screening: early leads towards a therapeutic for spinal muscular atrophy. Hum. Mol. Genet. 2005;14:2003–2018. doi: 10.1093/hmg/ddi205. doi:10.1093/hmg/ddi205. [DOI] [PubMed] [Google Scholar]
- 47.Thurmond J., Butchbach M.E., Palomo M., Pease B., Rao M., Bedell L., Keyvan M., Pai G., Mishra R., Haraldsson M., et al. Synthesis and biological evaluation of novel 2,4-diaminoquinazoline derivatives as SMN2 promoter activators for the potential treatment of spinal muscular atrophy. J. Med. Chem. 2008;51:449–469. doi: 10.1021/jm061475p. doi:10.1021/jm061475p. [DOI] [PubMed] [Google Scholar]
- 48.Singh J., Salcius M., Liu S.W., Staker B.L., Mishra R., Thurmond J., Michaud G., Mattoon D.R., Printen J., Christensen J., et al. DcpS as a therapeutic target for spinal muscular atrophy. ACS Chem. Biol. 2008;3:711–722. doi: 10.1021/cb800120t. doi:10.1021/cb800120t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chuang D.M., Leng Y., Marinova Z., Kim H.J., Chiu C.T. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009;32:591–601. doi: 10.1016/j.tins.2009.06.002. doi:10.1016/j.tins.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Avila A.M., Burnett B.G., Taye A.A., Gabanella F., Knight M.A., Hartenstein P., Cizman Z., Di Prospero N.A., Pellizzoni L., Fischbeck K.H., et al. Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy. J. Clin. Invest. 2007;117:659–671. doi: 10.1172/JCI29562. doi:10.1172/JCI29562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Narver H.L., Kong L., Burnett B.G., Choe D.W., Bosch-Marce M., Taye A.A., Eckhaus M.A., Sumner C.J. Sustained improvement of spinal muscular atrophy mice treated with trichostatin a plus nutrition. Ann. Neurol. 2008;64:465–470. doi: 10.1002/ana.21449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang J.G., Hsieh-Li H.M., Jong Y.J., Wang N.M., Tsai C.H., Li H. Treatment of spinal muscular atrophy by sodium butyrate. Proc. Natl Acad. Sci. USA. 2001;98:9808–9813. doi: 10.1073/pnas.171105098. doi:10.1073/pnas.171105098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai L.K., Tsai M.S., Ting C.H., Li H. Multiple therapeutic effects of valproic acid in spinal muscular atrophy model mice. J. Mol. Med. 2008;86:1243–1254. doi: 10.1007/s00109-008-0388-1. doi:10.1007/s00109-008-0388-1. [DOI] [PubMed] [Google Scholar]
- 54.Mercuri E., Bertini E., Messina S., Solari A., D'Amico A., Angelozzi C., Battini R., Berardinelli A., Boffi P., Bruno C., et al. Randomized, double-blind, placebo-controlled trial of phenylbutyrate in spinal muscular atrophy. Neurology. 2007;68:51–55. doi: 10.1212/01.wnl.0000249142.82285.d6. doi:10.1212/01.wnl.0000249142.82285.d6. [DOI] [PubMed] [Google Scholar]
- 55.Swoboda K.J., Scott C.B., Reyna S.P., Prior T.W., LaSalle B., Sorenson S.L., Wood J., Acsadi G., Crawford T.O., Kissel J.T., et al. Phase II open label study of valproic acid in spinal muscular atrophy. PLoS ONE. 2009;4:e5268. doi: 10.1371/journal.pone.0005268. doi:10.1371/journal.pone.0005268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garbes L., Riessland M., Holker I., Heller R., Hauke J., Trankle C., Coras R., Blumcke I., Hahnen E., Wirth B. LBH589 induces up to 10-fold SMN protein levels by several independent mechanisms and is effective even in cells from SMA patients non-responsive to valproate. Hum. Mol. Genet. 2009;18:3645–3658. doi: 10.1093/hmg/ddp313. doi:10.1093/hmg/ddp313. [DOI] [PubMed] [Google Scholar]
- 57.Riessland M., Ackermann B., Forster A., Jakubik M., Hauke J., Garbes L., Fritzsche I., Mende Y., Blumcke I., Hahnen E., et al. SAHA ameliorates the SMA phenotype in two mouse models for spinal muscular atrophy. Hum. Mol. Genet. 2010;19:1492–1506. doi: 10.1093/hmg/ddq023. [DOI] [PubMed] [Google Scholar]
- 58.Hua Y., Zhou J. Modulation of SMN nuclear foci and cytoplasmic localization by its C-terminus. Cell. Mol. Life Sci. 2004;61:2658–2663. doi: 10.1007/s00018-004-4300-z. doi:10.1007/s00018-004-4300-z. [DOI] [PubMed] [Google Scholar]
- 59.Mattis V.B., Rai R., Wang J., Chang C.W., Coady T., Lorson C.L. Novel aminoglycosides increase SMN levels in spinal muscular atrophy fibroblasts. Hum. Genet. 2006;120:589–601. doi: 10.1007/s00439-006-0245-7. doi:10.1007/s00439-006-0245-7. [DOI] [PubMed] [Google Scholar]
- 60.Wolstencroft E.C., Mattis V., Bajer A.A., Young P.J., Lorson C.L. A non-sequence-specific requirement for SMN protein activity: the role of aminoglycosides in inducing elevated SMN protein levels. Hum. Mol. Genet. 2005;14:1199–1210. doi: 10.1093/hmg/ddi131. doi:10.1093/hmg/ddi131. [DOI] [PubMed] [Google Scholar]
- 61.Sangkuhl K., Schulz A., Rompler H., Yun J., Wess J., Schoneberg T. Aminoglycoside-mediated rescue of a disease-causing nonsense mutation in the V2 vasopressin receptor gene in vitro and in vivo. Hum. Mol. Genet. 2004;13:893–903. doi: 10.1093/hmg/ddh105. doi:10.1093/hmg/ddh105. [DOI] [PubMed] [Google Scholar]
- 62.Mattis V.B., Ebert A.D., Fosso M.Y., Chang C.W., Lorson C.L. Delivery of a read-through inducing compound, TC007, lessens the severity of a SMA animal model. Hum. Mol. Genet. 2009;18:3906–3913. doi: 10.1093/hmg/ddp333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mattis V.B., Fosso M.Y., Chang C.W., Lorson C.L. Subcutaneous administration of TC007 reduces disease severity in an animal model of SMA. BMC Neurosci. 2009;10:142. doi: 10.1186/1471-2202-10-142. doi:10.1186/1471-2202-10-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kerem E., Hirawat S., Armoni S., Yaakov Y., Shoseyov D., Cohen M., Nissim-Rafinia M., Blau H., Rivlin J., Aviram M., et al. Effectiveness of PTC124 treatment of cystic fibrosis caused by nonsense mutations: a prospective phase II trial. Lancet. 2008;372:719–727. doi: 10.1016/S0140-6736(08)61168-X. doi:10.1016/S0140-6736(08)61168-X. [DOI] [PubMed] [Google Scholar]
- 65.Corti S., Nizzardo M., Nardini M., Donadoni C., Salani S., Del Bo R., Papadimitriou D., Locatelli F., Mezzina N., Gianni F., et al. Motoneuron transplantation rescues the phenotype of SMARD1 (spinal muscular atrophy with respiratory distress type 1) J. Neurosci. 2009;29:11761–11771. doi: 10.1523/JNEUROSCI.2734-09.2009. doi:10.1523/JNEUROSCI.2734-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Corti S., Nizzardo M., Nardini M., Donadoni C., Salani S., Ronchi D., Simone C., Falcone M., Papadimitriou D., Locatelli F., et al. Embryonic stem cell-derived neural stem cells improve spinal muscular atrophy phenotype in mice. Brain. 2009;133:465–481. doi: 10.1093/brain/awp318. [DOI] [PubMed] [Google Scholar]
- 67.Kerr D.A., Llado J., Shamblott M.J., Maragakis N.J., Irani D.N., Crawford T.O., Krishnan C., Dike S., Gearhart J.D., Rothstein J.D. Human embryonic germ cell derivatives facilitate motor recovery of rats with diffuse motor neuron injury. J. Neurosci. 2003;23:5131–5140. doi: 10.1523/JNEUROSCI.23-12-05131.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li J., Ma Y., Teng Y.D., Zheng K., Vartanian T.K., Snyder E.Y., Sidman R.L. Purkinje neuron degeneration in nervous (nr) mutant mice is mediated by a metabolic pathway involving excess tissue plasminogen activator. Proc. Natl Acad. Sci. USA. 2006;103:7847–7852. doi: 10.1073/pnas.0602440103. doi:10.1073/pnas.0602440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gamm D.M., Wang S., Lu B., Girman S., Holmes T., Bischoff N., Shearer R.L., Sauve Y., Capowski E., Svendsen C.N., et al. Protection of visual functions by human neural progenitors in a rat model of retinal disease. PLoS ONE. 2007;2:e338. doi: 10.1371/journal.pone.0000338. doi:10.1371/journal.pone.0000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nayak M.S., Kim Y.S., Goldman M., Keirstead H.S., Kerr D.A. Cellular therapies in motor neuron diseases. Biochim. Biophys. Acta. 2006;1762:1128–1138. doi: 10.1016/j.bbadis.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 71.Dimos J.T., Rodolfa K.T., Niakan K.K., Weisenthal L.M., Mitsumoto H., Chung W., Croft G.F., Saphier G., Leibel R., Goland R., et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. doi:10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- 72.Ebert A.D., Yu J., Rose F.F., Jr, Mattis V.B., Lorson C.L., Thomson J.A., Svendsen C.N. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature. 2009;457:277–280. doi: 10.1038/nature07677. doi:10.1038/nature07677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kaji K., Norrby K., Paca A., Mileikovsky M., Mohseni P., Woltjen K. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. doi:10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woltjen K., Michael I.P., Mohseni P., Desai R., Mileikovsky M., Hamalainen R., Cowling R., Wang W., Liu P., Gertsenstein M., et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. doi:10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yusa K., Rad R., Takeda J., Bradley A. Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nat. Methods. 2009;6:363–369. doi: 10.1038/nmeth.1323. doi:10.1038/nmeth.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou H., Wu S., Joo J.Y., Zhu S., Han D.W., Lin T., Trauger S., Bien G., Yao S., Zhu Y., et al. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–384. doi: 10.1016/j.stem.2009.04.005. doi:10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim D., Kim C.H., Moon J.I., Chung Y.G., Chang M.Y., Han B.S., Ko S., Yang E., Cha K.Y., Lanza R., et al. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–476. doi: 10.1016/j.stem.2009.05.005. doi:10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ichida J.K., Blanchard J., Lam K., Son E.Y., Chung J.E., Egli D., Loh K.M., Carter A.C., Di Giorgio F.P., Koszka K., et al. A small-molecule inhibitor of tgf-Beta signaling replaces sox2 in reprogramming by inducing nanog. Cell Stem Cell. 2009;5:491–503. doi: 10.1016/j.stem.2009.09.012. doi:10.1016/j.stem.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shi Y. Induced pluripotent stem cells, new tools for drug discovery and new hope for stem cell therapies. Curr. Mol. Pharmacol. 2009;2:15–18. doi: 10.2174/1874467210902010015. doi:10.2174/1874467210902010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lyssiotis C.A., Foreman R.K., Staerk J., Garcia M., Mathur D., Markoulaki S., Hanna J., Lairson L.L., Charette B.D., Bouchez L.C., et al. Reprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4. Proc. Natl Acad. Sci. USA. 2009;106:8912–8917. doi: 10.1073/pnas.0903860106. doi:10.1073/pnas.0903860106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gavrilina T.O., McGovern V.L., Workman E., Crawford T.O., Gogliotti R.G., DiDonato C.J., Monani U.R., Morris G.E., Burghes A.H. Neuronal SMN expression corrects spinal muscular atrophy in severe SMA mice while muscle-specific SMN expression has no phenotypic effect. Hum. Mol. Genet. 2008;17:1063–1075. doi: 10.1093/hmg/ddm379. doi:10.1093/hmg/ddm379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rose F.F., Jr, Mattis V.B., Rindt H., Lorson C.L. Delivery of recombinant follistatin lessens disease severity in a mouse model of spinal muscular atrophy. Hum. Mol. Genet. 2009;18:997–1005. doi: 10.1093/hmg/ddn426. doi:10.1093/hmg/ddn426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sumner C.J., Wee C.D., Warsing L.C., Choe D.W., Ng A.S., Lutz C., Wagner K.R. Inhibition of myostatin does not ameliorate disease features of severe spinal muscular atrophy mice. Hum. Mol. Genet. 2009;18:3145–3152. doi: 10.1093/hmg/ddp253. doi:10.1093/hmg/ddp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Duque S., Joussemet B., Riviere C., Marais T., Dubreil L., Douar A.M., Fyfe J., Moullier P., Colle M.A., Barkats M. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol. Ther. 2009;17:1187–1196. doi: 10.1038/mt.2009.71. doi:10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Foust K.D., Nurre E., Montgomery C.L., Hernandez A., Chan C.M., Kaspar B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. doi:10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]