Abstract
Background
Deregulated androgen receptor (AR) action is critical for prostate cancer (PCa) progression. Aberrant expression of AR-associated coregulators contributes to AR activity in PCa. The mechanisms underlying coregulator expression in PCa are under intense investigation as they may lead to alternative means of targeting AR activity in PCa cells. We have recently shown that over 30 percent of coregulator expression in the PCa cell line LNCaP is subject to androgen regulation.
Methods
Using multiple PCa cell lines as well as xenograft models, non-malignant prostate epithelial cell lines and androgen-responsive tissues derived from a male Wistar rat model system, we explored the effect of androgen stimulation and androgen deprivation on the expression of the core coactivators SRC1, SRC2, SRC3, CBP and p300.
Results
Androgen stimulation of model systems representing PCa led to a decrease in the expression of SRC1, SRC2, SRC3, CBP and p300, whereas androgen deprivation induced the expression of these coactivators. In contrast, expression of these coregulators remained largely unaffected following changes in the androgenic milieu in AR-positive models representing non-malignant prostate cells and tissues.
Conclusions
Our data indicate differences in the regulation of coregulator expression between neoplastic and normal prostate cells. These findings emphasize the important potential of targeting the mechanisms regulating coregulator expression for therapeutic intervention in PCa.
Keywords: prostate cancer, androgen receptor, coregulator, coactivator, corepressor, cofactor
Introduction
Androgen signaling plays a critical role in the progression of prostate cancer (PCa). Standard therapy for non-organ-confined disease targets the action of the androgen receptor (AR), the transcription factor mediating the cellular effects of androgens, by reducing the circulating levels of its natural ligands and/or by administration of anti-androgens which compete for binding to the AR (1). While androgen deprivation therapies lead to initial remission, eventually most tumors recur as castration-recurrent (CR) PCa (CRPC), which is almost invariably fatal. Nonetheless, evidence indicates that CRPC cells continue to rely on the AR for proliferation (2-8). Consequently, AR signaling is considered a valuable therapeutic target throughout disease progression, and significant efforts are being directed towards understanding the mechanisms by which AR activity is regulated in (CR) PCa cells.
The ability of the AR to execute its transcriptional program relies on the recruitment of a large number of coregulatory proteins (9,10). Over the last decade, approximately 50 AR-associated coregulators have been found to be aberrantly expressed during PCa progression (Heemers and Tindall, in press). Deregulated coregulator expression often correlates with a poor prognosis. Moreover, changes in coregulator expression have been demonstrated to profoundly affect (CR) AR activity (11,12). Accordingly, AR-associated coregulators have been suggested as potential targets for therapeutic intervention in PCa disease. Thus, signal transduction pathways that govern altered expression of these critical cofactors in PCa are subject to intense investigation.
Recently, we and others have shown that conditions of androgen stimulation and androgen deprivation lead to marked changes in the expression levels of more than a third of coregulators in PCa cells (13-19). Androgen-dependent alterations in coregulator expression affect PCa cell proliferation and selectively influence the normal level of androgen-induction or repression of target genes. Moreover, investigation into the molecular mechanisms by which changes in androgenic milieu influence coregulator expression point towards diverse regulation at the level of transcription, translation, protein stability as well as turnover (13-19). In addition, some coregulator genes appear under direct control of the AR, while transcriptional regulation of other coregulator genes relies on the action of secondary transacting factors (15-17). Overall, these studies indicate the existence of a multitude of feedforward and feedback mechanisms by which the AR is able to modulate its activity in PCa cells and suggest that a better understanding of these events could lead to novel therapeutic avenues.
Recent findings from our laboratory suggest that androgen modulation of coregulator expression is PCa cell-specific, which increases the appeal of targeting coregulator expression for therapy. Using the AR-positive and androgen-sensitive human bladder cancer cell lines UMUC3 and TCC-SUP, we demonstrated that the expression of SRC1, SRC2, SRC3, p300 and CBP, all coactivators which are core components of the AR transcriptional complex, is not affected by androgen treatment (20). These findings are in contrast to observations in the LNCaP PCa cell line, where the expression of these 5 coregulators is down-regulated by diverse molecular mechanisms following androgen stimulation (13-15, 17).
In this manuscript, using multiple AR-positive PCa cell line and xenograft models, benign prostatic epithelial cell lines and androgen-responsive tissues derived from a male Wistar rat model, we explore the generality of the concept of androgen modulation of coregulator expression. Our results provide evidence for differential regulation of coregulator expression in benign and malignant prostate cells and as such validate the potential of coregulators as attractive targets for PCa therapy.
Materials and Methods
Cell culture
LNCaP and VCaP cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and were cultured in phenol red free RPMI1640 medium (Invitrogen, Carlsbad, CA) or DMEM medium (Invitrogen) respectively. Medium contained 9% fetal bovine serum (FBS) (Biosource, Rockville, MD), 100 U/ml streptomycin and 0.25 μg/ml amphotericin B (Fungizone) (Invitrogen). In experiments assessing the effects of androgen treatment, cells were seeded in medium containing 9% charcoal stripped serum (CSS), 100 U/ml streptomycin and 0.25 μg/ml amphotericin B. The LNCaP-Rf cell line was established and maintained as described (16,17). RWPE1 cells were obtained from ATCC and cultured in Keratinocyte-Serum Free Medium (KSFM) supplemented with epidermal growth factor (EGF) and bovine pituitary extract (BPE) (Invitrogen). In experiments studying the effects of androgen stimulation, RWPE1 cells were maintained in KSFM with 0.1 % bovine serum albumine (BSA) (Sigma-Aldrich, StLouis, MO). PrEC cells were purchased from Clonetics (Walkersville, MD) and cultured in prostate epithelial cell basal medium (PrEBM, Lonza, Walkersville, MD) supplemented with PrEGM SingleQuot Kit Supplements and Growth Factors (Lonza) and 9% FBS or CSS, respectively. Cells were maintained at 37°C in a humidified atmosphere of 5% CO2 in air.
Reagents
Methyltrienolone (R1881) was purchased from DuPont (Boston, MA). Testosterone and testosterone propionate was obtained from Sigma. Antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA) (AR, p300, CBP), Cell Signaling (Beverly, MA) (beta-actin, beta-tubulin), BD Transduction laboratories (San Jose, CA) (SRC1, SRC2, SRC3). The specificity of the antibodies used has been verified previously (15,17).
Xenograft studies
The androgen-dependent (AD) and androgen-independent (AI) LuCaP23.1 and LuCaP 35 xenograft models (21,22) were kindly provided by Dr. Vessella (Department of Urology, University of Washington Medical Center). AD LuCaP models were propagated in BALB/c nu/nu mice and AI models were propagated in SCID mice. Mice were housed in the Mayo Clinic pathogen-free rodent facility. All procedures were approved by the Mayo Clinic Institutional Animal Care and Use Committee (IACUC).
Rat studies
Twelve-week-old male Wistar rats were divided into three groups for experiments assessing the effects of testosterone. Groups contained four rats. Rats belonging to group I were sham-operated. Rats belonging to groups II and III were castrated by surgically removing the testes. Immediately following surgery, androgens or vehicle were administrated subcutaneously in the neck. Treatment was continued daily for 4 days. Group III animals received a mixture of testosterone and testosterone propionate (0.25 mg each, dissolved in 10 μl ethanol and mixed with 90 μl olive oil). Doses and drug choice were based on previous studies (23). The duration of the treatment was limited to 4 days to avoid major changes in tissue composition of strictly androgen-dependent organs such as the prostate. Rats belonging to groups I and II received 10 % ethanol in olive oil. Four days later, rats were anesthetized and exsanguinated. For RNA and protein analysis, tissues were excised and immediately frozen and stored in liquid nitrogen. All experiments were conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals and with the approval of the Mayo Clinic IACUC committee.
Preparation of whole cell lysates
Cells were washed with ice-cold PBS. Whole cell lysis buffer (110mM SDS, 100mM DTT, 80mM Tris-HCL pH 6.9, 10% glycerol) was pipetted onto the culture dish. Cell lysates were boiled for 5 minutes and stored at -20°C until analysis.
Preparation of whole tissue protein extracts
To prepare protein extracts from rat tissues or xenograft material, pulverized tissues were collected in whole cell lysis buffer, homogenized using a Dounce homogenizer and boiled for 5 minutes. Debris was removed by centrifugation for 10 minutes at top speed in a microcentrifuge. The supernatant was stored at -20°C until analysis.
Cell fractionation
LNCaP cells were seeded in medium supplemented with CSS. Two days later, medium was replaced and cells were treated with 1nM R1881 or vehicle for 96 hours. Nuclear and cytoplasmic extracts were prepared using a Nuclear Extract Kit (Active Motif, Carlsbad, CA) following the manufacturer's instructions and stored in a -80C freezer until analysis.
Western blot analysis
Equal amounts of protein were subjected to 3-8% Tris-Acetate NuPAGE gel electrophoresis (Invitrogen) according to the manufacturer's instructions. Proteins were blotted onto nitrocellulose membranes (BioRad, Hercules, CA). Blots were reprobed with antibodies against beta-actin to evaluate potential differences in protein loading.
RNA isolation
RNA isolation from cultured cells was done as described before (16,17). To prepare total RNA from rat tissues or xenograft material, pulverized tissues were collected in Trizol (Invitrogen) and homogenized using a Dounce homogenizer. Debris was removed by centrifugation for 10 minutes at 12,000g in a chilled microcentrifuge. The supernatant was used to complete the RNA extraction according to the manufacturer's guidelines.
Real-time RT-PCR
cDNA was prepared and real time RT-PCR was performed as before (16,17). Primers targeting human SRC1, SRC2, SRC3, p300, CBP and PSA expression have been described (15,17). Primer sequences used to analyze rat tissues are listed in Table 1. Rodent GAPDH primers were purchased from Applied Biosystems (Foster City, CA).
| target gene | primer pair | target sequence |
|---|---|---|
| rSRC1 | 5′ | cagcgggagctgtacagtca |
| 3′ | ctgggagtccggatgaagga | |
| rSRC2 | 5′ | gcagcgaactttgatgatga |
| 3′ | cgtagttcggaggaaatgga | |
| rSRC3 | 5′ | gagcaaactcttccgcaatc |
| 3′ | caggattcgggtttggtcta | |
| rCBP | 5′ | gtacagccaccaaggagcat |
| 3′ | ctgccattagctgtgggttt | |
| rp300 | 5′ | ccagacttgggacctttctg |
| 3′ | cctggtttcacctccacagt |
Replication of results
Results shown are representative of 2 or more independent experiments.
Results
Differential coregulator expression in benign and malignant epithelial prostate cells
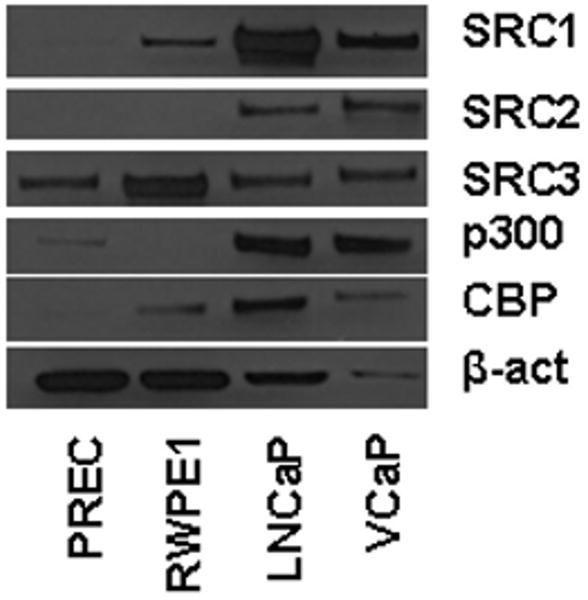
Previous studies have shown that the expression of the AR-associated coactivators SRC1, SRC2, SRC3, p300 and CBP is elevated in PCa compared to benign prostate (reviewed in 11). As expression of these coregulators tends to correlate with aggressive disease, these observations have prompted investigations into the signals and signal transduction that regulate their expression in in vitro model systems for PCa. Here, we further explore regulation of coregulator expression using, among others, cell-based models representative of normal and neoplastic epithelial prostate cells. To validate whether these model systems mimic the clinical situation in terms of coregulator expression patterns, we assessed and compared basal expression levels of SRC1, SRC2, SRC3, p300 and CBP in 2 cell line model systems that represent benign epithelial prostate cells (PrEC and RWPE1) and 2 PCa cell lines (LNCaP and VCaP). To this end, cells were grown in their regular medium and harvested when cultures reached 70-80% confluence. Equal amounts of protein were loaded side-by-side on a gel and analyzed by western blot. Expression of the house-keeping gene beta-actin was evaluated as an internal reference. As shown in Figure 1, the relative expression of all 5 coregulators was higher in the cancer cell lines LNCaP and VCaP compared to the benign cells. The differences in coregulator expression were especially pronounced for SRC1, SRC2 and p300, but also evident for SRC3 and CBP. Taken together, these data validate the use of these model systems for our studies.
Figure 1. Differential coregulator expression in benign and malignant epithelial prostate cells.
PrEC, RWPE1, LNCaP and VCaP cells were grown in their regular medium and harvested when cultures reached 70-80% confluence. Equal amounts of protein were analyzed by western blot using antibodies directed against SRC1, SRC2, SRC3, p300 and CBP. As an internal loading control, blots were stripped and reprobed with an antibody recognizing beta-actin (β-act).
Androgen regulation of coregulators is a common feature in PCa cell lines
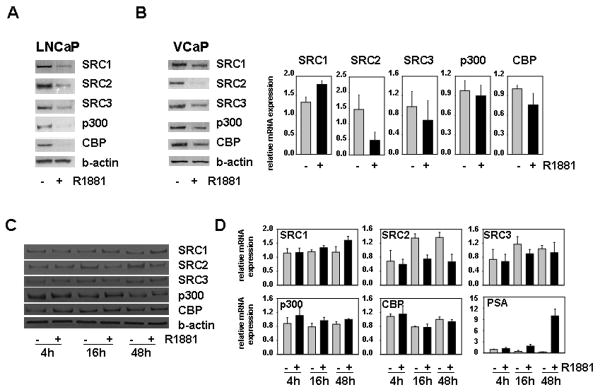
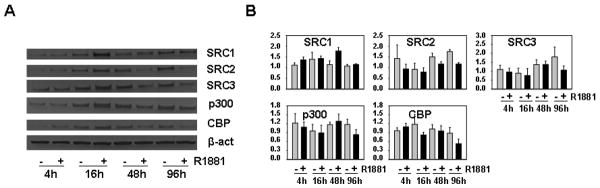
In previous studies, we and others showed that androgen stimulation of the androgen-responsive PCa cell line LNCaP leads to downregulation of the AR-associated coactivators SRC1, SRC2, SRC3, p300 and CBP (13-15, 17). To explore whether this regulation is a peculiarity of this particular cell line, we examined the effect of androgen treatment on expression of these coregulators in a second, independent AR-positive PCa cell line, VCaP. In contrast to LNCaP cells, in which the AR is characterized by a mutation in its ligand binding domain that results in broadened ligand specificity, VCaP cells express a wild-type AR (24,25). LNCaP and VCaP cells were treated for 4 days with 1 nM of the synthetic androgen R1881 or vehicle control. As shown in Figures 2A and B, western blot analysis of whole cell extracts confirmed androgen-induced decreases in the expression of SRC1, SRC2, SRC3, p300 and CBP in LNCaP cells and revealed that androgen exposure leads to downregulation of these coregulators also in VCaP cells. The relative level of repression of coactivators varied between the 2 cell lines. Specifically, the extent of androgen regulation of p300 and CBP appeared less pronounced in VCaP cells. These observations are in line with recent findings of androgen-induced suppression of p300 and SRC-2 expression in another AR-positive PCa cell line, LAPC-4 (14,15), and suggest that androgen regulation of coregulator expression is a common event in androgen-sensitive PCa cell lines. Similar to our previous findings in LNCaP cells (15,17), after 96 hours of androgen exposure VCaP cells appeared to decrease mRNA levels for SRC2, SRC3 and CBP, while leaving p300 messenger levels essentially unaltered. In contrast to LNCaP cells, VCaP cells did not respond to androgen stimulation by down-regulating SRC1 mRNA expression (Fig. 1B). To further explore the concept of generality of androgen-regulated coregulator expression in PCa cells and to evaluate the molecular mechanism(s) that may underlie these events, we performed time course studies in which we treated VCaP cells for 4, 16 and 48 hours with 1nM R1881 or ethanol vehicle. SRC2 was the only coregulator affected by androgen stimulation following shorter androgen exposure in VCaP cells : both at the protein level and at the RNA level decreases in SRC2 expression were observed after 16 hours of treatment (Figs. 2C and 2D). Consistent with observations done at 96 hours, SRC1 mRNA expression was increased at the 48h time point, whereas protein and mRNA expression of SRC3, CBP and p300 was not notably affected at any of the earlier time points. Based in part on similar time course studies in LNCaP cells, we and others proposed that SRC2, the expression of which is rapidly suppressed at the mRNA level after exposure androgens, is a direct, ARE-driven target for AR action (14,17) whereas androgen regulation of SRC1, SRC3, CBP and p300 involves other intermediary transcription factors as well as post-transcriptional events (15,17). The results in VCaP cells, where kinetics of SRC2 suppression and induction of PSA, a well-characterized ARE-driven gene, are remarkably similar (Figure 2D), suggest common mechanistic features in the androgen regulation of these AR-associated core coactivators in LNCaP and VCaP PCa cells.
Figure 2. Androgens downregulate expression of SRC1, SRC2, SRC3, CBP and p300 in PCa cell lines.
LNCaP (A) and VCaP cells (B,C,D) were seeded in medium supplemented with CSS. Two days later, medium was changed and cells were treated with 1nM of the synthetic androgen R1881 or ethanol vehicle for 4, 16, 48 (C,D) or 96 hours (A and B). Total protein extracts were prepared and equal amounts of protein were analyzed by western blotting using an antibody directed against SRC1, SRC2, SRC3, CBP and p300. To assess potential inter-sample loading differences, blots were stripped and reprobed with an antibody recognizing beta-actin (b-actin) (A, left panel B, C). RNA was isolated and converted into cDNA. Real time RT-PCR was performed with primers recognizing SRC1, SRC2, SRC3, p300, CBP and PSA (right panel B and D). mRNA levels were normalized with the values obtained from GAPDH. Values are expressed as relative expression levels, taking the value obtained from one of the untreated samples at the 96 hour (right panel B) or 4 hour (D) time point as 1. Columns, means of values obtained from three independent biological replicates; bars, SEM.
Androgen stimulation does not notably affect cellular localization of core coregulators in PCa cells
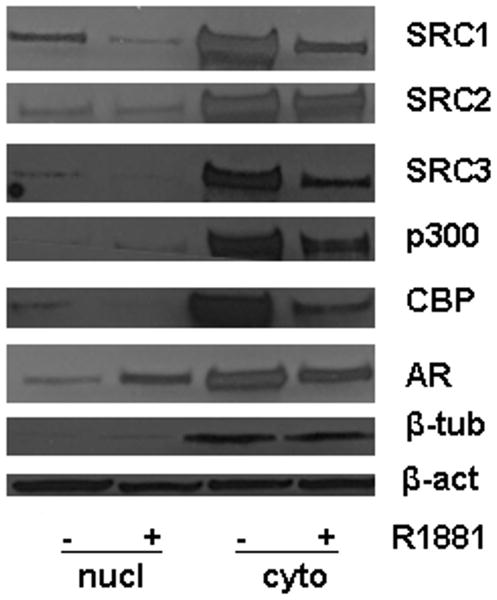
Reports for other AR-associated coregulators, such as Tip60 and Hey1 have indicated that changes in the androgenic milieu of PCa cells can induce relocalization of these important regulators of AR activity (26,27). We therefore explored whether androgen stimulation affects the subcellular distribution pattern of SRC1, SRC2, SRC3, p300 and CBP. To this end, nuclear and cytoplasmic extracts were prepared from LNCaP cells grown in the presence or absence of R1881 for 96 hours and analyzed by western blotting. Figure 3 shows that, as expected, androgen stimulation induced relocalization of AR from the cytoplasm to the nucleus. Noteworthy, except for p300, for which effects were more pronounced in the cytoplasm, androgen exposure led to similar decreases in the nuclear and cytoplasmic levels of the 5 core coregulators. Enrichment of beta-tubulin in the cytoplasmic fraction confirmed the effectiveness of the cell fractionation process. These data indicate that androgen stimulation does not induce major changes in the cellular distribution of SRC1, SRC2, SRC3, p300 and CBP.
Figure 3. Effects of androgens on the cellular distribution of SRC1, SRC2, SRC3, CBP and p300.
LNCaP cells were seeded in medium supplemented with CSS. Two days later, medium was changed and cells were treated with 1nM of the synthetic androgen R1881 or ethanol vehicle for 96 hours. Nuclear (nucl) and cytoplasmic (cyto) extracts were prepared and equal amounts of protein were analyzed by western blotting using antibodies directed against SRC1, SRC2, SRC3, CBP and p300. As controls for the efficacy of cell fractionation and androgen stimulation, expression of AR and beta-tubulin (β-tub) was also evaluated. To assess potential inter-sample loading differences, blots were stripped and reprobed with an antibody recognizing beta-actin (β-act).
Androgen deprivation affects coregulator expression in several PCa model systems
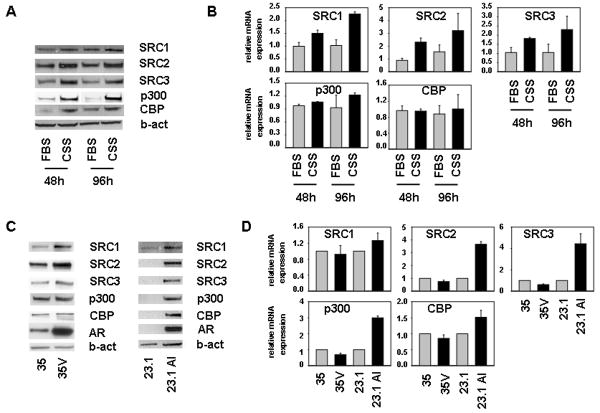
In view of the negative effects of androgen treatment on the expression levels of SRC1, SRC2, SRC3, p300 and CBP, we wondered whether androgen deprivation, which is clinically relevant, would affect the expression levels of these coactivators. To this end, we compared the effect of culturing LNCaP cells in medium supplemented with fetal bovine serum (FBS) or charcoal stripped serum (CSS), which is depleted of androgens. Figure 4A shows that after 2 and 4 days under androgen-deprived conditions the protein expression of these 5 coactivators was markedly increased in LNCaP cells. For SRC1, SRC2 and SRC3 similar effects were observed at the mRNA level (Fig. 4B). To verify the effect of androgen deprivation in model systems that more closely mimic the clinical situation, we examined expression of SRC1, SRC2, SRC3, p300 and CBP in two xenograft models representing the progression of androgen-stimulated PCa to CR PCa, LuCAP23.1 and LuCAP35 (21,22). Both xenograft models were derived from lymph node PCa metastases, are AR-positive, show androgen sensitivity and undergo CR growth post-castration. The resulting isogenic CR tumors are known as LuCAP23.1 AI and LuCAP35V, respectively (21,22). Comparison of coactivator expression levels between LuCAP35 and LuCAP35V tissues showed moderately increased expression of SRC1, SRC2 and SRC3 in the CR model (Figure 4C). Levels of SRC1, SRC2 and SRC3 protein were also considerably higher in LNCaP23.1 AI than in LNCaP23.1 specimens. In the LuCAP23.1 model, expression of p300 and CBP also markedly increased in the CR state (Figure 4C, right panel). Reminiscent of the clinical situation, AR protein levels were elevated in LuCAP35V and LuCAP 23.1 AI tumors in comparison with their androgen-sensitive counterparts. Expression levels of CBP and p300 appeared to be similar in LuCAP35 and LuCAP35V extracts. Real time RT-PCR analysis of these xenograft specimens indicated that, in line with observations at the protein level, androgen deprivation led to an increase in mRNA levels of the 5 coregulators studied here in LuCAP23.1/23.1AI isogenic model. In contrast, SRC1, SRC2, SRC3, p300 and CBP mRNA expression tended to be slightly lower in LuCAP35V samples than in to LuCAP35 tumors (Fig. 4D). Overall, these findings suggest a disconnection between regulation of coregulator expression at the mRNA and protein level, indicating a role for post-transcription events in the transition to the CR state in these isogenic models.
Figure 4. Effect of androgen deprivation on expression of SRC1, SRC2, SRC3, CBP and p300 in PCa models.
A,B. Effect of short-term androgen deprivation. LNCaP cells were seeded in medium supplemented with FBS (FBS) or CSS (CSS) medium. 48 and 96 hours later later, total protein extracts were prepared and equal amounts of protein were analyzed by western blotting (A) and real time RT-PCR (B) as described. mRNA values are expressed as relative expression levels, taking the value obtained from one of the CSS samples at the 48 hour time point as 1. Columns, means of values obtained from three independent biological replicates; bars, SEM. (B) C,D. Effect of long-term androgen deprivation. Whole tissue extracts were prepared from LuCAP35/35V (35/35V) and LuCAP23.1/23.1AI (23.1/23.1AI) xenograft tumors and subjected to immunoblotting analysis. To evaluate potential loading differences, blots were stripped and reprobed with an antibody against beta-actin (b-act) (C). Matching RNA samples were converted to cDNA and real time RT-PCR was performed as described. Columns, means of values obtained from three independent biological replicates; bars, SEM. (D).
Androgen regulation of coregulator expression is conserved in a CR cell line
In view of these findings and recent observations that androgen signaling is present in and critical for CR PCa cells (5,7,8), we explored the impact of androgen stimulation on coregulator expression in LNCaP-Rf cells, a model system for CRPC. The LNCaP-Rf cell line has been established in our laboratory by long term androgen ablation of LNCaP cells and shows increased expression of AR and faster proliferation than its parental line LNCaP (17), features that are reminiscent of the clinical situation. As shown in Figure 5, readministration of R1881 to LNCaP-Rf cells led to a decrease in expression of SRC2, SRC3, CBP and p300. These effects were notable at the protein level as well as on the mRNA level, and took a longer period of androgen exposure to occur when compared to LNCaP cells. In this experiment, effects on SRC1 expression were not readily observed. Noteworthy, in LNCaP-Rf cells effects were observed also for p300 mRNA, contrary to LNCaP cells where androgen regulation of p300 expression is limited to the protein level (15).
Figure 5. Androgen regulation of coregulator expression is conserved in LNCaP-Rf CRPC cells.
LNCaP-Rf cells were treated with 1nM of the synthetic androgen R1881 or ethanol vehicle for 4, 16, 48 or 96 hours. Total protein extracts were prepared and equal amounts of protein were analyzed by western blotting using antibodies directed against SRC1, SRC2, SRC3, CBP and p300. To assess potential inter-sample loading differences, blots were stripped and reprobed with an antibody recognizing beta-actin (β-act) (A). RNA was isolated and converted into cDNA. Real time RT-PCR was performed with primers recognizing SRC1, SRC2, SRC3, p300, CBP and PSA (B). mRNA levels were normalized with the values obtained from GAPDH. Values are expressed as relative expression levels, taking the value obtained from one of the untreated samples at the 4 hour time point as 1. Columns, means of values obtained from three independent biological replicates; bars, SEM.
Androgen-independence of coregulator expression in non-neoplastic prostate cells in culture
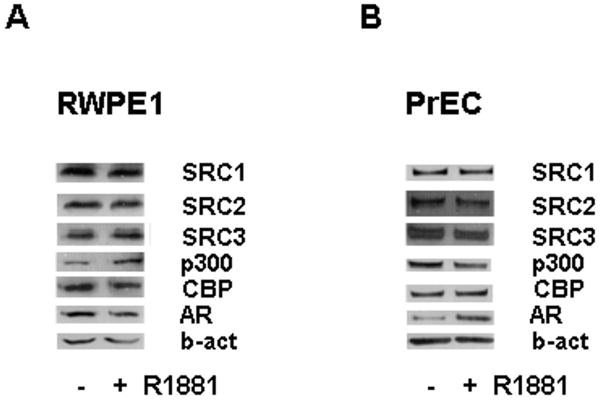
Taken together, our data suggest that androgen regulation of coregulator expression is a common event in AR-positive PCa models from different origins. We recently found that expression of SRC1, SRC2, SRC3, CBP and p300 is not subject to androgen regulation in the AR-positive and androgen-sensitive human bladder cancer cell lines UMUC3 and TCC-SUP (20). These findings indicate that androgen modulation of coregulator expression could be PCa cell-specific. To further explore this possibility, we investigated the effects of androgen stimulation on coactivator expression in AR-positive human non-malignant prostate epithelial cells. RWPE1 and PrEC cells were used for these studies. The androgen-responsive RWPE1 cell line is derived from non-neoplastic adult human prostatic epithelial cells that were immortalized by human papillomavirus 18 (28). PrEC cells represent a basal epithelial cell population whose basal phenotype has been reported to modulate in response to culturing conditions, consistent with a transient amplifying prostate epithelial population (29). While differences in the AR status of these cells have been described (29-31), under the conditions used in our laboratory PrEC cells are consistently AR-positive. As shown in Figure 6A, in contrast to our findings in PCa models, 4 days of androgen treatment of RWPE1 cells did not result in notable changes in coactivator expression. Similar observations of androgen-unresponsive coactivator expression were evident following identical experiments using PrEC cells (Fig.6B).
Figure 6. Effect of androgens on expression of SRC1, SRC2, SRC3, CBP and p300 in normal prostate epithelial cells.
RWPE1 and PrEC cells were seeded in their regular growth medium. Two days later, cells were washed once with medium supplemented with 0.1% BSA (RWPE1 cells) or 9% CSS (PrEC cells). Cells were grown in medium supplemented with 0.1% BSA (RWPE1 cells) or 9% CSS (PrEC cells) in the presence or absence of 1nM R1881 or ethanol vehicle for 4 days. Total protein extracts were prepared and equal amounts of protein were analyzed by western blotting using antibodies directed against SRC1, SRC2, SRC3, CBP, p300 and AR. To assess potential inter-sample loading differences, blots were stripped and reprobed with an antibody recognizing beta-actin (b-act).
Differential regulation of coactivator expression in normal androgen-responsive tissues in vivo
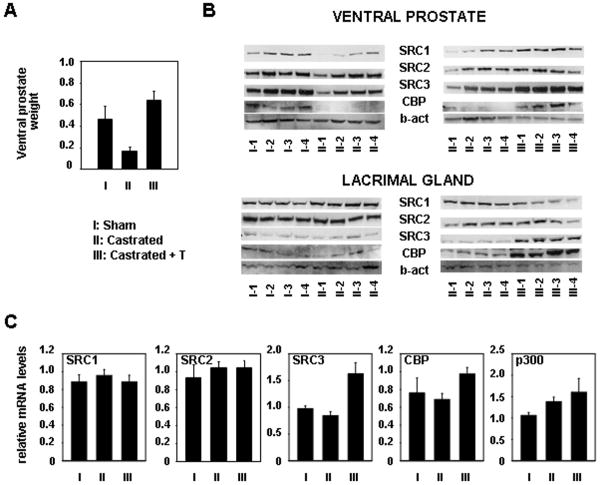
To further explore the possibility of differences in androgen dependency of coactivator expression between malignant and non-neoplastic prostate cells, we investigated the effects of androgen deprivation and androgen re-administration on coactivator expression in normal prostate tissue in vivo. To this end, 12-week old male Wistar rats were castrated and treated with a testosterone/testosterone propionate mixture (group III, 4 animals per group) or vehicle (group II) starting immediately following surgery for 4 days. A control group (group I) received sham-operations and vehicle treatment. As expected, castration-induced androgen withdrawal markedly reduced the weight of androgen-responsive ventral prostate tissues. Readministration of androgens restored and even slightly increased the weight of ventral prostates of group III above that of control animals (Figure 7A). Total tissue protein extracts were prepared from the ventral prostate from each animal and were analyzed for SRC1, SRC2, SRC3, CBP and p300 expression by western blotting. Specificity of the antibodies on rat tissues was verified with extracts from the rat prostate epithelial cell line NbeI (data not shown). As shown in Figure 7B (top panel), in ventral prostate tissues from castrated animals, a decrease in expression of SRC1, SRC2, SRC3 and CBP was observed. These effects were minimal for SRC2 and SRC3 and more pronounced for SRC1 and CBP. Re-administration of testosterone restored the expression levels of these 4 coactivators (Fig. 7B, top panel). Due to low expression levels, p300 protein expression could not be assessed in these tissues. Taken together, these findings again suggest a pattern of coregulator expression that is markedly different from that observed in PCa cells. In view of the well-known androgen-dependent cellular composition of prostate tissue, the possibility that these regulatory effects on coregulator expression are attributable to changes in the architecture of the gland could not be ruled out. Therefore, we also explored the effect of androgen withdrawal and restitution in a target tissue for androgen action that does not rely on androgens to maintain its structural integrity. To this end, we harvested the lacrimal glands from animals belonging to group I, II, and III. Western blot analysis of total protein extracts from these tissues revealed that androgen deprivation does not notably affect expression levels of SRC1, SRC2, SRC3 and CBP (Fig. 7B, lower panel). As for the ventral prostate extracts, expression of p300 was not detectable in these samples. Readministration of testosterone had no noticeable effect on expression of SRC1 and SRC2, but did induce protein expression of SRC3 and CBP to levels above those observed for group II animals. Noteworthy, the expression patterns for CBP and SRC3 were also observed at the mRNA level (Fig. 7C). Apparent modest effects on SRC1 expression may be attributable to slight loading differences (Figure 7B), and were not obvious at the mRNA level. Similarly, p300 mRNA expression levels in lacrimal glands did not appear subject to substantial changes upon variations in androgen concentrations. Taken together, our data suggest marked differences in the regulation of coregulator expression between normal and malignant prostate cells and emphasize the potential of targeting coregulator expression for the treatment of PCa.
Figure 7. Effect of androgen withdrawal and readministration on the expression of SRC1, SRC2, SRC3, p300 and CBP in androgen-responsive tissues in vivo.
Adult Wistar rats were castrated (groups II and III) or sham-operated (group I). Animals were treated with testosterone and testosterone propionate (group III) or vehicle (groups I and II) for 4 days. Thereafter, animals were sacrificed and ventral prostates and lacrimal glands were excised. Groups consisted of 4 animals. A. Effect of treatment on the weight of the ventral prostate tissues (in gram). B. For each animal, protein extracts were made from the ventral prostate (top panel) and lacrimal gland (bottom panel). Equal amounts of protein were analyzed by Western blotting using antibodies directed against SRC1, SRC2, SRC3 and CBP. To assess potential inter-sample loading differences, blots were stripped and reprobed with an antibody recognizing beta-actin (b-act). To further evaluate protein loading, blotting membranes were subjected to Ponceau S staining (data not shown). C. Matching RNA samples from lacrimal glands were analyzed by real time RT-PCR using primers amplifying rodent SRC1, SRC2, SRC3, CBP and p300 mRNA sequences. mRNA levels were normalized with the values obtained from rat GAPDH. Values are expressed as relative expression levels, taking the value obtained from one of the animals from group I as 1. Columns, means of values obtained from three independent biological replicates; bars, SEM.
Discussion
Deregulation of coregulator expression is a common event in PCa and is thought to contribute considerably to the sustained activity of the AR in the progression of this disease (11,12). Our studies are the first to report on differences in the regulation of coregulator expression between normal and neoplastic prostate epithelial cells. These findings validate ongoing efforts to understand the molecular mechanisms that govern coregulator expression in PCa and translate the resulting information into alternative means of targeting AR action in PCa.
Over 170 AR-associated coregulators have been described to date (9,10). These proteins structurally and functionally interact with the AR to modulate its transcriptional output. In the current study the regulation of 5 coactivators (SRC1, SRC2, SRC3, CBP and p300) that are core components of the AR transcriptional complex was evaluated. These proteins function as scaffolds to attract additional cofactors and serve as a bridge between the AR and the basal transcription factors. In addition, except for SRC2, these coactivators possess histone acetyl transferase (HAT) activity that induces acetylation of histone residues and, ultimately, increases accessibility of DNA to the transcriptional machinery (10). Expression of SRC1, SRC2, SRC3, p300 and CBP is elevated in PCa compared to benign prostate and often correlates with aggressive disease and poor prognosis (32-35). We and others have previously described androgen-dependent downregulation of these coactivators in the PCa cell line LNCaP (13-15,17). Here, using multiple independent and isogenic PCa cell lines and xenografts, we show that this regulation is a common event in PCa cells. Apart from p300 and CBP expression in the LuCaP 35/35V isogenic model and SRC1 expression in LNCaP-Rf cells, we noted that the presence of androgens leads to a decrease in the expression of this set of 5 coactivators in all PCa models. The observations in the LuCAP35/35V model may reflect PCa heterogeneity in the regulation of coregulator expression. In addition, while our results indicate that similar mechanisms underlie androgen regulation of the 5 coactivators studied here in the PCa cell lines LNCaP and VCaP, they also suggest substantial mechanistic variability may govern coregulator protein expression in PCa cells in general. Regulation that occurs at the post-transcriptional level may be particularly relevant to modulate coregulator expression in PCa.
Noteworthy, our results are in agreement with recent reports describing androgen-dependent regulation of p300 and SRC2 in LAPC-4 cells (14,15), another AR-positive cell line that was not included in our studies. Furthermore, expression of SRC1 and SRC2 has been described to increase following androgen deprivation in the CWR22 xenograft model (32), which closely recapitulates the transition from androgen-stimulated to CR PCa. Perhaps more relevant, the latter study also noted stronger immunostaining for SRC1 and SRC2 in clinical specimens derived from CR patients than in samples from patients with androgen-stimulated PCa (32). Also, very high levels of CBP have been found in advanced PCa that failed androgen ablation therapy (13), suggesting that androgen deprivation strategies may lead to elevated CBP expression. In contrast to these PCa models, our observations in model systems representing normal AR-positive prostate epithelial cells reveal striking differences in the regulation of coregulator expression. Overall, the RWPE1 and PrEC cell lines display a predominantly androgen-independent expression pattern of SRC1, SRC2, SRC3, CBP and p300. These results were not due to overall androgen unresponsiveness as evidenced by androgen regulation of AR expression in PrEC cells (Fig.6B). Furthermore, in non-malignant androgen-responsive tissues derived from a Wistar rat model, the expression patterns of these 5 core coactivators also did not follow the trend of androgen regulation seen in PCa cells. In these tissues, expression of SRC1, SRC2, SRC3 and CBP could be reliably detected by immunoblotting and was not strongly affected by androgen withdrawal. Studies of coregulator expression in ventral prostate tissue are hampered somewhat by the well-known androgen-dependency of its cellular composition. Even taking these conditions under consideration, our results suggest that regulation of coregulator expression in ventral prostate is markedly different from that observed in PCa cells. Importantly, these observations are not limited to androgen-dependent tissues derived from the urogenital tract such as the ventral prostate, but hold true also in the lacrimal gland. Unlike the prostate, the latter tissue does not undergo involution or major changes in its cellular composition following androgen deprivation, and therefore may more reliably reflect androgen-dependent changes in expression of genes of interest. Androgen re-administration immediately after castration did not have major effects on expression of SRC1 and SRC2 in the lacrimal gland and appeared to modestly increase levels of CBP and SRC3. These effects on CBP and SRC3 expression were confirmed at the mRNA level (data not shown). Whether these effects, which are not obvious between sham-operated control animals and their castrated control animals, are simply related to an apparent slight overshoot in androgen levels upon testosterone/testosterone propionate administration, as reflected by increased wet weight of ventral prostates in group III, cannot conclusively be deduced from our studies. Overall, the results from our animal studies clearly point to coregulator regulation that is distinct from that observed in PCa models.
The findings in non-malignant prostate or lacrimal gland cells are reminiscent of recent observations in our laboratory using AR-positive human bladder cancer cell lines (UMUC3 and TCC-SUP) (20). In these cells, the expression of the same set of 5 coactivators was unresponsive to androgen treatment. Nonetheless, these cofactors remained important for androgen-dependent cell features, as knockdown of the expression of any one of these 5 coactivators blunted androgen-responsive cell viability (20). Therefore, our findings do not rule out the possibility that SRC1, SRC2, SRC3, CBP and p300 may play important roles in the development or function of the normal prostate or for that matter in androgen-dependent physiology in general. This premise is supported by observations in knock-out mice models. For example, disruption of SRC1 leads to partial resistance to androgens with reduced testis weight and decreased growth and development of the prostate (36). Moreover, male mice that are deficient in SRC2 suffer from hypofertility, defects in spermiogenesis and testicular degeneration (37). The importance of these coregulators for non-malignant or non-PCa related androgen-dependent cellular events might be dependent on androgen regulation of AR-cofactor interactions or on androgen effects on post-translational modifications that determine coregulator activity. These are aspects of coregulator function that were not addressed in the current study. Similarly, the data shown do not take into account potential intracellular cofactor redistribution upon changes in the androgenic milieu. Such relocalization has been described previously for selected coregulators, (e.g. Tip60 and Hey1, an AR-associated coactivator and corepressor respectively (26,27)) in PCa cells.
Immunohistochemical assessment of SRC1, SRC2, SRC3, CBP and p300 in PCa specimens (32-35), as well as comparison of expression patterns for SRC1, SRC2 and CBP in human androgen-stimulated and CR PCa specimens has revealed a predominantly nuclear coactivator expression pattern that is not subject to change throughout disease progression (13,32,35). The results of our cell fractionation experiments (Figure 3) support these observations, as they do not suggest major cellular redistribution of the 5 coactivators upon androgen stimulation. In line with these findings, ongoing immunohistochemical evaluation of SRC1, SRC2, SRC3, CBP and p300 in androgen-dependent tissues derived from our Wistar rat model do not indicate androgen-dependent shifts in cofactor localization (data not shown).
Taken together, our data provide evidence for differential regulation of coregulator expression in normal and malignant prostate epithelial cells. In view of the large number of AR-associated coregulators that are subject to androgen modulation in PCa cells, it will be important to verify whether our findings can be extrapolated to other androgen-responsive coregulator genes. Ultimately, a better understanding of the regulation of coregulator expression may lead to alternative means of targeting AR action for therapy in PCa.
Acknowledgments
Grant support: This work was supported by NIH grants CA121277, CA91956, CA15083, CA125747, DK65236, the T.J. Martell Foundation, the Charlotte Geyer Foundation and the Belgian-American Educational Foundation (BAEF) (HVH).
Footnotes
Disclosure statement: The authors have no conflicting interests to declare.
References
- 1.Miyamoto H, Messing EM, Chang C. Androgen deprivation therapy for prostate cancer: current status and future prospects. Prostate. 2004;61:332–353. doi: 10.1002/pros.20115. [DOI] [PubMed] [Google Scholar]
- 2.Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. J Natl Cancer Inst. 2001;93:1687–1697. doi: 10.1093/jnci/93.22.1687. [DOI] [PubMed] [Google Scholar]
- 3.Litvinov IV, De Marzo AM, Isaacs JT. Is the Achilles' heel for prostate cancer therapy a gain of function in androgen receptor signaling? J Clin Endocrinol Metab. 2003;88:2972–2982. doi: 10.1210/jc.2002-022038. [DOI] [PubMed] [Google Scholar]
- 4.Debes JD, Tindall DJ. Mechanisms of androgen-refractory prostate cancer. N Engl J Med. 2004;351:1488–1490. doi: 10.1056/NEJMp048178. [DOI] [PubMed] [Google Scholar]
- 5.Mohler JL. Castration-recurrent prostate cancer is not androgen-independent. Adv Exp Med Biol. 2008;617:223–234. doi: 10.1007/978-0-387-69080-3_21. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol. 2008;8:440–448. doi: 10.1016/j.coph.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G, de Bono JS. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26:4563–4571. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 8.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;8(324):787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heinlein CA, Chang C. Androgen receptor (AR) coregulators: an overview. Endocr Rev. 2002;23:175–200. doi: 10.1210/edrv.23.2.0460. [DOI] [PubMed] [Google Scholar]
- 10.Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28:778–808. doi: 10.1210/er.2007-0019. [DOI] [PubMed] [Google Scholar]
- 11.Heemers HV, Tindall DJ. Androgen receptor coregulatory proteins as potential therapeutic targets in the treatment of prostate cancer. Curr Cancer Ther Rev. 2005;1:175–186. [Google Scholar]
- 12.Chmelar R, Buchanan G, Need EF, Tilley W, Greenberg NM. Androgen receptor coregulators and their involvement in the development and progression of prostate cancer. Int J Cancer. 2007;120:719–733. doi: 10.1002/ijc.22365. [DOI] [PubMed] [Google Scholar]
- 13.Comuzzi B, Nemes C, Schmidt S, Jasarevic Z, Lodde M, Pycha A, Bartsch G, Offner F, Culig Z, Hobisch A. The androgen receptor co-activator CBP is up-regulated following androgen withdrawal and is highly expressed in advanced prostate cancer. J Pathol. 2004;204:159–166. doi: 10.1002/path.1609. [DOI] [PubMed] [Google Scholar]
- 14.Agoulnik IU, Vaid A, Nakka M, Alvarado M, Bingman WE, 3rd, Erdem H, Frolov A, Smith CL, Ayala GE, Ittmann MM, Weigel NL. Androgens modulate expression of transcription intermediary factor 2, an androgen receptor coactivator whose expression level correlates with early biochemical recurrence in prostate cancer. Cancer Res. 2006;66:10594–10602. doi: 10.1158/0008-5472.CAN-06-1023. [DOI] [PubMed] [Google Scholar]
- 15.Heemers HV, Sebo TJ, Debes JD, Regan KM, Raclaw KA, Murphy LM, Hobisch A, Culig Z, Tindall DJ. Androgen deprivation increases p300 expression in prostate cancer cells. Cancer Res. 2007;67:3422–3430. doi: 10.1158/0008-5472.CAN-06-2836. [DOI] [PubMed] [Google Scholar]
- 16.Heemers HV, Regan KM, Dehm SM, Tindall DJ. Androgen induction of the androgen receptor coactivator four and a half LIM domain protein-2: evidence for a role for serum response factor in prostate cancer. Cancer Res. 2007;67:10592–10599. doi: 10.1158/0008-5472.CAN-07-1917. [DOI] [PubMed] [Google Scholar]
- 17.Heemers HV, Regan KM, Schmidt LJ, Anderson SK, Ballman KV, Tindall DJ. Androgen modulation of coregulator expression in prostate cancer cells. Mol Endocrinol. 2009;23:572–583. doi: 10.1210/me.2008-0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen PH, Tsao YP, Wang CC, Chen SL. Nuclear receptor interaction protein, a coactivator of androgen receptors (AR), is regulated by AR and Sp1 to feed forward and activate its own gene expression through AR protein stability. Nucleic Acids Res. 2008;36:51–66. doi: 10.1093/nar/gkm942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou JX, Guo L, Revenko AS, Tepper CG, Gemo AT, Kung HJ, Chen HW. Androgen-induced coactivator ANCCA mediates specific androgen receptor signaling in prostate cancer. Cancer Res. 2009;69:3339–3346. doi: 10.1158/0008-5472.CAN-08-3440. [DOI] [PubMed] [Google Scholar]
- 20.Boorjian SA, Heemers HV, Frank I, Farmer SA, Schmidt LJ, Sebo TJ, Tindall DJ. Expression and significance of androgen receptor coactivators in urothelial carcinoma of the bladder. Endocr Relat Cancer. 2009;16:123–137. doi: 10.1677/ERC-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis WJ, Vessella RL, Buhler KR, Bladou F, True LD, Bigler SA, Curtis D, Lange PH. Characterization of a novel androgen-sensitive, prostate-specific antigen-producing prostatic carcinoma xenograft: LuCaP 23. Clin Cancer Res. 1996;2:1039–1048. [PubMed] [Google Scholar]
- 22.Corey E, Quinn JE, Buhler KR, Nelson PS, Macoska JA, True LD, Vessella RL. LuCaP 35: a new model of prostate cancer progression to androgen independence. Prostate. 2003;55:239–246. doi: 10.1002/pros.10198. [DOI] [PubMed] [Google Scholar]
- 23.Heemers H, Vanderhoydonc F, Roskams T, Shechter I, Heyns W, Verhoeven G, Swinnen JV. Androgens stimulate coordinated lipogenic gene expression in normal target tissues in vivo. Mol Cell Endocrinol. 2003;205:21–31. doi: 10.1016/s0303-7207(03)00205-3. [DOI] [PubMed] [Google Scholar]
- 24.Veldscholte J, Ris-Stalpers C, Kuiper GG, Jenster G, Berrevoets C, Claassen E, van Rooij HC, Trapman J, Brinkmann AO, Mulder E. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990;173:534–540. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 25.van Bokhoven A, Varella-Garcia M, Korch C, Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ, Lucia MS. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:205–225. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 26.Halkidou K, Gnanapragasam VJ, Mehta PB, Logan IR, Brady ME, Cook S, Leung HY, Neal DE, Robson CN. Expression of Tip60, an androgen receptor coactivator, and its role in prostate cancer development. Oncogene. 2003;22:2466–2477. doi: 10.1038/sj.onc.1206342. [DOI] [PubMed] [Google Scholar]
- 27.Belandia B, Powell SM, García-Pedrero JM, Walker MM, Bevan CL, Parker MG. Hey1, a mediator of notch signaling, is an androgen receptor corepressor. Mol Cell Biol. 2005;25:1425–1436. doi: 10.1128/MCB.25.4.1425-1436.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bello D, Webber MM, Kleinman HK, Wartinger DD, Rhim JS. Androgen responsive adult human prostatic epithelial cell lines immortalized by human papillomavirus 18. Carcinogenesis. 1997;18:1215–1223. doi: 10.1093/carcin/18.6.1215. [DOI] [PubMed] [Google Scholar]
- 29.Garraway LA, Lin D, Signoretti S, Waltregny D, Dilks J, Bhattacharya N, Loda M. Intermediate basal cells of the prostate: in vitro and in vivo characterization. Prostate. 2003;55:206–218. doi: 10.1002/pros.10244. [DOI] [PubMed] [Google Scholar]
- 30.King KJ, Nicholson HD, Assinder SJ. Effect of increasing ratio of estrogen: androgen on proliferation of normal human prostate stromal and epithelial cells, and the malignant cell line LNCaP. Prostate. 2006;66:105–114. doi: 10.1002/pros.20327. [DOI] [PubMed] [Google Scholar]
- 31.Tran CP, Lin C, Yamashiro J, Reiter RE. Prostate stem cell antigen is a marker of late intermediate prostate epithelial cells. Mol Cancer Res. 2002;1:113–121. [PubMed] [Google Scholar]
- 32.Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, Wilson EM. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61:4315–4319. [PubMed] [Google Scholar]
- 33.Gnanapragasam VJ, Leung HY, Pulimood AS, Neal DE, Robson CN. Expression of RAC 3, a steroid hormone receptor co-activator in prostate cancer. Br J Cancer. 2001;85:1928–1936. doi: 10.1054/bjoc.2001.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Debes JD, Sebo TJ, Lohse CM, Murphy LM, Haugen DA, Tindall DJ. p300 in prostate cancer proliferation and progression. Cancer Res. 2003;63:7638–7640. [PubMed] [Google Scholar]
- 35.Comuzzi B, Lambrinidis L, Rogatsch H, Godoy-Tundidor S, Knezevic N, Krhen I, Marekovic Z, Bartsch G, Klocker H, Hobisch A, Culig Z. The transcriptional co-activator cAMP response element-binding protein-binding protein is expressed in prostate cancer and enhances androgen- and anti-androgen-induced androgen receptor function. Am J Pathol. 2003;162:233–241. doi: 10.1016/S0002-9440(10)63814-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J, Qiu Y, DeMayo FJ, Tsai SY, Tsai MJ, O'Malley BW. Partial hormone resistance in mice with disruption of the steroid receptor coactivator-1 (SRC-1) gene. Science. 1998;279:1922–1925. doi: 10.1126/science.279.5358.1922. [DOI] [PubMed] [Google Scholar]
- 37.Gehin M, Mark M, Dennefeld C, Dierich A, Gronemeyer H, Chambon P. The function of TIF2/GRIP1 in mouse reproduction is distinct from those of SRC-1 and p/CIP. Mol Cell Biol. 2002;22:5923–5937. doi: 10.1128/MCB.22.16.5923-5937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]