Abstract
Both testosterone and its nonaromatizable metabolite dihydrotestosterone (DHT) induce spermatogenesis in gonadotropin-deficient hpg mice. Surprisingly, because aromatization is not required, estradiol (E2) also induces spermatogenesis and increases circulating FSH in hpg mice, but the mechanism remains unclear. We studied E2-induced spermatogenesis in hpg mice on an estrogen receptor (ER)-α (hpg/αERKO) or ERβ (hpg/βERKO) knockout or wild-type ER (hpg/WT) background treated with subdermal E2 or DHT implants for 6 wk. In hpg/WT and hpg/βERKO, but not hpg/αERKO mice, E2 increased testis and epididymal weight, whereas DHT-induced increases were unaffected by ERα or ERβ inactivation. E2 but not DHT treatment increased serum FSH (but not LH) in hpg/WT and hpg/βERKO but not hpg/αERKO hpg mice. DHT or E2 alone increased (premeiotic) spermatogonia and (meiotic) spermatocytes without significant change in Sertoli cell numbers. DHT alone increased postmeiotic spermatids, regardless of ER presence, compared with variable ERα-dependent E2 postmeiotic responses. An ERα-mediated effect was confirmed by treating hpg mice for 6 wk by subdermal selective ER-α (16α-LE2) or ERβ (8β-VE2) agonist implants. ERα (but not ERβ) agonist increased testis and epididymal weight, Sertoli cell, spermatogonia, meiotic, and postmeiotic germ cell numbers. Only ERα agonist markedly increased serum FSH, whereas either agonist induced small rises in serum LH. Administration of ERα agonist or E2 in the presence of functional ERα induced prominent gene expression of specific Sertoli (Eppin, Rhox5) and Leydig cell (Cyp11a1, Hsd3b1) markers. We conclude that E2-induced spermatogenesis in hpg mice involves an ERα-dependent neuroendocrine mechanism increasing blood FSH and Sertoli cell function.
Although not necessary, estradiol is sufficient to induce spermatogenesis in the gonadotropin-deficient hpg mouse by an ERα-dependent mechanism that increases blood FSH and activates Sertoli cell function.
Both individual and synergistic actions of testosterone (T) and FSH are required for optimal regulation of spermatogenesis (1,2,3). The concerted roles of FSH and T are mediated via Sertoli cells, which express the FSH and androgen receptor (AR), both of which are absent in developing male germinal cells. The hormonal actions of each have been illustrated clearly by phenotypes exhibited in mouse models null for the respective hormone pathways. For example, hypogonadal (hpg) mice are functionally deficient in gonadotrophins and sex steroids due to a naturally occurring deletion in the Gnrh1 gene (4,5) and consequently exhibit immature testis with spermatogenic arrest at the pachytene stage of meiosis (6). Disruption of FSH action alone, by either inactivation of the FSHβ subunit (7) or receptor (8,9) genes, leads to reduced sperm production and mature testis size due primarily to an insufficient Sertoli cell population (4,5). Whereas FSH actions alone can maintain Sertoli cell number (10), androgen actions are indispensable for the completion of postmeiotic germ cell development (6). This critical role for androgen signaling is consistent across mouse models with defective androgen signaling globally (11) or in Sertoli cells only (12,13,14,15), with spermatogenesis blocked at the tetraploid pachytene spermatocytes stage. Hence, there is abundant evidence that induction of spermatogenesis depends on the complementary actions of FSH and androgens, the former to establish a sufficient Sertoli cell population and the latter for functional completion of meiosis and postmeiotic sperm differentiation/maturation.
Although an absolute requirement for androgen action in spermatogenesis is clear, the precise molecular mechanisms involved in steroid-induced testicular development are not. Qualitatively complete spermatogenesis can be restored in hpg mice by administration of either T or its metabolite, the potent nonaromatizable androgen dihydrotestosterone (DHT), in the absence of FSH stimulation (6). These data strongly indicate that the effects are exerted directly on the testis, did not require aromatization, and were mediated via the AR (6). However, later studies demonstrated that comparable spermatogenesis in hpg mice is induced after similar treatment with estradiol (E2) (16). Interestingly, this E2 effect is dependent on a functional AR (17,18) and coincides with increased circulating FSH levels. Prompted by the disparities in the available evidence on the mechanism and site of action by which E2 induces spermatogenesis in hpg mice, the present study set out to segregate the spermatogenic actions of E2 by two complementary pharmacogenetic experiments using: 1) selective estrogen receptor (ER)α or ERβ agonists in gonadotropin-deficient hpg mice, and 2) pure androgen (DHT) and estrogen (E2) treatments in novel compound hpg/ER knockout lines selectively lacking ERα or ERβ (hpg/αERKO or hpg/βERKO mice) on the hpg genetic background. Noting that spermatogenesis occurs normally in mice rendered estrogen deficient due to genetic disruption of estradiol synthesis (19) or either of its ERs (20,21,22,23), our results from this in vivo approach reveal that E2-induced spermatogenesis in hypogonadotrophic mice is dependent on functional ERα but not ERβ.
Materials and Methods
Mice
For study 1, hpg mice (Gnrh1−/− males, C3H/HeH × 101/H) were maintained under standard housing conditions (ad libitum access to food and water in a temperature and humidity controlled, 12-h light cycle environment) at the ANZAC Research Institute. All experiments were approved by the Sydney South West Area Health Service's Animal Welfare Committee within National Health and Medical Research Council ethical guidelines for animal experimentation. Genotype was confirmed by PCR (24). All procedures were performed under ketamine/xylazine anesthesia using additional analgesics if required for nonterminal procedures.
For study 2, hpg mice were crossed onto the ERα knockout (αERKO) and ERβ knockout (βERKO) lines to generate hypogonadal forms of each respective ER-null line. Compound hpg/αERKO and hpg/βERKO mice were obtained from individual colonies that were first established by crossing heterozygous Gnrh1+/− males (C3H/HeH × 101/H; Jackson Laboratory, Bar Harbor, ME) with heterozygous Esr1+/− (αERKO) or Esr2+/− (βERKO) females (C57BL/6) to generate compound heterozygous animals for general breeding. The hpg (Gnrh1−/−) littermate males expressing native ERα and ERβ (termed hpg/WT) derived from these heterozygous breeding pairs were considered comparable and used as controls. All animals were genotyped for targeted Esr1 or Esr2, and mutant Gnrh1 alleles as described previously (25) by PCR on DNA extracted from tail biopsies using the Wizard SV 96 Genomic DNA extraction kit (Promega, Madison, WI). Mice were individually identified by an implanted microchip transponder (BioMedic Data Systems, Seaford, DE) and maintained in plastic cages in a temperature-controlled room (21–22 C) under a 12-h light, 12-h dark schedule with access to NIH 31 mouse chow and fresh water ad libitum. Mice were killed by exsanguination under anesthesia with a sublethal dose of sodium pentobarbital. The Animal Care and Use Committee of the National Institute of Environmental Health Sciences preapproved all procedures involving animals.
Drugs
ERα (16α-LE2) or ERβ (8β-VE2) agonists were provided by Bayer Schering Pharma (previously Schering, Berlin, Germany) (26,27). Drug treatments were administered by subdermal implantation of 60-d release pellets (Innovative Research Associated, Sarasota, FL) containing the ERα agonist (16α-LE2, 15 and 45 μg/kg · d, nominally 0.375 and 1.125 μg per 25 g mouse per day) or ERβ agonist (8β-VE2, 150 and 450 μg/kg · d, nominally 3.75 and 11.25 μg per 25 g/mouse · d). Doses used were previously shown to induce maximal ER-specific effects of each agonist (28).
E2 and DHT were administered via subdermal implantation of single SILASTIC brand tubes (inner diameter 1.47 mm, outer diameter 1.95 mm, end sealed with SILASTIC brand adhesive; Dow Corning Corp., Midland, MI) filled to 1 cm with crystalline DHT (6) or E2 recrystallized into a homogenous powder after dilution (1:1000) with cholesterol (18). Control animals received no implant.
Study design
In study 1, weanling (3 wk old) hpg males (n = 11–23/group) were untreated or treated for 6 wk with selective ERα (16α-LE2) or ERβ (8β-VE2) agonists. Untreated age-matched wild-type littermate males were used as controls. After 6 wk of treatment, mice were weighed and blood collected (processed serum stored at −20 C) under anesthesia by cardiac exsanguination; then one testis was immediately excised, weighed, and snap frozen in liquid nitrogen. Animals were then perfused with heparinized 0.9% saline followed by Bouin's fixative via the left ventricle and the fixed contralateral testis collected for sectioning and stereological evaluation; prostate lobes (anterior, ventral, dorsolateral), epididymis, seminal vesicles, spleen, and pituitary were dissected and weighed.
In study 2, E2 or DHT induction of spermatogenesis was examined in hpg/WT, hpg/αERKO, and hpg/βERKO mice (n = 7–25/group) of 6–12 wk of age administered single subdermal SILASTIC brand implants (1 cm; Dow Corning) containing either crystalline E2 or DHT. Control animals received no implant. Due to limited animal availability, mice were treated in small cohorts, each cohort consisting of both control and treated littermate mice, and data then combined for analysis. Six weeks after implantation, mice were anesthetized and weighed, serum collected from retroorbital bleed and then perfused, and tissues collected as described in study 1. Weights of testes, epididymis, seminal vesicles, liver, spleen, heart, and kidneys were recorded and serum stored at −20 C.
Histology and stereology
After whole-body perfusion with modified Bouin's fixative, testes were dissected and placed in fresh Bouin's solution for an additional 24 h and then transferred to 70% ethanol and processed for histological examination. Sertoli and germ cell populations were quantified stereologically by the optical dissector technique as described previously (3,29) using CASTGRID (Olympus, Aarhus, Denmark) software.
Assays
Mouse serum FSH (30) and LH (31) levels were measured by immunofluorometric assays as described previously. Serum testosterone, DHT, and E2 were measured in a single assay batch using a novel liquid chromatography, tandem mass spectrometry method as described (32) as adapted for mouse samples (33). Briefly, 0.1 ml serum was extracted with hexane-ethyl acetate fortified with deuterated internal standards and run without derivatization. The limits of quantification were 0.1 ng/ml, 0.2 ng/ml, and 5 pg/ml, respectively.
Gene expression
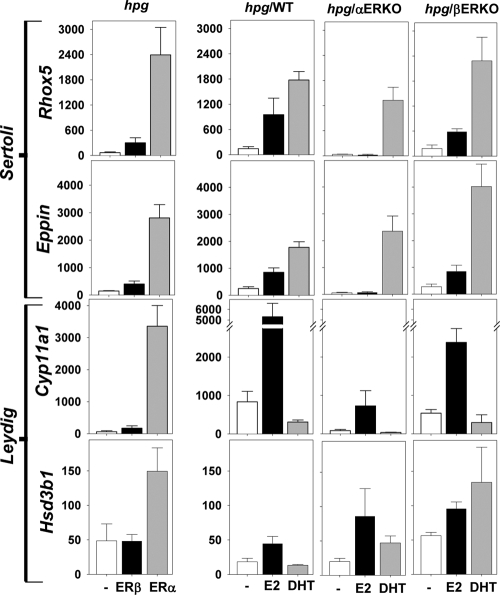
Total RNA from flash frozen testes was obtained after homogenization in TRI reagent (Sigma, St. Louis, MO) or TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. First-strand cDNA synthesis was performed using the SuperScript III first-strand synthesis system for RT-PCR (Invitrogen). Testis mRNA expression levels of Sertoli cell-specific Eppin (also known as Spinlw1) and Rhox5, and Leydig cell Cyp11a1 and Hsd3b1 were quantified by real-time quantitative PCR (qPCR) analysis of cDNAs using a Corbett RotorGene 6000 (Corbett Research, Sydney, Australia) and validated primers [Eppin and Rhox5 (15); Cyp11a1 (34); murine Hsd3b1 (SA Biosciences, Frederick, MD)] with RT2 SYBR Green qPCR master mix (SA Biosciences). Mouse Wbscr1 primers were used as a housekeeping gene and loading control to standardize relative transcript expression levels in different samples (n = 4–10/group) as described (15). Previous work showed that expression level of Wbscr1 is relatively high and consistent during murine testicular development, indicating its suitability as a housekeeping gene expressed at similar levels in somatic and germ cell populations (35).
To estimate total Sertoli or Leydig cell transcript expression levels in whole-testis RNA in which testis size differed according to treatments in each study, a two-step adjustment procedure was used. First, an adjustment for the housekeeping gene is required to correct for differences in loading of the cDNA on a per-cell basis in the qRT-PCR. By definition, this housekeeping gene is expressed to a similar degree in all cells, including germinal and nongerminal cells. This first adjustment provides a valid estimate of gene expression level in whole-testis RNA.
Because induction of spermatogenesis causes an intense proliferation of germ cells, this greatly dilutes the measured gene expression levels of the nonproliferating, specialist somatic cells when estimated from whole-testis RNA. To estimate the total RNA level in Sertoli (or Leydig) cells therefore requires a second adjustment to extrapolate the per-cell estimate from whole-testis RNA to Sertoli (or Leydig) cell RNA. This adjustment multiplies the whole-cell RNA by the tissue/cell proportion represented by the Sertoli (or Leydig) cells to obtain the total RNA in each of these target cells. Consequently, a correction factor to adjust for the dilution of nonproliferating somatic cell RNA by the proliferation of germinal cells is best approximated by the ratio of testis weights because germinal epithelium comprises the bulk (85%) of testis volume (5). This procedure is valid for expression of Sertoli or Leydig cell-specific genes because neither Sertoli (this study) nor Leydig cell (36) numbers in the testis are changed by exogenous sex steroid treatments. Therefore, this second adjustment allows estimation of RNA levels for specific genes in the relatively constant somatic (Sertoli, Leydig) cell populations after correcting for the dilutional effect of the intense proliferation of germ cells as also reported previously (35). All samples were tested in duplicate.
Data analysis
Data were analyzed by one- and two-way ANOVA as appropriate to the study design with Tukey-Kramer and linear contrasts as post hoc tests as required using NCSS software (NCSS, Kaysville, UT). The magnitude of the DHT effect was compared between genotypes by using the pooled se from suitable linear contrasts for DHT vs. no treatment. All data are expressed as mean ± sem.
Results
Study 1
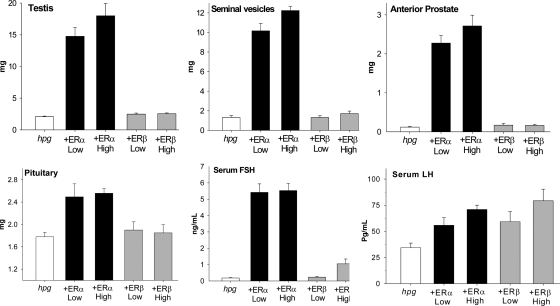
Male hpg mice were treated for 6 wk with selective ERα or ERβ agonists at low and high doses and then compared with untreated hpg mice. As shown in Fig. 1, the low (15 μg/kg) and high (45 μg/kg) doses of ERα agonist caused comparable increases in the weight of testis, seminal vesicle, anterior prostate lobe, and pituitary. Also increased in weight were the epididymis and ventral and dorsolateral prostate lobes, whereas spleen weight was unaffected and body weight decreased compared with values from untreated hpg animals (data not shown). In contrast, neither the low (150 μg/kg) nor high (450 μg/kg) doses of ERβ agonist had any effect on body or any organ weight examined (Fig. 1).
Figure 1.
Testis (upper left), seminal vesicle (upper middle), and anterior prostate lobe (upper right) weights together with pituitary (lower left), serum FSH (lower middle), and LH (lower right) panels in hpg mice treated with low or high doses of a selective ERα (black bars) or selective ERβ (gray bars) agonist or untreated (white bars). Data expressed as mean and sem. For further details, see text.
Both doses of ERα agonist equally and markedly induced serum FSH levels in hpg males (Fig. 1). The ERβ agonist also produced a significant rise in FSH (P = 0.019 vs. low dose, Fig. 1); however, this was observed in mice treated with the high dose only and was minimal compared with the effect of the ERα agonist. In contrast, small but significant increases in serum LH in hpg males were induced by both ERα or ERβ agonists, compared with untreated controls (Fig. 1).
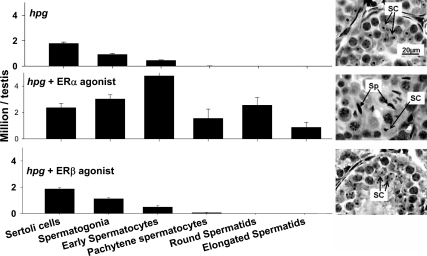
As expected, testes of untreated hpg mice displayed the immature germinal epithelium characteristic of stalled postnatal meiotic development, including random distribution of immature Sertoli cells (Fig. 2). The ERα agonist (45 μg/kg) stimulated the formation of haploid spermatids with elongating nuclei in hpg testis (Fig. 2). The testes of ERα agonist-treated mice contained haploid spermatids with elongating nuclei and mature Sertoli cells properly organized within the germinal epithelium, typically located adjacent to the basement membrane and featuring tripartite nucleoli (Fig. 2). The ERα agonist treatment increased tubular diameter, although tubular lumina were absent or poorly developed, and hypertrophy and increased vesiculation of the Leydig cells compared with testes of untreated hpg mice. In contrast, neither dose of the ERβ agonist had an effect on testis morphology in hpg mice (Fig. 2C).
Figure 2.
Sertoli (SC) and germ cell population of the testis in age-matched littermate hpg mice that were untreated (upper panel) or treated with a selective ERα agonist (16α-LE2, 45 μg/kg · d for 6 wk; middle panel) or a selective ERβ agonist (8β-VE2, 450 μg/kg · d for 6 wk; right panel) for 6 wk. Data expressed as mean and sem. At the right is representative testicular histology from age-matched littermate hpg mice that were untreated (upper panel) or treated with the selective ERα agonist (middle panel) or the selective ERβ agonist (right panel). Note elongated spermatids (Sp) present in tubules of hpg mice treated with selective ERα but not ERβ agonist. All photomicrographs are at the same magnification. For further details, see text.
Stereological evaluation of the testes from the different treatment groups showed that the administration of ERα agonist (45 μg/kg) produced an increase in Sertoli cell number and significant increases in numbers of spermatogonia, spermatocytes (early and pachytene), and spermatids (round and elongated) (Fig. 2). The results were the same when expressed in absolute terms (Fig. 2) or relative to Sertoli cell number (not shown). The low-dose ERα agonist (15 μg/kg) was not examined directly because the similar testis weight and histology indicated that the stereological enumeration would be similar. The administration of ERβ agonist (450 μg/kg) had no significant effect on numbers of Sertoli cells or any germ cell population, consistent with overall histology (Fig. 2).
Study 2
Male ERα knockout (αERKO), ERβ knockout (βERKO), or wild-type (WT) mice each on an hpg background were treated for 6 wk with either E2 or DHT and compared with untreated littermate controls of the same genotype.
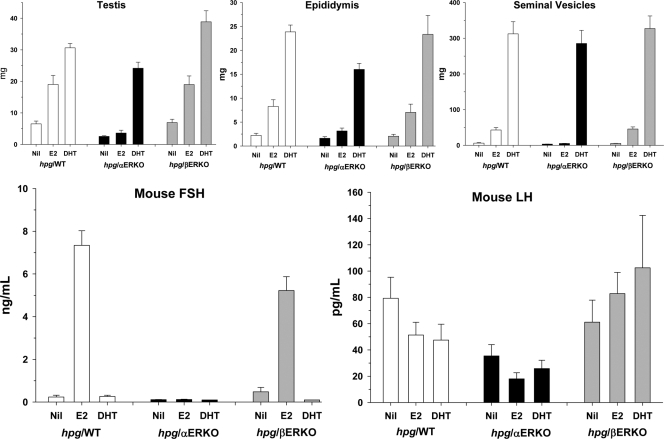
Administration of DHT increased testis, epididymal, and seminal vesicle weights in hpg/WT as well as hpg/αERKO and hpg/βERKO males, whereas E2 increased testis, epididymal, and seminal vesicle weights in hpg/ WT and hpg/βERKO but not hpg/αERKO males (Fig. 3). The magnitude of the E2 effects on testis, epididymal, and seminal vesicle weights was markedly less than the DHT effect.
Figure 3.
Testis (upper left), epididymis (upper middle), and seminal vesicles (upper right) together with serum FSH (lower left) and LH (lower right) in hpg mice on wild-type (hpg/WT, white bars), ERα knockout (hpg/αERKO, black bars), or ERβ knockout (hpg/βERKO, gray bars) genetic backgrounds. Data expressed as mean and sem. For further details, see text.
In contrast, DHT had no effect on circulating FSH levels in all of the three genotypes, whereas E2 significantly increased FSH levels in hpg/WT and hpg/βERKO but not in hpg/αERKO mice (Fig. 3). Serum LH levels were not significantly increased by either E2 or DHT in any line (Fig. 3). Serum T was undetectable (<0.1 ng/ml) in all 58 samples including 17 DHT-treated and 20 E2-treated mice. Serum DHT was detectable in all 17 DHT-treated mice (mean 1.67 ± 0.19 ng/ml) with no difference according to ER genotype (P = 0.64) and only detectable in one (0.23 ng/ml) of 41 non-DHT-treated mice. Serum E2 was detectable in 11 of 20 estradiol-treated mice (mean 6.4 ± 1.5 pg/ml, median 6.5 pg/ml) with no difference according to ER genotype (P = 0.92) but not detectable (<5 pg/ml) in any of 38 non-E2-treated mice.
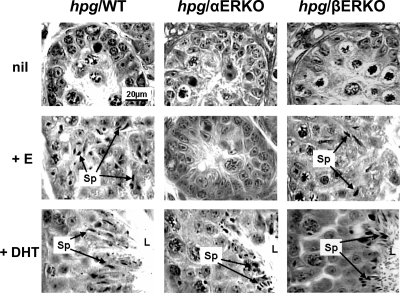
Testicular histology in untreated hpg/WT, hpg/αERKO, and hpg/βERKO males had a similar immature morphological appearance (Fig. 4). In both hpg/WT and hpg/βERKO males, E2 stimulated production of haploid spermatids, although sperm metamorphosis and spermiation appeared limited or incomplete, and tubular diameter was increased but lumina were poorly developed or absent, relative to DHT-induced effects. The E2-treated hpg/WT and hpg/βERKO testes displayed Sertoli cells with mature morphological characteristics (tripartite nucleoli and basal localization) and Leydig cells with increased vesiculation as compared with untreated hpg testis. In contrast, testes of hpg/αERKO mice appeared unresponsive to E2 treatment (Fig. 4), whereas DHT treatment induced the equivalent histological completion of spermatogenesis in all three hpg genotypes (Fig. 4).
Figure 4.
Testicular histology from age-matched littermate hpg mice on different genetic backgrounds comprising WT (left panels), αERKO (middle panels), and βERKO (right panels). The top row depicts the untreated, the middle row treated with E2, and the bottom row treated with DHT for 6 wk. Note elongated spermatids (Sp) indicated by arrows present in tubules of hpg mice treated with DHT or E2 for the mice on WT and βERKO but not αERKO background. Well-formed seminiferous tubular lumen (indicated by L) is present in all mice treated with DHT but not E2 or untreated. All photomicrographs are at the same magnification. For further details, see text.
Stereological analysis shows that neither genotype (WT, αERKO, βERKO) nor treatment (E2, DHT) significantly influenced Sertoli cell numbers in this study (Table 1). However, E2 increased all germ cell populations in hpg/WT and hpg/βERKO groups relative to respective controls but had no stimulatory effect in the hpg/αERKO group. In comparison, DHT increased all germ cell populations in each hpg genotype and had greater stimulating effect relative to E2, especially on the postmeiotic, haploid germ cell populations (Table 1).
Table 1.
Stereological enumeration of Sertoli and germ cell populations
| Group | n | Sertoli cells | Spermatogonia | Early spermatocytes | Pachytene spermatocytes | Round spermatids | Elongated spermatids |
|---|---|---|---|---|---|---|---|
| hpg/WTa | 6 | 1.60 ± 0.20 | 0.99 ± 0.11 | 0.81 ± 0.17 | 0.09 ± 0.04 | 0.05 ± 0.05 | 0.01 ± 0.01 |
| hpg/WT + E2b | 6 | 1.77 ± 0.18 | 1.99 ± 0.15 | 4.82 ± 0.57 | 1.03 ± 0.17 | 0.81 ± 0.19 | 0.09 ± 0.05 |
| hpg/WT + DHTc | 5 | 1.54 ± 0.12 | 1.74 ± 0.20 | 4.45 ± 0.24 | 1.51 ± 0.09 | 12.2 ± 0.52 | 11.2 ± 0.47 |
| hpg/αERKO | 5 | 1.39 ± 0.08 | 0.58 ± 0.07 | 0.53 ± 0.08 | 0.06 ± 0.02 | 0 | 0 |
| hpg/αERKO + E2b | 5 | 1.58 ± 0.08 | 0.62 ± 0.07 | 0.26 ± 0.08 | 0 | 0 | 0 |
| hpg/αERKO + DHTc | 5 | 1.45 ± 0.16 | 1.16 ± 0.10 | 3.18 ± 0.27 | 0.86 ± 0.18 | 6.20 ± 0.64 | 5.58 ± 1.02 |
| hpg/βERKO | 6 | 1.39 ± 0.08 | 1.20 ± 0.12 | 2.36 ± 0.42 | 0.36 ± 0.08 | 1.19 ± 0.44 | 0.45 ± 0.26 |
| hpg/βERKO + E2b | 6 | 1.79 ± 0.25 | 2.20 ± 0.34 | 4.81 ± 0.74 | 1.20 ± 0.20 | 4.68 ± 1.95 | 2.33 ± 1.28 |
| hpg/βERKO + DHTc | 5 | 1.47 ± 0.17 | 2.49 ± 0.30 | 5.27 ± 0.37 | 1.77 ± 0.53 | 14.5 ± 1.50 | 12.4 ± 1.47 |
| P value | |||||||
| Genotyped | 0.50 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Treatmente | 0.13 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| G × Tf | 0.97 | 0.038 | <0.001 | 0.133 | 0.002 | <0.001 | |
All data from study 2 expressed as mean ± sem and million cells per testis.
WT means wild-type genetic background with respect to ERα and ERβ;
E2 administered by 1 cm subdermal implants filled with recrystallized E2 diluted 1:1000 with cholesterol;
DHT administered by 1 cm subdermal implant filled with crystalline DHT;
Genotype (hpg/WT vs. hpg/αERKO vs. hpg/βERKO) main effect from two-way ANOVA;
Treatment (untreated vs. E2 vs. DHT) main effect from two-way ANOVA;
Genotype × treatment (G × T) interaction term from two-way ANOVA.
Testicular gene expression
Sertoli cell
In study 1, administration of ERα agonist markedly increased the testicular expression of Sertoli cell-specific genes, Rhox5 and Eppin, compared with expression in untreated hpg mice (Fig. 5, left upper panels). By contrast, there was little or no increase in Rhox5 and Eppin expression levels in testes from ERβ agonist-treated compared with untreated hpg mice (Fig. 5, left upper panels).
Figure 5.
Total testis Rhox5, Eppin, Hsd3b1, and Cyp11a1 mRNA expression levels. Data are shown as mean and sem for treatments (n = 4–10/group) including untreated (−) and ERβ and ERα agonists (left panel, study 1) in hpg mice and untreated (−), E2, or DHT in hpg/WT, hpg/αERKO, and hpg/βERKO mice (right three panels, study 2) along the x-axis. Gene expression levels (y-axis) were measured for two Sertoli cell markers Rhox5 and Eppin (upper two panels) and two Leydig cell markers Cyp11a1 and Hsd3b1 (lower two panels) by real-time qPCR analysis using whole-testis cDNA. The measured mRNA transcript levels are adjusted for Wbscr1 (housekeeping gene as loading control) and for testis weight (to account for dilution by germ cells) as detailed in text.
In study 2, E2 treatment increased the expression levels of Rhox5 and Eppin in hpg/WT and hpg/βERKO but not in hpg/αERKO mice, compared with expression levels in untreated controls (Fig. 5, right upper panels). By contrast, DHT administration markedly increased expression levels of Rhox5 and Eppin in hpg testis, regardless of whether ERβ or ERα was present or absent (Fig. 5, right upper panels).
Leydig cell
In study 1, treatment with the ERα agonist markedly increased testicular expression of Leydig cell gene markers Hsd3b1 and Cyp11a1 compared with untreated hpg controls, whereas treatment with the ERβ agonist had no effect (Fig. 5, left lower panels).
The effects of ERα agonist on gene expression observed in study 1 were largely replicated by E2 in the testes of hpg/WT in study 2 (Fig. 5). The hypogonadal ERβ-null (hpg/βERKO) mice were equally responsive to E2, supporting the absence of ERβ agonist effects in study 1. In contrast, all effects of E2 that were observed in hpg/WT testes, with the exception of increased Hsd3b1, were absent in testes of hpg/αERKO mice.
By contrast, DHT treatment had no stimulatory effect on testicular expression of Cyp11a1 (Fig. 5, right lower panels) in hpg males, regardless of the presence or absence of ERα or ERβ, whereas Hsd3b1 expression levels were significantly elevated in DHT-treated hpg/αERKO but not hpg/WT or hpg/βERKO males (Fig. 5, right lower panels).
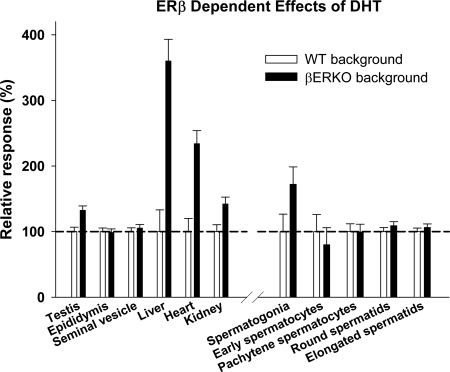
ERβ modulation of DHT effects on nonreproductive organ weights
Administration of DHT increased liver, kidney, and heart, but not spleen weight, in hpg/WT as well as hpg/αERKO and hpg/βERKO males (data not shown). However, the magnitude of the DHT effect on large nonreproductive organ weights (liver, kidney, heart) was markedly greater in the presence of ERβ inactivation (hpg/βERKO) compared with hpg mice having native (hpg/WT) genetic background (Fig. 6). By contrast, the modulating influence of the ERβ gene on DHT effects was less or absent in reproductive organs, in which there was a small but significant increase for the testis and spermatogonia but not other germ cell populations (Table 1 and Fig. 6).
Figure 6.
Magnitude of DHT effect in reproductive and nonreproductive organs in hpg mice on βERKO vs. WT (i.e. functional ERα and ERβ) genetic background. The magnitude of the DHT effect, defined as the increase in organ or tissue weight of the DHT-treated compared with the untreated controls on the same genetic background, with the DHT effect on βERKO background expressed as a percentage of DHT effect on the WT background (set at 100%). Data expressed as mean and sem. For further details, see text.
Discussion
Using two complementary pharmacogenetic approaches, the present study shows unequivocally that the E2 induction of spermatogenesis and increased serum FSH levels in hpg mice is dependent on an ERα mechanism. Both responses were abolished in mice with an inactivated ERα gene but remained unaffected by inactivation of the ERβ gene. Conversely, both responses were stimulated by a selective ERα agonist but not a selective ERβ agonist. In concert these experiments indicate that ERα activation is involved in both the increased serum FSH and induction of spermatogenesis in hpg mice, whereas an ERβ mechanism is involved in neither.
A prominent difference between androgen- and estrogen-mediated induction of spermatogenesis in gonadotropin-deficient hpg mice is that only the latter is accompanied by increased serum and pituitary FSH levels (6,16,17,18,30). Given that ERα is generally accepted to be expressed throughout the hypothalamic-pituitary-testicular axis, the concomitant increase in FSH by E2 raises the possibility of both direct and indirect pathways for estrogenic stimulation of spermatogenesis. Several lines of evidence argue against E2 induction of spermatogenesis being direct. These include: 1) the general premise that ERα is largely localized to Leydig and peritubular cells and only weakly in expressed Sertoli cells in mature wild-type and hpg testes (36,37,38) (Supplemental Fig. 1, published as supplemental data on The Endocrine Society's Journals Online web site at http://endo.endojournals.org); 2) that ERα-mediated effects in Leydig cells are predominantly inhibitory (18,39,40,41); 3) that spermatogenesis develops normally in mice null for estrogen synthesis (19) or estrogen action via ERα (20), ERβ (21,22), or both (23); and 4) that fully fertile germ cells develop in testis in which all somatic cells lack functional ERα (42,43). To this we add our present observations that the effects of ERα agonist alone on postmeiotic germ cell populations are small compared with DHT. Our findings do, however, show prominent effects of E2 via an ERα mechanism on gene expression in Sertoli and Leydig cells, which suggests the possibility for direct effects of E2 via specialized endocrine cells of the testis. It is unlikely that Leydig cells are directly involved in the ERα-mediated E2 induction of spermatogenesis because intratesticular steroid levels decline in E2-treated hpg males (18), and concurrent induction of circulating FSH is consistent with elevated Sertoli cell markers and limited postmeiotic germ cell development, typical of known FSH activity in androgen-deficient testes (3,44). The present findings confirm that E2 administration does not increase serum (16) or testicular (17,18) levels in hpg mice so that the E2-induced increase in seminal vesicle weight must be a direct E2 effect on seminal vesicles as reported previously (45). Therefore, the stimulatory effect of E2 for spermatogenesis in hpg mice most likely involves extratesticular ERα actions leading to increased circulating FSH and enhanced Sertoli cell maturation/function.
Nevertheless, given previous reports that AR is required for E2-induced spermatogenesis (17,18), we must also consider the possibility of ERα-AR heterodimers as a possible direct mechanism for our findings. Actions of an ERα-AR heterodimer has been reported in some breast and prostate tumor cells (46) but not confirmed in other studies (47).
Although the importance of increased FSH to E2-induced spermatogenesis in hpg mice is unclear, the current study clearly demonstrates that this effect is ERα dependent. Induction of increased pituitary weight and FSH secretion was observed in ERα agonist-treated hpg mice, and the increased circulating FSH was lacking in E2-treated hpg/αERKO mice. Because the hpg mouse lacks functional GnRH, the E2-induced increase in FSH must be due to effects directly on pituitary gonadotropes and independent of GnRH action. This is consistent with ERα being the predominant ER form expressed in the mouse pituitary (48). Although E2 classically exerts a solely negative feedback effect on the hypothalamic-pituitary axis of mature male mice, the paradoxical E2 stimulation of circulating FSH in hpg males resembles the positive estrogen feedback effects mediated by hypothalamic and pituitary mechanisms requiring the pituitary ERα receptor (49). The neuroendocrine mechanism underlying the ovulatory LH surge (with a concomitant FSH surge) characteristic of the mature female reproductive system is usually extinguished in males by neonatal androgen imprinting (50), although such a latent mechanism may still be evoked by toxicological doses of synthetic estrogens (51). Beyond establishing the existence of this pathway, whether it is a physiological mechanism or vestigial pharmacological reflex cannot be resolved by the present study.
This study finds no evidence for ERβ involvement in E2-induced increased serum FSH or spermatogenesis. Genetic loss of ERβ activity on the hpg background had no effect on E2-induced FSH levels, testicular growth, and spermatogenesis. Similarly, a selective ERβ agonist failed to stimulate testis, seminal vesicle, prostate, and pituitary growth or to increase Sertoli cell and spermatogonial numbers, unlike the ERα agonist response. Furthermore, loss of ERβ had no effect on DHT-induced spermatogenesis. Our present data suggest that any immunohistochemical evidence of ERβ expression in the testis reflects functional redundancy, as is also the case for ERα expression in germ cells (43). Furthermore, the normal development of spermatogenesis and fertility in mice with genetic inactivation of ERβ (21,22,23) supports the concept that any essential, nonredundant role for ERβ in postnatal testicular function remains to be established.
The present findings show that E2 actions in hpg testis increased testicular expression of Eppin and Rhox5, known AR-dependent, Sertoli cell-specific genes in the postnatal testis (15,52,53). The present study also reveals that up-regulated Eppin and Rhox5 expression involves an ERα mechanism. Prominent stimulation of these Sertoli cell transcripts is reproduced by an ERα but not an ERβ agonist and abolished by genetic inactivation of ERα but not ERβ genes. Considering our findings of ERα immunoreactive expression in Sertoli cells (Supplemental Fig. 1) together with the requirement for AR (17,18), this ERα-mediated E2 effect may involve direct activation of ERα in Sertoli cells or AR-ERα heterodimers, alone or together with FSH. However, this ERα selectivity was distinct from the DHT effect, which, manifested via AR, is unaffected by the inactivation of either ER, which tends to exclude the possibility of a DHT metabolite with ERα or ERβ agonist activity. The ERα-mediated rise in Sertoli cell expression of Rhox5 and Eppin could be due to the concomitant increases in serum FSH, as exogenous FSH treatment increases Rhox5 and Eppin expression in hpg testes (54). However, increased circulating FSH levels alone are not sufficient to complete postmeiotic germ cell development in hpg mice (3), consistent with the limited spermatogenic development induced by the ERα agonist in the present experiments. Neither Sertoli cell marker genes had well-defined ER response elements in their promoters.
The present work also demonstrated complex and distinct steroidal regulation of two Leydig cell genes involved in testicular steroidogenesis, Cyp11a1 (cytochrome P450 side chain cleavage enzyme) and Hsd3b1 (3β-hydroxysteroid dehydrogenase type 1). Estradiol increased testicular Cyp11a1 and Hsd3b1 expression in hpg mice, an effect ablated in the absence of ERα activity. This may reflect direct effects of E2 on Leydig cells because circulating levels of LH, the major Leydig cell trophic hormone, are fully suppressed in hpg mice, supported by failure of E2 to increase Leydig cell number (36). Alternatively, E2-mediated stimulation of Leydig cell transcripts may be due to FSH-mediated paracrine signaling between Sertoli and Leydig cells (34). By contrast to E2 stimulatory actions, DHT did not stimulate testicular Cyp11a1 expression in hpg/WT testes, revealing distinct E2 and androgenic regulation of expression of this steroidogenic enzyme. Furthermore, DHT-induced elevation of testicular Hsd3b1 expression in ERβ-deficient but not control hpg/WT males indicates complex regulation of Hsd3b1 involving AR and ERβ. Therefore, these in vivo models have demonstrated distinct and complex involvement of ER pathways, and AR interaction, in the steroidal regulation of Leydig cell Cyp11a1 and Hsd3b1 expression. Our findings provide strong evidence for ERβ-mediated effects of DHT, possibly via its 3β-diol metabolite (55), in nonclassical androgen target tissues like the liver, heart, and kidneys, whereas the effects on reproductive tissues are minimal (testis) or absent (accessory reproductive glands), which warrants further investigation.
In conclusion, the present findings indicate that E2 induction of spermatogenesis in the gonadotropin-deficient mouse depends on an ERα, but not an ERβ, mechanism involving increased pituitary secretion of FSH. The overall biological significance of this mechanism in the normal development and maintenance of spermatogenesis requires further exploration.
Supplementary Material
Acknowledgments
We thank Dr. Katja Prelle (Bayer Schering Pharma) for the supply of ERα and ERβ agonists.
Footnotes
This work was supported by the National Health and Medical Research Council (464857 to C.M.A. and D.J.H.), Australian Research Council (DP0881690 to C.M.A. and D.J.H.), and the Division of Intramural Research, National Institute of Environmental Health Sciences (Z01ES70065 to K.S.K.).
Disclosure Summary: The authors have nothing to disclose relative to this work.
First Published Online April 21, 2010
Abbreviations: AR, Androgen receptor; DHT, dihydrotestosterone; E2, estradiol; ER, estrogen receptor; ERKO, knockout lacking ER; hpg, hypogonadal; qPCR, quantitative PCR; T, testosterone; WT, wild type.
References
- O'Donnell L, Meachem SJ, Stanton PG, McLachlan RI 2006 Endocrine regulation of spermatogenesis. In: Neill JD, ed. Physiology of reproduction. 3rd ed. Amsterdam: Elsevier; 1017–1069 [Google Scholar]
- Walker WH, Cheng J 2005 FSH and testosterone signaling in Sertoli cells. Reproduction 130:15–28 [DOI] [PubMed] [Google Scholar]
- Haywood M, Spaliviero J, Jimemez M, King NJ, Handelsman DJ, Allan CM 2003 Sertoli and germ cell development in hypogonadal (hpg) mice expressing transgenic follicle-stimulating hormone alone or in combination with testosterone. Endocrinology 144:509–517 [DOI] [PubMed] [Google Scholar]
- Orth JM, Gunsalus GL, Lamperti AA 1988 Evidence from Sertoli-cell depleted rats indicates that spermatid number depends on numbers of Sertoli cells produced during perinatal development. Endocrinology 122:787–794 [DOI] [PubMed] [Google Scholar]
- Russell LD, Ren HP, Sinha Hikim I, Schulze W, Sinha Hikim AP 1990 A comparative study in twelve mammalian species of volume densities, volumes, and numerical densities of selected testis components, emphasizing those related to the Sertoli cell. Am J Anat 188:21–30 [DOI] [PubMed] [Google Scholar]
- Singh J, O'Neill C, Handelsman DJ 1995 Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology 136:5311–5321 [DOI] [PubMed] [Google Scholar]
- Kumar TR, Wang Y, Lu N, Matzuk MM 1997 FSH is required for ovarian follicle maturation but not for male fertility. Nat Genet 15:201–204 [DOI] [PubMed] [Google Scholar]
- Dierich A, Sairam MR, Monaco L, Fimia GM, Gansmuller A, LeMeur M, Sassone-Corsi P 1998 Impairing follicle-stimulating hormone (FSH) signalling in vivo: targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc Natl Acad Sci USA 95:13612–13617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abel MH, Wootton AN, Wilkins V, Huhtaniemi I, Knight PG, Charlton HM 2000 The effect of a null mutation in the follicle-stimulating hormone receptor gene on mouse reproduction. Endocrinology 141:1795–1803 [DOI] [PubMed] [Google Scholar]
- Allan CM, Garcia A, Spaliviero J, Zhang FP, Jimenez M, Huhtaniemi I, Handelsman DJ 2004 Complete Sertoli cell proliferation induced by follicle-stimulating hormone (FSH) independently of luteinizing hormone activity: evidence from genetic models of isolated FSH action. Endocrinology 145:1587–1593 [DOI] [PubMed] [Google Scholar]
- Notini AJ, Davey RA, McManus JF, Bate KL, Zajac JD 2005 Genomic actions of the androgen receptor are required for normal male sexual differentiation in a mouse model. J Mol Endocrinol 35:547–555 [DOI] [PubMed] [Google Scholar]
- Holdcraft RW, Braun RE 2004 Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development 131:459–467 [DOI] [PubMed] [Google Scholar]
- De Gendt K, Swinnen JV, Saunders PT, Schoonjans L, Dewerchin M, Devos A, Tan K, Atanassova N, Claessens F, Lécureuil C, Heyns W, Carmeliet P, Guillou F, Sharpe RM, Verhoeven G 2004 A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc Natl Acad Sci USA 101:1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Chen YT, Yeh SD, Xu Q, Wang RS, Guillou F, Lardy H, Yeh S 2004 Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc Natl Acad Sci USA 101:6876–6881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim P, Robson M, Spaliviero J, McTavish KJ, Jimenez M, Zajac JD, Handelsman DJ, Allan CM 2009 Sertoli cell androgen receptor DNA binding domain is essential for the completion of spermatogenesis. Endocrinology 150:4755–4765 [DOI] [PubMed] [Google Scholar]
- Ebling FJ, Brooks AN, Cronin AS, Ford H, Kerr JB 2000 Estrogenic induction of spermatogenesis in the hypogonadal mouse. Endocrinology 141:2861–2869 [DOI] [PubMed] [Google Scholar]
- Baines H, Nwagwu MO, Furneaux EC, Stewart J, Kerr JB, Mayhew TM, Ebling FJ 2005 Estrogenic induction of spermatogenesis in the hypogonadal (hpg) mouse: role of androgens. Reproduction 130:643–654 [DOI] [PubMed] [Google Scholar]
- Lim P, Allan CM, Notini AJ, Axell AM, Spaliviero J, Jimenez M, Davey R, McManus J, MacLean HE, Zajac JD, Handelsman DJ 2008 Oestradiol-induced spermatogenesis requires a functional androgen receptor. Reprod Fertil Dev 20:861–870 [DOI] [PubMed] [Google Scholar]
- Robertson KM, O'Donnell L, Jones ME, Meachem SJ, Boon WC, Fisher CR, Graves KH, McLachlan RI, Simpson ER 1999 Impairment of spermatogenesis in mice lacking a functional aromatase (cyp 19) gene. Proc Natl Acad Sci USA 96:7986–7991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy EM, Washburn TF, Bunch DO, Goulding EH, Gladen BC, Labahn DB, Korach KS 1996 Targeted disruption of the estrogen receptor gene in male mice causes alteration of spermatogenesis and infertility. Endocrinology 137:4796–4805 [DOI] [PubMed] [Google Scholar]
- Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O 1998 Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal MC, Krust A, Chambon P, Mark M 2008 Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERβ-null mutant. Proc Natl Acad Sci USA 105:2433–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M 2000 Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development 127:4277–4291 [DOI] [PubMed] [Google Scholar]
- Lang J 1991 Assay for deletion in GnRH (hpg) locus using PCR. Mouse Genome 89:857 [Google Scholar]
- Couse JF, Yates MM, Walker VR, Korach KS 2003 Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol Endocrinol 17:1039–1053 [DOI] [PubMed] [Google Scholar]
- Fritzemeier KH, Hillisch A, Elger W, Kaufmann U, Kollenkirchen U, Kosemund D, Lindenthal B, Muller G, Muhn P, Nubbemeyer R, Peters O, Siebel P, Hegele-Hartung C 2004 Biological effects of ERα- and ERβ-selective estrogens. Ernst Schering Res Found Workshop 127–150 [PubMed] [Google Scholar]
- Hillisch A, Peters O, Kosemund D, Muller G, Walter A, Schneider B, Reddersen G, Elger W, Fritzemeier KH 2004 Dissecting physiological roles of estrogen receptor α and β with potent selective ligands from structure-based design. Mol Endocrinol 18:1599–1609 [DOI] [PubMed] [Google Scholar]
- McPherson SJ, Ellem SJ, Simpson ER, Patchev V, Fritzemeier KH, Risbridger GP 2007 Essential role for estrogen receptor β in stromal-epithelial regulation of prostatic hyperplasia. Endocrinology 148:566–574 [DOI] [PubMed] [Google Scholar]
- Handelsman DJ, Spaliviero JA, Simpson JM, Allan CM, Singh J 1999 Spermatogenesis without gonadotropins: maintenance has a lower testosterone threshold than initiation. Endocrinology 140:3938–3946 [DOI] [PubMed] [Google Scholar]
- Jimenez M, Spaliviero JA, Grootenhuis AJ, Verhagen J, Allan CM, Handelsman DJ 2005 Validation of an ultrasensitive and specific immunofluorometric assay for mouse follicle-stimulating hormone. Biol Reprod 72:78–85 [DOI] [PubMed] [Google Scholar]
- van Casteren JI, Schoonen WG, Kloosterboer HJ 2000 Development of time-resolved immunofluorometric assays for rat follicle-stimulating hormone and luteinizing hormone and application on sera of cycling rats. Biol Reprod 62:886–894 [DOI] [PubMed] [Google Scholar]
- Harwood DT, Handelsman DJ 2009 Development and validation of a sensitive liquid chromatography-tandem mass spectrometry assay to simultaneously measure androgens and estrogens in serum without derivatization. Clin Chim Acta 409:78–84 [DOI] [PubMed] [Google Scholar]
- McNamara KM, Harwood DT, Simanainen U, Walters KA, Jimenez M, Handelsman DJ 2010 February 6 Measurement of sex steroids in murine blood and reproductive tissues by liquid chromatography-tandem mass spectrometry. J Steroid Biochem Mol Biol 10.1016/j.jsbmb.2010.02.001 [DOI] [PubMed] [Google Scholar]
- Allan CM, Lim P, Robson M, Spaliviero J, Handelsman DJ 2009 Transgenic mutant D567G but not wild-type human FSH receptor overexpression provides FSH-independent and promiscuous glycoprotein hormone Sertoli cell signaling. Am J Physiol Endocrinol Metab 296:E1022–E1028 [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Willerton L, Baker PJ 2002 Changes in Leydig cell gene expression during development in the mouse. Biol Reprod 66:966–975 [DOI] [PubMed] [Google Scholar]
- Baines H, Nwagwu MO, Hastie GR, Wiles RA, Mayhew TM, Ebling FJ 2008 Effects of estradiol and FSH on maturation of the testis in the hypogonadal (hpg) mouse. Reprod Biol Endocrinol 6:4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson WN, Couse JF, Banks EP, Korach KS, Newbold RR 2000 Expression of estrogen receptor β is developmentally regulated in reproductive tissues of male and female mice. Biol Reprod 62:310–317 [DOI] [PubMed] [Google Scholar]
- Zhou Q, Nie R, Prins GS, Saunders PT, Katzenellenbogen BS, Hess RA 2002 Localization of androgen and estrogen receptors in adult male mouse reproductive tract. J Androl 23:870–881 [PubMed] [Google Scholar]
- Hsueh AJ, Dufau ML, Catt KJ 1978 Direct inhibitory effect of estrogen on Leydig cell function of hypophysectomized rats. Endocrinology 103:1096–1102 [DOI] [PubMed] [Google Scholar]
- Delbès G, Levacher C, Duquenne C, Racine C, Pakarinen P, Habert R 2005 Endogenous estrogens inhibit mouse fetal Leydig cell development via estrogen receptor α. Endocrinology 146:2454–2461 [DOI] [PubMed] [Google Scholar]
- Strauss L, Kallio J, Desai N, Pakarinen P, Miettinen T, Gylling H, Albrecht M, Mäkelä S, Mayerhofer A, Poutanen M 2009 Increased exposure to estrogens disturbs maturation, steroidogenesis, and cholesterol homeostasis via estrogen receptor α in adult mouse Leydig cells. Endocrinology 150:2865–2872 [DOI] [PubMed] [Google Scholar]
- Mahato D, Goulding EH, Korach KS, Eddy EM 2000 Spermatogenic cells do not require estrogen receptor-α for development or function. Endocrinology 141:1273–1276 [DOI] [PubMed] [Google Scholar]
- Mahato D, Goulding EH, Korach KS, Eddy EM 2001 Estrogen receptor-α is required by the supporting somatic cells for spermatogenesis. Mol Cell Endocrinol 178:57–63 [DOI] [PubMed] [Google Scholar]
- Allan CM, Haywood M, Swaraj S, Spaliviero J, Koch A, Jimenez M, Poutanen M, Levallet J, Huhtaniemi I, Illingworth P, Handelsman DJ 2001 A novel transgenic model to characterize the specific effects of follicle-stimulating hormone on gonadal physiology in the absence of luteinizing hormone actions. Endocrinology 142:2213–2220 [DOI] [PubMed] [Google Scholar]
- Bianco JJ, Handelsman DJ, Pedersen JS, Risbridger GP 2002 Direct response of the murine prostate gland and seminal vesicles to estradiol. Endocrinology 143:4922–4933 [DOI] [PubMed] [Google Scholar]
- Migliaccio A, Di Domenico M, Castoria G, Nanayakkara M, Lombardi M, de Falco A, Bilancio A, Varricchio L, Ciociola A, Auricchio F 2005 Steroid receptor regulation of epidermal growth factor signaling through Src in breast and prostate cancer cells: steroid antagonist action. Cancer Res 65:10585–10593 [DOI] [PubMed] [Google Scholar]
- Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, Jindal S, Segara D, Jia L, Moore NL, Henshall SM, Birrell SN, Coetzee GA, Sutherland RL, Butler LM, Tilley WD 2009 Androgen receptor inhibits estrogen receptor-α activity and is prognostic in breast cancer. Cancer Res 69:6131–6140 [DOI] [PubMed] [Google Scholar]
- Couse JF, Lindzey J, Grandien K, Gustafsson JA, Korach KS 1997 Tissue distribution and quantitative analysis of estrogen receptor-α (ERα) and estrogen receptor-β (ERβ) messenger ribonucleic acid in the wild-type and ERα-knockout mouse. Endocrinology 138:4613–4621 [DOI] [PubMed] [Google Scholar]
- Singh SP, Wolfe A, Ng Y, DiVall SA, Buggs C, Levine JE, Wondisford FE, Radovick S 2009 Impaired estrogen feedback and infertility in female mice with pituitary-specific deletion of estrogen receptor α (ESR1). Biol Reprod 81:488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Garcia-Segura LM, Naftolin F 1997 Lack of gonadotropin-positive feedback in the male rat is associated with lack of estrogen-induced synaptic plasticity in the arcuate nucleus. Neuroendocrinology 65:136–140 [DOI] [PubMed] [Google Scholar]
- Atanassova N, McKinnell C, Turner KJ, Walker M, Fisher JS, Morley M, Millar MR, Groome NP, Sharpe RM 2000 Comparative effects of neonatal exposure of male rats to potent and weak (environmental) estrogens on spermatogenesis at puberty and the relationship to adult testis size and fertility: evidence for stimulatory effects of low estrogen levels. Endocrinology 141:3898–3907 [DOI] [PubMed] [Google Scholar]
- Lindsey JS, Wilkinson MF 1996 Pem: a testosterone- and LH-regulated homeobox gene expressed in mouse Sertoli cells and epididymis. Dev Biol 179:471–484 [DOI] [PubMed] [Google Scholar]
- Sivashanmugam P, Hall SH, Hamil KG, French FS, O'Rand MG, Richardson RT 2003 Characterization of mouse Eppin and a gene cluster of similar protease inhibitors on mouse chromosome 2. Gene 312:125–134 [DOI] [PubMed] [Google Scholar]
- Abel MH, Baban D, Lee S, Charlton HM, O'Shaughnessy PJ 2009 Effects of FSH on testicular mRNA transcript levels in the hypogonadal mouse. J Mol Endocrinol 42:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira AG, Coelho PH, Guedes FD, Mahecha GA, Hess RA, Oliveira CA 2007 5α-Androstane-3β,17β-diol (3β-diol), an estrogenic metabolite of 5α-dihydrotestosterone, is a potent modulator of estrogen receptor ERβ expression in the ventral prostrate of adult rats. Steroids 72:914–922 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.