Abstract
A number of previous studies documented the angiogenic potential of outgrowth endothelial cells in vitro and in vivo and provided evidence that therapeutic success could depend on coculture or coimplantation strategies. Thus, deeper insight into the molecular mechanisms underlying this pro-angiogenic effect of cocultures might provide new translational options for tissue engineering and regenerative medicine. One promising signaling pathway in bone repair involved in neoangiogenesis and bone formation is the sonic hedgehog (Shh) pathway. In this article, we focus on the effect of Shh on the formation of microvessel-like structures and osteoblastic differentiation in cocultures of primary osteoblasts and outgrowth endothelial cells. Already after 24 h of treatment, Shh leads to a massive increase in microvessel-like structures compared with untreated cocultures. Increased formation of angiogenic structures seems to correlate with the upregulation of vascular endothelial growth factor or angiopoietins (Ang-1 and Ang-2) studied at both the mRNA and protein levels. In addition, treatment with cyclopamine, an inhibitor of hedgehog signaling, blocked the formation of microvessel-like structures in the cocultures. However, exogenous Shh also resulted in the upregulation of several osteogenic differentiation markers in real-time polymerase chain reaction, as well as in an increased mineralization and alkaline phosphatase activity. The present data highlight the central role of the Shh pathway in bone regeneration and vascularization. Further, Shh might have the potential to improve both angiogenesis and osteogenesis in clinical applications in the future.
Introduction
Coculture systems constitute a promising instrument to mimic physiological processes in bone regeneration and vascularization and may provide new insights into the underlying molecular mechanisms. Further, several studies demonstrated that coculture or coimplantation strategies are essential, especially in the context of prevascularization strategies involving endothelial cells or vascular structures that aim to improve the neovascularization in tissue constructs after implantation.1–3 The generation of complex bioengineered tissues such as prevascularized bone constructs, for instance, still represents a major challenge with respect to both the selection of suitable cell sources for cellular therapy and the identification of new molecular targets for further therapeutic intervention.
In a series of studies outgrowth endothelial cells (OECs), a subpopulation of endothelial progenitor cells from peripheral blood has proven their therapeutic potential by contributing to the formation of actively perfused vessels within the implanted constructs or the peri-implant tissue.4–6 Based on these observations OECs are currently being discussed as a potential cell source for therapeutic applications aiming to enhance neovascularization during tissue repair. Nevertheless, perfused vessels formed by OECs were only observed when OECs were coimplanted with other cell types such as mesenchymal stem cells,1 smooth muscle cells,7 or primary osteoblasts (pOBs).8 It is widely accepted that coculture or coimplantation of endothelial cells with other cell types induces the formation of microvessel-like structures and positively influences their angiogenic potential in vitro9–12 or in vivo. Induction of the angiogenic phenotype of endothelial cells by coculture approaches seems to be mediated by proangiogenic growth factors such as vascular endothelial growth factor (VEGF),13 but matrix components and direct cellular communication via gap junctions14 are additional essential elements involved in angiogenesis.
However, the detailed mechanisms and molecular signaling pathways guiding angiogenic activation are still poorly understood. In addition, identification of molecular signaling pathways capable of influencing both fundamental processes in bone regeneration, namely, osteogenesis and angiogenesis, might lead to new translational approaches. One interesting group of signaling pathways is mediated by hedgehog morphogens known to play a pivotal role in numerous tissues during embryonic development.15–17
Three human homologs of the Drosophila hedgehog gene are currently recognized, Indian hedgehog, Desert hedgehog, and sonic hedgehog (Shh).18 Shh signaling occurs through the interaction of the morphogen with the patched1 (Ptch1) receptor, which then activates the Gli family of transcription factors. There is increasing evidence from the literature that the Shh pathway plays a significant role in vasculogenesis. During embryogenesis, for example, transgenic overexpression of Shh in the dorsal neural tube of zebrafish results in hypervascularization of the neuroectoderm. Further, zebrafish embryos lacking Shh activity show no arterial differentiation.19 Recently, several publications indicated the importance of Shh in postnatal vascularization processes.20,21 In addition, there is also evidence that Shh is involved in bone regeneration processes in adults and initiates osteoblastic differentiation during endochondral bone formation.22,23
Due to the importance of Shh signaling in angiogenesis as well as in osteogenic differentiation, this morphogen might be useful as a new therapeutic approach in bone tissue engineering. The purpose of this present study was to investigate Shh signaling in a coculture system consisting of human pOBs and OECs with regard to its influence on angiogenesis or osteogenesis, respectively.
Materials and Methods
Isolation and expansion of OECs
Mononuclear cells were isolated from peripheral blood buffy coats by Ficoll Histopaque density gradients as previously described.24 The mononuclear cell fraction was cultured in endothelial cell growth medium-2 (EGM-2) (CC-3162; Lonza, Verviers, Belgium) with supplements from the kit (5% fetal calf serum [FCS; Gibco Life Technologies, Karlsruhe, Germany] and 1% penicillin/streptomycin [P/S]) on collagen-coated (BD Europe, Heidelberg, Germany) well plates. A total number of 5 × 106 cells per well were seeded on 24-well plates. After 3–4 weeks, colonies with a cobblestone-like morphology appeared. These cells, the so-called late OECs, were collected and expanded over several passages.
Isolation of pOBs
Human pOBs were isolated from human cancellous bone fragments as previously published25,26 from healthy donors in accordance with rules of the local ethics committee. Bone explants were washed with phosphate-buffered saline (PBS) several times, and collagenase type IV (C-5138; Sigma, Deisenhofen, Germany) at a concentration of 1 mg/mL was added for 1 h at 37°C for enzymatic digest. After washing in PBS, bone fractions were placed onto six-well plates cultured in Dulbecco's modified Eagle's medium (DMEM-Ham F12; Gibco) containing 20% FCS and 1% P/S. During culture, cells were fed with DMEM–Ham F12 with 10% FCS. Cells were used from several donors up to the third passage.
Coculture of OECs and pOBs
Cocultures were grown on fibronectin-coated Thermanox coverslips (12 mm in diameter) by seeding pOBs at a density of 300,000/well in a first step. After 24 h 200,000 OECs were added per well and cocultures were further cultured in EGM-2 with supplements from the kit (5% FCS and 1% P/S) for different time periods.
Shh stimulation of cocultures consisting of pOBs and OECs
Cocultures were seeded as previously described. After 1 week of cocultivation, cells were treated with 5 μg/mL recombinant human Shh-N (R&D Systems, Wiesbaden, Germany) in EGM-2 with supplements from the kit (5% FCS and 1% P/S) for various time points as indicated in the text. In control experiments, cocultures were treated with the Shh inhibitor cyclopamine (Merck, Darmstadt, Germany) at different concentrations (5, 10, and 20 μM) in EGM-2 with supplements from the kit (5% FCS and 1% P/S) for 3 weeks. Additionally, cocultures were treated for 24 h simultaneously with 5 μg/mL Shh plus cyclopamine at different concentrations (5, 10, and 20 μM) in EGM-2 with supplements from the kit (5% FCS and 1% P/S).
Immunofluorescent staining
Cultures seeded on Thermanox coverslips were prepared for immunofluorescent staining for the endothelial marker PECAM-1 (CD31). After fixation with 3.7% paraformaldehyde (Merck), cells were washed with PBS and permeabilized using 0.1% Triton-X/PBS. Cells were incubated with primary antibodies: anti-human CD31 (Dako, Hamburg, Germany) diluted 1:50 in 1% bovine serum albumin/PBS for 45 min at room temperature. After washing three times with PBS, cells were incubated with the secondary antibody Alexa 488 anti-mouse (Molecular Probes, MoBiTec, Göttingen, Germany) diluted 1:1000 in 1% bovine serum albumin/PBS for 45 min at room temperature. Finally, cell nuclei were counterstained by Hoechst stain, and cells were mounted with Gelmount (Biomeda, Foster City, CA) and examined using a confocal laser scanning microscope (LeicaTCS-NT, Leica Microsystems, Wetzlar, Germany).
Quantitative real-time reverse transcriptase–polymerase chain reaction
RNA was extracted from Shh-treated and untreated pOB and OEC monocultures as well as from cocultures using RNeasy Kit (Qiagen, Hilden, Germany). One microgram of extracted RNA was used to transcribe RNA into cDNA according to a standard protocol using Omniscript RT kit (Qiagen). Quantitative real-time polymerase chain reaction (PCR; 7300 Real Time PCR System; Applied Biosystems, Applera Deutschland GmbH, Darmstadt, Germany) was performed in triplicate with the following cycler program: 95°C for 15 min, 94°C for 15 s, 55°C for 30 s, 72°C for 35 s, 95°C for 15 s, 60°C for 1 min, and 95°C for 15 s, 40 cycles. Four nanograms of cDNA was used for one reaction. Then, real-time primers were used: VEGF A (VEGFA), angiopoietin-1 (Ang-1), angiopoietin-2 (Ang-2), Ptch1, von Willebrand factor (vWF), osteocalcin, osteonectin, and alkaline phosphatase (ALP) (all primers were ordered from Qiagen). CD31 was synthetisized and purchased from Microsynth (Belgach, Switzerland): forward 5′- CCGGATCTATGACTCAGGGACCAT-3′; rev 3′GGATGGCCTCTTTCTTGTCCAG-5′. Ribosomal protein 13A was used as endogenous standard. Gene expression of Shh-stimulated and nonstimulated co- and monocultures was compared by setting control cultures to 1 (reference value) as indicated in the relevant figures.
Enzyme-linked immunosorbent assay
Culture supernatants from Shh-treated pOB monocultures and OEC monocultures as well as from Shh-treated cocultures were collected 24 h and 14 days after stimulation. The concentration of VEGF, Ang-1, and Ang-2 was measured and compared with that of untreated cells using enzyme-linked immunosorbent assay (ELISA) DuoSets® (R&D Systems). ELISA was performed according to the manufacturer's protocol in triplicates. A streptavidin–horseradish peroxidase colorimetric reaction was used to observe protein concentrations. The optical density of each well was measured using a microplate reader (GENios plus, TECAN, Crailsheim, Germany) and a wavelength of 450 nm.
Quantification of mineralization using alizarin red
To quantify the mineralization of Shh-treated pOB monocultures or cocultures compared with nontreated controls, an Osteogenesis Quantitation Kit (Chemicon® International, Hofheim, Germany) was used according to the manufacturer's protocol. Quantitative analysis of alizarin red staining was performed by the extraction of the stain, and concentrations were measured in triplicate at OD405 using a microplate reader (TECAN; GENios plus). Alizarin red concentration was defined as μM alizarin red/mg protein. A bicinchoninic acid protein Assay Reagent Kit (Pierce, Thermo Fischer, Bonn, Germany) was used to determine the protein concentration according to the manufacturer's protocol.
Determination of ALP activity within the cell lysate
Quantitative determination of ALP activity within the cell lysates of Shh-stimulated and unstimulated cocultures and pOB monocultures was performed using p-nitrophenyl phosphate (Sigma Aldrich, Steinbach, Germany). Briefly, cells were lysed with 0.1% Triton X-100 in 0.1 M Tris buffer (pH 7.2), harvested with a cell scraper, and incubated at room temperature for 45 min while vortexing every 15 min. Twenty microliters of each sample was incubated with 40 μL of substrate solution (0.2% p-nitrophenyl phosphate in 1 M diethanolamine HCl) in triplicates in a 96-well plate for 30 min at 37°C. After incubation period, 80 μL of stop solution (2 M NaOH/0.2 mM EDTA) was added to each well, and absorbance was measured at 405 nm using a microplate reader (TECAN; GENios plus). ALP activity was standardized to the protein content and results were depicted as mM pNP/mg protein.
Determination of angiogenesis-related proteins in response to Shh stimulation
Cells from three different donors were pooled in equal ratios and lysed for protein extraction. The total protein concentration of Shh-stimulated and unstimulated cocultures and monocultures was determined as described above and measured at 550 nm using a microplate reader (TECAN; GENios plus). Subsequently, a Proteome Profiler™ Human Angiogenesis Array study (R&D Systems) was performed according to the manufacturer's protocol to detect angiogenesis-related proteins (55 different proteins in total) within the treated and nontreated cultures. Samples were analyzed using Array-Pro Analyzer Version 4.5 (Media Cybernetics, Bethesda, MD). The protein content of Shh-stimulated and nonstimulated cocultures and monocultures was compared by setting control cultures to 100% (reference value).
Image quantification
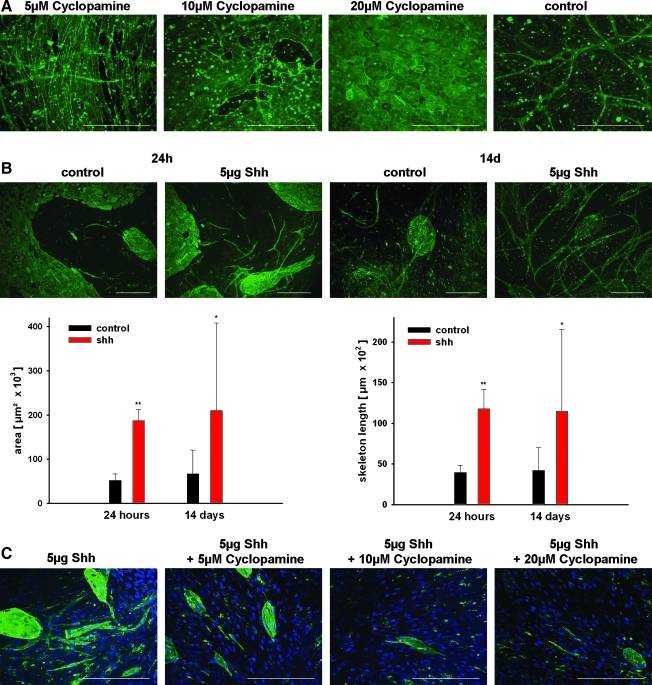
Microscopic images were analyzed using the software ImageJ 1.4327 as described previously.28 Statistical analysis was performed with MS-Excel (Student's t-test, paired, two-tailed distribution), and significant differences of means are indicated in Figure 1B (*p < 0.05 and **p < 0.01).
FIG. 1.
Effect of Shh treatment on cocultures consisting of pOBs and OECs. (A) Immunofluorescent staining for CD31 after treatment of cocultures consisting of OECs and pOBs with cyclopamine in different concentrations (5, 10, and 20 μM) for 3 weeks compared with a nontreated coculture (n = 6). (B) Cells were cocultured 1 week before stimulation with 5 μg/mL recombinant Shh for 24 h (n = 6) and 14 days (n = 6) as described previously and stained immunohistochemical for the endothelial marker CD31. The area and the total skeleton length of vascular structures formed by stimulated and nonstimulated cocultures (n = 3) after 24 h and 14 days were assessed quantitatively (diagrams lower row). Significance was assessed by p-value *p < 0.05 and **p < 0.01. (C) Twenty-four-hour treatment of cocultures simultaneously with 5 μg Shh and cyclopamine in different concentrations (5–20 μM) stained for CD31. Cell nuclei were counterstained with Hoechst. Scale bars (A–C): 300 μm. OECs, outgrowth endothelial cells; pOBs, primary osteoblasts; Shh, sonic hedgehog.
Statistical analysis
All data are presented as mean values ± standard error of the mean. Statistical significance was evaluated using the paired Student's t-test. This test was chosen to compare treated and untreated samples or samples at different time points from the same individuals. Statistical analyses were performed with Excel (Microsoft Office; Microsoft, München, Germany), and significance was assessed by p-value < 0.03 or < 0.05, respectively.
Results
Shh enhances the formation of capillary-like structures in a coculture system of pOBs and OECs
Cocultures of OECs and pOBs under control conditions are characterized by an abundant appearance of microvessel-like structures that seem to increase with progressing culture (Fig. 1A). In our initial experiments this vessel formation in cocultures was blocked by the incubation with the Shh inhibitor cyclopamine in a concentration-dependent manner, as indicated in Figure 1A. Cocultures treated with 10 or 20 μM cyclopamine showed practically no formation of angiogenic structures. On the other hand, stimulation of cocultures with 5 μg recombinant Shh resulted in a considerable increase in the formation of vessel-like structures compared with control cells cultured in EGM-2 (Fig. 1B). The increase in angiogenic structures in the coculture in response to Shh was already observed after 24 h, as indicated by the formation of tube-like structures and interconnected networks (Fig. 1B, upper row). Quantitative analysis of angiogenic structures in Shh-treated cocultures documented a significantly enhanced formation of angiogenic structures after 24 h and 14 days compared with untreated controls (Fig. 1B, lower row). The effect of the angiogenic stimulation by Shh was reduced when cocultures were incubated simultaneously for 24 h with Shh and cyclopamine, depending on the cyclopamine concentration (Fig. 1C). In control experiments using OEC monocultures seeded on Matrigel®, Shh also stimulated the formation of angiogenic structures in OEC monocultures (data not shown).
Analysis of angiogenic factors in response to Shh on the RNA and protein level
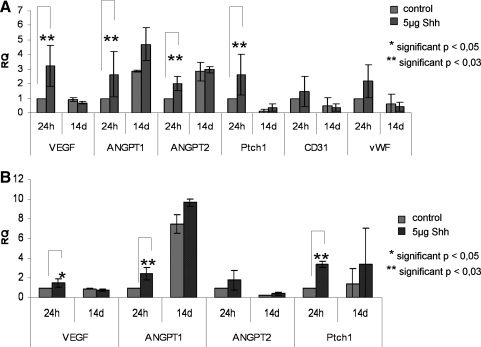
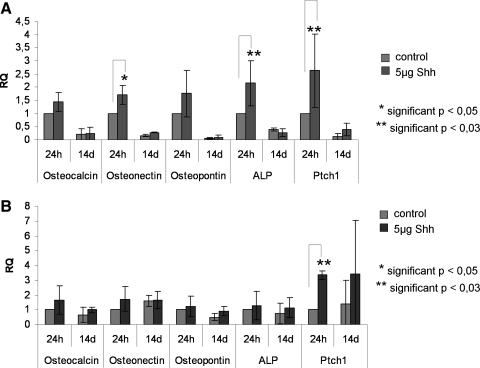
We investigated several proangiogenic molecules on the RNA and on the protein level in the cocultures in response to Shh stimulation. In addition, we studied Shh-stimulated or unstimulated monocultures and used indirect cocultures on Transwell® systems (Supplemental Fig. S1, available online at www.liebertonline.com/ten) to gain insight into the potential origin of the growth factors. In quantitative real-time PCR, expression of VEGF, Ang-1, Ang-2, and the hedgehog receptor Ptch1, used as control to document hedgehog pathway activation, was significantly upregulated in Shh-treated cocultures after 24 h of stimulation (p < 0.03), as depicted in Figure 2A. In addition, relative gene expression of the endothelial markers, CD31 and vWF, seems to increase slightly after 24 h of stimulation. During long-term stimulation with Shh for 14 days, VEGF expression was downregulated compared with 24 h of treatment, whereas Ang-1 or Ang-2 expression, respectively, was not significantly changed after 14 days (Fig. 2A).
FIG. 2.
Shh treatment of cocultures (A) and pOB monocultures (B) results in an upregulation of proangiogenic factors at the mRNA level. (A) Mean values of relative gene expression of the proangiogenic factors VEGF, Angiopoietin1 (ANGPT1), and Angiopoietin2 (ANGPT2), the Shh receptor Ptch1 and the endothelial markers CD31 and in Shh-treated and untreated cocultures for 24 h (n = 6) and 14 days (n = 3). (B) Mean values of relative gene expression of VEGF, Angiopoietin1, Angiopoietin2, and Ptch1 in Shh-treated and untreated pOB monocultures for 24 h (n = 6) and 14 days (n = 3). RPL13A was taken as endogenous standard in both experiments (A, B). Gene expression of Shh-stimulated and nonstimulated cocultures and monocultures was compared by setting control cultures to 1 (reference value). Ptch1, Patched1; RPL13A, ribosomal protein 13A; VEGF, vascular endothelial growth factor; vWF, von Willebrand factor.
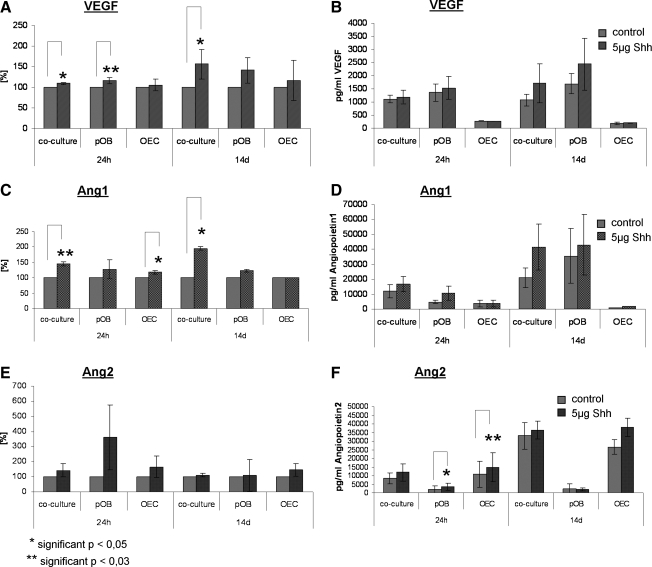
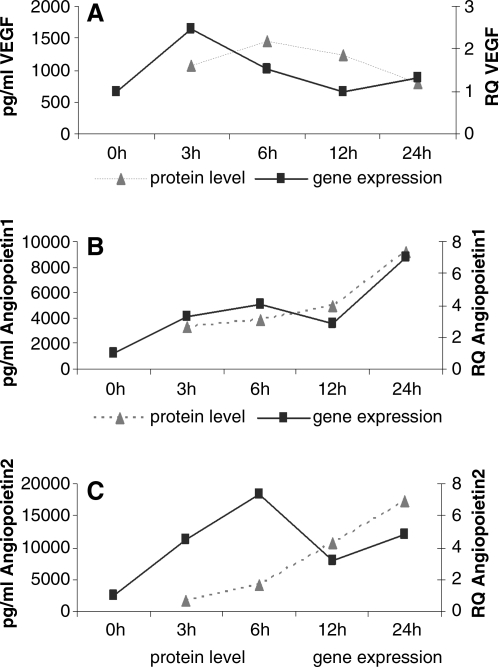
Similar findings for the cocultures were observed on the protein level after determining the free VEGF levels in the cell culture supernatants by ELISA. Results from ELISA for the angiogenic factors VEGF, Ang-1, and Ang-2 are depicted as percentage change compared with untreated cultures (Fig. 3A, C, E), as well as absolute concentrations (Fig. 3B, D, F) to document differences in mono- and cocultures or culture time-dependent changes. The temporal relation of gene expression and protein concentrations found in the cell culture supernatants of Shh-treated cocultures is also depicted in Figure 4 for the first 24 h and documented the induction of VEGF, Ang-1, and Ang-2 release timely delayed to increased expression of the related genes. The release of VEGF (Fig. 3A) was significantly increased in Shh-treated cocultures after 24 h and after 14 days of stimulation (p < 0.05). In addition, Ang-1 concentrations (Fig. 3C) were significantly higher in Shh-stimulated cocultures than in controls after 24 h (p < 0.03) and 14 days of stimulation (p < 0.05). Cocultures of pOBs and OECs as well as pOBs in monocultures showed a higher concentration of Ang-1 in the supernatant after 14 days compared with 24 h (Fig. 3D). The release of Ang-2 in the cocultures was higher in response to Shh treatment compared with nonstimulated controls after 24 h and 14 days of treatment (Fig. 3F).
FIG. 3.
Effect of Shh treatment on the release of proangiogenic factors in cocultures, pOB monocultures, and OEC monocultures. The concentrations of VEGF (A, B), Angiopoietin1 (C, D), and Angiopoietin2 (E, F) in the supernatants of pOBs, OECs, and cocultures consisting of both cell types were measured in an enzyme-linked immunosorbent assay after 24 h and after 14 days of stimulation. Results are demonstrated in percentage ratio (control = 100%; A, C, E) and additionally shown as absolute values (B, D, F). *p < 0.05 and **p < 0.03.
FIG. 4.
Time course of gene and protein expression in response to Shh. The effect of Shh on expression of VEGF (A), Angiopoietin1 (B), and Angiopoietin2 (C) at the mRNA level as well as the protein amount of these molecules in the supernatants of cocultures (n = 3) were determined after 3, 6, 12, and 24 h of Shh stimulation. Gene expression was compared by setting control cultures to 1 (reference value). RPL13A was taken as endogenous standard. Protein release is shown as absolute values (pg/mL).
In pOB monocultures we observed similar effects of Shh treatment on VEGF expression, comparable to the results of the cocultures. In real-time PCR VEGF expression was significantly upregulated in Shh-treated pOBs after 24 h (p < 0.05), followed by downregulation of VEGF expression after 14 days (Fig. 2B) of Shh treatment. On the protein level, monocultures of pOBs showed a significant increase of free VEGF in the supernatant in response to Shh treatment for 24 h (p < 0.03) (Fig. 3A). At the same time expression of Ang-1 and Ptch1 in real-time PCR was significantly higher in Shh-stimulated pOBs after 24 h of treatment (p < 0.03), whereas no significant effect on expression of Ang-2 was observed (Fig. 2B). On the protein level the release of Ang-2 (Fig. 3E, F) was increased in pOB and OEC monocultures already 24 h after stimulation with Shh (p < 0.05; p < 0.03).
In general, the concentration of free VEGF in the supernatants of pOB monocultures was higher compared with OEC and cocultures (Fig. 3B). These observations suggest that VEGF originates mainly from pOBs and are in accordance with experimental data from indirect cocultures on Transwell filter systems that show a secretion of VEGF by osteoblasts but not by OECs (Supplemental Fig. S1). We assumed that in the coculture, free VEGF concentrations might be lower compared with the corresponding monocultures of pOBs due to binding of VEGF by endothelial cells or matrix components. Thus, we also performed Proteome Profiler Human Angiogenesis studies to detect surface bound or intracellular protein levels of VEGF as well as other angiogenic factors in response to Shh treatment. Three different donors of cocultures of pOBs and OECs as well as pOB monocultures and OEC monocultures were stimulated with Shh for 24 h, as previously described. The Shh-stimulated cocultures showed an enhanced VEGF protein content (+23.7%) in cell lysates compared with nonstimulated cocultures (Fig. 5A), whereas Shh-treated pOB monocultures and OEC monocultures exhibited decreased levels of VEGF (Fig. 5A) in the lysate. Shh treatment of pOBs in monoculture resulted in lower amounts of VEGF, Ang-1 (Fig. 5B), and Ang-2 (Fig. 5C) compared with unstimulated pOBs in the cell lysates, but higher amount of free VEGF, Ang-1, and Ang-2 released to the culture supernatant (Fig. 3). This suggests that Shh leads to the secretion of growth factors to the cell culture medium.
FIG. 5.
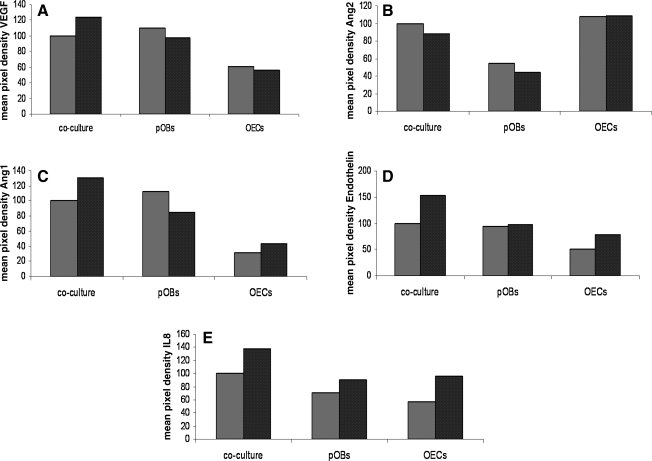
Determination of the intracellular protein level of proangiogenic factors in cocultures, pOB monocultures, and OEC monocultures in response to Shh stimulation (black bars) compared to untreated controls (grey bars). The amount of VEGF (A), Angiopoietin1 (B), Angiopoietin2 (C), endothelin (D), and IL-8 (E) in the cell lysates of cocultures, pOB monocultures, and OEC monocultures was evaluated using a Proteome Profiler Human Angiogenesis Array (n = 3). Results are referred to a positive control and exhibited as mean pixel densities, which were finally converted into percentage values. All individual values were referred to control coculture set to 100%.
Protein profiler arrays also indicated an influence of Shh on other molecules involved in the cellular crosstalk during angiogenic activation and vessel stabilization. Endothelin protein amounts in cell lysates were found to be highly enriched in response to Shh in OEC monocultures and in cocultures. In addition, IL-8 protein synthesis was increased in all types of cultures by stimulation with Shh (Fig. 5).
Shh treatment results in an upregulation of osteogenic markers at the mRNA level
To assess the effect of Shh treatment on osteogenic differentiation, quantitative real-time PCR detecting expression of several molecules involved in osteogenic differentiation was performed for cocultures (Fig. 6A), as well as for pOB (Fig. 6B) and OEC monocultures (data not shown) serving as controls. After 24 h of Shh stimulation, cocultures as well as pOB monocultures showed a tentative upregulation of the osteogenic markers osteocalcin, osteonectin, osteopontin, and ALP, whereas statistical significance was only documented for the upregulation of osteonectin (p < 0.05) and ALP (p < 0.03) in cocultures after 24 h of treatment (Fig. 6A). Nevertheless, Shh stimulation for 14 days resulted in a decrease in expression of osteogenic markers compared with 24 h in cocultures (Fig. 6A) as well as in pOB monocultures (Fig. 6B).
FIG. 6.
Shh treatment of cocultures (A) and pOB monocultures (B) results in an upregulation of osteogenic factors at the mRNA level. (A) Mean values of relative gene expression of different genes involved in osteogenesis in Shh-treated and untreated cocultures for 24 h (n = 6) and 14 days (n = 3). (B) Mean values of relative gene expression of different genes involved in osteogenesis in Shh-treated and untreated pOB monocultures for 24 h (n = 6) and 14 days (n = 3). RPL13A was taken as endogenous standard in both experiments (A, B). Gene expression of Shh-stimulated and nonstimulated co- and monocultures was compared by setting control cultures to 1 (reference value).
Shh stimulation promotes osteoblastic differentiation in the coculture of pOBs and OECs
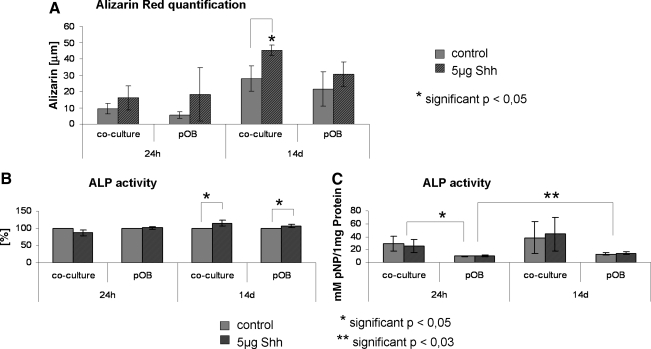
The potential effect of Shh on osteoblastic differentiation as indicated by real-time PCR was further investigated by analyzing the effect of Shh on the mineralization of monocultures and cocultures after 24 h, 7 days (data not shown), and 14 days using alizarin red staining and quantification (Fig. 7A). In general, mineralization was consistently higher in Shh-treated cocultures and pOB monocultures compared with nontreated cultures at all time points of investigation (24 h, 7 days, and 14 days), as indicated by alizarin assays and depicted in μM alizarin red/mg protein. The mineralization of the culture increased continuously during the course of Shh stimulation from 24 h to 7 days (data not shown) then to 14 days in cocultures and pOB monocultures and was found to be statistically significant in the coculture after 14 days of Shh treatment (p < 0.05; Fig. 7A).
FIG. 7.
Effect of Shh on osteoblastic differentiation in the coculture system consisting of pOBs and OECs. (A) Quantification of calcification in Shh-treated cocultures and pOB monocultures after 24 h (n = 6) and 14 days (n = 3) of Shh stimulation compared with unstimulated cultures evaluated by quantitative analyses of alizarin red staining. Values represent averages and alizarin red concentration is defined as μM alizarin red/mg protein. (B, C) ALP activity within the cell lysate in Shh-treated cocultures and pOB monocultures compared with untreated cultures after 24 h (n = 3) and 14 days (n = 3) of stimulation. Results are represented in averages. The standardized ALP activity is defined as mM pNP/mg protein and is depicted in (C). (B) The results in percentage to control = 100%. ALP, alkaline phosphatase.
This positive effect of Shh on osteogenic markers was further confirmed by comparing ALP activity in the stimulated and unstimulated cocultures or pOB monocultures (Fig. 7B, C) investigated after 24 h, 7 days (data not shown), and 14 days. ALP activity is depicted as percentage change compared with controls (Fig. 7B), as well as in standardized concentrations given as mM/mg protein to identify influences by mono- or coculture or culture time, respectively (Fig. 7C). ALP activity was significantly increased in Shh-treated cocultures and pOB monocultures during 14 days of stimulation compared with the corresponding controls (Fig. 7B, p < 0.05). In general, the cocultures showed a higher ALP activity than the pOB monoculture after 24 h as well as after 14 days (p < 0.05) (Fig. 7C).
Discussion
New approaches simultaneously enhancing the two fundamental processes in bone regeneration, namely, osteogenesis and angiogenesis, could be of considerable therapeutic potential. One of the most promising molecular targets for therapeutic intervention is the Shh signaling pathway due to its pivotal role in the initiation of angiogenesis and osteogenic differentiation. Using a coculture system based on human OECs isolated from the peripheral blood and pOBs, we demonstrated that the formation of microvessel-like structures in vitro depends on the functional activity of the Shh signaling pathway. In addition, we provide experimental evidence for a beneficial influence of Shh treatment on the formation of microvessel-like structures, as well as on osteogenic differentiation in the coculture model.
The effect of angiogenic activation of OECs by coculturing with pOBs was the focus of our previous studies.8,25,28 These studies implied a proangiogenic effect on OECs by the coculture with pOBs leading to the formation of luminal vascular structures in vitro and perfused microvessels in vivo connected to the host's vasculature. In this study, treatment with the Shh inhibitor cyclopamine reduced the formation of vascular structures by OECs in the coculture system, indicating that the Shh pathway might be involved in the angiogenic activation of OECs in the coculture system.
The stimulation of the angiogenic activity of OECs in the coculture system might be due to direct effects of Shh on OECs, as previously reported for other endothelial cell types29 or endothelial progenitor cells.30 A direct effect of Shh on the angiogenic activity of OECs was clearly observed in our control experiments using OEC monocultures seeded on Matrigel. In these controls Shh induced capillary-like structures in OECs. Similar observations were reported in the literature and seem to correlate with upregulation of molecules such as ICAM-1 and RhoA or the activation of Focal adhesion kinase31,32 involved in the migratory activity of angiogenic activated endothelial cells. A direct influence of Shh was documented in our study by the upregulation of Ptch1 in response to Shh treatment in OECs as well as in osteoblasts and their cocultures, thus indicating the activation of Shh signaling pathway in all investigated cell types. On the other hand, treatment of cocultures with cyclopamine reduced the formation of angiogenic structures by OECs and the simultaneous treatment with Shh and its inhibitor cyplopamine reduced the angiogenic effect in cocultures in a concentration-dependent manner.
Direct effects of Shh on the angiogenic activity might also be present in the Shh-treated coculture systems, but it is difficult to distinguish between direct and indirect mechanisms in the coculture system. Nevertheless, there are several indications that the angiogenic activation of OECs in this study is at least partly mediated by indirect paracrine mechanisms in the cocultures. In this study Shh exerts positive effects on expression and secretion of proangiogenic factors such as VEGF and angiopoietins in the coculture system. These observations were proven on the mRNA level using quantitative real-time PCR and on the protein level by ELISA and protein array studies. By comparing the effects of Shh treatment on monocultures and cocultures utilizing ELISA and protein arrays, we assume that Shh treatment results in the increased secretion of VEGF by pOBs, which in turn might be responsible for the activation of endothelial cells. In addition, increased levels of Ang-1 in the cocultures after Shh treatment seem to originate from the pOBs. These observations are in accordance with reports from the literature documenting increased levels of VEGF and Ang-1 in Shh-treated mesenchymal cells21,33 and expression of Ang-134–36 and VEGF in osteoblasts.35 The upregulation of Ang-2 gene expression or its increased release into cell culture supernatant is probably mediated by OECs in the cocultures in accordance with the origin of Ang-2 from endothelial cells, as reported in the literature.37 Additional experiments using indirect cocultures of pOBs and OECs on Transwell filter systems analyzed by ELISA further confirmed the origin of VEGF and Ang-1 as products from pOBs and Ang-2 as product from OECs (Supplemental Fig. S1).
VEGF and angiopoietins cooperatively orchestrate the individual steps during vessel formation and stabilization. VEGF induces angiogenic activation of endothelial cells, which is facilitated in the presence of Ang-2, leading to the destabilization of cell–cell and cell–matrix interactions in endothelial cells.38,39 On the other hand, successful vascularization and formation of functional vessels depends on stabilization of vascular structures. This vessel stabilization is promoted by Ang-1 through recruitment of smooth muscle cells and pericytes40,41 often summarized as mural cells. In accordance with the temporal increase in vascular structures with culture time as reported previously and indicated in the present study, VEGF, Ang-1, and Ang-2 levels increased with progressing culture time and were further enhanced by Shh treatment. Although the coculture process itself is beneficial for the angiogenic process leading to angiogenic structures, Shh might be an interesting therapeutic option to further accelerate the formation of capillary structures in coculture systems leading to an effect on the angiogenic activation of OECs already after 24 h of treatment. This might be of particular importance for a fast connection to the vascular supply of the host after the implantation of a tissue-engineered construct. Further, therapeutic application of Shh might contribute to the reduction of the preculture time of prevascularized tissue constructs in vitro. Shh treatment provides several advantages in comparison to stimulation with other angiogenic factors by exerting effects on multiple factor families that influence both angiogenesis and vessel stabilization. Besides its effects on VEGF and angiopoietins, Shh enhanced expression of other angiogenic factors and cytokines in the cocultures, such as endothelin-1 and IL-8, as shown by our protein arrays data. IL-8 is known to play an important role in endothelial cell survival42 and exerts pro-angiogenic effects on endothelial cells,43 whereas endothelin-1 is involved in the control of the cellular cross talk of endothelial cells and osteoblasts controlling the levels of VEGF.44 Further, endothelin is thought to be involved in the differentiation of osteoprogenitors.45
In addition to the effects of Shh on the angiogenic potential of endothelial cells in the coculture, Shh further enhanced multiple key features involved in osteogenic differentiation, suggesting a synergistic effect of Shh on both processes, namely, angiogenesis and osteogenic differentiation. Positive influence of Shh on gene expression of osteogenic markers such as ALP, osteonectin, osteopontin, and osteocalcin1,46–48 was observed in real-time PCR. The assessment of calcification in the cocultures using alizarin red and the quantification of ALP activity further supported the improvement of osteogenic differentiation as a result of Shh treatment.
The stimulatory action of Shh on osteogenic differentiation was already reported in previous studies,22,49 suggesting a close interaction of Shh with BMP-250 or with parathyroid-hormone-related peptide.51 As discussed above we also observed increased levels of Ang-1 and VEGF in response to Shh treatment enhancing the vascularization process. Improving the vascularization process in bone tissues in turn enhances bone formation in vivo as reported by several studies from the literature.52–54
Further, the coculture procedure of OECs and osteogenic cells itself exerts a positive effect on ALP activity compared with monocultures of pOBs and is in agreement with recent reports.55 Collectively with results from previous studies, the present data suggest several advantages of cocultures for bone tissue engineering applications.
In summary, new concepts to stimulate and control bone regeneration or to develop constructs for bone replacement are needed that take into consideration both osteogenesis and angiogenesis. This is of particular importance in terms of their potential clinical application. Coculture techniques in combination with additional treatment with growth factors or morphogens represent a promising approach with several advantages with respect to the formation of a stable vasculature and osteogenic differentiation. The present study highlighted the potential of Shh to stimulate both processes in a human coculture model of OECs and pOBs.
Supplementary Material
Acknowledgments
The authors would like to thank B. Pavic for her excellent technical assistance. This work was financially supported by grants from the European commission (EXPERTISSUES Contract number 500283-2) and BMBF, for German-Chinese Young Investigator Group (grant number 0315033).
Disclosure Statement
No competing financial interests exist.
References
- 1.Au P. Tam J. Fukumura D. Jain R.K. Bone marrow derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111:4551. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levenberg S. Rouwkema J. Macdonald M. Garfein E.S. Kohane D.S. Darland D.C. Marini R. van Bitterswijk C.A. Mulligan R.C. D'Amore P.A. Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 3.Sanz L. Santos-Valle P. Alonso-Camino V. Salas C. Serrano A. Vicario J.L. Cuesta A.M. Compte M. Sanchez-Martin D. Alvarez-Vallina L. Long-term in vivo imaging of human angiogenesis: critical role of bone marrow-derived mesenchymal stem cells for the generation of durable blood vessels. Microvasc Res. 2008;75:308. doi: 10.1016/j.mvr.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Gulati R. Jevremovic D. Peterson T.E. Chatterjee S. Shah V. Vile R.G. Simari R.D. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93:1023. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 5.Hur J. Yoon C.H. Kim H.S. Choi J.H. Kang H.J. Hwang K.K. Oh B.H. Lee M.M. Park Y.B. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 6.Silva E.A. Kim E.-S. Kong H.J. Mooney D.J. Material-based deployment enhances efficacy of endothelial progenitor cells. Proc Natl Acad Sci U S A. 2008;105:14347. doi: 10.1073/pnas.0803873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melero-Martin J.M. Khan Z.A. Picard A. Wu X. Paruchuri S. Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 8.Fuchs S. Ghanaati S. Orth C. Barbeck M. Kolbe M. Hofmann A. Eblenkamp M. Gomes M. Reis R.L. Kirkpatrick C.J. Contribution of outgrowth endothelial cells from human peripheral blood on in vivo vascularization of bone tissue engineered constructs based on starch polycaprolactone scaffolds. Biomaterials. 2009;30:526. doi: 10.1016/j.biomaterials.2008.09.058. [DOI] [PubMed] [Google Scholar]
- 9.Rouwkema J. de Boer J. Van Blitterswijk C.A. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng. 2006;12:2685. doi: 10.1089/ten.2006.12.2685. [DOI] [PubMed] [Google Scholar]
- 10.Elbjeirami W.M. West J.L. Angiogenesis-like activity of endothelial cells co-cultured with VEGF-producing smooth muscle cells. Tissue Eng. 2006;12:381. doi: 10.1089/ten.2006.12.381. [DOI] [PubMed] [Google Scholar]
- 11.Choong C.S. Hutmacher D.W. Triffitt J.T. Co-culture of bone marrow fibroblasts and endothelial cells on modified polycaprolactone substrates for enhanced potentials in bone tissue engineering. Tissue Eng. 2006;12:2521. doi: 10.1089/ten.2006.12.2521. [DOI] [PubMed] [Google Scholar]
- 12.Unger R.E. Sartoris A. Peters K. Motta A. Migliaresi C. Kunkel M. Bulnheim U. Rychly J. Kirkpatrick C.J. Tissue-like self-assembly in cocultures of endothelial cells and osteoblasts and the formation of microcapillary-like structures on three-dimensional porous biomaterials. Biomaterials. 2007;28:3965. doi: 10.1016/j.biomaterials.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 13.Guillotin B. Bareille R. Bourget C. Bordenave L. Amedee J. Interaction between human umbilical vein endothelial cells and human osteoprogenitors triggers pleiotropic effect that may support osteoblastic function. Bone. 2008;42:1080. doi: 10.1016/j.bone.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Villars F. Guillotin B. Amedee T. Dutoya S. Bordenave L. Bareille R. Amedee J. Effect of HUVEC on human osteoprogenitor cell differentiation needs heterotypic gap junction communication. Am J Physiol Cell Physiol. 2002;282:C775. doi: 10.1152/ajpcell.00310.2001. [DOI] [PubMed] [Google Scholar]
- 15.Nagase T. Nagase M. Machida M. Yamagishi M. Hedgehog signaling: a biophysical or biomechanical modulator in embryonic development? Ann N Y Acad Sci. 2007;1101:412. doi: 10.1196/annals.1389.029. [DOI] [PubMed] [Google Scholar]
- 16.Nagase T. Nagase M. Yoshimura K. Machida M. Yamagishi M. Defects in aortic fusion and craniofacial vasculature in the holoprosencephalic mouse embryo under inhibition of sonic hedgehog signaling. J Craniofac Surg. 2006;17:736. doi: 10.1097/00001665-200607000-00026. [DOI] [PubMed] [Google Scholar]
- 17.Heine P. Dohle E. Schulte D. Sonic hedgehog signaling in the chick retina accelerates Meis2 downregulation simultaneously with retinal ganglion cell genesis. Neuroreport. 2009;20:279. doi: 10.1097/WNR.0b013e32832000ae. [DOI] [PubMed] [Google Scholar]
- 18.Fietz M.J. Concordet J.P. Barbosa R. Johnson R. Krauss S. McMahon A.P. Tabin C. Ingham P.W. The hedgehog gene family in Drosophila and vertebrate development. Dev Suppl. 1994;43:43. [PubMed] [Google Scholar]
- 19.Lawson N.D. Vogel A.M. Weinstein B.M. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- 20.Straface G. Aprahamian T. Flex A. Gaetani E. Biscetti F. Smith R.C. Pecorini G. Pola E. Angelini F. Stigliano F. Castellot J.J., Jr. Losordo D.W. Pola R. Sonic hedgehog regulates angiogenesis and myogenesis during post-natal skeletal muscle regeneration. J Cell Mol Med. 2008;13:2424. doi: 10.1111/j.1582-4934.2008.00440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pola R. Ling L.E. Silver M. Corbley M.J. Kearney M. Pepinsky R.B. Shapiro R. Taylor F.R. Baker D.P. Asahara T. Isner J.M. The morphogen sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 22.van der Horst G. Farih-Sips H. Lowik C.W. Karperien M. Hedgehog stimulates only osteoblastic differentiation of undifferentiated KS483 cells. Bone. 2003;33:899. doi: 10.1016/j.bone.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura T. Aikawa T. Iwamoto-Enomoto M. Iwamoto M. Higuchi Y. Pacifici M. Kinto N. Yamaguchi A. Noji S. Kurisu K. Matsuya T. Induction of osteogenic differentiation by hedgehog proteins. Biochem Biophys Res Commun. 1997;237:465. doi: 10.1006/bbrc.1997.7156. [DOI] [PubMed] [Google Scholar]
- 24.Fuchs S. Hermanns M. Kirkpatrick C. Retention of a differentiated endothelial phenotype by outgrowth endothelial cells isolated from human peripheral blood and expanded in long-term cultures. Cell Tissue Res. 2006;1:79. doi: 10.1007/s00441-006-0222-4. [DOI] [PubMed] [Google Scholar]
- 25.Fuchs S. Hofmann A. Kirkpatrick C.J. Microvessel-like structures from outgrowth endothelial cells from human peripheral blood in 2-dimensional and 3-dimensional co-cultures with osteoblastic lineage cells. Tissue Eng. 2007;13:2577. doi: 10.1089/ten.2007.0022. [DOI] [PubMed] [Google Scholar]
- 26.Hofmann A. Konrad L. Gotzen L. Printz H. Ramaswamy A. Hofmann C. Bioengineered human bone tissue using autogenous osteoblasts cultured on different biomatrices. J Biomed Mater Res A. 2003;67:191. doi: 10.1002/jbm.a.10594. [DOI] [PubMed] [Google Scholar]
- 27.Rasband W.S. ImageJ. 1997–2007. http://rsb.info.nih.gov/ij/ http://rsb.info.nih.gov/ij/ Cited 2008.
- 28.Fuchs S. Jiang X. Schmidt H. Dohle E. Ghanaati S. Orth C. Hofmann A. Motta A. Migliaresi C. Kirkpatrick C.J. Dynamic processes involved in the pre-vascularization of silk fibroin constructs for bone regeneration using outgrowth endothelial cells. Biomaterials. 2009;30:1329. doi: 10.1016/j.biomaterials.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 29.Soleti R. Benameur T. Porro C. Panaro M.A. Andriantsitohaina R. Martinez M.C. Microparticles harboring sonic hedgehog promote angiogenesis through the upregulation of adhesion proteins and proangiogenic factors. Carcinogenesis. 2009;30:580. doi: 10.1093/carcin/bgp030. [DOI] [PubMed] [Google Scholar]
- 30.Madoka Y. Kazumasa N. Yusuke M. Masaaki I. Junpei S. Yoshiaki S. Tomoya N. Yasuhiro N. Noboyuki Y. Kazuya S. Atsuo M. Satoshi T. Toshikatsu O. Hidenori K. Toru K. Mikihiro K. Toshifumi A. Daniel C.C. Yutaka K. Sonic hedgehog derived from human pancreatic cancer cells augments angiogenic function of endothelial progenitor cells. Cancer Sci. 2008;99:1131. doi: 10.1111/j.1349-7006.2008.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soleti R. Benameur T. Porro C. Panaro M.A. Andriantsitohaina R. Martinez M.C. Microparticles harboring sonic hedgehog promote angiogenesis through the upregulation of adhesion proteins and proangiogenic factors. Carcinogenesis. 2009;30:580. doi: 10.1093/carcin/bgp030. [DOI] [PubMed] [Google Scholar]
- 32.Soleti R. Martinez M.C. Microparticles harbouring sonic hedgehog: role in angiogenesis regulation. Cell Adh Migr. 2009;3:293. doi: 10.4161/cam.3.3.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Asai J. Takenaka H. Kusano K.F. Ii M. Luedemann C. Curry C. Eaton E. Iwakura A. Tsutsumi Y. Hamada S. Thorne T. Kishore R. Losordo D.W. Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation. 2006;113:2413. doi: 10.1161/CIRCULATIONAHA.105.603167. [DOI] [PubMed] [Google Scholar]
- 34.Kasama T. Isozaki T. Odai T. Matsunawa M. Wakabayashi K. Takeuchi H.T. Matsukura S. Adachi M. Tezuka M. Kobayashi K. Expression of angiopoietin-1 in osteoblasts and its inhibition by tumor necrosis factor-alpha and interferon-gamma. Transl Res. 2007;149:265. doi: 10.1016/j.trsl.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Horner A. Bord S. Kelsall A.W. Coleman N. Compston J.E. Tie2 ligands angiopoietin-1 and angiopoietin-2 are coexpressed with vascular endothelial cell growth factor in growing human bone. Bone. 2001;28:65. doi: 10.1016/s8756-3282(00)00422-1. [DOI] [PubMed] [Google Scholar]
- 36.Park J.H. Song H.I. Rho J.M. Kim M.R. Kim J.R. Park B.H. Park T.S. Baek H.S. Parathyroid hormone (1–34) augments angiopoietin-1 expression in human osteoblast-like cells. Exp Clin Endocrinol Diabetes. 2006;114:438. doi: 10.1055/s-2006-924400. [DOI] [PubMed] [Google Scholar]
- 37.Thomas M. Augustin H.G. The role of the Angiopoietins in vascular morphogenesis. Angiogenesis. 2009;12:125. doi: 10.1007/s10456-009-9147-3. [DOI] [PubMed] [Google Scholar]
- 38.Asahara T. Chen D. Takahashi T. Fujikawa K. Kearney M. Magner M. Yancopoulos G.D. Isner J.M. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83:233. doi: 10.1161/01.res.83.3.233. [DOI] [PubMed] [Google Scholar]
- 39.Sato T.N. Tozawa Y. Deutsch U. Wolburg-Buchholz K. Fujiwara Y. Gendron-Maguire M. Gridley T. Wolburg H. Risau W. Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 40.Suri C. Jones P.F. Patan S. Bartunkova S. Maisonpierre P.C. Davis S. Sato T.N. Yancopoulos G.D. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 41.Suri C. McClain J. Thurston G. McDonald D.M. Zhou H. Oldmixon E.H. Sato T.N. Yancopoulos G.D. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282:468. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- 42.Li A. Dubey S. Varney M.L. Dave B.J. Singh R.K. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 43.Heidemann J. Ogawa H. Dwinell M.B. Rafiee P. Maaser C. Gockel H.R. Otterson M.F. Ota D.M. Lugering N. Domschke W. Binion D.G. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 44.von Schroeder H.P. Veillette C.J. Payandeh J. Qureshi A. Heersche J.N.M. Endothelin-1 promotes osteoprogenitor proliferation and differentiation in fetal rat calvarial cell cultures. Bone. 2003;33:673. doi: 10.1016/s8756-3282(03)00215-1. [DOI] [PubMed] [Google Scholar]
- 45.Veillette C.J.H. von Schroeder H.P. Endothelin-1 down-regulates the expression of vascular endothelial growth factor-A associated with osteoprogenitor proliferation and differentiation. Bone. 2004;34:288. doi: 10.1016/j.bone.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Tepper O.M. Galiano R.D. Capla J.M. Kalka C. Gagne P.J. Jacobowitz G.R. Levine J.P. Gurtner G.C. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 47.Liu F. Malaval L. Gupta A.K. Aubin J.E. Simultaneous detection of multiple bone-related mRNAs and protein expression during osteoblast differentiation: polymerase chain reaction and immunocytochemical studies at the single cell level. Dev Biol. 1994;166:220. doi: 10.1006/dbio.1994.1309. [DOI] [PubMed] [Google Scholar]
- 48.Aubin J.E. Advances in the osteoblast lineage. Biochem Cell Biol. 1998;76:899. [PubMed] [Google Scholar]
- 49.Nakamura T. Aikawa T. Iwamoto-Enomoto M. Iwamoto M. Higuchi Y. Maurizio P. Kinto N. Yamaguchi A. Noji S. Kurisu K. Matsuya T. Induction of osteogenic differentiation by hedgehog proteins. Biochem Biophys Res Commun. 1997;237:465. doi: 10.1006/bbrc.1997.7156. [DOI] [PubMed] [Google Scholar]
- 50.Yuasa T. Kataoka H. Kinto N. Iwamoto M. Enomoto-Iwamoto M. Iemura S. Ueno N. Shibata Y. Kurosawa H. Yamaguchi A. Sonic hedgehog is involved in osteoblast differentiation by cooperating with BMP-2. J Cell Physiol. 2002;193:225. doi: 10.1002/jcp.10166. [DOI] [PubMed] [Google Scholar]
- 51.Jemtland R. Divieti P. Lee K. Segre G.V. Hedgehog promotes primary osteoblast differentiation and increases PTHrP mRNA expression and iPTHrP secretion. Bone. 2003;32:611. doi: 10.1016/s8756-3282(03)00092-9. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki T. Miyamoto T. Fujita N. Ninomiya K. Iwasaki R. Toyama Y. Suda T. Osteoblast-specific Angiopoietin 1 overexpression increases bone mass. Biochem Biophys Res Commun. 2007;362:1019. doi: 10.1016/j.bbrc.2007.08.099. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y. Wan C. Deng L. Liu X. Cao X. Gilbert S.R., et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerber H.P. Vu T.H. Ryan A.M. Kowalski J. Werb Z. Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 55.Usami K. Mizuno H. Okada K. Narita Y. Aoki M. Kondo T., et al. Composite implantation of mesenchymal stem cells with endothelial progenitor cells enhances tissue-engineered bone formation. J Biomed Mater Res A. 2008;90:730. doi: 10.1002/jbm.a.32142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.